Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although the earliest study of electrical stimulation of the gut was reported more than 40 years ago, the development of the field is slow when compared to cardiac pacing or other areas of neuromodulation including spinal cord stimulation, deep brain stimulation, and motor cortex stimulation. In the earliest study regarding intestinal stimulation, published in , Bilgutay et al. reported the use of intraluminal electrical stimulation via the tip of a nasogastric tube to induce peristalsis and shorten the recovery period from ileus after laparotomy; an increase in gastric contractions as well as gastric emptying was reported in both dogs and humans. However, subsequent randomized controlled studies failed to produce consistent or promising results ( ). Before the 1960s, there was a lack of understanding of gastrointestinal (GI) electrophysiology, which only became a topic of interest in the later 1960s and early 1970s ( ). From 1970s to early 1990s, Kelly and colleagues made significant contributions to our understanding of the electrical stimulation of the gut; numerous studies in both dogs and humans were reported by this group ( ).
Starting from 1990s, more progress has been made on the methodologies, effects, mechanisms, and clinical applications of GI electrical stimulation. Numerous reports are available in the literature on electrical stimulation of various organs of the GI tract, such as the stomach, small intestine, colon, and rectum for the treatment or therapeutic potentials of various conditions such as gastroparesis, short bowel syndrome, intestinal pseudo-obstruction, and fecal incontinence ( ). This chapter will focus on intestinal electrical stimulation (IES). Topics on gastric electrical stimulation can be found in a separate chapter of this book and a number excellent reviews ( ).
The major difference between electrical stimulation of the gut and neuromodulation lies in the target of stimulation. Neuromodulation typically has its target on nerves or nervous systems to change functions of an organ, whereas electrical stimulation of the gut does not necessarily target on specific nerves or nervous systems and may directly change various functions of an organ in the gut, such as neural, hormonal, and mechanical functions. In addition, the stimulation methodologies of IES may be different from other aspects of neuromodulation, mainly because of the following two reasons: (1) the GI organ is composed of smooth muscles. The response of smooth muscles to electrical stimulation is slow, and therefore long pulses (in the order of msec instead of μs) are typically required to alter the function of the organ being stimulated; (2) the GI organ has intrinsic myoelectrical activity and therefore the electrical stimulation of the gut may be designed to enhance or alter this intrinsic myoelectrical activity.
The main function of the stomach is to accommodate ingested food, grind and fix the food, and finally pump it out of the stomach to the small intestine. The accommodation of the ingested food is achieved by relaxation of the proximal stomach via the release of an inhibitory neurotransmitter called nitric oxide, whereas emptying of the stomach is accomplished by sequentially propagated contractions, called peristalsis, from the corpus to the distal antrum.
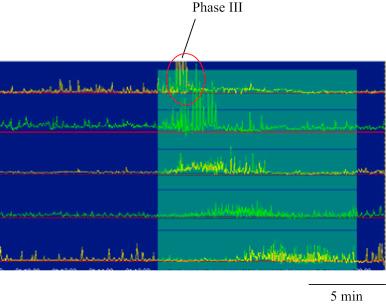
The main function of the small intestine is to absorb various nutrients from the ingested food emptied from the stomach. Intestinal motility is organized in such a way that the organ has sufficient time to absorb needed nutrients from the ingested food and then transport the remaining down to the ileum and the colon. Accordingly, intestinal motility after a meal is characterized by segmental contractions with different propagation directions, including antegrade, retrograde, and simultaneous contractile patterns. The force of postprandial intestinal contractions is moderate, about 50%–70% of the maximum strength. The intestinal motility pattern in the fasting state undergoes cycles of periodic fluctuation divided into three phases, called migrating motor complex (MMC): Phase I (no contractions, 40–60 min), Phase II (intermittent contractions, 20–40 min), and Phase III (regular rhythmic contractions, 2–10 min). Typical gastric and intestinal contractions in the fasting state are shown in Fig. 117.1 . The maximum frequency of the stomach is about 3 cycles/min (cpm) and 12 cpm in the most proximal small intestine, duodenum and 8–9 cpm in the most distal intestine, ileum ( ).
It is important to understand electrophysiology of the small intestine for IES because (1) intestinal motility is regulated by intestinal myoelectrical activity, and (2) to alter the function of the small intestine, electrical stimuli may have to be designed to enhance or inhibit this intrinsic, intestinal myoelectrical activity.
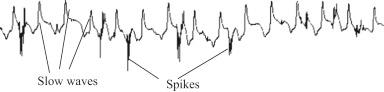
Myoelectric activity of the small intestine is similar to that of the stomach. It consists of two components: pacesetter potentials or slow waves and spike potentials. A typical recording showing intestinal slow waves and spikes are shown in Fig. 117.2 . Small intestinal slow waves originate from a region in the proximal 1 cm of the duodenum and propagate as an annular wave front in an abroad direction ( ). These slow waves determine the frequency and the direction of propagation of intestinal contractions. Spike potentials are superimposed on the slow waves and are electrical counterparts of contractions ( ).
In the dog, the proximal 10%–30% of the small intestine (30–115 cm of duodenum and jejunum) maintains the same slow-wave frequency, 18–20 cpm, in a region called the “frequency plateau” ( ). Aborad 1
1 Aborad: direction away from the mouth.
to this point, there is a diminishing slow-wave frequency gradient along the small bowel to a rate of 14 cpm in the distal ileum ( ). In humans, slow waves in the duodenum and proximal jejunum occur at about 12 cpm with an aborad gradient to about 9 cpm in the terminal ileum ( ). Whether a proximal plateau of identical frequencies is present in the human duodenum and proximal jejunum has not been clearly shown ( ). Transection and reanastomosis of the small bowel decreases the slow-wave frequency in the distal segment in both dogs ( ) and man ( ). In addition, at least in dogs, the propagation of slow waves in the distal segment becomes abnormal, with a high percentage of these slow waves propagating in an orad 2
2 Orad: direction to the mouth.
, rather than an aborad direction ( ).
Studies have indicated that intestinal myoelectrical dysrhythmia is associated with intestinal motor disorders in some clinical settings ( ). It is known that abnormalities in the frequency of intestinal slow waves are associated with intestinal hypomotility and that uncoupled or dysrhythmic intestinal myoelectrical activity leads to a lack of coordinated intestinal contractions or peristalsis.
Intestinal dysmotility is often seen in patients with irritable bowel syndrome (IBS) and patients with chronic, intestinal pseudo-obstruction 3
3 http://en.wikipedia.org/wiki/Pseudo-obstruction Intestinal pseudo-obstruction is the decreased ability of the intestines to push food through, and often causes dilation of various parts of the bowel. It can be a primary condition (idiopathic or inherited from a parent) or caused by another disease (secondary). The clinical and radiological findings are often similar to true intestinal obstruction. Retrieved from wikipedia on January 29, 2008.
. Intestinal dysmotility is featured with altered MMC patterns in the fasting state and hypotensive or hypertensive postprandial contractions. Altered MMC patterns include a complete loss of the fasting MMC pattern, a loss of Phase III and a prolonged Phase II, and abnormal propagation of Phase III activities. Postprandial contractile abnormalities include a continuation of fasting MMC pattern, absence or decreased amplitude of contractions and abnormal propagation of contractions. Uncoordinated hypertensive contractions may also occur in patients with neuropathy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here