Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors thank Audrys G. Pauza, MSc, for his graphical assistance in preparing this chapter. The authors are also exceptionally grateful to Prof. José Jalife for his careful reading of the manuscript and friendly editorial assistance. Funding for this study was partly allocated from Grants MIP-11184 and MIP-13037 provided by the Research Council of Lithuania.
The mammalian sinoatrial node (SAN), including the human one, is a wedge-shaped structure situated at the junction of the superior vena cava (SVC) with the musculature of the terminal crest ( Fig. 38.1A ). The SAN is located beneath the epicardium of the terminal crest with a layer of atrial muscle interspersed between it and the endocardium. The node is always arranged about a central SAN artery. The SAN has a wide body and a thin head and tail, which are located more toward the endocardial surface than toward the body ( Fig. 38.2 ). The human SAN measures 30 mm in length, 2 mm in height, and up to 7 mm in width. Nodal cells are fascicular in nature and are embedded in a prominent fibrous tissue matrix. The cells around the SAN artery are more densely packed and less eosinophilic than those at the nodal borders. Nodal cells are smaller than the adjacent atrial myocardial cells, and a distinct border is observed between the node and adjacent atrial myocardium. Rabbit SAN cells are homogenously distributed in the head and body of the SAN but are patchily arranged in the tail. The mammalian SAN is a complex three-dimensional (3D) structure that is anatomically and functionally isolated from the atrial myocardium (crista terminalis and interatrial septum) except for several SAN exit pathways. , This conduction barrier is formed by the SAN arteries, connective tissue, and fat and by an abrupt change in connexin43 (Cx43) expression between atrial and nodal cells. , Cardiac conduction system (CCS) cells that are immunoreactive for hyperpolarization-activated cyclic nucleotide-gated potassium channel 4 (HCN4) and Cx45 but negative for Cx43 extend downward from the SVC in the interatrial groove and are capable of independent pacemaker activity. In the rabbit SAN, HCN4 immunoreactive cells spread as “cellular sleeves” from the main mass toward the right auricle and lateral wall of right atrium.
In the mouse heart, HCN4-immunoreactive SAN cardiomyocytes (CMs) are also widely distributed around the root of the SVC and form a well-defined horseshoe-shaped boundary by the side of the terminal groove and along the course of the SAN artery. Slender and narrow HCN4-immunoreactive myocytes from the main mass of the SAN extend toward the right auricle, the root of the right pulmonary vein (PV), and the root of the caudal vein. On electron microscopy, the SAN CMs, described as pale cells (P cells), contain sparse myofibrils, scanty sarcoplasmic reticulum (SR), and numerous glycogen particles and are evidently smaller in size compared with regular atrial CMs. The mouse SAN also involves another type of CMs called transitional cells (T cells), which contain a slightly increased number of myofibrils. Frequently, however, the structure of the SAN cells in rats and mice do not correspond with the classic description because the SAN pacemaker cells of these animals lack glycogen particles, their transverse profiles vary in size and shape, and the morphologic pattern of myofibrils of numerous conductive myocytes may be similar to myofibrils in regular atrial CMs. The nodal cells are positive for acetylcholinesterase (AChE), in contrast to the surrounding atrial myocytes, but this staining is much less intense than that displayed by neural AChE activity. Thus the structure and architecture of SAN cells in mammals appear to be more complex than previously considered.
Autonomic innervation of the heart involves both the extrinsic and intrinsic cardiac nervous systems. The former collectively includes the autonomic neurons in the brainstem or along the spinal cord (e.g., the vagosympathetic trunk) and their axons en route to the heart; the latter consists of the autonomic ganglia and axons located in the heart itself or along the great vessels in the thorax. Studies in different animal species demonstrate significant variations in morphologic pattern of cardiac innervations. In humans, as well as in other mammalians, extrinsic cardiac nerves access the heart via the heart hilum (HH). From the arterial part of the HH (i.e., around the ascending aorta and pulmonary trunk), nerves extend predominantly into the ventricles, whereas from the venous part of the HH (i.e., around the PVs and venae cavae), accessing nerves proceed on both the atria and ventricles ( Figs. 38.3 and 38.4 ). In the arterial part of the HH, plentiful nerves are regularly located in a fat pad between the ascending aorta and pulmonary trunk, but occasionally these nerves may be found in front of, behind, and on both sides of the aorta and pulmonary trunk. Within the venous part of the human HH, neural access sites are concentrated at the following locations: the left atrial nerve fold (ligament of Marshall), between the right superior PV (RSPV) and SVC, between the RSPV and left superior PV, between the left superior PV and left inferior PV, between the RSPV and right inferior PV, and, rarely, from the left and right sides of the inferior vena cava. Rarely, human hearts contain thin accessing nerves in front of and dorsal to the SVC. The number of nerves in any access and the thickness of the nerves on the HH level vary from heart to heart, but commonly, two to five nerves proceed through any neural access site.
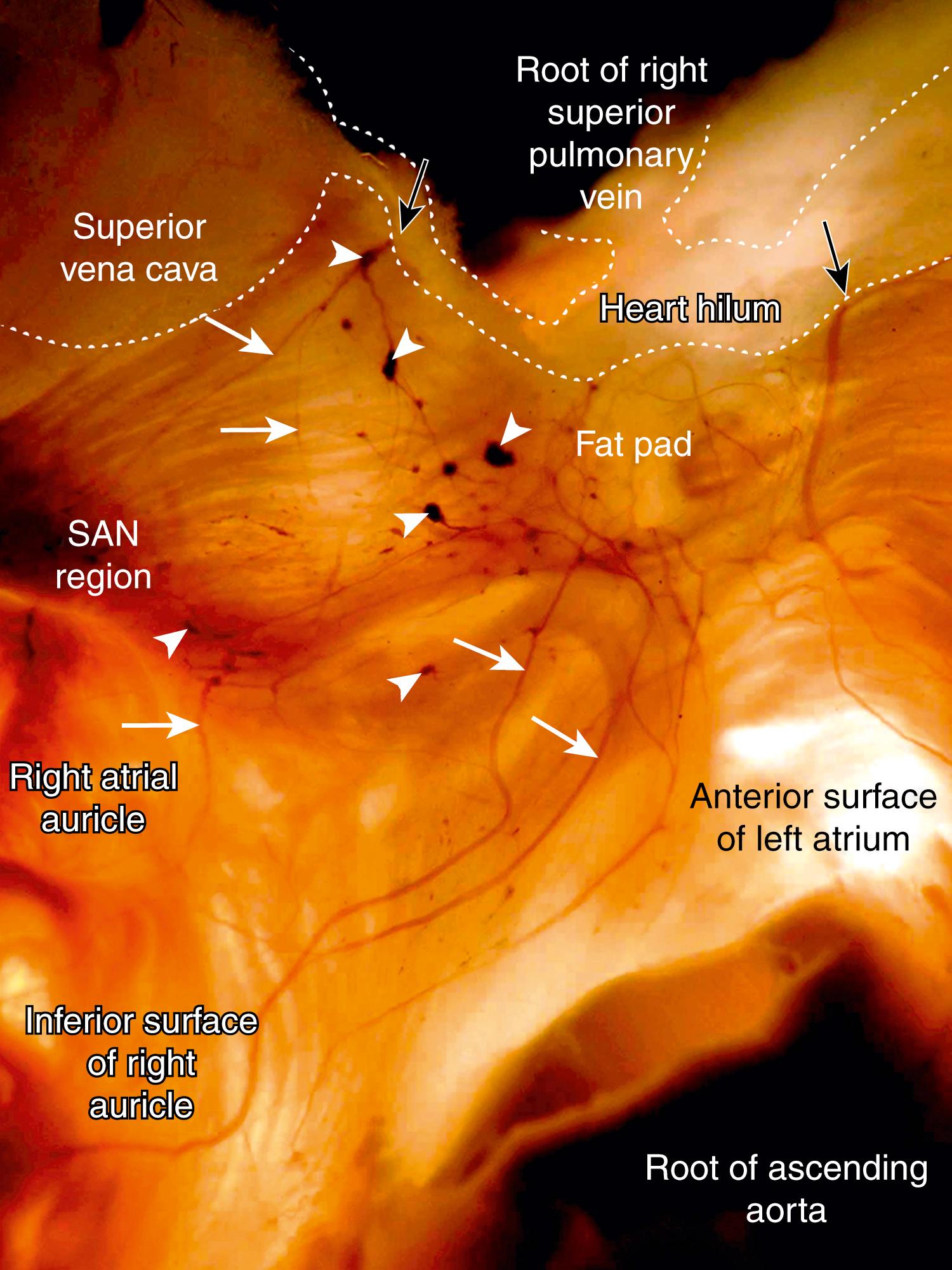
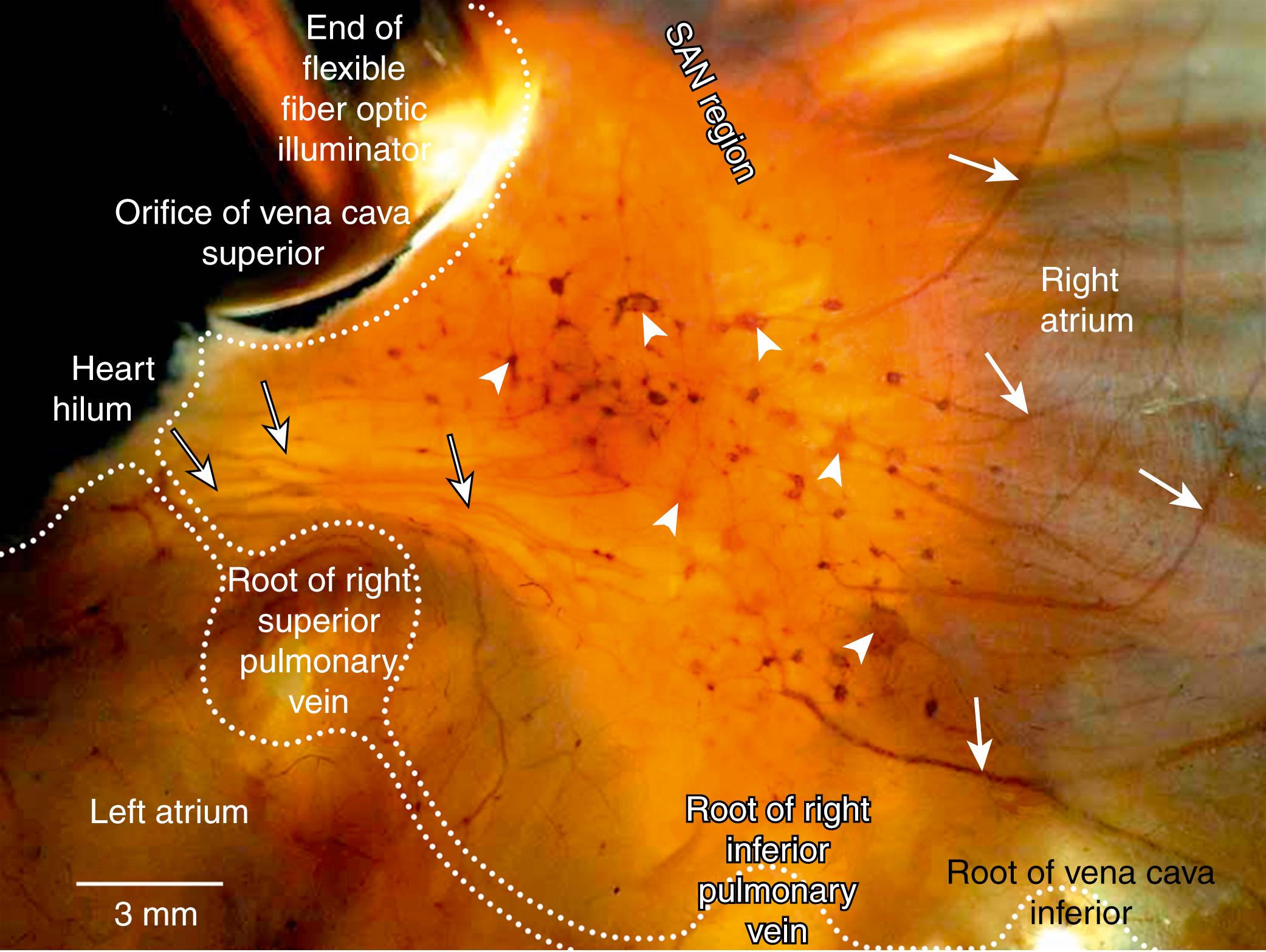
In the human heart, nerves from the accessing sites in the HH extend into the epicardium, in which they course by seven pathways. Two nerve pathways start from the arterial part of the HH. From there, the nerves extend to the left side by the left ventral coronary groove and to the right side by the right ventral coronary groove. Nerves that pass onto the heart through the venous part of the HH proceed by five pathways: the anterior interatrial groove and the groove situated dorsally between the roots of the SVC and RSPV nerves extending mainly to the right atrium, the left atrial nerve fold (ligament of Marshall) to the lateral surface of the left atrium, and the ventral and dorsal surfaces of the left atrium epicardial nerves, which course to the left atrium and dorsal wall of the left ventricle (LV). Very close to both parts of the HH, the nerves proceeding by these pathways reach the epicardial ganglia, which as ganglionated fields (GFs) are distributed in consistent regions, interconnecting complexly among themselves via thin nerves. Although sparse thin epicardial nerves also interconnect ganglia situated on different neural pathways, all GFs are clearly separated by wide regions of the heart surface, in which the epicardial neural plexus is devoid of ganglia. Within the boundaries of the GFs, epicardial nerves extending from the HH branch out, become thin, and pass into the ganglia. In GFs, some epicardial nerves originate from numerous intrinsic ganglia, appear thicker, and course away from GFs to pass directly into the structures they innervate, in which they gradually disappear from view in two ways: either via penetration into myocardium or by becoming gradually thinner in the epicardium (see Figs. 38.3 and 38.4 ). Because the latter nerves are mostly devoid of ganglia, they are called postganglionated nerves (postGNs), whereas others that proceed from the HH into the GFs are called preganglionated nerves (preGNs). Because of characteristic disposition, appearance, and interrelations of the preGNs, GFs, and postGNs, it has been suggested that the entire epicardial neural plexus of humans may be considered to be composed of seven ganglionated subplexuses, each of which contains its own preGNs, GF, and postGNs. The subplexal organization of the epicardial neural plexus has been confirmed in other mammalian species examined so far by employing the technique of histochemical staining of nerves in total heart preparations. , , , , The largest GFs, involving up to 84% of all cardiac ganglia, are distributed throughout the atria. The GFs of the different epicardial ganglionated subplexuses contain unequal numbers of ganglia, but their architecture is generally similar; that is, ganglia of various shapes and sizes are complexly interconnected in a network of thin epicardial nerves.
The human intrinsic cardiac ganglia range in size from those containing a few neurons to large ganglia measuring up to 0.5 mm by 1 mm and involving more than 2800 nerve cell bodies. , On average, the adult human heart contains about 800 epicardial ganglia that involve more than 43,000 neurons. Depending on the age, the number of intrinsic ganglia in one heart may significantly fluctuate. For instance, in human adult hearts, the mean number is 700, whereas in human fetuses, neonates, infants, and children, the mean is larger than 900. Importantly, the number of intrinsic cardiac ganglia and nerve cells strictly depends on species. The sheep heart contains 769 plus or minus 52 epicardial ganglia in which 17,000 neurons reside. The estimated total number of the intrinsic ganglia per porcine heart is 362 plus or minus 52, comprising about 12,000 neurons. The intracardiac neurons in adult guinea pigs are concentrated within 329 plus or minus 15 ganglia that enclose approximately 2500 nerve cells. The mean number of intrinsic cardiac neurons in old rats is nearly 6600, but juvenile rats contain significantly fewer such neurons at only 5000. Correspondingly, rabbit and mouse hearts involve nearly 2100 and 1100 intrinsic cardiac neurons, respectively. , ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here