Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Infectious diarrhea is a major cause of illness throughout the world with 2.39 billion episodes of diarrhea estimated in 2015, of which 957.5 million occurred in children under the age of 5 years. Despite the number of deaths attributed to diarrhea declining by 20.8% from 2005 to 2015, over 1.3 million people die of diarrheal diseases each year worldwide with the greatest impact on infants and children. Globally, the leading pathogens with the highest mortality are rotavirus, Shigella spp., and Salmonella spp.; in children under the age of 5 years, the leading pathogens with the highest mortality are rotavirus, Cryptosporidium spp., and Shigella spp. Although the total number of deaths attributed to diarrhea in the USA is low in comparison to the global burden, a recent study has shown diarrheal diseases caused by infection to be the only infectious disease with increasing mortality from 2000 to 2014 (2.41 deaths per 100,000 persons) in the USA. The leading diarrheal pathogens are Clostridioides difficile , rotavirus, Shigella , Campylobacter , and enterotoxigenic Escherichia coli (ETEC) with CDC surveillance programs estimating 47.8 million foodborne illnesses, most often diarrheal illnesses, to occur annually in the USA.
This chapter focuses on bacterial and viral diarrhea and infectious proctocolitis in adults. Disease due to many of the pathogens discussed is linked to ingestion of contaminated food or water ( Table 110.1 ). Other key topics in enteric infectious disease are covered in Chapter 16, Chapter 35, Chapter 36, Chapter 111, 112, Chapter 113, Chapter 114 .
| Pathogen | Foodborne (%) | Travel Related (%) |
|---|---|---|
| Nontyphoidal salmonellae | 94-95 | 11 |
| Shigella spp. | 58 | 15 |
| Campylobacter spp. | 80 | 20 |
| STEC ∗ | 68-85 | 3.5-18 |
| ETEC ∗ | 100 | 55 |
| Yersinia spp. | 90 | 7 |
| Noncholera Vibrio spp. | 50-57 | 7 |
| Noroviruses | 26-40 | 15 |
∗ ETEC , Enterotoxigenic Escherichia coli ; STEC , Shiga toxin-producing E. coli .
Acquisition of an enteric infection is the result of the interaction of host factors that typically protect against infection and microbial virulence factors that function to overcome host defenses. Emerging studies are exploring how host genetics and diet, acting through microbiota-dependent and -independent mechanisms, change host susceptibility to infection.
Gastric acidity is a crucial first-line host defense that ingested pathogenic bacteria and other pathogens must survive to infect the small or large intestine. In general, bacterial pathogens are highly susceptible to low pH with a pH below 4.0 being rapidly bactericidal. In contrast, achlorhydria facilitates bacterial survival during gastric passage. Consistent with the importance of gastric acid as a host defense, treatment with PPIs, and to a lesser degree the shorter-acting and less potent H2RAs, is a risk factor for bacterial gastroenteritis, including Salmonella , Campylobacter , and C. difficile infection, and viral gastroenteritis, including norovirus.
The intestinal epithelium provides multiple components that contribute to host protection against potential enteric pathogens. Commensal microbiota as well as potential enteric pathogens first encounter the mucus layer that coats the epithelium. The outer loosely organized luminal mucus layer serves as a habitat for commensal microbiota in the colon, whereas the inner gel-like mucus layer largely excludes direct bacterial-epithelial cell contact. Bacteria that do penetrate this layer are thought to be cleared rapidly by the host mucosal immune system.
Multiple cell types within the intestinal epithelium—including enterocytes, Paneth cells, goblet cells, and M cells—all help protect the host against enterocolitis. Mechanisms of protection include barrier formation and water efflux via the tight junctions of enterocytes, enterocyte and Paneth cell secretion of antimicrobial molecules, goblet cell secretion of mucins, and M-cell presentation of antigens to the mucosal immune system, initiating, in many instances, a protective mucosal immune response. Specific molecules on intestinal epithelial cells also contribute to resistance to pathogens. Of particular importance are pattern recognition receptors, which mediate the recognition of microbes and lead to the activation of innate and adaptive immune responses in the intestinal mucosa. The inflammatory responses that follow can serve to protect the host or, conversely, contribute to disease development. A key host protective mechanism is the production of secretory immunoglobulin A, which can be both nonspecific (e.g., through microbial agglutination) or specific, as part of a pathogen-specific adaptive immune response. Members of the commensal microbiota, as well as potential enteric pathogens, can initiate specific mucosal immune responses. Studies of these mucosal immune mechanisms have provided abundant and evolving insights into the complexity of host immune responses. Lastly, the enteric nervous system (ENS), which is involved with normal intestinal motility (see Chapters 99 and 100 ), also contributes to protect the host. Impaired intestinal motility in the setting of enteric infections, as well as hormones and molecules produced by the host, are known to influence disease severity.
The luminal resident microbiota population in the colon comprises 10 13 to 10 14 bacteria per gram of stool and has increasingly been recognized as a powerful contributor to resistance to colonization and disease development with enteric pathogens. This is arguably best illustrated by the marked clinical responses of recurrent C. difficile colitis to intestinal microbiota transplant therapy (see Chapter 112 ). Finally, emerging data from human and murine models are beginning to uncover ways in which host genetics and diet, functioning by microbiota-dependent and -independent mechanisms, change host susceptibility to intestinal infections (e.g., Card9 , a key innate immunity gene and IBD-associated risk gene controls virulence of Citrobacter rodentium in mouse models; human genetic variation in FUT2 is associated with a 9- to 26-fold increase risk of infection with some strains of norovirus and rotavirus ).
Bacterial pathogens have evolved various virulence factors and mechanisms that enable them to overcome host defenses, including adherence factors, enterotoxin and cytotoxin elaboration, and mucosal invasion among others. The ability of bacteria to adhere to host mucosal cells is critical to the initial interaction of each enteric pathogen with the intestinal epithelium. Numerous adhesins that differ in morphologic features and receptor specificities have been identified and vary in their capacity to mediate colonization in human compared with animal hosts. The complexity of adherence mechanisms is enhanced by the observation that particular bacteria express and use more than 1 adhesin, a redundancy that likely enhances bacterial virulence. Many bacterial adhesins recognize oligosaccharide residues of glycoproteins or glycolipids displayed in the mucus or surface of intestinal epithelial cells.
Enteropathogenic Escherichia coli (EPEC) cause disease, in part, because of tight intestinal epithelial adherence and serve as a classic model of the potential stages of adherence for enteric pathogens and their mechanistic complexity. EPEC initially exhibit nonintimate attachment to intestinal epithelial cells. This initial attachment is mediated by a bundle-forming pilus associated with a large plasmid common to EPEC isolates. Next, EPEC induce signal transduction events in the intestinal epithelial cells that lead to cytoskeletal changes in the enterocyte. Ultimately, intimate attachment of EPEC to the host cell membrane results, which is mediated by an EPEC outer membrane protein called intimin that is encoded by the eaeA gene cluster on the EPEC chromosome. This classic ultrastructural change, known as an attaching-effacing lesion , leads to elongation and destruction of microvilli, with classic pedestal formation ( Fig. 110.1 ). The role of the eaeA gene as a virulence factor that causes diarrhea in human EPEC infection has been confirmed in volunteer challenge studies.
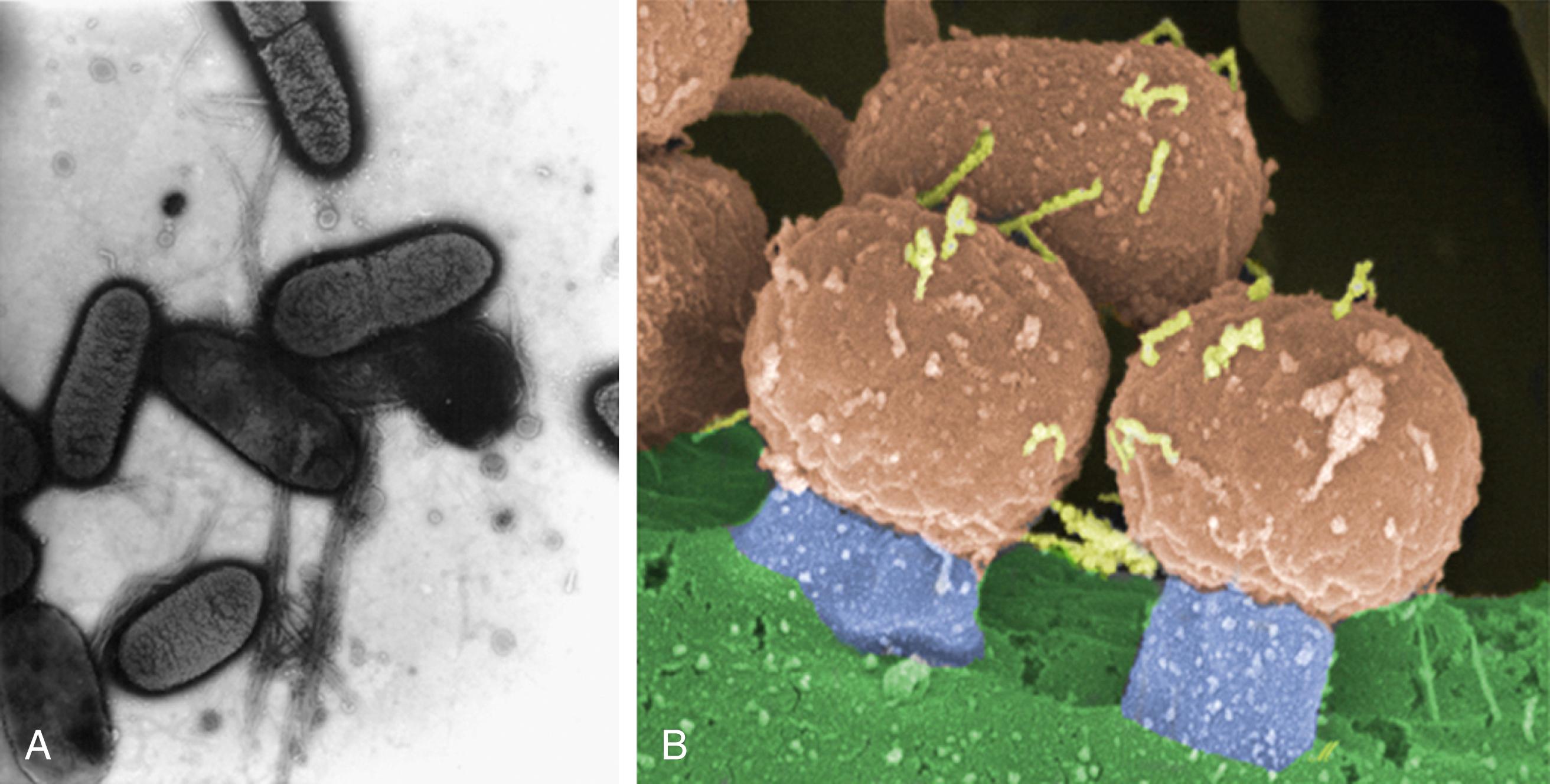
Nonintimate attachment using other adhesin molecules is typical of other noninvasive bacteria such as ETEC, and molecular studies have demonstrated that nonintimate attachment through surface protein antigens known as pili or fimbriae (also referred to as adherence- or colonization-factor antigens ) is required for a bacterium to colonize and be fully pathogenic. These adhesins bind to specific receptor sites on the surface of the intestinal cell via specific ligand-receptor interactions. Classic studies by Moon and coworkers, in which loss or gain of fimbriae by genetic manipulation resulted in the loss or gain of the ability to adhere to and colonize the intestine, identified that these colonization factors (CFs) are important to the pathogenesis of E. coli diarrheal disease in animals. Adherence not only permits colonization but also can facilitate delivery of enterotoxin to the epithelium.
Production of toxins, usually proteins, by enteric pathogens is a key mechanism that contributes to the expression of diarrhea after infection. Nonetheless, asymptomatic colonization by enteric pathogens known to produce toxins and other virulence factors is common. Enteric toxins can be classified by their functional effect on the intestinal epithelium or by their precise molecular mechanism of action. From a functional perspective, there are 2 major groups of enteric bacterial toxins: enterotoxins and cytotoxins. Classic enterotoxins such as cholera toxin or the enterotoxins of ETEC induce intestinal secretion largely without altering the morphology of the intestinal epithelium. The predominant site of action of most enterotoxins is thought to be the small intestine. In contrast, cytotoxins such as the C. difficile toxins or Shiga toxins (Stx) of Shigella dysenteriae and E. coli (e.g., E. coli O157:H7 among others) often act in the colon, where morphologic changes in the intestinal epithelium result through a variety of mechanisms such as by inducing epithelial cell injury or death, altering the cytoskeleton of the epithelial cells, or inducing an inflammatory response that results in injury to the epithelium. In addition, the actions of several enteric bacterial toxins, such as the C. difficile toxins, are known to be complemented by their ability to alter ENS activity, also likely contributing to disease pathogenesis.
Although the biologic activity of many potential enteric bacterial toxins has been abundantly identified, precise molecular mechanisms of action have been identified for relatively few. Classic mechanisms of action include alteration of intestinal epithelial cell cyclic nucleotide levels (e.g., cholera toxin, E. coli LT [heat-labile] or STa [heat-stable] toxins); inhibition of protein synthesis (e.g., Stx); modifications of actin cytoskeleton (e.g., C. difficile toxins [see Chapter 112 ]); and pore formation (e.g., Clostridium perfringens enterotoxin [see Chapter 111 ]). Other mechanisms likely contributing to induction of disease by enteric bacterial toxins include alteration of epithelial cell calcium signaling and changes in arachidonic acid metabolism among others. Ultimately, proof of the role of a particular enteric bacterial toxin in human disease is via volunteer studies and is infrequent. Examples of enteric bacteria and their toxins studied in humans include Vibrio cholerae , Shigella spp., and pathotypes of E. coli among others.
Disease induction by some enteric pathogens requires their ability to invade and multiply within intestinal epithelial cells, with resultant cell injury and possibly cell death that incites a mucosal inflammatory response. Shigella species, Salmonella species, Campylobacter jejuni , Yersinia enterocolitica , and some (enteroinvasive) strains of E. coli are classic examples of bacteria for which invasion contributes to disease pathogenesis. Although the colon is most often the primary site of pathology with invasive enteric bacteria, non- dysenteriae Shigella spp. also produce enterotoxins that stimulate small bowel secretion and contribute to the first phase of watery diarrhea seen in shigellosis. Subsequent colon colonization and cellular invasion by non dysenteriae Shigella spp. then results in colon inflammation with colitic symptoms and, occasionally, bloody diarrhea.
The Infectious Diseases Society of America (2017) and the American College of Gastroenterology (2016) have both published guidelines in recent years for the evaluation and management of acute diarrheal infections. Only about 10% of patients who develop diarrheal disease present for medical consultation. The initial step in the diagnostic evaluation of a patient with acute diarrhea is a thorough history and physical examination, the goals of which are to identify patients who may be at risk of severe illness or susceptible to complications, and to identify risk factors for infection and those who will benefit from specific therapy. Most patients simply need rehydration therapy. Consideration of the patient’s general health, severity and duration of illness, and the setting in which the illness was acquired should enable the clinician to determine who needs further evaluation ( Fig. 110.2 ).
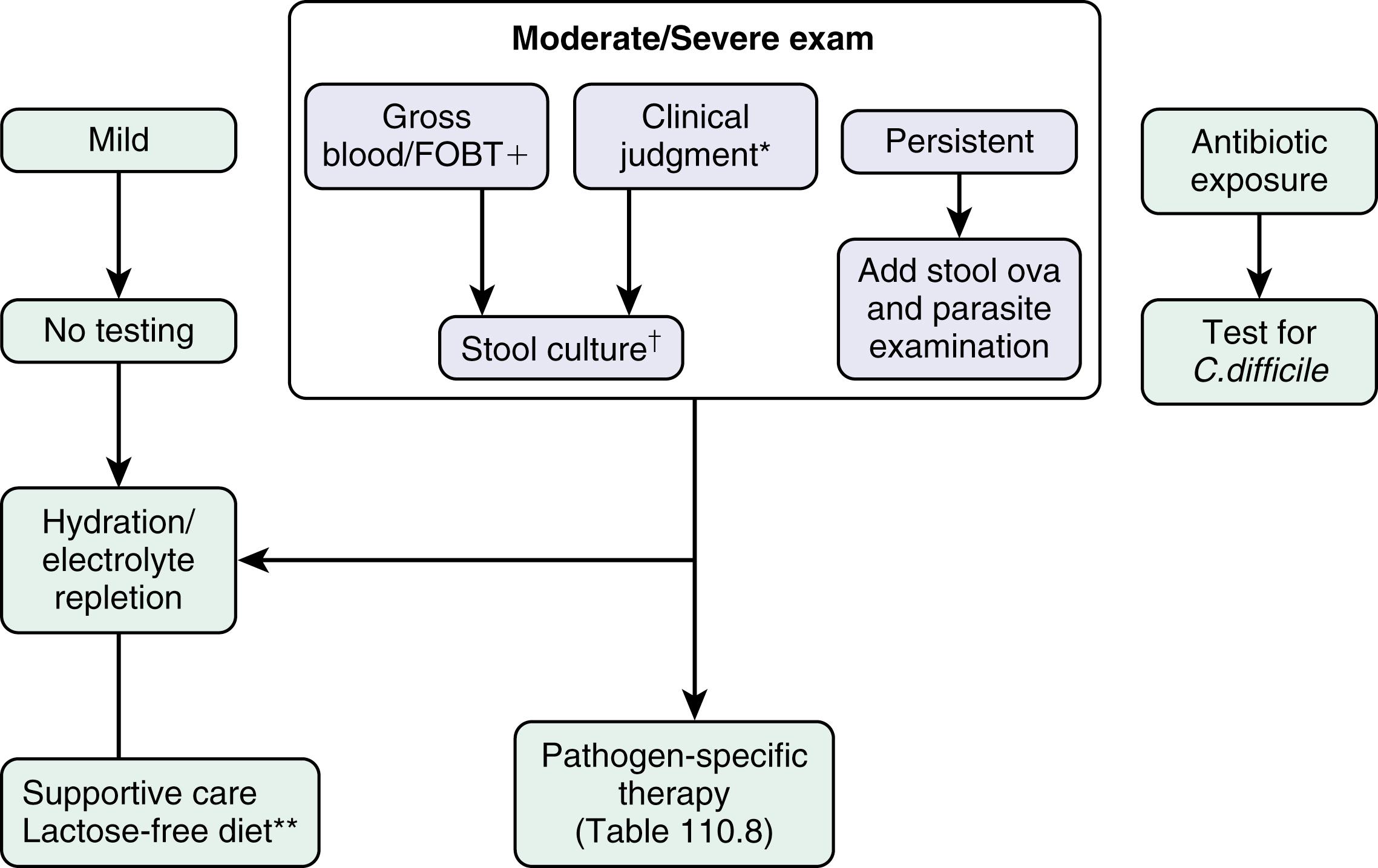
Patients who are debilitated, malnourished, or immunocompromised, and those who have severe comorbid illnesses, are at increased risk for complications of diarrhea and infection. The morbidity and mortality of infectious diarrheal diseases are highest in children younger than 5 years of age (particularly severe in those <2 years old ) and older adults. These high-risk patient groups may require hospitalization for diagnosis and treatment. Other patients who also require a more aggressive approach include those with systemic signs and evidence of inflammatory diarrhea, illnesses lasting greater than 3 to 5 days, a history or physical examination suggesting specific pathogens that will benefit from specific therapy ( Table 110.2 ), and infection with certain specific organisms (e.g., V. cholerae , Salmonella Typhi ). Bloody diarrhea can be considered a medical emergency and often requires hospitalization for diagnosis and management ; for example, Stx (Shiga toxin)-producing E. coli (STEC) infection can present with bloody diarrhea, and early, aggressive fluid management may diminish complications (e.g., renal failure) of this infection.
| Finding | Causative Organisms |
|---|---|
| Hemolytic-uremic syndrome/thrombotic thrombocytopenic purpura | STEC; most common with Shigella dysenteriae among Shigella spp., but S. dysenteriae is not endemic in the USA |
| Reactive arthritis ∗ | Salmonella spp., Shigella spp., Campylobacter spp., Yersinia spp. |
| Bone marrow suppression | Salmonella serovars Typhi and Paratyphi |
| Guillain-Barré syndrome | Campylobacter jejuni |
| Toxic megacolon | Shigella spp., Clostridioides difficile, Salmonella (rarely) |
| Aortitis/endovascular infection | Nontyphoidal salmonellae |
| Intestinal hemorrhage/perforation | Salmonella serovars Typhi and Paratyphi, TB enteritis |
| RLQ tenderness | Yersinia spp. |
| Cellulitis | Vibrio vulnificus and alginolyticus (see text) |
| Post-infection IBS ∗ | All, including viral gastroenteritis; viruses typically yield less severe post-infection IBS |
| Small bowel lymphoproliferative disease | Campylobacter jejuni |
∗ Can occur with any enteric pathogen. STEC, Shiga toxin-producing E. coli .
A traditional approach to help guide clinical diagnostic considerations has been the division of acute, presumably infectious, diarrheal disease into 2 broad clinical syndromes: a watery, noninflammatory diarrheal syndrome and an inflammatory diarrheal syndrome ( Table 110.3 ); a subgroup of the latter is the proctitis diarrheal syndrome. Classically, patients with noninflammatory diarrhea present with watery stools without visible blood or pus, and sometimes complain of severe abdominal pain. These patients generally have few systemic signs or symptoms, and fever often is absent; abdominal cramping, nausea, and vomiting can occur. This syndrome is most frequent in individuals infected with enterotoxigenic pathogens or viruses (see later). Many pathogens that cause inflammatory disease, however, can mimic this syndrome, particularly in the early phases of disease development (see later Shigella section).
| Characteristic | Inflammatory | Noninflammatory |
|---|---|---|
| Clinical presentation | Bloody, small-volume diarrhea; lower quadrant abdominal cramps; patients may be febrile and toxic | Large-volume, watery diarrhea; patients may have nausea, vomiting, generalized abdominal cramps |
| Site of involvement | Colon | Small intestine |
| Diagnostic evaluation | Indicated | Indicated if the patient is severely volume depleted or appears ill |
| Fecal leukocytes | Frequently present | Small numbers often present |
| Causes | Shigella spp., Salmonella spp., Entamoeba histolytica, Campylobacter spp., Yersinia spp., invasive Escherichia coli, Clostridioides difficile | Viruses, Vibrio spp., Giardia lamblia , enterotoxigenic E. coli , other enterotoxin-producing bacteria |
Classically, patients with inflammatory diarrhea present with numerous small-volume stools that may be visibly mucoid, grossly bloody, or both. Such patients may appear toxic and are more often febrile. Abdominal cramping may be severe. Because of the small stool volumes, these patients are less likely to be dehydrated than those with noninflammatory diarrhea. Organisms causing inflammatory diarrheas may vary in their clinical presentations ( Table 110.4 ), are typically invasive, and usually affect the colon (see Invasive Pathogens, later). The acute inflammatory diarrheal syndrome also can have a noninfectious etiology, such as UC, Crohn disease, radiation or ischemic colitis, diverticulitis, and idiosyncratic reactions to medications. Fig. 110.3 A-C shows the endoscopic appearance of selected inflammatory diarrheas.
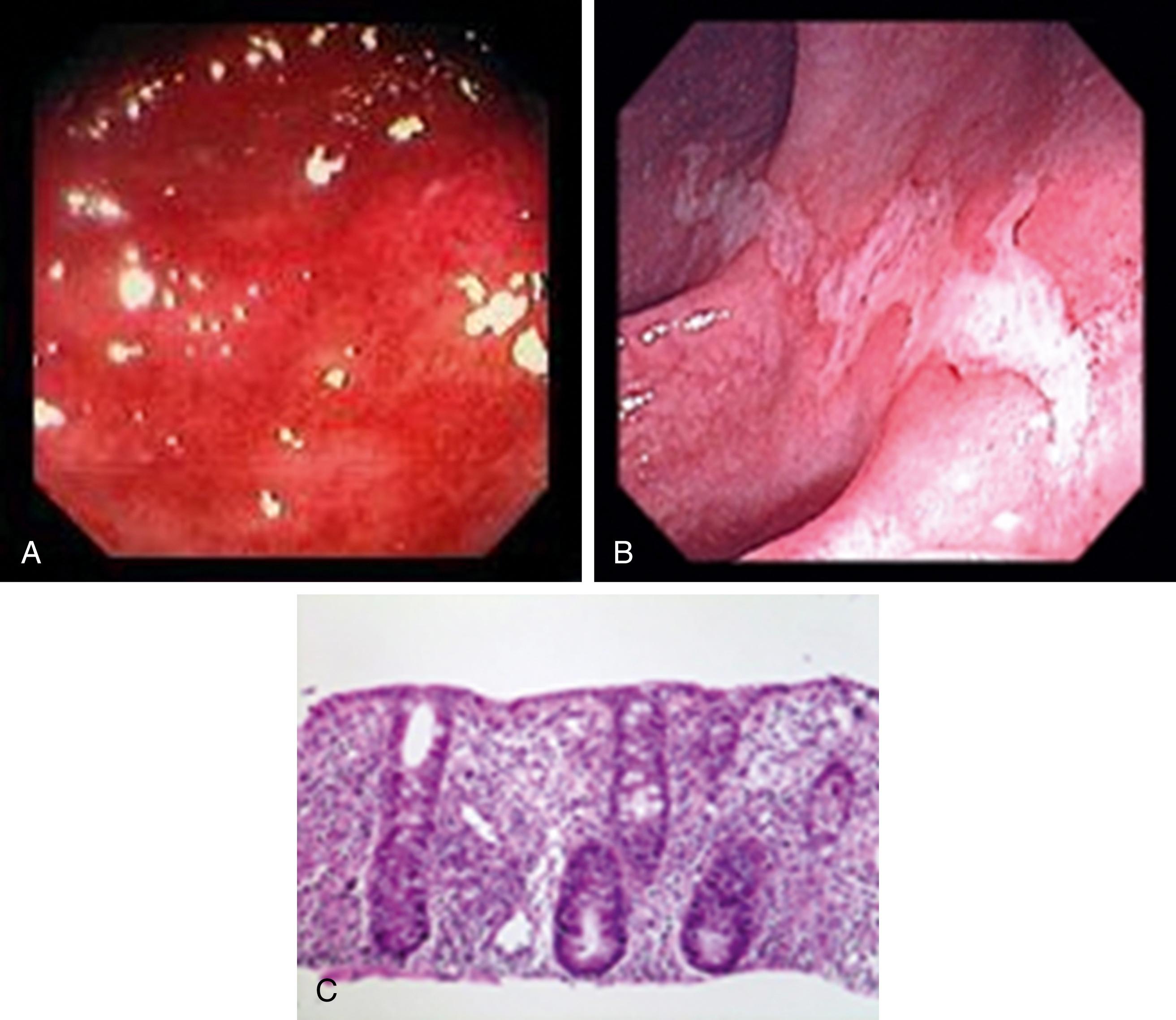
Proctitis syndrome is characterized by frequent painful bowel movements that contain blood, pus, and mucus. Tenesmus, often with rectal pain, usually is prominent. When acute, the most likely cause is one or more infectious agents (see Sexually Transmitted Infectious Proctitis, later). When a careful history reveals persistent, intermittent symptoms, noninfectious causes such as IBD (see Chapter 115 ) become diagnostic considerations.
Our growth in understanding the pathophysiology of infectious diarrheal diseases has led us to appreciate that inflammation is common to all infectious enterocolitides, with some infections (e.g., S. dysenteriae type 1) inducing dramatic colonic inflammation, and others (e.g., V. cholerae and ETEC ) inducing more modest small bowel or colon inflammation. Thus, the classic division of enteric pathogens into those inducing noninflammatory or inflammatory diarrhea is imperfect, and inflammation in response to enteric pathogens represents a continuum. Critical to the clinician, however, is identifying the patient at risk for morbidity or mortality due to infectious diarrheal disease. Most cases of watery diarrhea result in self-limited illnesses that require only advice on hydration maintenance and possibly diet. Evaluation of such patients who have mild diarrhea for less than 3 to 5 days is typically unnecessary. By contrast, patients who often require diagnostic evaluation are those with risk factors such as extremes of age, one or more immunocompromising conditions, marked stool frequency, bloody stools, or dehydration. Thus, the clinician’s assessment of the degree of illness, combined with the patient’s history, are key contributors that guide clinical judgment regarding diagnostic evaluations in diarrheal disease. Clinical or fecal evidence of acute inflammatory diarrhea or bloody diarrhea always deserves a diagnostic evaluation, and diagnostic evaluations, whenever possible, should precede initiation of empiric antibiotic therapy of diarrheal disease.
As already discussed, age is a primary determinant of diarrheal disease morbidity, with those at the extremes of age (i.e., <5 years and >65 years), particularly those living in long-term care facilities, at increased risk for poorer outcomes. Gastroenteritis outbreaks are common in nursing homes in high-income countries, with notable pathogens including norovirus, rotavirus, STEC, and C. difficile among others. Enhanced risk of diarrheal disease in older adults is due to numerous factors, such as comorbid medical conditions, altered immunity with senescence, intestinal dysmotility, exposure to medications including antibiotics, and drug-induced or acquired hypochlorhydria.
Immunocompromising conditions influence the risk of acquisition and disease severity for numerous enteric pathogens. Classic immunocompromising conditions include malignancy and its treatment, hemopoietic or solid organ transplantation, medications such as glucocorticoids or other immune modulators, and HIV infection. For example, lymphoproliferative diseases, glucocorticoid therapy, and HIV infection markedly increase the risk for salmonellosis. However, a number of other conditions, the immune effects of which are less well characterized, also enhance the risk of infection with enteric pathogens, including diabetes mellitus or liver disease (e.g., Vibrio vulnificus ), hemolysis (e.g., salmonellosis), and iron overload (e.g., yersiniosis).
Food exposures or ingestion of contaminated water also constitute major risk factors for acquisition of enteric pathogens (see Table 110.1 and Chapter 111 ). Ingestion of undercooked or raw foods (e.g., meats such as hamburger or chicken, oysters, clams, other seafood) and unpasteurized milk or juices (e.g., apple cider) is of particular concern. Travel, largely because of exposure to contaminated food or water, is a classic risk factor for acquiring infectious diarrheal disease (see “Travelers’ Diarrhea,” later). Animal exposures such as on farms or in petting zoos at county or state fairs (e.g., Campylobacter , Salmonella , STEC) or recreational exposures (e.g., camping or adventure tourism) also pose risk for enteric infections. Exposure to health care facilities, including hospitalization and treatment with antibiotics, increase the risk for C. difficile infection (see Chapter 112 ). Both gastroenteritis or asymptomatic carriage of enteric pathogens are common in children in day care centers and serve as a source for infections in adults caring for the children.
Pregnancy and the peripartum period enhance risk for transmission of enteric pathogens. A pregnant woman may transmit enteric pathogens carried asymptomatically to the newborn child, who may then develop clinical disease. Conversely, neonates or infants can transmit infection, particularly C. difficile , to their mothers or caretakers, because C. difficile infection is typically asymptomatic in children under the age of 1 year. Ingestion of delicatessen foods by pregnant women may lead to bacteremia, septicemia, meningoencephalitis, fetal loss, or neonatal infection from Listeria monocytogenes . Onset of these serious illnesses may occur up to 30 days after the implicated food exposure. Older adults and those with impaired immune systems are also at increased risk for invasive L. monocytogenes infection after contaminated food ingestion. In contrast, the immunocompetent host ingesting a food highly contaminated with L. monocytogenes develops abrupt but a short-lived diarrheal illness with fever.
Clinical distinction between infectious dysentery (diarrhea with blood and mucus in the stool) and IBD (particularly UC) can be difficult because diarrheal stools in both illnesses contain mucus and blood. Two features of infectious dysentery that distinguish it from UC are detection of a pathogen on diagnostic studies and a self-limited course that responds to antimicrobial therapy without relapse. Positive diagnostic tests for a pathogen in adults with diarrhea, however, are obtained in only about 50% of those studied.
Longitudinal studies in the 1990s provided some guidance in differentiating acute infectious colitis and early-onset IBD. Most patients with infectious colitis present early (within 1 week) and with fever to the physician; histopathology of the colon in infectious colitis also is different from that of chronic colitis in IBD (see later). In contrast, patients with IBD often have prior abdominal symptoms upon a careful history, less fever, and present later (>1 week after symptom onset). Travel may cause a patient to manifest either an acute exacerbation of IBD because chronic symptoms worsen as a result of travel-acquired enteric infection or de novo IBD that develops as a result of an altered intestinal microbiome superimposed on a genetic predisposition (see Chapter 115 ). Lastly, higher volume or frequency of diarrhea (especially >10 stools per day) is more commonly due to an infectious pathogen than IBD.
Histopathologic examination of colonic mucosa obtained by endoscopic biopsy can be helpful. Both the microbial form (dysentery) and various types of acute colitis typically show edema, neutrophils throughout the lamina propria, and superficial cryptitis with preservation of the normal tubular crypt pattern. Idiopathic UC, however, shows signs of chronicity, including lymphoplasmacytosis that typically involves the lower third of the lamina propria, and architectural crypt distortion (e.g., colonic glands with signs of regeneration such as branching). Resolving dysentery may have lymphocytes infiltrating the lamina propria, similar to UC, but crypt distortion and regeneration will not be present. Ultimately, there is no substitute for correlating clinical, endoscopic, and histopathologic features of disease to arrive at the proper diagnosis.
Laboratory diagnosis of acute diarrheal disease should be pursued in any patient judged as moderately to severely ill by the clinician (e.g., fever, toxicity); in patients with risk factors (see earlier) including exposure history (e.g., high-risk foods, travel, antibiotics); in patients whose symptoms suggest inflammatory diarrhea or who have bloody diarrhea; and in some patients with persistent diarrhea beyond 7 days. In general, most episodes of acute diarrheal illness in the USA are self-limited; diagnostic testing may be kept to a minimum, and treatment is aimed at preventing dehydration.
Recently published guidelines on the use of the microbiology laboratory for the diagnosis of infectious diseases provide a comprehensive resource on fecal testing to identify enteric pathogens including bacterial, viral, and parasitic agents). Crucial points in diagnostic testing are: (1) Only diarrheal stools (i.e., those that take the form of the container) should be tested; (2) Rectal swabs are inferior to stool culture for testing in adults (and rejected by many clinical microbiology laboratories) but perform similarly in children; (3) Communication with the clinical microbiology laboratory is essential because the diagnostic testing capabilities of clinical microbiology laboratories vary. In particular, if the clinician is concerned about a particular organism(s), this should be communicated to the laboratory; specific examples are STEC, Vibrio , and Yersinia testing, which may only be done on special request and may require the assistance of public health authorities; (4) With improving technology and increasing use of molecular, culture-independent tests, single stool specimens in adults detect approximately 90% of enteric pathogens and, in children, 98%. Thus, one sample for children and a second for selected adult patients is reasonable for diagnosis. In patients with the proctitis syndrome, 1 sample is typically adequate for diagnosis.
A range of tests are used to identify enteric pathogens, including bacterial and viral cultures, various pathogen-specific nucleic acid amplification tests (NAATs), and immunoassays (e.g., enzyme- or fluorescent-based; see later STEC section), ova and parasite examinations, and certain specialized stains. In recent years, multiplex molecular assays, testing for an array of bacterial, parasitic, and viral pathogens, have increasingly been used for diagnosis with greater than 97% specificity and greater than 98% sensitivity . Multiplex assays reduce cost and turnaround time for most pathogens compared with conventional methods, yet require selection of a pre-focused panel of pathogens and will detect viable and nonviable organisms. Of note, re-analysis of the Global Enteric Multicenter Study (GEMS) specimens using quantitative molecular diagnostics ascribed 89.3% of childhood diarrheal cases to a pathogen compared with 51.5% of cases using conventional diagnostics. These techniques changed the population-level characterization of disease incidence and are an important illustration of how historical and contemporary incidence data needs to be considered in the context of the assays used.
Conventional biomarkers to differentiate noninflammatory and inflammatory diarrhea are unreliable. Fecal examination for leukocytes is limited by the ability of most enteric pathogens to induce some inflammation and observations that even classic inflammatory pathogens (e.g., Salmonella , Shigella , C. difficile ) often do not induce a marked fecal leukocyte response ( Table 110.4 ); fecal calprotectin may be emerging as more useful , but is not elevated in all cases. Serum markers of inflammation, including ESR and CRP, are also insufficient for all cases.
| Symptom/Sign/Test | STEC O157:H7 | Campylobacter spp. | Salmonella spp. | Shigella spp. |
|---|---|---|---|---|
| % | ||||
| Fever | 41.4 | 50.9 | 69.4 | 56.6 |
| Abdominal pain | 72.0 | 45.4 | 28.8 | 33.5 |
| Bloody stool | 91.3 | 37.0 | 33.8 | 54.3 |
| Gross blood | 63.0 | 7.8 | 4.8 | 14.7 |
| Occult blood | 82.8 | 52.0 | 43.4 | 59.1 |
| Fecal leukocytes | 70.5 | 42.9 | 29.4 | 37.8 |
| Peripheral leukocyte count > 10,000/mm 3 | 70.9 | 42.0 | 45.3 | 58.0 |
More invasive investigations, including flexible sigmoidoscopy with biopsies and upper GI endoscopy with duodenal aspirate and biopsies, are reserved for special situations such as the immunocompromised host in whom stool examination has not yielded a diagnosis. Flexible sigmoidoscopy can be useful in evaluating patients with proctitis, tenesmus, or sexually transmitted diseases (STDs), or in identifying the pseudomembranes of C. difficile infection.
The prototypic organisms in this group are V. cholerae and ETEC, both of which elaborate enterotoxins that cause dehydrating diarrhea. The salient characteristic of diarrhea caused by V. cholerae and ETEC is that disease primarily results from intestinal fluid loss, which is related to the action of the enterotoxin on the small intestinal epithelial cells. These organisms usually do not invade the mucosal surface, and thus, mucosal architecture remains intact. The fecal effluent is watery and often voluminous, producing clinical features of dehydration. Bacteremia is rarely a complication of toxigenic diarrhea.
Cholera, the prototypical toxigenic diarrhea, can cause dehydration and death within a few hours of onset. Stool output can exceed 1 L/hr, with daily fecal outputs of 15 to 20 L if parenteral fluid replacement keeps up with losses. The acutely ill patient typically has marked signs of dehydration: poor skin turgor, “washerwoman’s” hands, absent pulses, reduced renal function, and hypovolemic shock.
More has been learned about pathophysiology—and normal intestinal function—from cholera than from any other intestinal disease.
First described in choleric stool by Filippo Pacini in 1854, V. cholerae is a gram-negative, short, curved rod that looks like a comma. It is actively motile by means of a single polar flagellum. Vibrios are strongly aerobic and prefer alkaline and high-salt environments.
More than 200 serogroups of V. cholerae have been described, based on the O antigen, a cell wall lipopolysaccharide. Epidemic and pandemic disease are only caused by the O1 and O139 serogroups. The clinical features of infection with the V. cholerae O139 strain are virtually indistinguishable from infection caused by V. cholerae O1. To date, V. cholerae O139 has remained confined to Southeast Asia, and the incidence has declined in most areas, save for pockets in China and Thailand. V. cholerae strains that fail to agglutinate in O1 or O139 antisera are referred to as non-O1 non-O139 V. cholerae and, in addition to diarrhea, occasionally cause severe extraintestinal infections, particularly in compromised hosts.
The O1 serogroup is composed of 2 biotypes, classical and El Tor, which are differentiated on the basis of biochemical characteristics, biotype-specific genes, or both. Further differentiation into 3 serotypes is based on type-specific O antigens (A, B, C: Ogawa [A, B]; Inaba [A, C]; Hikojima [A, B, C]). The major serotypes associated with clinical disease are Inaba and Ogawa, and rarely Hikojima.
Toxigenic V. cholerae that agglutinates in O1 antiserum is the main cause of epidemic cholera. El Tor biotype V. cholerae is responsible for the current pandemic that began in 1961 in Indonesia. El Tor vibrios are somewhat hardier than others in nature. Compared with the classical biotype, the El Tor biotype generally causes a milder disease with a higher frequency of inapparent infection—although there are increasing reports of El Tor variants demonstrating hypervirulence {MS Son 2011 Characterization of V Cholerae O1 El Tor biotype}. A hypervirulent variant El Tor strain caused the Haitian outbreak and ongoing endemic disease following the devastating earthquake in 2010.
Among the more than 200 serogroups that comprise the V. cholerae species, only serogroups O1 and O139 typically carry the cholera toxin genes. Cholera toxin (CT) is an 84-kd heterodimer composed of 5 B subunits that encircle a single A subunit. The B subunit is responsible for binding to the monoganglioside GM1 receptor on intestinal epithelial cells. The A subunit is responsible for activation of adenylate cyclase located on the basolateral cellular membrane. The genetic material for CT is contained on a filamentous bacteriophage, CTXÖ, which integrates into the bacterial chromosome or replicates as a plasmid. V. cholerae also produces additional toxins that may contribute to diseases, including the zonula occludens toxin, that alter intestinal permeability by acting on intestinal epithelial cell tight junctions, and the accessory cholera enterotoxin.
Seven cholera pandemics have occurred in the last 200 years. The seventh pandemic is ongoing and originated in Indonesia in 1961, spread through Asia, Africa, and South America, and is caused by the El Tor biotype. Cholera cases are underreported, but a common World Health Organization (WHO) estimate of the global disease burden is 1.4 to 4.0 million cases with 21,000 to 143,000 deaths annually, primarily focused in Sub-Saharan Africa, Asia, and the Caribbean. V. cholerae is the leading cause of diarrhea-associated death in children aged 5 to 14 years. The largest current outbreak and humanitarian crisis is in Yemen and caused by the El Tor serotype Ogawa, with estimates of over 1 million people infected to date in the wake of the ongoing civil war. Cases of toxigenic V. cholerae infection remain few in the USA with only 7 patients reported to the CDC in 2014; all infections were travel-associated.
Cholera has both endemic and epidemic phases, with epidemics often superimposed on existing endemic cholera. The primary vehicle for spread of cholera is contaminated food and water, and a high inoculum dose (~10 8 to 10 11 organisms) is typically required for infection. There is also a complex aquatic environment reservoir, likely including copepods, zooplankton, aquatic vegetation, and water fowl, which maintains low levels of V. cholerae . Cholera has marked seasonal variation and may emerge as a public health problem as a result of increased growth with warming of the environmental reservoir. Spikes in aquatic V. cholerae counts are associated with human disease. Individuals with mild hypochlorhydria may represent a high-risk population. Once cholera enters the human population, transmission will often become direct (human-to-human) via contact with feces or immediate contamination of food or water in the household. So-called rice water feces (see later) contains high concentrations of V. cholerae organisms, which are hyperinfectious for about 5 to 24 hours after passage. In epidemics, it appears that the majority of infections are due to direct transmission. Locations with dense populations, poor sanitation, limited health infrastructure, and logistical issues, such as the conditions present in Haiti following the 2010 earthquake or currently in Yemen, all contribute to direct transmission.
The clinical syndrome of cholera is caused by the action of the toxin on intestinal epithelial cells. Cholera toxin increases adenylate cyclase activity, resulting in elevated levels of cyclic adenosine monophosphate in the intestinal epithelial cells, which, in turn, causes intestinal secretion. Fluid loss in cholera originates in the small intestine. The most sensitive areas are the upper intestine, particularly the duodenum and upper jejunum; the ileum is less affected, and the colon is relatively insensitive to the toxin. Diarrhea results because the large volume of fluid produced in the upper intestine overwhelms the absorptive capacity of the colon.
Attachment of V. cholerae to the intestinal mucosa is mediated by various surface components, including a fimbrial CF known as toxin-coregulated pilus . The toxin-coregulated pilus attachment protein might play an important role in producing naturally occurring protective antibodies against V. cholerae .
Despite the derivation of the term cholera (Greek: chole , bile), the appearance of choleric stools resembles rice water; that is, the stool has lost all pigment and becomes a clear fluid with small flecks of mucus. The electrolyte composition ( Table 110.5 ) is isotonic with plasma, and the effluent has a low protein concentration. On microscopic examination of stool during V. cholerae O1 infection, there are typically few inflammatory cells and only small numbers of shed mucosal cells; V. cholerae O139, however, induces more intestinal inflammation.
| Type of Fluid | Electrolyte Concentrations (mmol/L) ∗ | |||
|---|---|---|---|---|
| Sodium | Potassium | Chloride | Bicarbonate | |
| Choleric Stool | ||||
| Adult | 124 | 16 | 90 | 48 |
| Child | 101 | 27 | 92 | 32 |
| Fecal Fluid in Nonspecific Diarrhea (Child) | 56 | 25 | 55 | 14 |
| IV Therapy Solutions | ||||
| Lactated Ringer’s | 130 | 4 | 109 | 28 † |
| 5:4:1 ‡ , § | 129 | 11 | 97 | 44 |
| 2:1 || | 141 | — | 94 | 47 |
∗ mmol/L = mEq/L for univalent ions
† Equivalent concentration after lactate conversion to bicarbonate.
‡ Add glucose, 110 mmol/L (20 g/L).
§ IV solution that is 5 g of sodium chloride, 4 g of sodium bicarbonate, and 1 g of potassium chloride per liter.
Cholera vibrios (other than O139) do not invade the mucosal surface, and bacteremia is virtually unknown in this disease. A biopsy specimen taken from the mucosa during acute cholera largely shows normal architecture, in sharp contrast to the inflammatory and ulcerating lesions associated with Salmonella and Shigella .
Like many other infectious diseases, there is a spectrum of clinical manifestations with V. cholerae , ranging from an asymptomatic carrier state to a desperately ill patient with severe dehydration. Notably, in field situations, the clinical case rate is approximately 0.26%; that is, for every clinical case of cholera, there are approximately 400 asymptomatic people who have had contact with the organism, as demonstrated by an elevation in vibriocidal antibody titers. Thus, acquired immunity, genetic determinants (e.g., blood type), and intestinal microbiota modulate disease expression. In clinical cholera, the initial stage is characterized by vomiting and abdominal distention and is followed rapidly by diarrhea that accelerates over the next few hours to frequent large-volume rice-water stools. All the clinical symptoms and signs can be ascribed to fluid and electrolyte losses. Patients present with profound dehydration and hypovolemic shock, often leading to kidney failure. The stool is isotonic with plasma, although there is an inordinate loss of potassium and bicarbonate, with resultant hypokalemic acidosis (see Table 110.5 ). Mild fever may be present, but there are no signs of sepsis.
Treatment of acute cholera is based on the physiologic principles of restoring fluid and electrolyte balance and maintaining intravascular volume. These objectives are accomplished with IV solutions or oral fluids that contain electrolytes in isotonic concentrations (see Table 110.5 ). Particular attention is paid to administration of bicarbonate and potassium, which are lost excessively in choleric stool. Various oral rehydration solutions (ORS) have been developed for treating mild-to-moderate cases ( Table 110.6 ).
| ORS | Carbohydrate (g/L) | Sodium (mmol/L) | Potassium (mmol/L) | Chloride (mmol/L) | Base (mmol/L) ∗ | Osmolarity (mOsm/L) |
|---|---|---|---|---|---|---|
| WHO (2002) † | 13.5 | 75 | 20 | 65 | 30 | 245 |
| WHO (1975) | 20 | 90 | 20 | 80 | 30 | 311 |
| Enfalyte ‡ | 30 | 50 | 25 | 45 | 34 | 167 |
| Pedialyte § | 25 | 45 | 20 | 35 | 30 | 250 |
| Naturalyte || | 25 | 45 | 20 | N/A | 48 | 265 |
| Pediatric Electrolyte ¶ | 25 | 45 | 20 | N/A | 30 | 250 |
| CeraLyte ∗∗ | 40 | 50-90 | 20 | N/A | 30 | 220 |
| Commonly Used Beverages (Not Appropriate for Diarrhea Treatment) | ||||||
| Apple juice †† | 120 | 0.4 | 44 | 45 | N/A | 730 |
| Coca-Cola Classic ‡‡ | 112 | 1.6 | N/A | N/A | 13.4 | 650 |
| Gatorade §§ | 58.3 | 20 | 3.2 | 11 | N/A | 299 |
∗ Actual or potential bicarbonate (e.g., lactate, citrate, or acetate). As presented, mmol/L = mEq/L.
† The European Society of Pediatric Gastroenterology, Hepatology and Nutrition recommends reduced osmolarity ORS (50-60 mmol/L Na+) as first-line therapy for children with acute gastroenteritis due to increased effectiveness for rehydration in children (Guarino A, Ashkenazi S, Gendrel D, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014; 59(1):132-52. https://doi.org/10.1097/MPG.0000000000000375 ).
‡ Mead-Johnson Laboratories, Princeton, NJ. Additional information is available at http://www.mjn.com/app/iwp/HCP/Content2.do?dm=mjid=/HCP_Home/Product_Information/Product_Descriptions/Enfalyteiwpst=B2Cls=0csred=1r=342 .
§ Ross Laboratories, Abbott Laboratories, Columbus, OH. Data regarding flavored and freezer pop Pedialyte are identical. Additional information is available at http://www.pedialyte.com .
|| Unico Holdings, Lake Worth, FL. Additional information is available at http://www.unico-holdings.com .
¶ Nutramax Products, Gloucester, MA. Additional information available at http://www.nutramax.com/ .
∗∗ Cera Products, L.L.C., Jessup, MD. Additional information available at http://www.ceralyte.com/index.html .
†† Meeting U.S. Department of Agriculture minimum requirements.
‡‡ Coca-Cola Corporation, Atlanta, GA. Figures do not include electrolytes that might be present in local water used for bottling. Base=phosphate.
§§ Pepsico, Purchase, NY. Additional information available at http://www.gatorade.com .
The simple therapeutic principles of fluid replacement and antibiotic use save many lives. This knowledge has been available only since 1970; before then, the mortality rate for cholera was 50% to 75%. Application of these physiologic principles reduces the mortality rate in adults to less than 1%, as exemplified by such a mortality rate in the Peruvian epidemic in 1991. Children with cholera still have a mortality rate of 3% to 5% because fluid reserves are limited in young children.
Antimicrobial agents are helpful ancillary measures to treat cholera, because their use reduces stool output, duration of diarrhea, fluid requirements, and Vibrio excretion. The CDC and WHO recommend doxycycline as a single oral dose of 300 mg for nonpregnant adults. A single oral dose of azithromycin 1 g is recommended for pregnant women, and a dose of 20 mg/kg is recommended for children, not to exceed 1 g. Antimicrobial therapy usage should be tailored to local V. cholerae susceptibility patterns when known. Zinc supplementation, through complex actions including improvement of immune responses, has been shown to reduce the duration and volume of diarrhea among children with cholera in Bangladesh and may be a useful adjunct to standard therapy. Antimicrobial resistance (e.g., to fluoroquinolone antibiotics) is an increasing concern, but because of risk of direct transmission, antibiotics are indicated for moderate and severe cholera.
The WHO has prequalified a 2-dose regimen killed oral cholera vaccine and currently recommends its use in endemic areas and areas at risk for outbreaks. Since the global stockpile was amassed in 2013, almost 13 million doses have been delivered. Recent experience with reactive vaccination in Zambia has supported use of the first dose alone for short-term protection. The current focus of the WHO is to deploy vaccines and improve local infrastructures to ensure clean water supply and sanitation, committing to a goal of eliminating cholera outbreaks worldwide by 2030. Currently, no vaccines for the prevention of cholera are available in the USA, nor is cholera vaccination recommended by the CDC for most travelers.
In general, non-O1/O139 serogroups do not carry cholera toxin genes and cannot cause epidemic diarrheal disease, but rather typically cause mild sporadic diarrheal illnesses. There are, however, examples of non-O1/O139 serogroups that do produce cholera toxin and have caused outbreaks of cholera-like illness in the USA. Strains within the same species can produce different enterotoxins, cytotoxins, and hemolysins. The diversity of toxin production is matched by the diversity of clinical symptoms: diarrhea ranges from watery diarrhea to frank dysentery; some strains penetrate the intestinal mucosa, causing bacteremia or septicemia, sometimes with secondary end-organ involvement; others have been incriminated in wound or ear infections after exposure to ocean water or handling raw seafood.
The most common antecedent history is consumption of raw oysters within the preceding 72 hours. Other seafood such as clams, mollusks, and crab all have been implicated in non-O1/O139 vibrio disease. In outbreaks, there is a high attack rate, with incubation periods that range from as short as 6 to 12 hours to as long as 3 days. Over 90% of non-O1/O139 vibrios produce a polysaccharide capsule, and heavily encapsulated strains are associated with greater septicemia rates relative to unencapsulated strains. Bacteremia can occur and is most common in patients with cirrhosis, diabetes, or other immunocompromising conditions. Because the GI disease is typically self-limited and relatively benign in the USA, antibiotics are not recommended; however, septicemia, wound infections, and deep organ infections should be treated with appropriate antibiotics.
V. parahaemolyticus causes an acute diarrheal disease after consumption of contaminated raw fish or shellfish. Strains of V. parahaemolyticus produce a number of distinct hemolysins, the most significant of which is responsible for the Kanagawa phenomenon (i.e., hemolysis of human red blood cells in Wagatsuma bacteriologic medium). Kanagawa-positive isolates are pathogenic for humans, whereas Kanagawa-negative strains are nonpathogenic members of the marine environment.
V. parahaemolyticus gastroenteritis is the most common cause of seafood-borne illness worldwide, and the leading cause of foodborne outbreaks in Japan, India, China, Taiwan, Korea, and Malaysia. According to the CDC’s Cholera and Other Vibrio Illness (COVIS) Annual Summary, 2014, there were 605 reported cases of V. parahaemolyticus and 4 deaths. Cases of all vibrio disease (excluding toxigenic V. cholerae ) have been increasing in the USA since the mid-1990s, an increase that has been driven largely by increasing rates of V. parahaemolyticus disease. Cases tend to be clustered along coastal states where shellfish consumption and seawater exposure are common. Rising seawater temperature has been proposed to promote outbreaks.
The median attack rate of V. parahaemolyticus in foodborne outbreaks in the USA is reported as 56% and varied from 3% to 100% of exposed persons with a median incubation of 17 hours (range, 4 to 90 hours). Seafood, or cross-contamination with seafood, was the food vehicle in all outbreaks.
V. parahaemolyticus causes both foodborne and nonfoodborne disease, and the clinical presentation may vary depending on the route of exposure. In the USA, nonfoodborne V. parahaemolyticus infection represents only 11% of all V. parahaemolyticus infection, but constitutes 19% of all nonfoodborne Vibrio spp. infections. Bacteria were most commonly isolated from wounds (79%), blood (10%), and the ear (6%). Cases tended to have fever (42%) and cellulitis (67%) and were often associated with swimming (61%), walking (49%), and boating (29%). Only 6 deaths (3%) among 216 V. parahaemolyticus infections were reported.
Foodborne outbreaks in the USA are characterized by diarrhea with associated cramping, nausea, and vomiting. Outbreaks are most common between April and November. The median reported duration of illness was 2.4 days (range, 8 hours to 12 days). A single death was reported out of 1064 cases in outbreaks from 1973 to 1998. In the USA from 1973 to 1998, 5% of sporadic V. parahaemolyticus infections presented as primary septicemia, of which over 90% had a history of recent oyster consumption; 29% of these septicemic patients died. In Asia, V. parahaemolyticus infection has presented as a dysentery-like syndrome, but this is rarely reported in the USA.
V. vulnificus is perhaps the most important noncholera Vibrio species in the USA because it is the most lethal species, especially in patients with underlying liver disease, diabetes mellitus, or other compromising conditions. In 2014, COVIS reported 124 cases, of which 79% were hospitalized and 18% died. V. vulnificus can be acquired as a wound infection, often with characteristic bullous, even hemorrhagic, necrotizing, skin lesions; through salt water exposure; or by direct consumption of seafood, usually raw oysters; the mortality rate of resulting septicemia exceeds 50%. Because this infection can be fatal in patients with underlying liver disease, such persons should be warned to avoid eating raw seafood, especially oysters.
In the USA, Vibrio alginolyticus is the second leading cause of wound infection and the leading cause of ear infections among Vibrio species; it is not commonly associated with foodborne vibriosis. Vibrio mimicus acquires its name from its similarity to cholera vibrios, even in producing an enterotoxin that resembles CT, and is now believed to share a common ancestor with sixth pandemic V. cholerae. The organism has been isolated from patients in the USA with diarrhea, septicemia, or wound infections. Vibrio fluvialis has a wide geographic distribution, although human disease is less common than from other Vibrio species. Other pathogenic Vibrio species include Grimontia hollisae (formerly V. hollisae ) , Photobacterium damselae subsp. damselae ( formerly V. damsel), V. harveyi, V. metschnikovii, and V. furnissii . Disease expression is similar to other non-O1/O139 vibrios and includes watery, even bloody, diarrhea, wound infections, and/or bacteremia/septicemia.
Recommendations for antimicrobial therapy for noncholera vibrio infections are primarily based on animal studies. The role of antimicrobial therapy in V. parahaemolyticus gastroenteritis is not clear because symptoms tend to be mild and self-limiting; treatment instead is focused on repletion of fluid losses. Antibiotics can be considered in severe cases, based on organism sensitivity. In wound infection or septicemia, establishing control of local infection and systemic antibiotics are warranted. Recent analysis of surveillance data from the COVIS dataset, including infections of all Vibrio species from 1990 to 2010, concluded that a treatment regimen inclusive of quinolones is associated with lower mortality. V. vulnificus infections treated with quinolone or tetracycline were associated with lower mortality than treatment with cephalosporin alone.
Aeromonas species are ubiquitous environmental organisms found principally in fresh and brackish water, especially in the summer months, with clinical manifestations of infection similar to those caused by Vibrio species. Aeromonas species are divided into 2 groups: psychrophilic (Greek: psychros , cold) aeromonads, which grow optimally at temperatures ranging from 22°C to 25°C, and mesophilic aeromonads, which grow best between 35°C and 37°C. Psychrophilic strains usually are isolated from environmental water sources and fish; Aeromonas salmonicida is the most common strain in this group. Based on their phenotypic features, the mesophilic aeromonads are further grouped into 3 complexes: Aeromonas hydrophila, Aeromonas caviae, and Aeromonas veronii . All 3 of these Aeromonas species have been associated with human infection. In order to cause disease, aeromonads must adhere to and colonize the intestinal epithelium. Aeromonas strains produce an array of toxins, including heat-labile enterotoxin, hemolysin, and cytotoxin. There also may be some degree of invasion of the epithelial cells, with resultant dysentery or colitis.
Aeromonas infections often are associated with drinking untreated water, such as well or spring water, or eating contaminated foods. Estimates of the disease burden vary widely and may differ by seasonal distribution and the predominant species. Aeromonas infection is not a reportable disease in the USA, so incidence data are limited; the GEMS found the vast majority of infections are in Latin America, Southeast Asia, North Africa, and Middle East. In the USA, cases peak around July and August ; studies in other countries have failed to demonstrate a seasonal pattern. Although Aeromonas infection has been associated with diarrheal disease, other studies have found similar rates of aeromonad isolation from diarrhea cases and asymptomatic controls, leading some to question the pathogenicity of Aeromonas .
Aeromonas gastroenteritis can vary in clinical presentation from watery diarrhea to dysentery; diarrhea is seen in 75% to 89% of cases. Duration of diarrhea is typically 3 to 10 days, but chronic diarrhea for longer than 1 year has been reported; the frequency of chronic diarrhea is unknown. Fever and abdominal pain are variable. Complications associated with Aeromonas gastroenteritis include segmental colitis, ischemic colitis, and hemolytic-uremic syndrome (HUS). Aeromonas septicemia may have a GI portal of entry with or without associated symptoms, particularly in immunocompromised patients, although up to 30% of septicemia cases have no underlying disorders. Furthermore, Aeromonas has long been recognized as a cause of wound infections after swimming in fresh or brackish water and of bacteremia or deep organ infections in immunocompromised hosts.
Supportive care, particularly rehydration therapy, is sufficient intervention in many cases. The diarrhea is typically self-limiting and does not require antibiotics. In chronic diarrhea or in immunocompromised patients, there may be a role for antibiotics in reducing the duration of symptoms. Aeromonads may carry several different α-lactamases and are consistently resistant to α-lactam antibiotics (e.g., penicillin, ampicillin, first- or second-generation cephalosporins). Aeromonads tend to be sensitive to trimethoprim/sulfamethoxazole (TMP/SMX), third-generation cephalosporins, fluoroquinolones, tetracycline, chloramphenicol, and aminoglycosides. Given current susceptibility patterns, fluoroquinolones or third-generation cephalosporins are the preferred therapies. Carbapenems are also effective, but there have been reports of aeromonads expressing metallo-α-lactamases active against carbapenems. Therapy should be altered based on antibiotic susceptibility data when available.
Plesiomonas shigelloides is a motile gram-negative rod, member of the family Enterobacteriaceae, and ubiquitous freshwater organism . Most cases are associated with consumption of raw seafood, and the organism has been reported to cause outbreaks. P. shigelloides also causes travelers’ diarrhea and constitutes 1% to 3% of such cases in Latin America and Africa and about 5% of such cases in Asia. The pathogenesis of P. shigelloides infection is poorly understood. There is evidence that potential virulence factors include cytotoxic hemolysin, iron acquisition systems, and lipopolysaccharide. Diarrhea ranges from mild and watery to severe colitis with visible blood. Abdominal pain often is prominent, and fever and vomiting are common. Extraintestinal manifestations, usually sepsis or meningitis, are rare and are more common in children and immunosuppressed patients. The diarrhea is usually self-limiting, so antibiotics are likely of limited use. Chronic diarrhea and extraintestinal disease may benefit from antibiotic therapy. P. shigelloides is commonly resistant to aminopenicillins and tetracyclines. Sporadic resistance to other antibiotics may occur. Potential effective treatment regimens are likely similar to those for other microbiological causes of dysentery and involve a fluoroquinolone, third-generation cephalosporin, or even carbapenems in severe or resistant infections, but little information is available on the efficacy of treatment.
E. coli are common, but constitute a minority of the commensals of the intestinal microbiota in humans and animals. Although most strains are relatively innocuous in the bowel, others possess virulence factors that cause diarrheal disease. At least 6 types of E. coli intestinal pathovars have been recognized ( Table 110.7 ). Their virulence factors include toxin production, adherence to epithelial cells, and invasiveness, each of which is encoded by specific genetic elements (plasmids or chromosomal genes) that determine pathogenicity.
| Strains | Pathogenic Mechanisms | Persons Affected | Clinical Features |
|---|---|---|---|
| DAEC | Diffuse adherence to Hep-2 cells | Children in developing countries | Watery diarrhea (acute) and persistent diarrhea |
| EAEC | Aggregative adherence to Hep-2 cells | Children in developing countries | Watery diarrhea (acute) and persistent diarrhea |
| STEC O157:H7 Non-O157:H7 O104:H4 ∗ |
Stxs 1 and 2 | Children and adults Persons who ingest contaminated food, especially hamburger (outbreaks) |
Watery diarrhea Bloody diarrhea (classic) |
| EIEC | Epithelial cell invasion | Children and adults | Watery diarrhea Dysentery |
| EPEC Typical Atypical |
Attaching and effacing Bundle-forming pilus, attachment and effacement lesions Atypical adherence pattern |
Children | Watery diarrhea (acute) Persistent diarrhea |
| ETEC | Heat-labile and/or heat-stable toxin Adherence |
Children in developing countries; travelers | Watery diarrhea |
EPEC was initially recognized as causing severe neonatal diarrhea and remains a common cause of diarrheal illness and associated morbidity in children globally. EPEC induces classic attaching and effacing lesions, in which bacteria attach to the intestinal cell membrane and cause effacement of the microvilli (see Fig. 110.1 B ). A common pathogenicity island (LEE, locus for enterocyte effacement) is responsible for these lesions and serves as the basis for molecular identification of EPEC. Historically, EPEC was further divided into typical (tEPEC) and atypical (aEPEC); tEPEC produces bundle-forming pili, which causes a characteristic adherence pattern on cultured epithelial cells, whereas aEPEC lacks the gene to produce bundle-forming pili and exhibits an atypical adherence pattern. Once adherent, EPEC forms a pore and secretes multiple effector proteins directly into the enterocyte, which results in a complex cascade of changes within the cell. A diverse array of genes encode the effector proteins, the functions of which are not fully understood. Some of the mechanisms described, however, include disruption of tight junctions, stimulation of interleukin (IL)-8 release, stimulation of adenosine release, and inhibition of fluid resorption through disruption of NaCl transport mechanisms; tEPEC causes more severe disease than aEPEC. The pathogenicity of aEPEC became controversial after an aEPEC infection study in healthy volunteers showed no clinical disease, yet multiple examples of diarrheal outbreaks attributed to aEPEC exist in the literature.
EPEC has been reported as a major cause of diarrhea in children, particularly those younger than 1 year and in HIV-infected subjects. In general, the burden of EPEC diarrheal disease has been declining in more recently published studies; this may be due to interventions such as breast-feeding, which is particularly effective against EPEC infection. Notably, however, the GEMS study found a 2.8-fold elevated risk of death among infants with tEPEC infection; this was the highest pathogen-attributable risk of death, although other pathogens (rotavirus, Cryptosporidium , ETEC-producing heat-stable toxin, and Shigella ) were more common; these findings support the need for attention to the severity of disease outcomes attributed to tEPEC. EPEC is also reported to be the most frequent pathogen detected in mixed infections, but, importantly, can often be recovered from asymptomatic individuals.
Clinical presentation is characterized by acute diarrhea with vomiting and dehydration, but EPEC is also strongly associated with persistent diarrhea; this is an important distinction compared with other diarrheal pathogens. Little data exist on preferred antimicrobial treatment regimens. Most infections resolve spontaneously and require neither a definitive diagnosis nor antibiotic treatment. Supportive care, notably rehydration, remains imperative in treating diarrhea, particularly in infants and children. The role of antibiotics in severe or persistent disease is unknown.
Inspired by the discoveries in cholera, investigators directed their attention to E. coli as a cause of acute toxigenic diarrheal disease. Originally in India, and thereafter in many parts of the world, strains of E. coli were found that elaborated an enterotoxin similar to that of V. cholerae . ETEC is a group of E. coli distinct from EPEC serotypes. ETEC infections mostly are sporadic, although outbreaks of ETEC do occur.
ETEC is acquired by consuming contaminated foods and liquids. Infection first requires adherence and then toxin production. Adherence is mediated primarily by CFs, which are carried on plasmids. More than 25 distinct CFs have been identified and are designated as CFA (colonization factor antigen) or CS (coli surface antigen), followed by a number. Worldwide, CFA/I, CFA/II, and CFA/IV are most common; historically 30% of ETEC clinical isolates lacked a known CF, although a recent study using whole genome sequencing identified a novel CF, CS30, found in many isolates previously unassigned to known CFs. ETEC colonizes the surface of small intestinal epithelium without penetrating the epithelial layer, and bacterial chromosomal loci tia and tib are believed to play a role in adherence to enterocytes; as in cholera, there is neither mucosal damage nor bacteremia. Two types of enterotoxins are produced by ETEC. The heat–labile toxin (LT) is an approximately 84-kd protein that is destroyed by heat and acid and, similar to cholera toxin, is composed of 1 A subunit and a pentameric ring of 5 B subunits. There are 2 groups of LT, LT-I and LT-II, that are differentiated on the basis of the target membrane receptor that binds the B subunit. Through its A subunit, LT acts pathophysiologically like cholera toxin by activating adenylate cyclase, thereby causing secretion of fluid and electrolytes into the small intestinal lumen. The second ETEC toxin is heat stable (ST) and is able to withstand heating to 100°C. ST is a family of low molecular weight toxins with 2 primary classes, STa and STb. Only STa has been associated with human disease; it is an approximately 2-kd peptide. STa activates membrane-spanning guanylate cyclase; the resultant increase in cyclic guanosine monophosphate induces intestinal secretion from both the small and large intestine. ETEC strains may elaborate LT only, ST only, or both LT and ST.
The major vehicles of infection appear to be contaminated foods and drinking water. The burden of disease, particularly in children, remains significant, although the specific estimates vary across epidemiologic studies of the global burden of diarrheal disease in children. ST-producing ETEC has been associated with an increased risk of death in infants aged 0 to 11 months, whereas LT-ETEC has been associated with persistent diarrhea. In the Global Burden of Diarrheal Disease study (2015), 23,649 deaths of children younger than 5 years of age were attributed to ETEC infection. ETEC has also been implicated in severe dehydrating diarrhea in adults. In the USA, there were 39,718 cases of food-borne ETEC, of which 55% were acquired during travel. Indeed, ETEC is the most common cause of travelers’ diarrhea worldwide (30% to 60% of cases). Strains vary by country and, multiple ETEC outbreaks with varying strains have been reported across the USA since the mid-1970s; most are related to consumption of contaminated food or on cruise ships docking in USA ports.
In the endemic and outbreak setting, ETEC infection is typically a secretory diarrhea that can vary widely in severity but, like cholera, can cause severe dehydration. Associated vomiting is common, but fever is rare. Presentation is similar in adults and children. In a review of 17 ETEC outbreaks, 81% manifested more diarrhea than vomiting compared with viral gastroenteritis outbreaks, where vomiting tends to be a more prominent symptom. The median incubation period was typically 24 to 50 hours and duration of illness greater than 3 days.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here