Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
While risk of bleeding of an unruptured brain arteriovenous malformation (AVM) is close to 2%, rupture rate of a previously ruptured AVM is considerably higher; thus, treatment of the latter is in most cases indicated whereas treatment of unruptured aneurysm remains a matter of debate.
Brain AVMs may become symptomatic with hemorrhage but also with non-hemorrhagic deficits or symptoms that can be related to arterial steal, venous congestion, hydrodynamic disorders, or mass effect; these features often have characteristic angiographic features enabling identification and targeted treatment.
Endovascular treatment has evolved considerably over the past years; however, a thorough understanding of the angioarchitecture—including feeding arteries, nidus-type veins, and draining veins—remains the hallmark for a safe endovascular approach.
Cerebrofacial arteriovenous metameric syndromes and cerebral proliferative angiopathy are disease entities that may mimic diffuse brain AVMs; however, their separate natural history mandates a different therapeutic approach as compared to other pial brain AVMs.
Spinal cord pial arteriovenous malformations are rare vascular disorders that can become symptomatic due to hemorrhage, venous congestion, and, rarely, mass effect.
Endovascular treatment options have better outcome in fistulous AVMs as compared to nidus-type AVMs.
Partial targeted embolization appears to reduce the risk for future hemorrhage, but it does not eliminate it.
One of the basic principles in medicine is that the understanding of a disease should precede its treatment. Unfortunately, in the case of spinal and brain arteriovenous malformations (AVMs), little is known about their etiology and natural history. For this reason, it is difficult to confidently prescribe guidelines regarding treatment, especially in incidentally found AVMs. The numerous different classification schemes that should aid in the understanding of arteriovenous shunts testify to this lack of knowledge. To further complicate matters, advances in diagnostic tools for pretreatment risk assessment as well as continuously improved treatment modalities (catheterization and embolization materials and techniques) are likely to further change the way we will manage these vascular malformations.
We have subdivided this chapter into two main sections, the first one dealing with the brain and the second one with the spine. We will confine ourselves to the discussion of endovascular treatments of pial AVMs only (i.e., AVMs arising from arteries that would normally supply brain tissue and veins that would normally drain brain tissue). We will leave discussion of dural (both brain and spinal) arteriovenous shunts for a different chapter.
When considering endovascular treatment of pial brain AVMs, a classification that is based upon the size, the pattern of venous drainage, and the eloquence of the portions of brain adjacent to the AVM is of limited use, for three reasons. First, such a classification cannot predict the natural history of a specific AVM for an individual patient; second, it does not anticipate the risk of treating brain AVMs by endovascular techniques; and, third, it does not enhance our understanding of this disease.
Concerning the last point, a classification based upon the etiology of vascular malformations may be a more useful approach to assess vascular malformations in general, of which the AVMs are but a subgroup. This etiologic classification takes into account the part of the vascular tree where the malformation originates as well as the timing and triggering event that initiates the malformation. The first determinant is the part of the vascular tree where the malformation begins. Since arteries and veins are already differentiated early, during vasculogenesis, the location of the malformation can begin anywhere along the vascular tree: from the arterial side to the arterial-capillary, the venous junction venules, veins, sinuses, and lymphatics. The second determinant will be the timing of when the trigger initiates the malformation within the vascular structure. An early hit during vasculogenesis (such as a germinal mutation) will affect more cells and will lead to a metamerically arranged defect, whereas a late hit (such as a somatic mutation that occurs late during the fetal life or even postnatally) will have a more focal impact (such as failed localized remodeling during vascular renewal). Finally, the nature of the triggering event—intrinsic (i.e., genetic) versus extrinsic (i.e., environmental), traumatic or infectious—will add another level of complexity to this schematic approach to classifying brain vascular malformations. Although there is likely to be a continuous spectrum of vascular diseases rather than clear-cut disease entities, the scheme that is based on the above-mentioned assumptions may help to define vascular malformations from an etiologic standpoint.
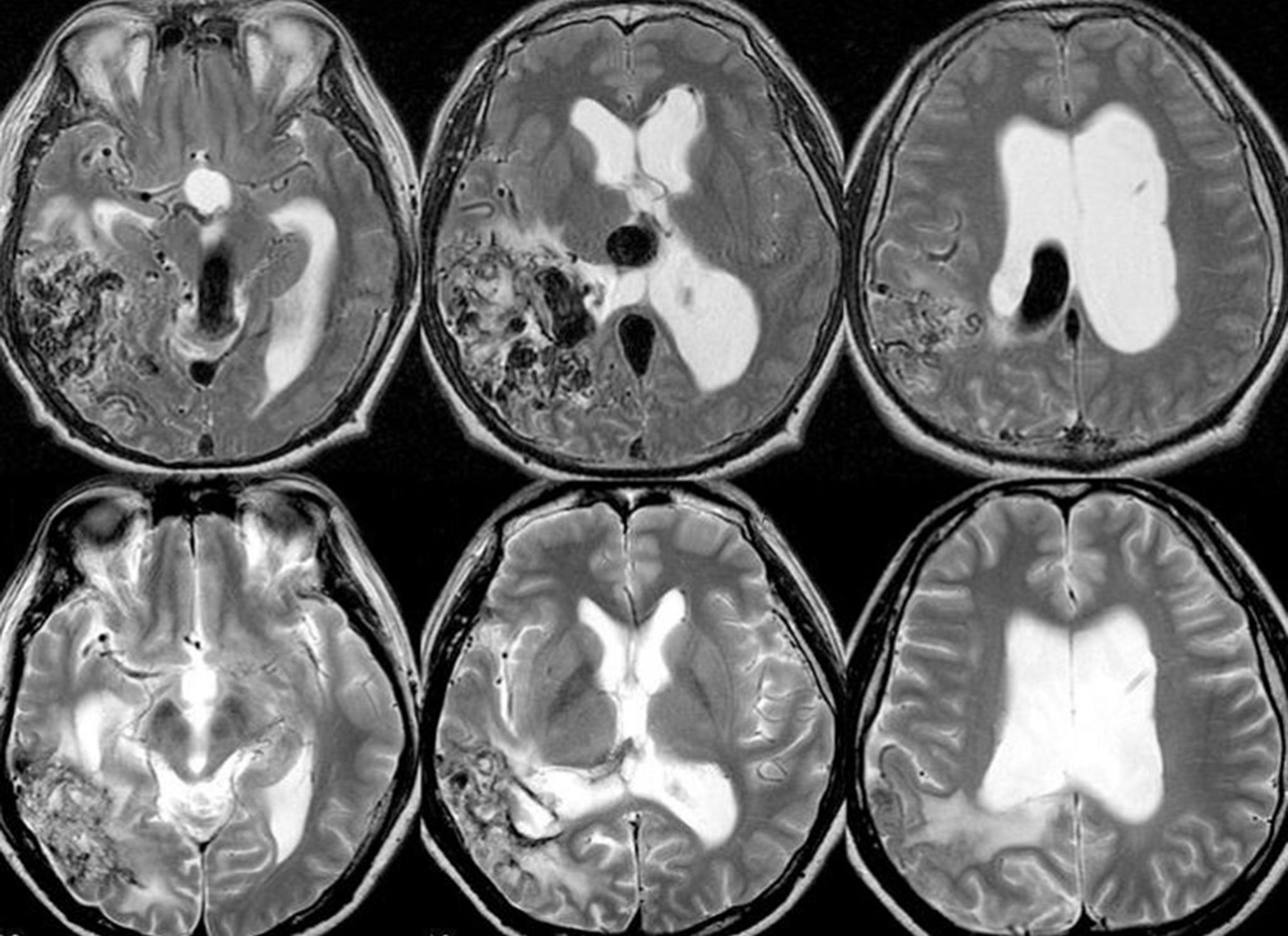
A classification that can predict the natural history first has to distinguish those AVMs that have bled from those that have not bled, which in most instances is possible by the review of the clinical history. In asymptomatic patients, T2-weighted gradient echo sequences (GRE) or susceptibility-weighted imaging (SWI) that can identify signs of older hemorrhage may assist in identifying those rare cases in whom a subclinical hemorrhage may have occurred.
The future risk of bleeding from a brain AVM has been the subject of many studies. In 1983, Graf et al. published their results concerning the risk for future hemorrhage; these were calculated to be 37% in 20 years for unruptured AVMs and 47% for ruptured AVMs. Crawford et al. found similar 20-year cumulative risks of hemorrhage (33% for unruptured and 51% for ruptured AVMs respectively); however, they added that older age was a major risk factor, with patients older than 60 years harboring a risk for rupture of 90% in 9 years. Both studies demonstrated an annual average risk for hemorrhage of approximately 2%, which was confirmed in the study of Brown et al. in 1988, who investigated only unruptured AVMs. He added that the risk for permanent morbidity and mortality following a hemorrhage of a brain AVM was 29% and 23%, respectively. Ondra in 1990 calculated a slightly higher risk of 5% per year for unruptured brain AVMs with a mortality risk of 1% per year (i.e., 25% for all hemorrhages) and a morbidity of 2.7% per year (more than 50% for all hemorrhages). In a prospective series published by Mast in 1997, the yearly risk of hemorrhage for previously ruptured brain AVMs was calculated as 17% per year, whereas in unruptured AVMs, the risk of hemorrhage was 2% per year. They found male gender, deep venous drainage, and previous hemorrhages to be the major determinants for future hemorrhages. More recently, the ARUBA trial provided us with more information regarding the natural history of these unruptured brain AVMs, giving an estimated risk of rupture of 2.2% per year for unruptured brain AVMs.
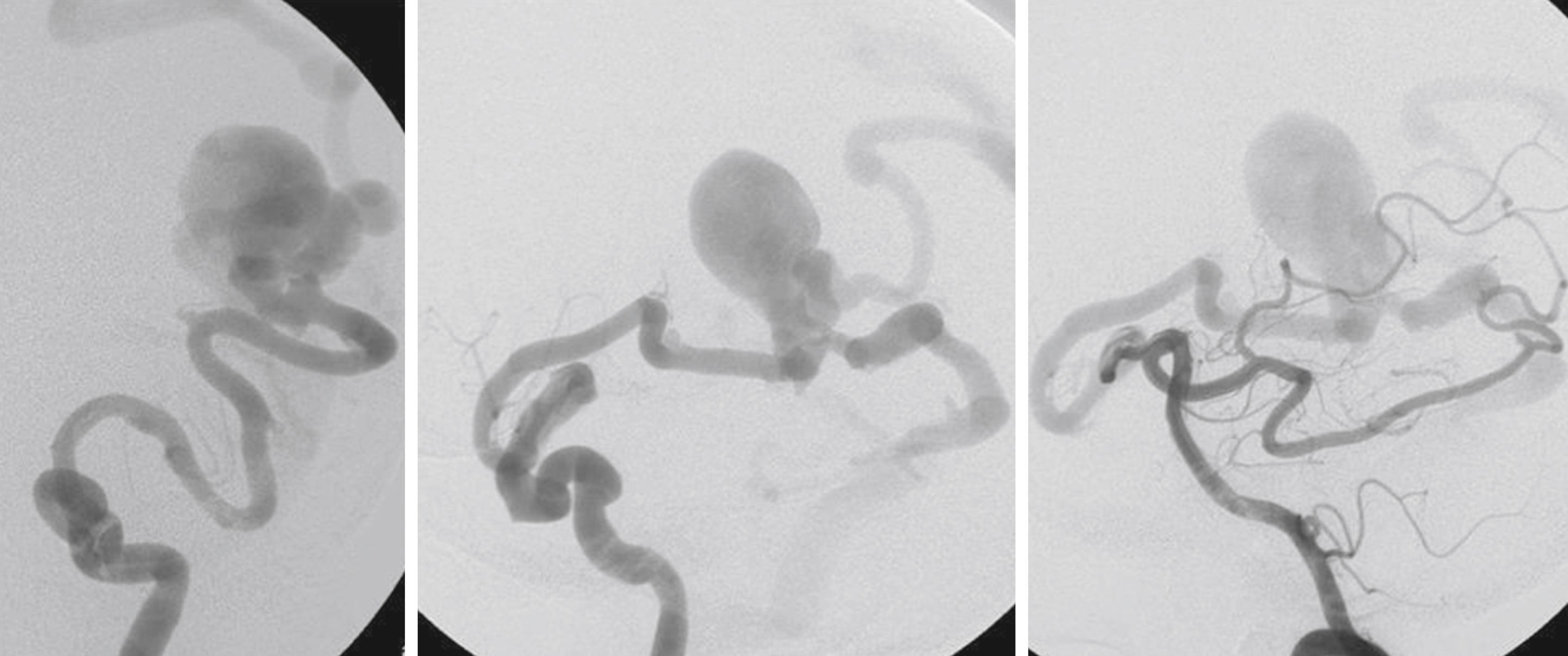
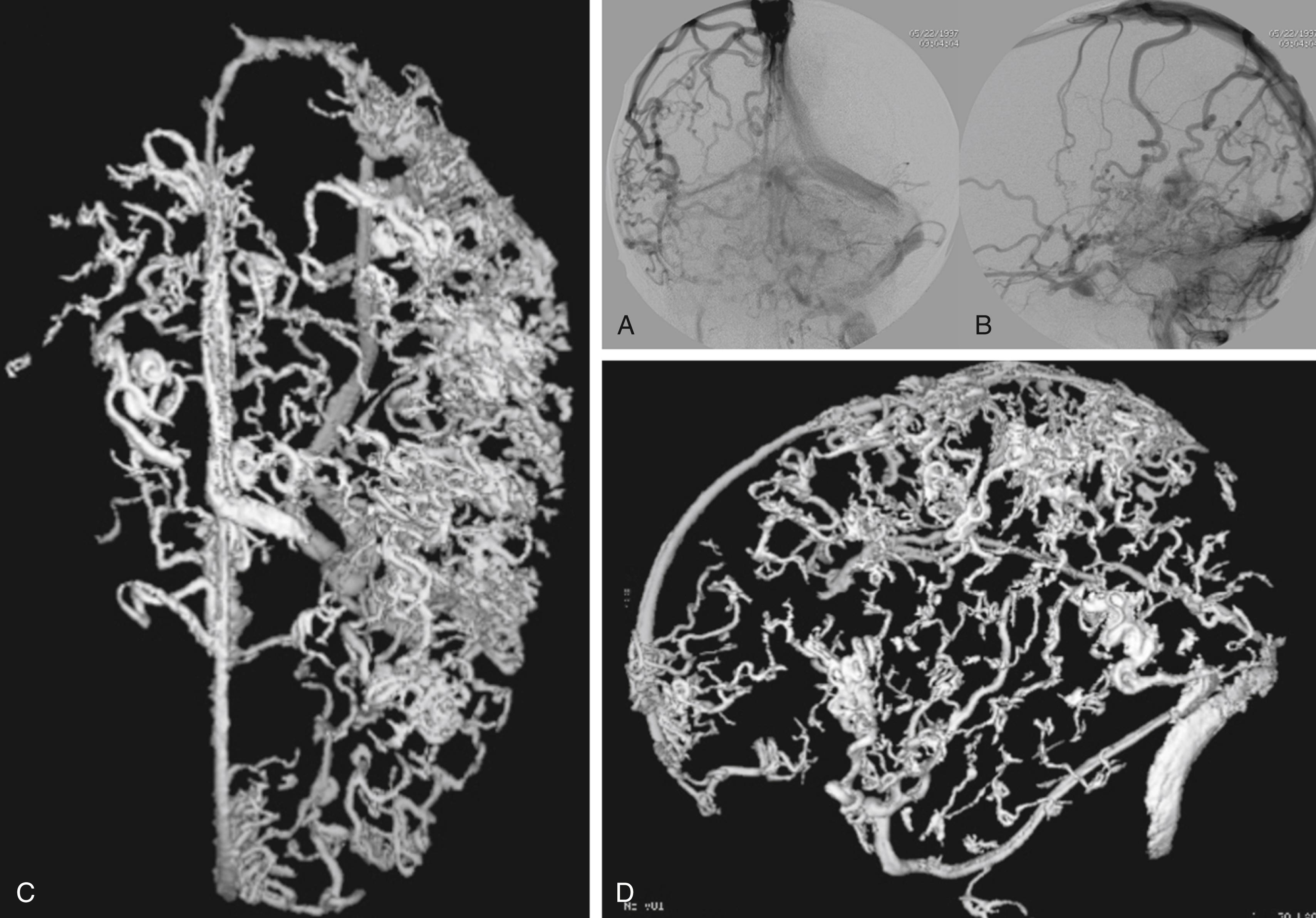
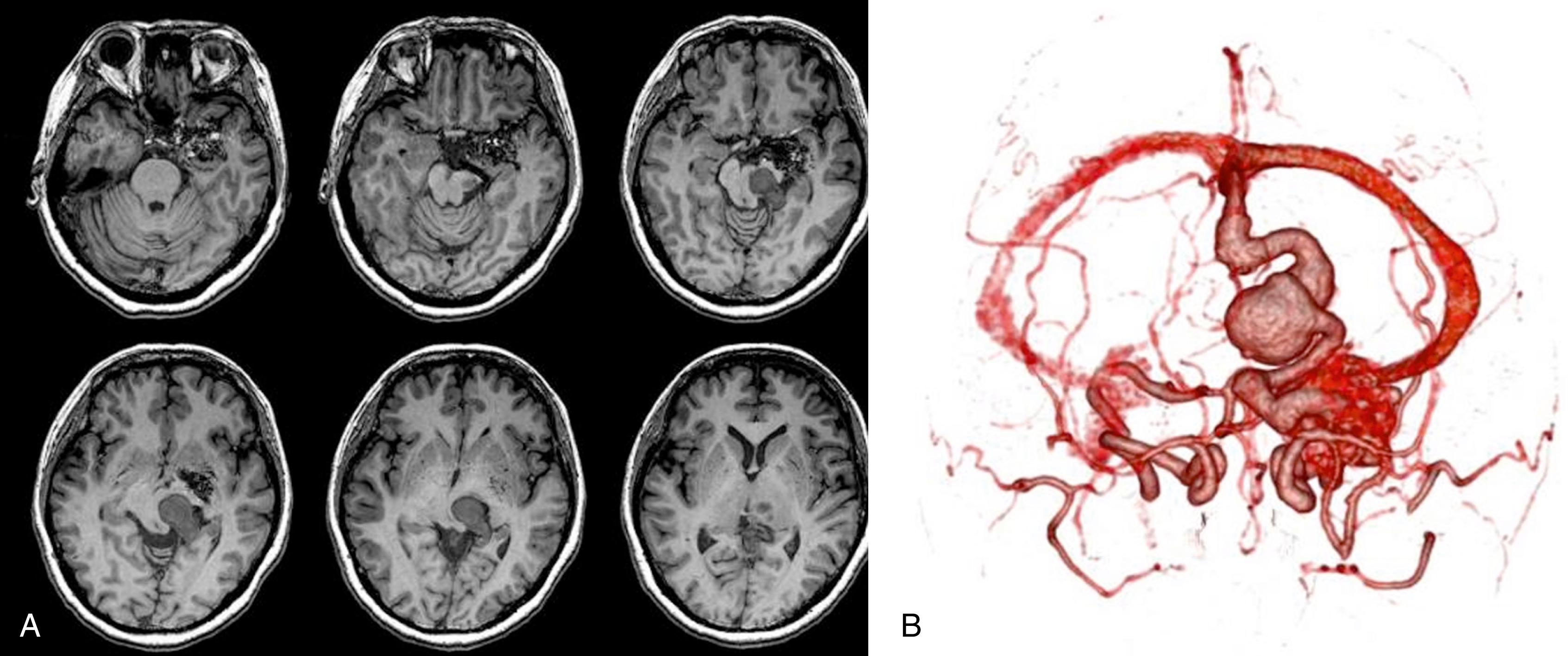
While most physicians would agree about the necessity of treating pial AVMs that have bled due to their higher rebleeding risk, pial AVMs that have not bled should be further subdivided to select those patients in whom therapy may be indicated; that is, where the therapeutic risk is believed to be lower than the natural history risk. As hemorrhage is but one presentation of a brain AVM, symptomatic AVMs that have not bled can nonetheless cause significant quality of life issues for the patient. In our practice, we try to identify the symptomatic but unruptured AVMs according to their pathophysiology as it pertains to the angioarchitecture (i.e., the structure of blood vessels within and related to the AVM). Due to their high-flow shunt , fistulous-type pial AVMs (especially when present in childhood) can lead to psychomotor developmental delay, cardiac insufficiency and, if present later in life, to dementia. These lesions therefore merit treatment. In these cases, endovascular treatment should reduce the arteriovenous shunting ( Fig. 69.1 ). Venous congestion ( Figs. 69.2 and 69.3 ) that can be due to a high input (fistulous AVMs) or a reduced outflow (secondary stenosis or thrombosis of the draining vein[s], Figs. 69.4 and 69.5 ) may be associated with cognitive decline, focal neurologic deficits, or epilepsy. Interventional treatment may be considered for these patients with venous congestive edema with increased risk of subsequent hemorrhage. Even if signs of venous congestion are not overtly present, a long pial course of the draining vein may indicate that a degree of venous drainage restriction is present, potentially affecting a large area of brain parenchyma, and increasing the risk of venous congestion and subsequent epilepsy. The likelihood of developing seizures in patients with a long course of the pial draining vein is significantly increased. Conversely, a short vein that drains almost directly into a dural sinus is unlikely to interfere with the normal pial drainage. If a patient with this kind of angioarchitecture was to have epilepsy, the magnetic resonance imaging (MRI) should be scrutinized for signs of perinidal gliosis . In the former case (epileptic patient harboring an AVM with a long pial draining vein) endovascular treatment is warranted to reduce the interference with the normal pial drainage and hopefully to reduce the seizure frequency or severity. In the case of epilepsy associated with perinidal gliosis, endovascular therapies are unlikely to change the seizure frequency or severity and we would suggest abstention from endovascular treatment. Mass effect is a relatively rare event that may result from large venous ectasias or the nidus itself compressing critical structures. This in turn may lead to epilepsy, neurologic deficits, and even hydrocephalus. Arterial steal has been associated with clinical findings such as migraine and focal neurologic symptoms that most often are transitory in nature. With the advent of imaging modalities such as functional MRI and perfusion MRI/computed tomography (CT), it has now become possible to demonstrate whether or not the symptoms of a given patient can be attributed to a true arterial steal, which can be treated by endovascular means with the aim of reducing the shunt if the symptoms are disabling.
Understanding the genetic underpinnings of brain AVMs is an active area of research, including screening of candidate genes, genome-wide association studies (GWAS), and whole-exome screening (WEs). Our group has recently published work looking at the genetic changes found in sporadic brain AVMs. Specifically, we found that many pial AVMs are related to somatic mutations in the KRAS (Kirsten rat sarcoma viral oncogene homolog) pathway. Somatic mutations are non-inheritable mutations acquired in non-germline cells. Germinal mutations occur in non-somatic cells and can be transmitted to progeny. Hong et al. independently found BRAF (a gene that encodes a protein called B-Raf) and KRAS pathways mutations in patients with brain and spinal AVMs. These preliminary findings potentially open the door to therapies targeting these specific mutations.
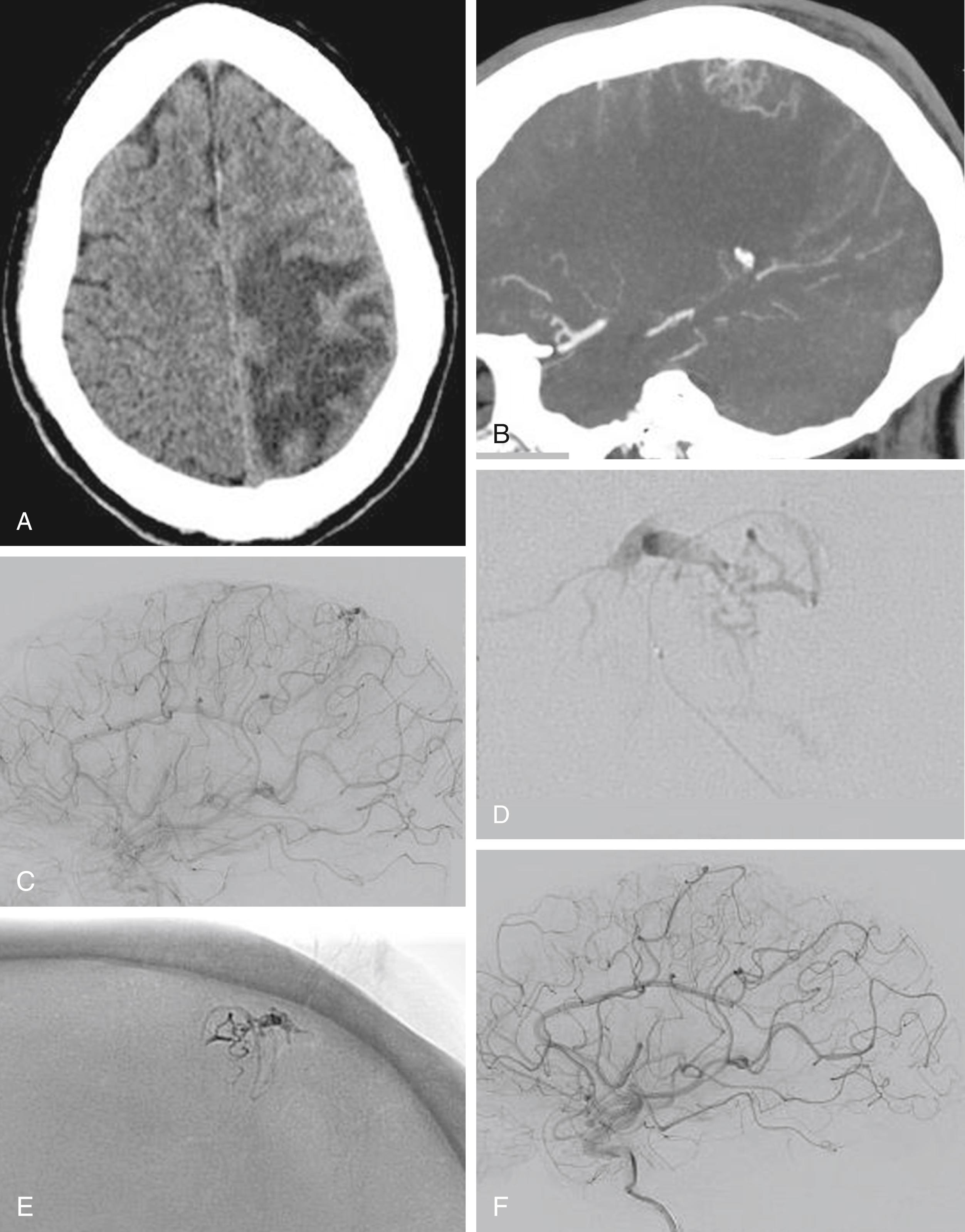
The underlying rationale for “partially targeted embolization” of brain AVMs is the hypothesis that specific angioarchitectonic features of a pial brain AVM can be regarded as “weak points” that may predispose to hemorrhage. Although this principle is not proven by randomized prospective trials, we have used it for more than 30 years. When treating patients with these weak points, we were able to show an improved outcome on follow-up when compared to the natural history. Specifically, these angioarchitectonic weak points consist of (a) intranidal aneurysms and venous ectasias and (b) venous stenosis. The first group to suggest that specific angiographic features present in brain AVMs make them more prone to future hemorrhage were Brown et al. in 1988, who found that the annual risk of future hemorrhage was 3% in brain AVMs alone, and 7% per year in brain AVMs with associated aneurysms ( Fig. 69.6 ). Meisel et al. found that among 662 patients with brain AVMs, the 305 patients who had AVMS with intranidal aneurysms had significantly increased episodes of rebleeding as compared to those that did not ( P < .002). In the Toronto series of 759 brain AVMs, those patients with aneurysms had increased risk of future bleeding ( P = .015). However, it can be difficult to distinguish between intranidal arterial aneurysms and intranidal venous ectasias. Therefore, these two entities are often grouped together as one entity in most series. Venous stenoses, on the other hand, are a separate angiographic weak point that are often present in ruptured AVMs ( Fig. 69.7 ). Potential causative factors of venous stenosis include high-flow vessel wall changes, failure in remodeling, or shear stress induced by the arterialization. A stenotic venous outflow pathway will lead to an imbalance of pressure in various compartments of the AVM, which may induce subsequent rupture of the AVM. In addition to these two previous angioarchitectonic risk factors, other factors that may lead to an increased risk of hemorrhage include isolated deep venous drainage, advanced age, and male gender.
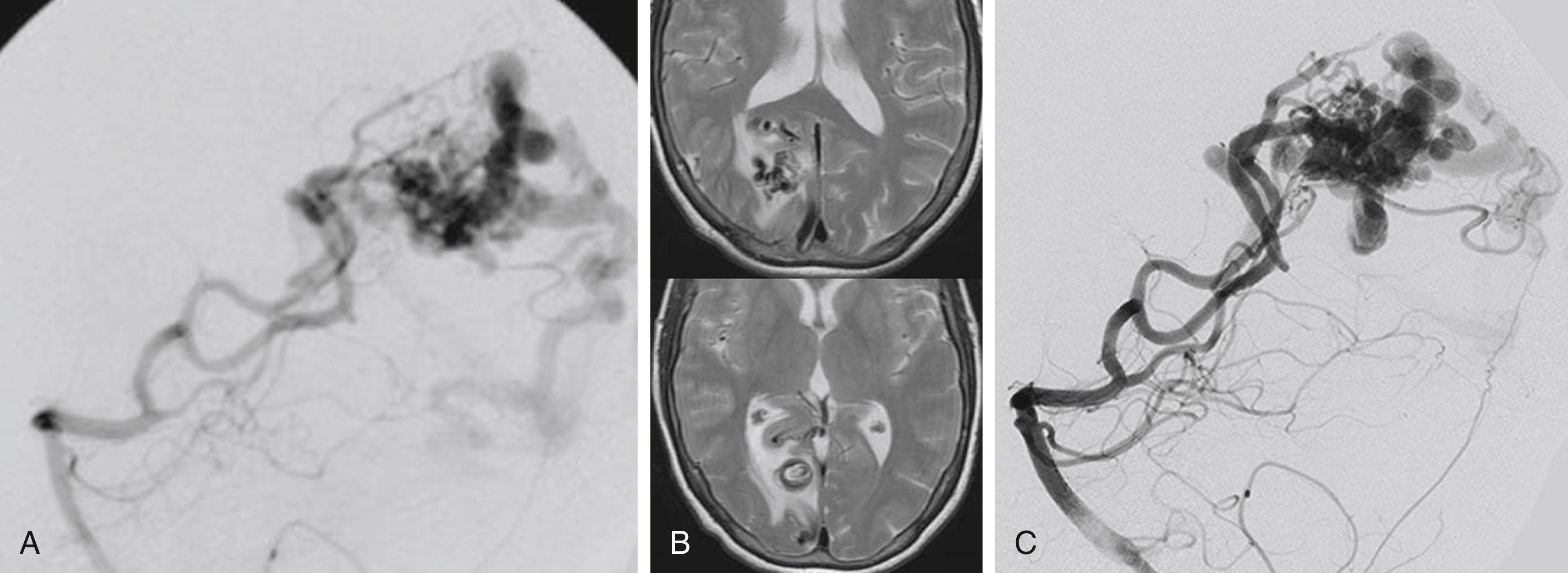
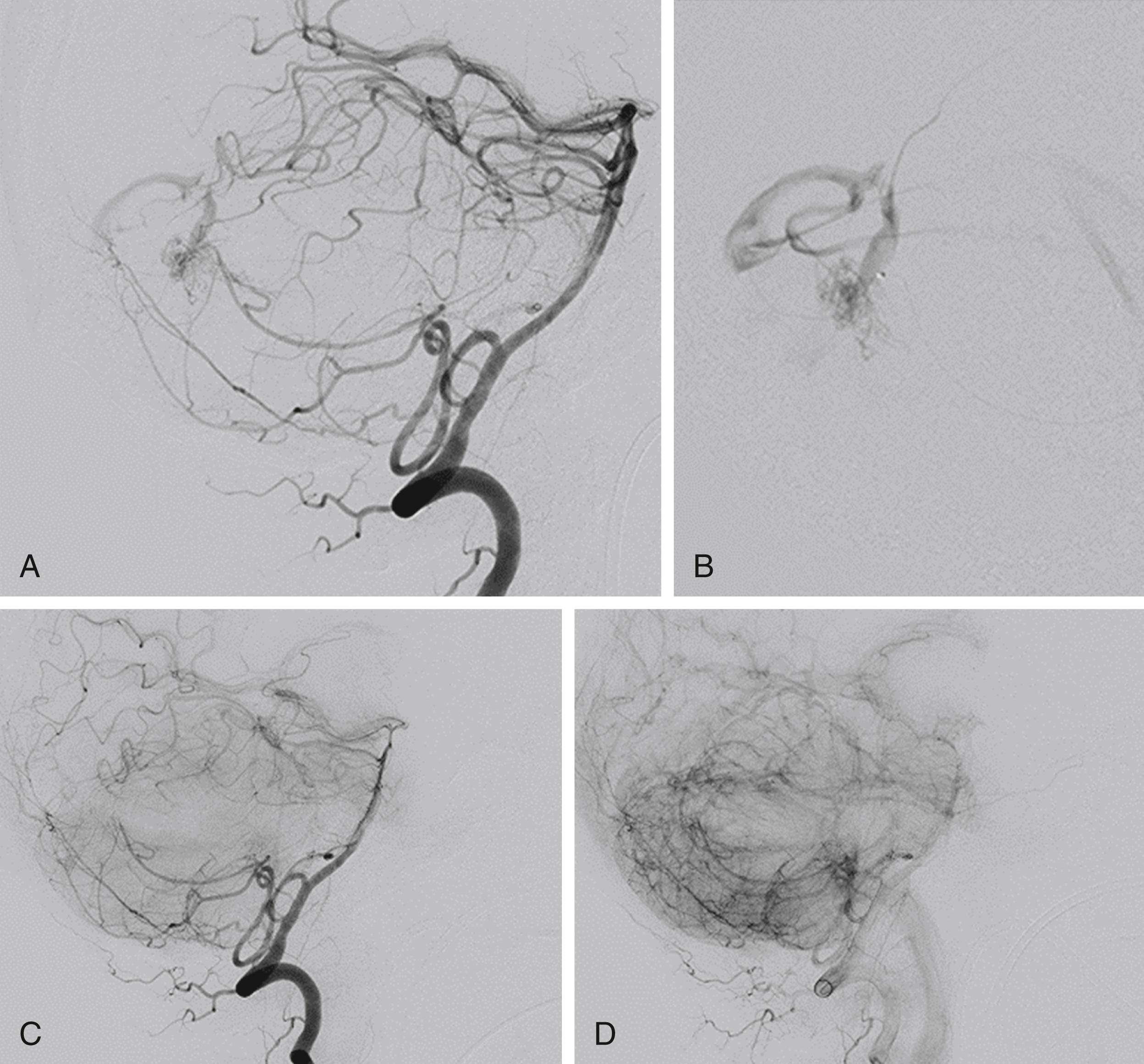
To determine the therapeutic plan for an AVM, an angiogram must determine the following: the presence or absence of flow-related aneurysms, the nature and number of the feeding arteries, the number of separate compartments of the malformation, any arterial or venous ectasias near to or within the malformation, and the nature of the venous drainage. On the arterial side, flow-related aneurysms typically arise at the branching points of the major feeding arteries. These aneurysms are due to vascular remodeling following increased shear stress and usually resolve following treatment of the AVM. Although such aneurysms are not a contraindication for endovascular treatment, the neurointerventionalist should take special care, since flow-directed catheters are prone to enter the aneurysm rather than the distal vessels. Both the number and the nature of the feeding arteries need to be assessed during angiography, since these features determine the best endovascular approach. An AVM with a single large feeder is a much more attractive interventional target than one with a large number of minimally dilated feeders.
There are two types of feeding arteries. Direct arterial feeders end in the AVM. Indirect arterial feeders go on to supply the normal cortex but also supply the AVM via small “ en passage ” vessels that branch off from these normal arteries. While direct feeders are safe targets for an endovascular therapy, an en passage feeder may carry the risk of inadvertent migration of an embolic agent into distal healthy vessels. In addition, liquid embolic agents can reflux proximally at the end of the injection, which can result in non-target embolization. Depending on the agent, the microcatheter, the injection technique, and the skills of the operator, this reflux may be as far as 1 cm proximal to the tip of the catheter. Therefore, safe deposition of liquid embolic agent is only possible if the catheter tip is beyond any vessel that supplies normal brain tissue. In case of en passage feeders, this may not be possible, especially if the catheter is hooked into the feeding artery and will jump backward due to the jet-effect when injecting a liquid embolic agent.
Moving from the artery to the nidus of the AVM itself, the angiographer needs to identify intranidal arterial aneurysms and venous varices, the number of compartments, and their nature (nidal vs. fistulous).
Finally, on the venous side of the AVM, the angiographer should identify the number of draining veins per compartment, whether there is drainage into the deep venous system (higher risk for hemorrhage, more difficult surgical treatment), and stenoses that restrict venous outflow. At the present time, conventional digital subtraction angiography is required to obtain this information, which is necessary for the treatment plan. In our experience, transvenous angiographic routes to brain AVMs are limited to brain AVMs with a single draining vein.
The treatment of brain AVMs hinges on the key distinction of whether they are ruptured or unruptured.
Treatment of unruptured AVMs occurs in our institution on an individual-case basis, following multidisciplinary discussion of each case with all of the relevant specialties. The elective treatment of unruptured AVMs in many institutions has decreased following the publication of the ARUBA trial. The ARUBA trial (A Randomized Trial of Unruptured Brain Arteriovenous Malformations) remains the only prospective, multicenter, parallel, non-blinded randomized controlled trial of unruptured brain AVMs. The primary outcome was time to the composite event of death from any cause or symptomatic stroke (defined as any new neurologic symptom associated with any imaging finding). Halted after 6 years after an interim analysis, the trial showed that this primary outcome was reached in 35 (30.7%) of the 114 patients assigned to an intervention, versus 11 (10.1%) of the 109 patients assigned to medical treatment. Published in 2014, the authors of ARUBA concluded that medical management alone was preferable to interventional therapy for the prevention of stroke in patients with an unruptured brain AVM. As a result, many authors recommended that all unruptured AVMs should be treated conservatively. We feel that this approach may be too simplistic, and that the treatment of unruptured AVMs requires a more nuanced approach. This is because despite it being a randomized controlled trial, the ARUBA trial has limitations and potential biases. The limitations of the ARUBA trial have been extensively discussed elsewhere. For example, ARUBA included AVMs of many different grades, whereas we only offer treatment to certain grades of AVM. Similarly, all types of interventional treatments (surgery, endovascular treatment, and radiosurgery) were assessed together, limiting the applicability of the results to any one modality. The choice of primary outcome raises also concerns—any “symptom” associated with a new CT or MRI finding following the treatment. This primary endpoint was reached in 30.7% of patients, which is a higher number for centers experienced in the treatment of these patients. Moreover, transient neurologic symptoms are can occur following treatment of AVMs, and it is not clear from ARUBA whether the primary endpoints they defined were transient or permanent. Thus, the lack of detail regarding this primary outcome may have skewed the results in favor of the conservative management arm. On the other hand, clinical trials are often more detailed regarding documentation of adverse events and outcomes than registries of clinical practice and the differences between the two groups were significant in favor of medical therapy. Finally, the initial goal of ARUBA was to follow 800 patients for between 5 and 7 years. Following the interim analysis, the trial was terminated, and 223 patients were followed for a mean of 33 months. Clearly, the up-front risks associated with treatment would be more likely to occur in this shorter period, leading to a potential bias against treatment. Our own group has published work showing significant disparity between the results for microsurgical resection of AVMs and the outcomes in ARUBA, further strengthening the argument that this was a flawed trial that cannot be blindly applied to justify non-treatment of all brain AVMs.
In particular, we feel that there are certain subgroups of patients in whom interventional treatment should be considered:
Pediatric patients (given the increased lifetime risk for hemorrhage when compared with elderly patients), especially those with high-flow fistulae that can result in high-output cardiac failure. These patients were not represented in the ARUBA trial.
Unruptured but symptomatic patients with an identifiable pathophysiologic mechanism as discussed above.
Change in imaging appearance of a conservatively managed AVM (new edema, formation or new aneurysmal outpouching).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here