Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Intra-aortic balloon pump counterpulsation increases coronary artery perfusion pressure, increases coronary artery blood flow (particularly in cardiogenic shock), and augments cardiac output by approximately 5% to 15%.
Catheter-mounted ventricular assist devices (VADs) are available for isolated right ventricular and left ventricular support. Left ventricular support devices can provide between 2.5 and 5.5 L/min of blood flow, depending on the product. Echocardiography should be performed daily when possible to confirm correct VAD position.
For left-sided catheter-mounted VADs (e.g., Impella 2.5, 5.0, CP) the inflow should be positioned approximately 3.5 cm below the aortic valve. For the Impella 5.5, the inflow should be positioned approximately 5 cm below the aortic valve.
Starting settings for the total artificial heart are left ventricular drive pressure of 180 to 200 mm Hg, right ventricular drive pressure of 30 to 60 mm Hg, heart rate of 100 to 120, and 50% systole. No native cardiac rhythm is required for proper device function.
The incidence of true heparin-induced thrombocytopenia (HIT) appears to be low during mechanical circulatory support. The 4T score can be used to categorize patients as low, intermediate, and high probability as in nonmechanical circulatory support patients.
Health care–associated infections are common in mechanical circulatory support patients, but there is no strong evidence to support use of prophylactic antibiotics. When signs of infection are present, targeted cultures should be performed, empiric antibiotics should be initiated, and tailored based on culture results.
A hemoglobin transfusion trigger of 7 g/dL is appropriate for most mechanical circulatory support patients, but exceptions may be appropriate in some clinical scenarios. There is no strong evidence to guide appropriate platelet transfusion during mechanical circulatory support. In extracorporeal membrane oxygenation patients with bleeding, it is reasonable to maintain a platelet count at least >50 × 10 9 /L and perhaps >100 × 10 9 /L because of qualitative platelet dysfunction.
Early mobility should be promoted as soon as is feasible in mechanical circulatory support patients, even those with peripheral cannulation. Mobility helps maintain strength, reduces muscle atrophy, and improves patient experience. A multidisciplinary approach is necessary to limit complications during ambulation and physical therapy.
Complex ethical dilemmas can arise in temporary mechanical circulatory support patients. Early discussions with patients and families about what temporary mechanical circulatory support can and cannot accomplish are critical to help create reasonable expectations.
In the early 2000s, a renaissance in mechanical circulatory support (MCS) occurred with multiple new temporary and durable devices entering clinical practice. By 2018, cardiogenic shock mortality fell to a historic low of 35%. Today, multiple MCS devices are available for right ventricular (RV) support, left ventricular (LV) support, or biventricular support. At present, the only durable left ventricular assist device (LVAD) that is commercially available in the United States is the HeartMate 3 (Abbott Cardiovascular, Plymouth, MN). In early 2021, the HVAD (HeartWare, Framingham, MA), which had been implanted in more than 19,000 patients worldwide was removed from the market by its manufacturer because of concerns about its high stroke rate and other factors.
Patients with refractory cardiogenic shock should be evaluated for MCS unless they have other end-stage diseases (e.g., metastatic cancer) or are of extreme age. There should be consideration of whether temporary MCS will be used as a bridge to recovery, transplantation, or durable LVAD implantation. There is no universal definition for cardiogenic shock, but accepted criteria include impaired LV or RV systolic function with corroborating findings such as (1) sustained systolic blood pressure <90 mm Hg or mean arterial pressure (MAP) <65 mm Hg; (2) pulmonary congestion with elevated LV end-diastolic pressure (>16 mm Hg); (3) signs of organ malperfusion; (4) cardiac index < 2.2 L/min/m 2 ; (5) mixed venous oxygen saturation (Sv o 2 ) less than 60%; or (6) elevated biomarkers indicative of tissue hypoxemia (e.g., elevated blood lactate concentration). , Low cardiac power output (cardiac output [CO, L/min] × MAP [mm Hg] × 0.0022) is indicative of cardiogenic shock. Patients with cardiac power output < 0.5 have mortality that exceeds 50% and should be considered for MCS if they do not improve with inotropy. ,
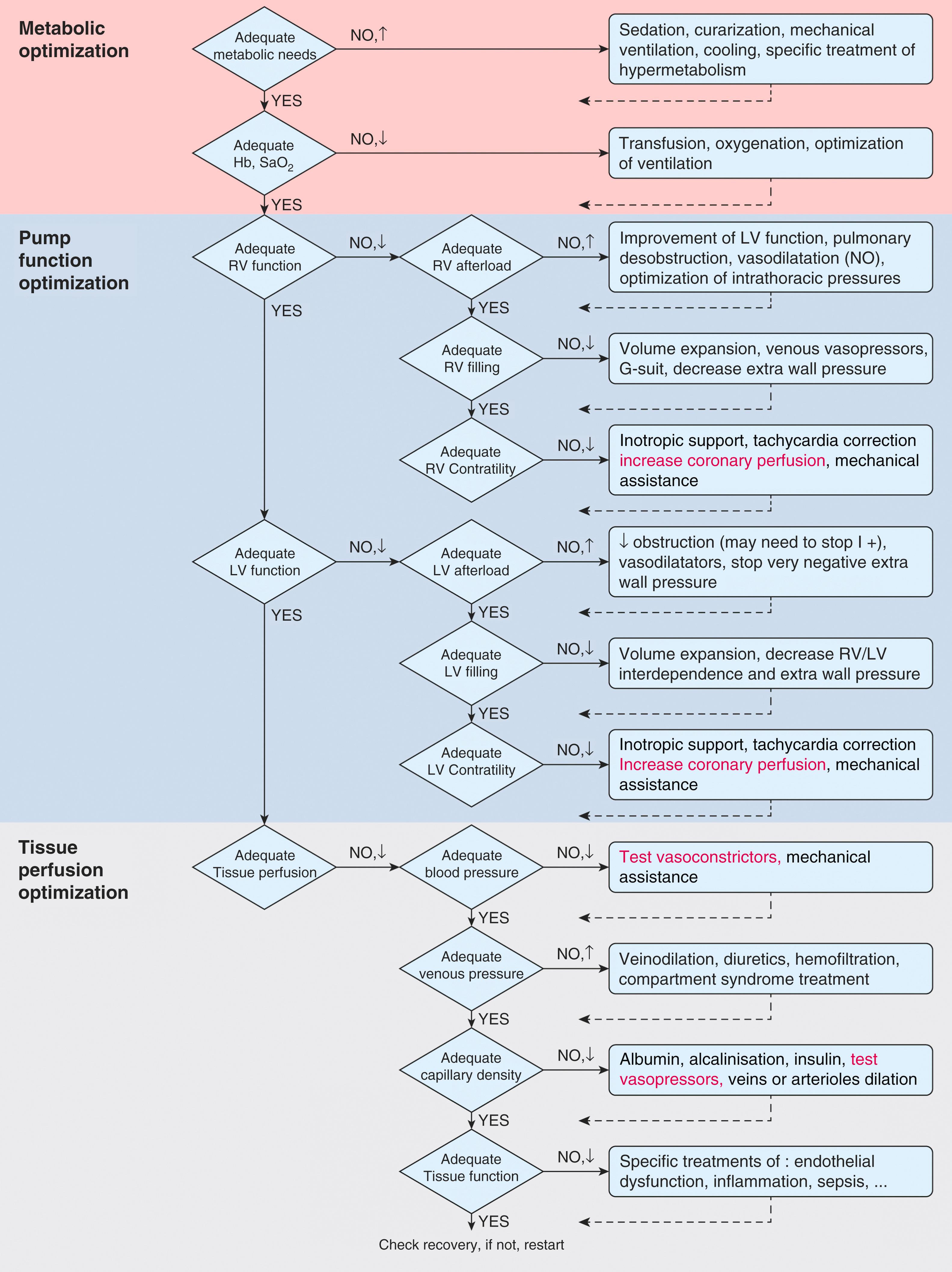
Cardiogenic shock is initially treated with inotropes with a goal to increase MAP > 65 mm Hg and improve RV and/or LV systolic function (see Chapter 34 ). Other interventions include metabolic optimization (e.g., optimization of mechanical ventilation and treatment of hypermetabolism) and optimization of tissue perfusion (e.g., reduction in central venous pressure [CVP]) ( Fig. 35.1 ). When cardiogenic shock cannot be reversed with inotropes and medical interventions, MCS should be considered. Early MCS is associated with superior outcomes when compared to using high-dose vasopressors and/or inotropes (e.g., norepinephrine > 0.2 μg/kg/min). The initial modality depends on multiple factors, but is mostly related to whether isolated RV, LV, or biventricular support is needed, what vascular access sites are available, and the shock team’s experience with various devices. Although the intra-aortic balloon pump (IABP) was historically the “gold standard” for initial MCS, there has been a steady trend toward using devices that can provide higher CO augmentation. In a 2015 expert consensus statement, catheter-mounted axial continuous-flow VADs and venoarterial (VA) extracorporeal membrane oxygenation (ECMO) were recommended as first-line MCS therapies, except in patients with myocardial infarction (MI) who could quickly be revascularized, in whom an IABP might be equivalent.
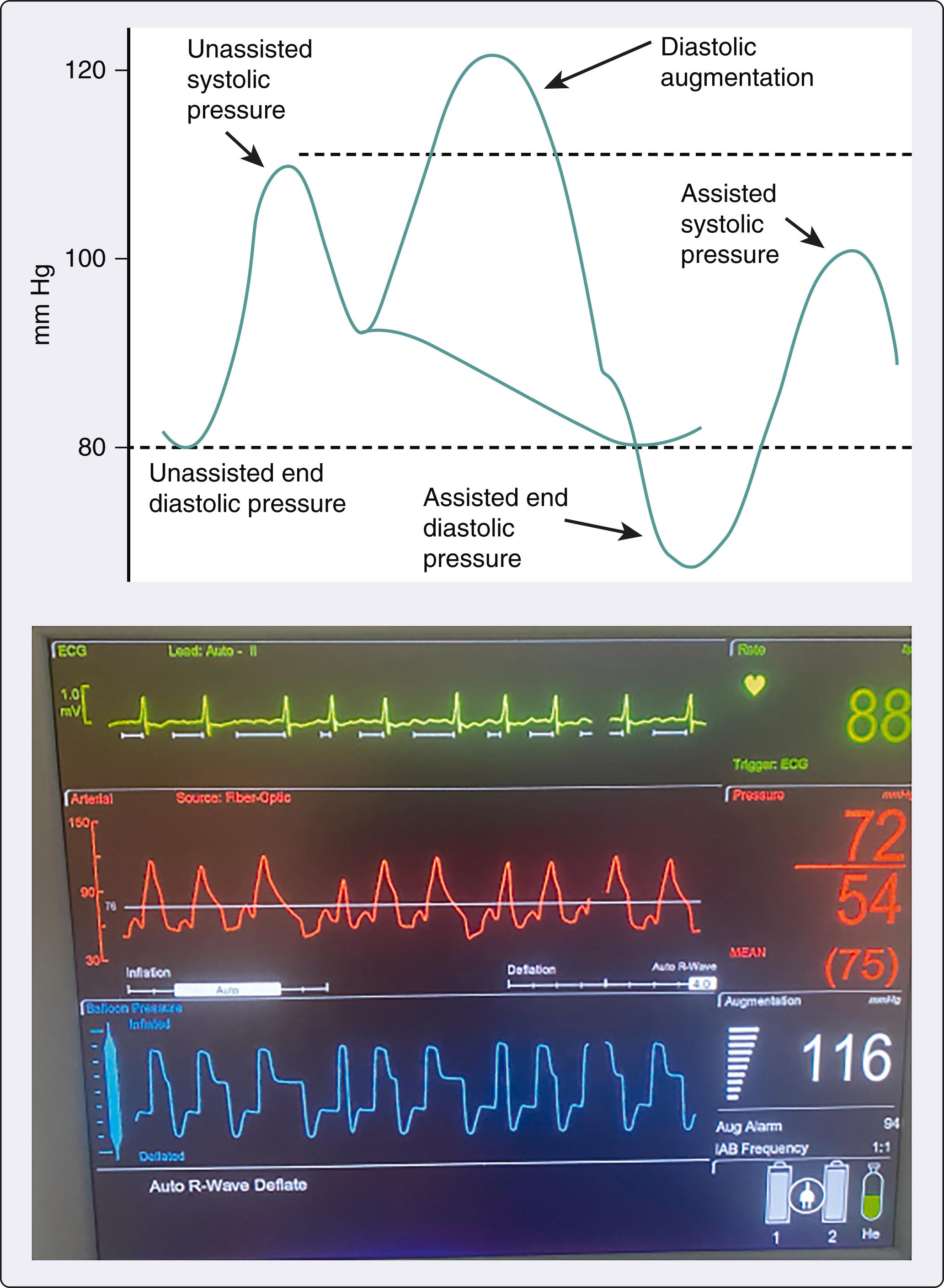
IABP counterpulsation was introduced by Kantrowitz in the 1960s. Using a percutaneous Seldinger technique, an IABP (usually 7–8 Fr catheter) is placed through a large artery, usually the common femoral artery, into the descending thoracic aorta with or without a vascular introducer sheath. IABP placement can be performed at the bedside, in the operating room, or in the cardiac catheterization laboratory where fluoroscopy can be used to confirm correct position. Chest x-ray and transesophageal echocardiography (TEE) can be used to confirm correct IABP position when fluoroscopy is not available. Modern IABP catheters have a polyurethane cylindrical balloon that inflates with helium during diastole and deflates during systole. They also have a fiberoptic tip that allows for rapid transfer of arterial pressure data to the console and in vivo autocalibration.
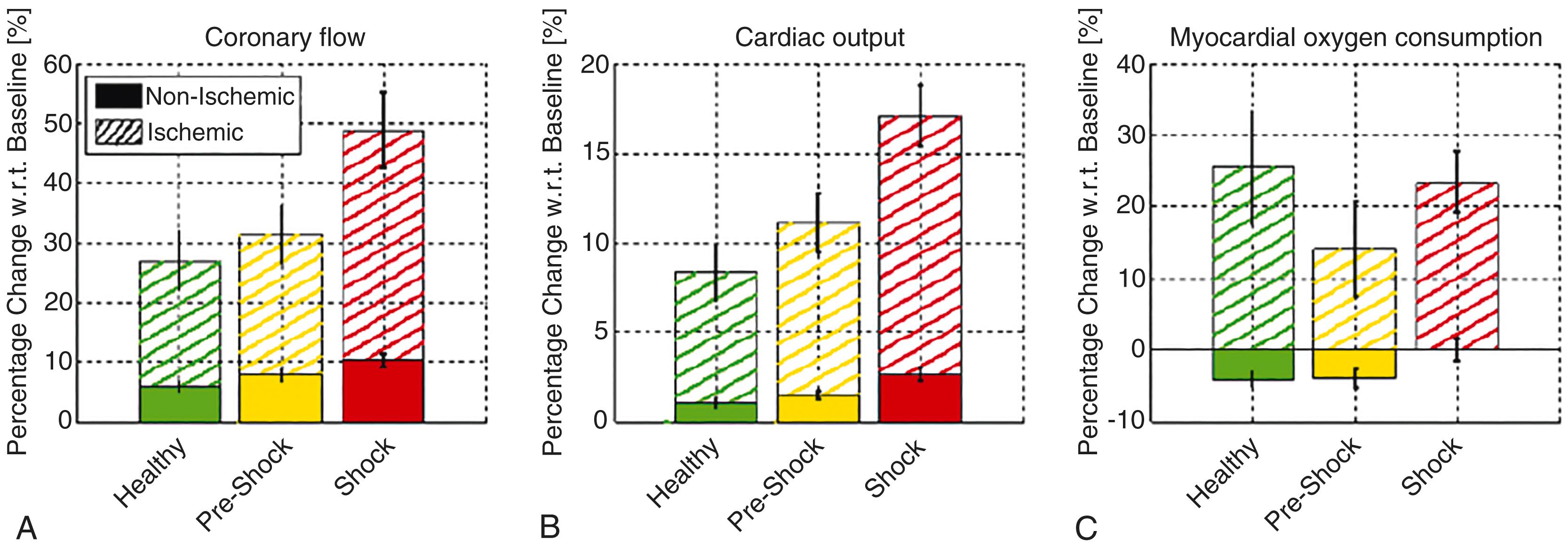
IABP counterpulsation has two main hemodynamic benefits ( Box 35.1 ). First, coronary artery perfusion pressure increases during diastole when the IABP inflates. Because coronary blood flow is autoregulated, increased perfusion pressure does not guarantee increased coronary blood flow. Increased coronary artery perfusion pressure only increases coronary blood flow when autoregulation is impaired, as in shock. Second, cardiac afterload is reduced during systole when the IABP deflates. Rapid IABP deflation increases stroke volume and reduces myocardial stroke work. In animal studies, this modestly increases CO (5%–10%) in the absence of shock, while there is an increase of up to 15% in shock ( Fig. 35.2 ). The alternating pattern of IABP inflation and deflation that occurs leads to a characteristic arterial waveform ( Fig. 35.3 ), which has two pressure peaks or a “bunny ear” pattern.
Inflates during diastole and deflates during systole
Three pressures measured: assisted systolic, assisted end-diastolic, and balloon-augmented diastolic
Late deflation and early inflation of IABP increase myocardial work
Late inflation and early deflation of IABP reduce its efficacy
Highest level of evidence for IABP is in complex percutaneous coronary intervention and in patients with ST elevation myocardial infarction
IABP increases coronary artery perfusion pressure and coronary artery blood flow, especially in cardiogenic shock
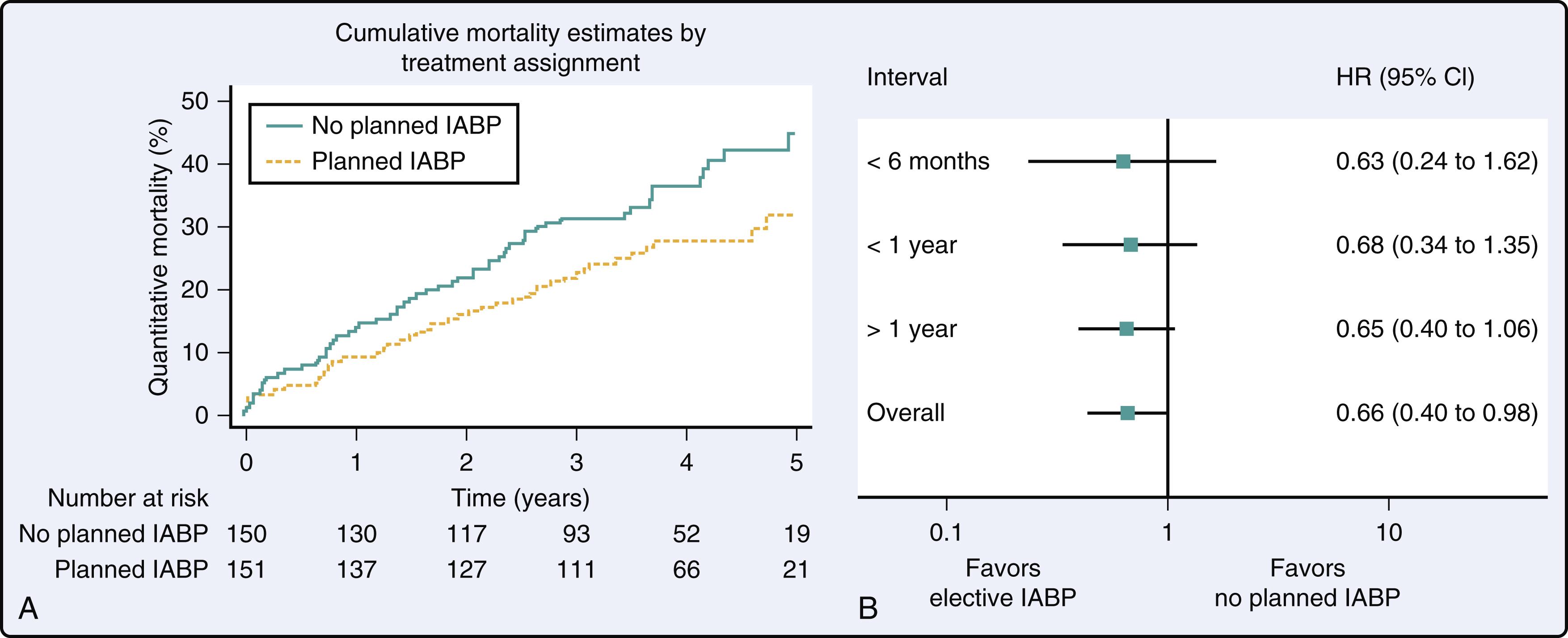
The strongest evidence to support IABP use is in patients having complex percutaneous coronary interventions (PCIs) and in patients with large ST elevation MIs (STEMIs) (see Chapter 2 ). The balloon pump–assisted coronary intervention study, which included over 300 patients having complex PCI, demonstrated a long-term survival advantage in patients who had an IABP placed before PCI ( Fig. 35.4 ). The Counterpulsation to Reduce Infarct Size pre-PCI acute MI study (CRISP-AMI) found that STEMI patients who were randomized to have an IABP placed had significantly less death, shock, and new congestive heart failure (CHF) (5% vs 12% when compared to controls). In this study, the benefit of IABP counterpulsation was particularly high in patients with large MIs (ST elevation ≥ 15 mm). Of note, in the landmark IABP in cardiogenic shock II trial (IABP-SHOCK II), which included 600 patients, both non-ST elevation MI (NSTEMI) and STEMI patients with cardiogenic shock were included and there was no reduction in short or long-term mortality with IABP placement at the time of PCI. , Recently, there has been a trend toward using more axillary IABPs, as this configuration allows patients with decompensated CHF or cardiogenic shock to stand, ambulate, and improve their priority on the cardiac transplant allocation list to Status 2.
Catheter-mounted axial continuous-flow VADs are designed to have a miniaturized blood pump as part of the catheter. Because of their design, the inflow and outflow must be inline, and hence axial-flow is mandated. In contrast, radial-flow pumps have an orthogonally oriented inflow and outflow. The Impella (Abiomed, Danvers, MA) line of catheter-mounted axial continuous-flow VADs is the only Food and Drug Administration (FDA)–approved system in the United States ( Fig. 35.5 ). Various pump sizes have proportional blood flow capacities for a given pump speed and afterload. It is important to note that axial-flow VADs have a linear inverse relationship between the transpump pressure difference, which when divided by the product of fluid density and acceleration of gravity yields the “head pressure,” and VAD flow. Head pressure is the primary determinant of VAD flow at any point in the cardiac cycle. In contrast, radial-flow pumps, often referred to as “centrifugal-flow pumps,” have a nonlinear head pressure–blood flow relationship.
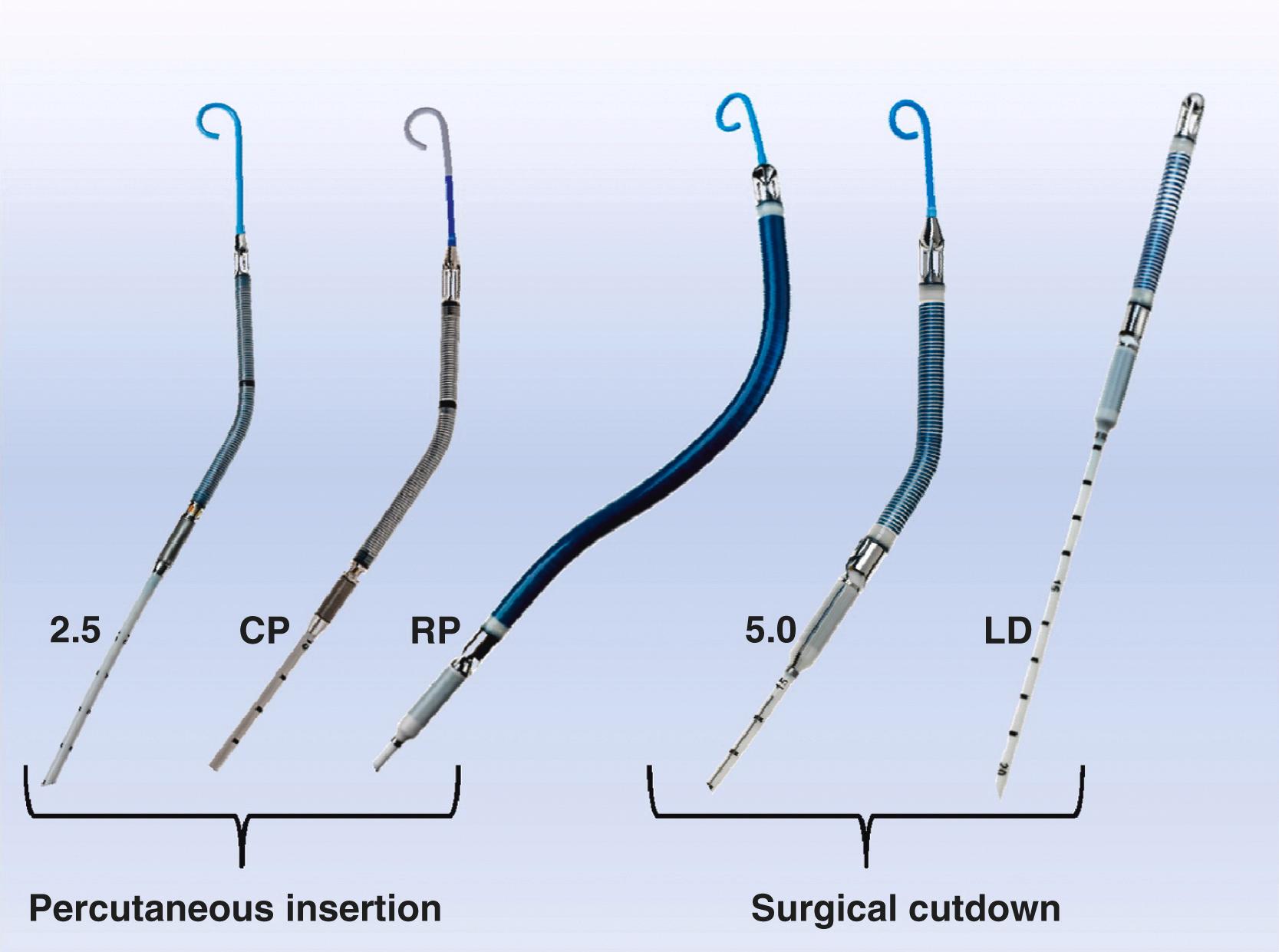
VADs capable of maximum blood flows of 2.5, 3.5 (CP), 5.0, and 5.5 L/min are available ( Fig. 35.5 and Table 35.1 ), with the Impella RP, available for RV support ( Box 35.2 ). The smaller devices (2.5, CP, and RP) are most commonly placed percutaneously, via transfemoral approaches. In contrast, the “full-support” 5.0 and 5.5 devices require surgical placement. Generally, this is via a side graft technique. With such an approach, surgical exposure of a systemic artery is performed. A side artery to end Dacron graft anastomosis is then created using a polypropylene suture. A temporary introducer sheath for the device is placed through the free end of the graft. The guidewire and catheter are advanced retrograde, crossing the aortic valve, and into the LV cavity. The catheter is removed, and the VAD is advanced into correct position over the guidewire. The guidewire is removed and device support is initiated. Placement of full-support devices can be performed peripherally (e.g., axillary artery) or centrally via the ascending thoracic aorta.
| Impella 2.5 | Impella CP | Impella 5.0/LD | Impella RP | |
|---|---|---|---|---|
| Access | Percutaneous, femoral | Percutaneous, femoral | Surgical, axillary/fem or ascend aorta | Percutaneous, femoral vein |
| Output (max; L/min ) | 2.5 | 4.0 | 5.0 | 4.6 |
| Guiding catheter size (Fr) | 9 | 9 | 9 | 11 |
| Motor size (Fr) | 12 | 14 | 21 | 22 |
| Introducer size | 13 Fr peel away | 14 Fr peel away | Dacron graft 10 mm recommended | 23 Fr peel away |
| RPM (max) | 51,000 | 46,000 | 33,000 | 33,000 |
| EU approval | 5 days CE mark | 5 days CE mark | 10 days CE mark | 14 days CE mark |
Devices available for right ventricular and left ventricular support
Left-sided devices include Impella 2.5, 5.0, 5.5, CP, and LD
Right-sided device is the Impella RP
Daily echocardiography should be performed when feasible to confirm correct device position
The inflow should be approximately 3.5 cm below the aortic valve for the Impella 2.5, 5.0, CP, and LD
The inflow should be approximately 5 cm below the aortic valve for the Impella 5.5
Placement signal varies by device, with Impella 5.0, LD, and RD having a differential pressure sensor, Impella 2.5 and CP having a fluid filled pressure sensor, and Impella 5.5 having a fiberoptic sensor
Impellas have 10 speeds between P0 and P9 and blood flow increases proportionally with pump speed
Blood flow is inversely related to the head pressure (inflow cannula pressure − outflow cannula pressure), with blood flow being highest during systole and lowest during diastole
Impellas have 10 speed settings (P0–P9), compared to the greater number of pump speeds and smaller stepwise titrations available with large platform devices. The blood flow and extent of LV unloading is proportional to the P number. For example, for the 5.5 device, P0 is 0 revolutions per minute (RPM), P1 is 12,000 RPM, P2 is 17,000 RPM, and each further iteration increases RPM by 2000 to 3000.
Impellas have been used extensively to support patients with cardiogenic shock in the post-MI setting, in postcardiotomy shock, and as a venting strategy in VA ECMO patients. In a systematic review that included over 600 cardiogenic shock patients from 11 studies, complication rates were relatively low with a major bleeding rate of 19.9%, hemolysis rate of 10.5%, limb ischemia rate of 5.0%, and stroke rate of 3.8%. When evaluating only high-risk PCI patients (over 7000 patients), complication rates were lower with a major bleeding rate of 6%, stroke rate of 2.8%, and vascular complication rate of 2.5%. When used as an adjunct for LV venting in VA ECMO patients, Impellas may reduce mortality by up to 50%, but prospective randomized trials are needed to confirm these findings.
While catheter-mounted VADs are capable of generating relatively high blood flows, miniaturization leads to high shear stress, which increases blood trauma. Larger pumps can achieve equivalent blood flow with lower pump speeds and higher maximum blood flow. In cases where patients require a high level of circulatory support (e.g., blood flow > 4–5 L/min) these features may be advantageous.
Peripheral cannulation can be used with paracorporeal VADs. For LV support, the archetypal example is the TandemHeart LVAD (TandemLife, Pittsburgh, PA). To place the device’s inflow, a long femoral venous cannula is placed through a common femoral vein, up the inferior vena cava (IVC) and across the atrial septum into the left atrium (LA). The device’s outflow is placed into a common femoral artery. The proprietary blood pump is frequently used, but other blood pumps can be employed in concert with the cannulas. The TandemHeart LVAD has been used both to support patients in cardiogenic shock and patients having high-risk PCI procedures. The device is easily inserted and can provide blood flow up to 4 L/min at a maximum RPM of 7500.
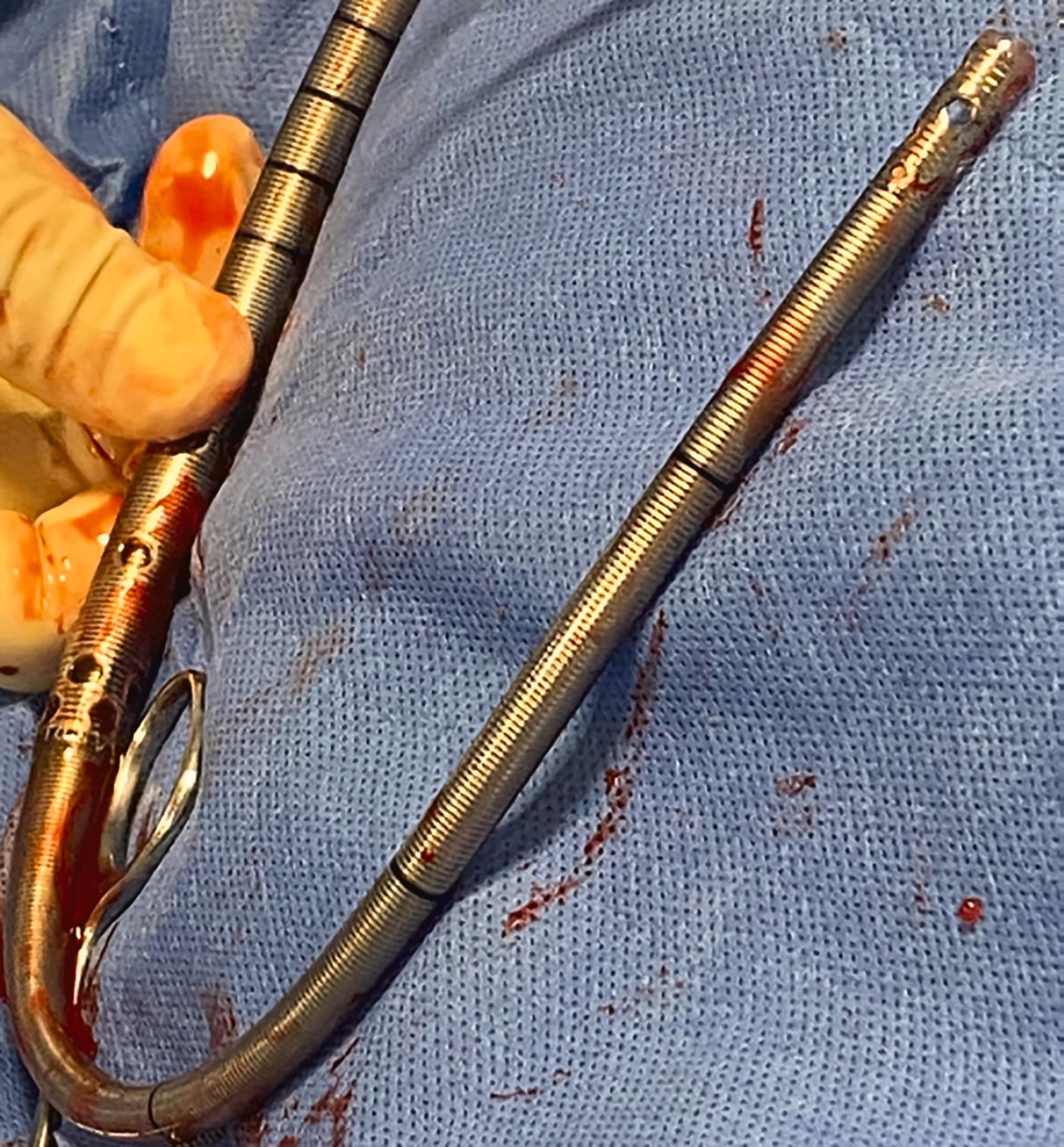
For isolated RV support, a single cannula approach can be employed. A dual-lumen right ventricular assist device (RVAD)-ECMO cannula ( Fig. 35.6 ), draws blood from the right atrium (RA) through three sets of inflow holes and returns it into the main pulmonary artery (PA). These devices have been used both to manage postcardiotomy RV failure and non-surgical RV failure from multiple causes including acute respiratory distress syndrome (ARDS), pulmonary embolism (PE), and acute MI.
VA ECMO can be used to support patients with cardiogenic shock from multiple etiologies including end-stage heart failure, acute MI, PE, drug intoxication, and refractory ventricular arrhythmia. It provides biventricular support, reduces cardiac preload, and increases afterload modestly. VA ECMO can be performed centrally (e.g., RA-ascending aorta) or peripherally (e.g., common femoral vein-common femoral artery). VA ECMO management is covered extensively in Chapter 27 .
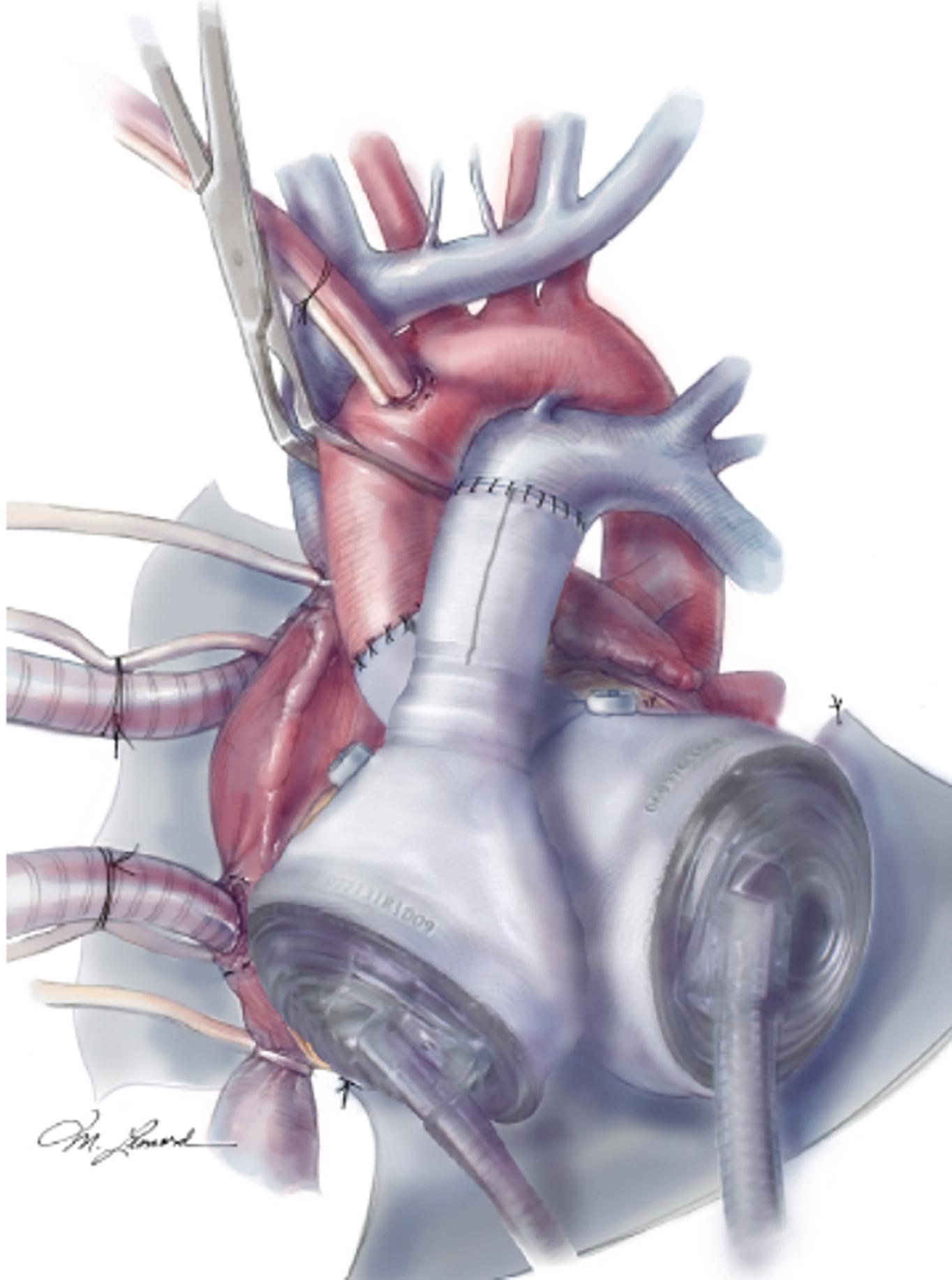
The CardioWest total artificial heart (Syncardia, Tucson, AZ) is a pneumatically driven biventricular VAD that has been implanted in more than 1800 patients worldwide ( Fig. 35.7 ). It is approved by the United States FDA as a bridge-to-cardiac transplantation. The device has two polyurethane artificial ventricles that each have a 70-mL stroke volume and two tilting disk valves (one inflow and one outflow) ( Box 35.3 ). The 70-mL device can deliver a CO of up to 9.5 L/min. Body size is an important consideration for patients who have total artificial heart implantation and a body surface area > 1.7 m 2 is required for the 70-mL device. In addition, the anterior-posterior chest distance (from posterior sternum to vertebral body) should be at least 10 cm. The total artificial heart can be used to treat biventricular failure from multiple causes, and is particularly useful in patients with refractory ventricular arrhythmias, as no cardiac rhythm is required for the device to function properly.
Two pneumatic artificial ventricles
Maximum stroke volume is 70 mL for each ventricle
Inotropes not necessary, vasopressors used to maintain a normal mean arterial pressure
No intrinsic cardiac rhythm necessary
Can tolerate lower hemoglobin concentration of approximately 6 g/dL
Durable LVAD management primarily with the HeartMate 3 is covered in detail in Chapter 22 .
The American Society of Echocardiography maintains detailed recommendations for the use of echocardiography in MCS management. Limitations include operator skill and experience and the quality of imaging windows. The latter is impacted by body habitus, patient positioning, implanted hardware, chest tubes, or air in the subcutaneous, pleural or mediastinal spaces, which occurs commonly after cardiothoracic surgery. In cases where transthoracic echocardiography (TTE) images are poor, TEE can produce superior images, but TEE is invasive and requires specialized training and certification. Regardless of the modality that is used, it is useful to have baseline reference images for comparison, so that changes in loading conditions, RV and LV function, and device position can be assessed.
TTE or TEE can be used for pump speed adjustment and assessment of cardiac recovery during bedside weaning (ie, ramp study). Both RV and LV function can easily be assessed, as device speed is decreased and preload increases. With TTE, the apical four-chamber view is useful for assessing both RV and LV systolic function simultaneously. Systolic function should be assessed subjectively and using quantitative measures such as tricuspid annular plane systolic excursion (TAPSE), the myocardial performance index, tissue Doppler annular velocity, and fractional area change. Global longitudinal strain and three-dimensional assessment of LV ejection fraction can be performed with appropriate hardware and software packages. Quantitative measurements of RV and LV internal diameters at end-diastole should be performed to assess chamber dilation.
Tricuspid regurgitation (TR) and mitral regurgitation (MR) can be assessed from the TTE apical four-chamber view. If severe functional TR or MR occurs as pump speed is decreased, it can be a sign of poor RV or LV function. RV outflow tract (RVOT) and LV outflow tract (LVOT) velocity time integrals (VTI) can be used to trend changes in RV and LV stroke volume as pump speed is changed. The RVOT VTI can be measured from a TTE parasternal RVOT view or TEE transgastric RVOT view. The LVOT VTI can be measured from a TTE apical five-chamber view or TEE deep transgastric view.
Adequate ventricular decompression is critical in MCS patients to reduce metabolic demand and prevent pulmonary (LV failure) and abdominal organ congestion (RV failure). While invasive hemodynamic monitors (e.g., PA catheter) or the MCS device itself (e.g., Impellas) can measure ventricular pressures, echocardiography allows for visualization of chamber size, comparison of RV and LV diameter, and quantification of valvular regurgitation, making it an important adjunct to invasive hemodynamic monitoring. When the LV is adequately decompressed, its end-diastolic diameter (LVEDD) should ideally be below the upper limit of normal (<5.5 cm). For a chronically dilated heart, this may not be possible, but current LVAD management guidelines recommend at least a 15% reduction in LVEDD with decompression. RV decompression can be assessed with TTE in the apical four-chamber or subxyphoid views. If RV or LV decompression is not adequate, potential corrective actions include increasing pump speed, LV venting during peripheral VA ECMO, or surgical closure of the aortic valve in patients with aortic insufficiency (AI) who have an LVAD.
Excessive unloading of the RV or LV can be problematic because it can cause ventricular suckdown. Suckdown can cause ventricular arrhythmias and also compromise ventricular function when the septal contribution to systole is impaired in the contralateral ventricle. In cases of suspected over-decompression or hypovolemia, TTE can be used to help adjust pump speed to a level where the interventricular septum is midline and there is no further suckdown. Ideally, the interventricular septum should be midline between the two ventricles when viewed in a TTE or TEE four-chamber view and in a normal position when the ventricles are viewed in a parasternal mid-papillary short-axis TTE view or transgastric mid-papillary short-axis TEE view.
Finally, all MCS devices require appropriate positioning for optimal function, and some devices can be repositioned using bedside echocardiographic guidance. TTE can be used to evaluate inflow cannula position for VADs in a variety of views and to confirm laminar blood flow (with low peak velocity) using color-flow and continuous-wave Doppler. IABP position is generally not confirmed using TTE, as visualizing the aortic-arch and descending thoracic aorta is challenging in most adults. However, TEE can be used to confirm or adjust IABP position. When correctly positioned, the IABP tip should be located approximately 2 to 3 cm below the left subclavian artery.
Other MCS devices for which echocardiography can be used to confirm correct position include Impellas, the TandemHeart VAD, and percutaneous RVAD-venovenous (VV) ECMO cannulas. The venous drainage cannula position in VA ECMO patients may be evaluated using TTE in the subxyphoid IVC view, but there is not much advantage over chest x-ray, except for evaluating IVC collapsibility around the cannula and thrombus on the cannula tip.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here