Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Infancy, considered as the time of birth until erect posture is assumed, is a highly vulnerable period of life, especially where nutrition is concerned. Infants have high nutrient requirements, are unable to secure food for themselves, and have immature digestive and absorptive functions. In the narrow sense, the focus of nutrition is on meeting nutritional needs to assure health of the infant. In fact, growth is well recognized as a sensitive, but not specific, indicator of the overall health and nutritional status of infants. It is likely, however, that infant nutrition has long-term health effects. Some parameters that may be affected by nutrition in infancy include cardiovascular health, blood pressure, bone mineralization, low-density lipoprotein cholesterol, split pro-insulin, and cognitive development. , Although these observations are tantalizing, they are observational: a causal relationship has not been established. It is likely that genetics and environmental factors also have an effect on health parameters, but present knowledge does not permit us to understand the relative importance of these factors or how they might interact. This chapter will use the definition of a nutrient requirement enunciated by Fomon “that because of practical difficulties in determining the influence of diet on the achievement of optimal health, the requirement for a nutrient usually is defined in a much more limited context: the quantity of the nutrient that will prevent all evidences of under nutrition attributable to the deficiency of the nutrient.”
Even this limited definition is problematic, because it is not always possible to factor out influences of the environment, genetics, nutrient-nutrient interactions, or nutrient-infant/child interactions.
Several approaches have been used to determine nutritional requirements. These include direct experimental evidence, extrapolation from experimental evidence relating to human subjects of other ages, analogy with the breast-fed infant, metabolic balance studies, clinical observations, and theoretically based calculations. Most recently, in setting the dietary reference intakes (DRI), the National Academy of Sciences Engineering and Medicine (NASEM) relied heavily on clinical trials including dose-response, balance, depletion-repletion, prospective observational, case-control studies, and clinical observations in humans. Greater emphasis was placed on studies that measured actual dietary and supplement intake than those that depended on self-reported food and supplement intake. All studies were published in peer-reviewed journals. Nevertheless, for some nutrients the available data did not provide a basis for proposing different requirements for various life stages or gender groups, most notably children less than 6 months of age. For infants 0 to 6 months of age only, adequate intakes (AI; Table 85.1 ) exist. The AI is based on the reported intake of human milk (780 mL/day), determined by test-weighing of full-term infants in three studies and by the reported average human milk concentration of a specified nutrient after 1 month of lactation. Although this is an intuitively logical approach, it provides information only for breast-fed infants. Human milk is a matrix of interacting factors, and each factor may be more or less biologically available in this matrix compared with the biologic availability of the factor when not in the human milk matrix. This means that there are no reference values applicable to non–breast-fed infants ( Tables 85.2 and 85.3 ). The AIs, based solely on estimates of nutrients in human milk, will result in frank deficiency for some nutrients if those nutrients are fed to non–breast-fed infants at the level of AI. Further, this approach assumes that the mother has no nutrient deficiency, that all events surrounding the birth were optimal (cord clamping, etc.), and that the mother’s milk has at least the average amount of nutrients. If any of these is not optimal and the infant is not supplemented, nutrient deficiency can occur.
| Term | Abbreviation | Definition |
|---|---|---|
| Estimated average requirement | EAR | The average daily nutrient intake level estimated to meet the requirement of half the healthy individuals in a particular life stage and gender group |
| Recommended dietary allowance | RDA | Average daily nutrient intake level sufficient to meet the nutrient requirement of nearly all (97%–98%) healthy individuals in a particular life stage and gender group |
| Adequate intake | AI | Recommended average daily nutrient intake level based on observed or experimentally determined approximations or estimates of nutrient intake by a group (or groups) of apparently healthy people that are assumed to be adequate–used when an RDA cannot be determined |
| Tolerable upper intake level | UL | The highest average daily nutrient intake level likely to pose no risk of adverse health effects to almost all individuals in the general population. As intake increases above the UL, the potential risk of adverse effects increases. |
| Acceptable macronutrient distribution range | AMDR | Range of macronutrient intakes for a particular energy source that are associated with reduced risk of chronic disease while providing adequate intakes of essential nutrients |
| Age (Months) | 5th Percentile | 50th Percentile | 95th Percentile |
|---|---|---|---|
| Males | |||
| 0 | 306 | 395 | 467 |
| 4 | 437 | 561 | 686 |
| 7 | 572 | 670 | 830 |
| 13 | 703 | 830 | 1,041 |
| 25 | 899 | 1,059 | 1,291 |
| 35 | 988 | 1,184 | 1,451 |
| Females | |||
| 0 | 252 | 378 | 520 |
| 4 | 401 | 508 | 686 |
| 7 | 518 | 616 | 759 |
| 13 | 650 | 792 | 952 |
| 25 | 846 | 1,006 | 1,237 |
| 35 | 944 | 1,148 | 1,433 |
| EER = TEE + energy deposition | |||
|
|||
|
|||
|
|||
|
|||
| Where: | |||
| TEE (kcal/day) = 89 (± SE 3) × Weight of the child (kg) – 100 (± SE 56) | |||
| EER = Estimated energy requirements | |||
| TEE = Total energy expenditure | |||
| ED = Energy deposition | |||
| Nutrient | Age | RDA | AI | UL |
|---|---|---|---|---|
| Carbohydrate (g/day) | 0–6 months | 60 | ||
| Total digestible; acceptable macronutrient distribution range: 45–65 | 7–12 months | 130 | 95 | Sugars ≤25% of calories |
| 1–3 years | 130 | |||
| 4–8 years | ||||
| Total fiber (g/day) | 0–6 months | ND | ||
| 7–12 months | ND | 19 | ||
| 1–3 years | 25 | |||
| 4–8 years | ||||
| Total fat (g/day) | 0–6 months | 31 | ||
| 7–12 months | 30–40 | 30 | ||
| 1–3 years | 25–35 | |||
| 4–8 years | ||||
| n -6 PUFAs (g/day) (linoleic acid) | 0–6 months | ND | 4.4 | |
| 7–12 months | ND | 4.6 | ||
| 1–3 years | 5–10 | 7 | ||
| 4–8 years | 5–10 | 10 | ||
| n -3 PUFAs (g/day) (α-linolenic acid) | 0–6 months | ND | 0.5 | |
| 7–12 months | ND | 0.5 | ||
| 1–3 years | 0.6–1.2 | 0.7 | ||
| 4–8 years | ||||
| Saturated and trans fatty acids, and cholesterol | 0–6 months | ND | ||
| 7–12 months | ND | |||
| 1–3 years | ||||
| 4–8 years | ||||
| Protein (g/day) | 0–6 months | ND | 9.1 | |
| 7–12 months | 13.5 | |||
| 1–3 years | 13 | |||
| 4–8 years | 19 | |||
| Biotin (μg/day) | 0–6 months | 5 | ||
| 7–12 months | 6 | |||
| 1–3 years | 8 | |||
| 4–8 years | 12 | |||
| Choline (mg/day) | 0–6 months | 125 | ND | |
| 7–12 months | 150 | ND | ||
| 1–3 years | 200 | 1,000 | ||
| 4–8 years | 250 | 1,000 | ||
| 2,000 | ||||
| Folate (μg/day) | 0–6 months | 65 | ND | |
| 7–12 months | 150 | 80 | ND | |
| 1–3 years | 200 | 300 | ||
| 4–8 years | 400 | |||
| Niacin (mg/day) | 0–6 months | 2 | ND | |
| 7–12 months | 6 | 4 | ND | |
| 1–3 years | 8 | 10 | ||
| 4–8 years | ||||
| Pantothenic acid (mg/day) | 0–6 months | 1.7 | ||
| 7–12 months | 1.8 | |||
| 1–3 years | 2 | |||
| 4–8 years | 3 | |||
| Riboflavin (mg/day) (vitamin B 2 ) | 0–6 months | 0.3 | ||
| 7–12 months | 0.5 | 0.4 | ||
| 1–3 years | 0.6 | |||
| 4–8 years | ||||
| Thiamin (mg/day) (vitamin B 1 ) | 0–6 months | 0.2 | ||
| 7–12 months | 0.5 | 0.3 | ||
| 1–3 years | 0.6 | |||
| 4–8 years | ||||
| Vitamin A (μg/day) | 0–6 months | 400 | 600 | |
| 7–12 months | 300 | 500 | 600 | |
| 1–3 years | 400 | 600 | ||
| 4–8 years | 900 | |||
| Vitamin B 6 (mg/day) (pyridoxine) | 0–6 months | 0.1 | ND | |
| 7–12 months | 0.5 | 0.3 | ND | |
| 1–3 years | 0.6 | 30 | ||
| 4–8 years | 40 | |||
| Vitamin B 12 (μg/day) (cobalamin) | 0–6 months | 0.4 | ||
| 7–12 months | 0.9 | 0.5 | ||
| 1–3 years | 1.2 | |||
| 4–8 years | ||||
| Vitamin C (mg/day) (ascorbic acid) | 0–6 months | 5 | 25 | |
| 7–12 months | 5 | 25 | ||
| 1–3 years | 5 | 50 | ||
| 4–8 years | 5 | 50 | ||
| Vitamin D (μg/day) (calciferol) 1 μg calciferol = 40 IU vitamin D | 0–6 months | 5 | 25 | |
| 7–12 months | 5 | 25 | ||
| 1–3 years | 5 | 50 | ||
| 4–8 years | 5 | 50 | ||
| Arsenic | 0–6 months | ND | ND | |
| 7–12 months | ND | ND | ||
| 1–3 years | ND | ND | ||
| 4–8 years | ND | ND | ||
| Boron (mg/day) | 0–6 months | ND | ND | ND |
| 7–12 months | ND | ND | ND | |
| 1–3 years | ND | ND | 3 | |
| 4–8 years | ND | ND | 6 | |
| Calcium (mg/day) | 0–6 months | 210 | ND | |
| 7–12 months | 270 | ND | ||
| 1–3 years | 500 | 2,500 | ||
| 4–8 years | 800 | 2,500 | ||
| Chromium (μg/day) | 0–6 months | 0.2 | ||
| 7–12 months | 5.5 | |||
| 1–3 years | 11 | |||
| 4–8 years | 15 | |||
| Copper (μg/day) | 0–6 months | 200 | ND | |
| 7–12 months | 340 | 220 | ND | |
| 1–3 years | 440 | 1,000 | ||
| 4–8 years | 1,300 | 3,000 | ||
| Fluoride (mg/day) | 0–6 months | 0.01 | 0.7 | |
| 7–12 months | 0.5 | 0.9 | ||
| 1–3 years | 0.7 | 1.3 | ||
| 4–8 years | 1 | 2.2 | ||
| Iodine (μg/day) | 0–6 months | 110 | ND | |
| 7–12 months | 90 | 130 | ND | |
| 1–3 years | 90 | 200 | ||
| 4–8 years | 300 | |||
| Iron (mg/day) | 0–6 months | 0.27 | 40 | |
| 7–12 months | 11 | 40 | ||
| 1–3 years | 7 | 40 | ||
| 4–8 years | 10 | |||
| Magnesium (mg/day) | 0–6 months | 30 | ND | |
| 7–12 months | 80 | 75 | ND | |
| 1–3 years | 130 | 65 | ||
| 4–8 years | 110 | |||
| Manganese (mg/day) | 0–6 months | 0.003 | ND | |
| 7–12 months | 0.6 | ND | ||
| 1–3 years | 1.2 | 2 | ||
| 4–8 years | 1.5 | 3 | ||
| Molybdenum (μg/day) | 0–6 months | 2 | ND | |
| 7–12 months | 17 | 3 | ND | |
| 1–3 years | 22 | 300 | ||
| 4–8 years | 600 | |||
| Nickel (mg/day) | 0–6 months | ND | ND | ND |
| 7–12 months | ND | ND | ND | |
| 1–3 years | ND | ND | 0.2 | |
| 4–8 years | ND | 0.3 | ||
| Phosphorus (mg/day) | 0–6 months | 100 | ND | |
| 7–12 months | 460 | 275 | ND | |
| 1–3 years | 500 | 3000 | ||
| 4–8 years | 3000 | |||
| Selenium (μg/day) | 0–6 months | 15 | 45 | |
| 7–12 months | 20 | 20 | 60 | |
| 1–3 years | 30 | 90 | ||
| 4–8 years | 150 | |||
| Silicon | 0–6 months | ND | ND | |
| 7–12 months | ND | ND | ||
| 1–3 years | ND | ND | ||
| 4–8 years | ND | ND | ||
| Vanadium (mg/day) | 0–6 months | ND | ND | |
| 7–12 months | ND | ND | ||
| 1–3 years | ND | ND | ||
| 4–8 years | ND | ND | ||
| Zinc (mg/day) | 0–6 months | 2 | 4 | |
| 7–12 months | 3 | 5 | ||
| 1–3 years | 3 | 7 | ||
| 4–8 years | 5 | 12 |
Growth within the first year is remarkable for its velocity, variations in the velocity, and demand for nutrients. The first 6 months of life are marked by the most rapid changes in physical growth, cognitive development, and nutrient intake, and overall health is intimately associated with growth. In fact, physical growth has been used as a marker of overall health. During the first 6 months of life, growth is more rapid than at any other time. Table 85.4 shows the increase in weight in the first 12 months of life. A substantial proportion of nutrient intake is allocated for growth—accretion of body mass. Over time, less and less of dietary intake is used for growth.
| At Birth | Age (Months) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight (kg) | 3.6 | 4.4 | 5.2 | 6.0 | 6.8 | 7.4 | 8.0 | 8.4 | 8.8 | 9.2 | 9.6 | 10.0 | 10.4 |
| Weight increase (kg) | 0.8 | 0.8 | 0.8 | 0.8 | 0.6 | 0.6 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | |
| % Increase | 18 | 15 | 13 | 12 | 8 | 8 | 5 | 4.7 | 4.5 | 4.3 | 4.2 | 4 |
The source of nutrients—human milk or formula—may have an effect on the rate of weight gain and body composition. For example, in a pooled analysis of 453 breast-fed infants from seven longitudinal observational studies of infant growth in North America and northern Europe, Dewey et al. showed that breast-fed infants grow more rapidly in the first 2 months and less rapidly from 3 to 12 months of age than formula-fed infants. A longer duration of breastfeeding was associated with a greater decline in weight for age and weight for length, but not in length for age. The only randomized study of breastfeeding and growth included 17,046 mother-infant pairs and, like the Dewey study, showed that breast-fed infants gained more weight in the first 3 months of life than formula-fed infants. Unlike the Dewey study, however, Kramer et al. showed there was no detectable deficit at 12 months. It is unclear whether human milk offers a true biologic value that results in different growth compared with formula-fed infants, or that human milk is limiting in calories and/or other nutrients after 4 to 6 months of age. For example, using an oxygen-18 dilution technique to estimate lean body mass and body fat in breast-fed and formula-fed infants, Motil et al. showed that length and weight gains and lean body mass and body fat accretion during the first 24 weeks of life were similar between the two groups, despite significantly higher nitrogen and energy intakes in the formula-fed group. This suggests that human infants can adapt to achieve normal growth despite variability of nutrient intake. Most investigators believe there is a difference in growth between formula-fed and breast-fed infants, and some believe that the weight gain in formula-fed infants is excessive. It appears, however, that the increased weight gain seen at 7 months of age is lean body mass and not fat mass. This may be important, as it suggests that infant feeding practices cannot predict later obesity risk. The World Health Organization (WHO) collected data on exclusively breast-fed infants from six countries on which curves representing growth standards for human infants were developed. The Centers for Disease Control (CDC) and the American Academy of Pediatrics (AAP) recommend that the WHO Growth Standard curves be used to monitor growth for infants and children ages 0 to 2 years of age.
Breastfeeding is strongly recommended as the preferred feeding for all infants, including premature newborns, by many health organizations, including the AAP, the Canadian Paediatric Society, the WHO, the NASEM, and the US Department of Health and Human Services. The Agency for Healthcare Research and Quality (AHRQ) reviewed infant outcomes in the industrialized world and found that breastfeeding is associated with a reduced risk of acute otitis media, nonspecific gastroenteritis, severe lower respiratory tract infections, atopic dermatitis, asthma in young children, obesity, diabetes type 1 and 2, childhood leukemia, sudden infant death, and necrotizing enterocolitis. No relationship was found between cognitive performance and a history of breastfeeding, and the relationship between breastfeeding and cardiovascular diseases and infant mortality were unclear. The review cautions, however, that almost all the available data were from observational studies, and there was a wide range of quality among the data sets. Thus causality between breastfeeding and these outcomes cannot be inferred.
Human milk is dynamic. Nutrient concentrations vary over time (months, and within a single day), within a single feed, and among women. The tremendous variability of human milk contents makes it difficult to assume average nutrient content, as the NASEM has in establishing AI for nutrients. Human milk is generally considered as colostrum, transitional milk, and mature milk.
Colostrum is the fluid secreted by the mammary gland for the first 7 days after birth. The volume varies from 2 to 20 mL per feeding, and colostrum consists of a mixture of mammary duct contents, newly secreted milk, and immunologically active cells. Colostrum is recognized by its intense yellow color, due to a high concentration of carotenoids, α-carotene, β-carotene, β-cryptoxanthin, lutein, and zeaxanthin; its high levels of vitamin E, protein, and immunoglobulin, especially IgA ; and its low fat levels.
Transition milk is that produced between colostrum and mature milk, from about 7 to 10 days postpartum. Its nutrient content gradually changes from that of colostrum to mature milk. The protein, immunoglobulin, and fat-soluble vitamin content decreases, while the lactose, water-soluble vitamin, fat, and total caloric content increases. Interestingly, the total fat content may have predictive value as 90% of women whose milk contained 20 g or more fat per feeding on the seventh day of lactation successfully breast-fed for at least 3 months, whereas those whose milk contained 5 to 10 g of fat had an 80% dropout rate by 3 months.
In general, mature human milk is a reliable source of all nutrients for healthy, term infants, except for vitamin D and iron, for the first 4 to 6 months. Exclusively breast-fed infants must be supplemented with vitamin D, 400 IU commencing within the first days after birth, to prevent rickets, and with iron, 1 mg/kg/d, starting at 4 months of age, to prevent iron-deficiency anemia; infants of vegan mothers must be supplemented with vitamin B 12 .
At 4 to 6 months, the concentrations of calories, iron, and zinc may become limiting. The WHO and UNICEF recommend exclusive breastfeeding for the first 6 months of life. The AAP supports exclusive breastfeeding for approximately 6 months, but recognizes that infants are developmentally ready to accept complementary foods between 4 and 6 months. A systematic review of the literature examining the length of time for which exclusive breastfeeding can provide the nutritional needs of infants was recently published.
There are few contradictions to breastfeeding. The AAP recommends that in the United States, women who are infected with human immunodeficiency virus (HIV) or human T-cell lymphotropic virus (HTLV type 1 and 2) or have active pulmonary tuberculosis who have not completed 2 weeks of treatment should not breast-feed. Infants with inborn errors of metabolism, such as galactosemia, should not be breast-fed.
Many medications taken by lactating women are safe for their infants. Before advising a woman on medication and breastfeeding, the specific medication can be reviewed on the Drugs and Lactation Database (LactMed). LactMed is supported by the US National Library of Medicine and has up-to-date, peer-reviewed information for pediatricians and mothers on prescribed medications and recreational drugs. This information can be accessed at http://toxnet.nlm.nih.gov and is the preferred source for information on medications for nursing mothers.
The CDC monitors chemical contaminants in human tissues and notes that while some women may have detectable levels of chemical agents in their breast milk, no established normal or abnormal levels exist. Human milk is not routinely tested for environmental pollutants. The CDC recommends, however, that breastfeeding continue despite the presence of chemical toxins. ,
The Centers for Disease Control maintains a website ( http://www.cdc.gov/breastfeeding/ ) that contains information on breastfeeding promotion, support for breastfeeding activities, and national policies on breastfeeding.
Infant formulas are liquids or reconstituted powders fed to infants and young children to serve as substitutes for human milk. They are safe and efficacious complete foods. No vitamin or mineral supplementation is necessary for healthy infants who are solely formula-fed. Infant formulas are regulated under the Federal Food, Drug, and Cosmetic Act of 1938; the Federal Meat Inspection Act of 1907; and the Poultry Products Inspection Act of 1957. The Federal Drug Administration (FDA), an agency in the Department of Health and Human Services, regulates infant formulas and evaluates the safety of food and color additives. This information is outlined and updated at https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/InfantFormula/ucm136118.htm on a regular basis. In 1989 infant formula vendors initiated direct marketing to consumers, and in 2008 the FDA permitted the addition of substances to infant formulas under the Generally Regarded as Safe (GRAS) exception legislation from 1961. These two events led to a proliferation of infant formulas designed to capitalize on caregiver perceptions of infant needs.
Formula feeding is indicated if the infant or mother has a medical problem (e.g., galactosemia, HIV infection), the family chooses not to breast-feed, or the infant fails to thrive on exclusive breastfeeding. For breast-fed infants who develop failure to thrive, infant formula should be provided as a supplement and not a replacement for human milk. It is important to note, however, that if formula replaces breastfeeding, the mother’s milk supply will decrease, making it likely that complete cessation of breastfeeding will occur. If an infant is formula-fed, the formula should be provided to the infant for the full first year to avoid malnutrition.
Infant formulas have undergone considerable changes in the recent past, and they continue to be reevaluated and reformulated in the light of new knowledge. While the makers of infant formulas have sought to replicate the content of human milk in their formulas, more recently infant formula makers have sought to replicate the performance of human milk. In making this concept shift, they have recognized that it is not possible to replicate all the contents of human milk; moreover, it is not possible to reconstruct the complex matrix in which nutrients and other factors interact to alter absorption, bioavailability, and function.
Formulas can be grouped according to protein, carbohydrate, or fat content. With respect to protein, there are four types of formula: cow’s milk based, soy, hydrolyzed cow’s milk, and chemically defined. Only cow’s milk formula is available with low iron content, and no indication exists for its use. Formulas are produced with and without lactose, with sucrose, hydrolyzed cornstarch, glucose polymers, with and without fiber, tapioca, and maltodextrins. Formulas can contain more or less medium-chain triglycerides, soybean oil, safflower oil, sunflower oil, canola oil, structured lipids, lecithin, corn oil, and coconut oil.
Standard, iron fortified cow’s milk formula is the formula of choice when breastfeeding is not used or is stopped before 1 year of age. The AAP Nutrition Handbook notes that cow’s milk formulas from different producers have similarities, but differ substantially from one another in the quality and quantity of nutrients. Although manufacturers offer rationales for the composition of their formula, the AAP notes that physiologically significant differences have not been clearly demonstrated among the various products. The FDA offers an incomplete list of infant formulas that do not require a medical statement for their use. It is important to note that the content of infant formulas is in constant flux, and for up-to-date information, it is best to contact the formula maker.
Soy formulas were developed during the 1960s for infants who could not tolerate milk proteins or lactose. Currently soy formulas constitute about 25% of the sales of all infant formulas in the USA. Soy formulas support growth and development equivalent to that of breast-fed and cow’s milk–based formula-fed infants. Soy formulas have added methionine to compensate for the low concentration of this essential amino acid in soy protein, and a trypsin inhibitor is added. Soy formulas generally have corn syrup solids as the carbohydrate source, and palm olein, soy, coconut, and high-oleic safflower oils as the fat source. Minerals and vitamins are added, as are taurine and carnitine. Soy formulas contain phytoestrogens that have physiologic activity in rodents, but to date no significant effects have been found on growth or pubertal development in humans. The AAP recommends the use of soy formulas for term infants whose nutritional needs are not met from breast milk, term infants with galactosemia or hereditary lactase deficiency, term infants with documented transient lactase deficiency, infants with documented IgE-associated allergy to cow’s milk, and parents who wish to feed a vegetarian diet to their term infant. Soy formulas are not recommended for preterm infants with birth weights of less than 1800 g, for infants with cow’s milk protein-induced enterocolitis or enteropathy, for the prevention of colic or allergy, or for infants who have sucrose-isomaltase deficiency.
Protein hydrolysate formulas were developed for infants who could not digest or were intolerant to intact cow’s milk protein and are recommended for infants intolerant to cow’s milk and soy proteins, those who have cow’s milk protein-induced enterocolitis, and those with significant malabsorption due to gastrointestinal or hepatobiliary disease such as cystic fibrosis, short gut, biliary atresia, cholestasis, or protracted diarrhea. The hydrolysates have the disadvantage of poor taste due to the presence of sulfated amino acids, high cost, and high osmolality. There are several protein hydrolysates available. To produce the hydrolysate, either casein or whey is heat-treated and enzymatically hydrolyzed. The resulting hydrolysate consists of free amino acids and peptides of various lengths. In some instances, the peptides are so small that they are incapable of eliciting an immunologic response in infants. The formula is then fortified with amino acids to compensate for the amino acids lost in the manufacturing process. The various products contain differing amounts of peptides of various chain lengths. These formulas are free of lactose and contain sucrose, tapioca starch, corn syrup solids, and cornstarch as the carbohydrate source. The formulas contain varying amounts of medium-chain triglycerides and polyunsaturated vegetable oil to supply essential fatty acids (EFAs).
Chemically defined formulas are those produced from single amino acids and formulated specifically for infants with extreme protein hypersensitivity whose symptoms persist on hydrolyzed protein formulas.
There are no definitive in vitro tests that quantify the allergenicity of a formula. Studies of antigenicity in animals are not indicative of antigenicity in human infants. The only way to test the allergenicity accurately is in studies in infants.
Long-chain polyunsaturated fatty acids, those with a chain length of more than 18 carbons and two or more double bonds, particularly arachidonic acid (AA) and docosahexaenoic acid (DHA), are found in breast milk. Most formulas manufactured for normal term infants in the US contain DHA and AA.
Because ingredients can now be added to formulas under GRAS, there has been a proliferation of formulas that contain various items, including prebiotics, composed of a diverse group of complex, nondigested carbohydrates that are fermented by gut bacteria; probiotics; live bacteria that alter the colonic microflora; and symbiotics, the combination of a probiotic and prebiotic. The benefits of these ingredients are unproven. The risks have not been clearly defined.
Cow’s milk, full-fat, skim, 1% or 2% fat, goat’s milk, evaporated milk, and other “milks” not specifically formulated to meet infant nutritional requirements are not recommended for use during the first 12 months of life. Infants fed these milks are at risk of iron-deficiency anemia because of low iron concentration, low bioavailability of iron, and possible intestinal blood loss. , These milks also contain higher protein, sodium, potassium, and chloride concentrations and increase renal solute load. The EFAs, vitamin E, and zinc are so low that deficiency may result. Low-fat milks may cause the infant to consume excessive amounts of protein to satisfy caloric needs.
The AAP recommends human milk as the sole nutrient for healthy, term infants for approximately the first 6 months of life and supports continued breastfeeding for at least 12 months. Longer exclusive breastfeeding protects against the exposure to potentially contaminated and/or low-nutrient dense foods that put infants at risk for diarrhea and malnutrition. If a safe supply of water and complementary foods exist, then the focus for the timing of introduction of complementary foods is on nutrients themselves. Exclusively breast-fed infants are at risk of developing iron, zinc, and calorie deficiency after 4 to 6 months of age and may benefit from the introduction of complementary feedings at that time. There are no studies that compare neurocognitive development or behavior in exclusively breast-fed infants and those on mixed feedings. There is no evidence that prolonged exclusive breastfeeding protects against allergy. In an attempt to prevent food allergy, parents have been advised to delay introduction of allergenic foods (milk, eggs, fish, nuts, etc.). The concept of prevention by avoidance was brought into question by the LEAP (Learn Early About Peanut Allergy) study, which clearly demonstrated that early introduction rather than avoidance prevented peanut allergy. Perkin et al. substantiated the findings of the LEAP study and added egg-white protein to the list of food allergies potentially prevented by early introduction. However, the study could find no advantage to the early introduction of milk, sesame, fish, or wheat. No information exists on the optimal time at which to introduce complementary foods for formula-fed infants. Developmentally, infants are ready to receive complementary foods when they have some hand-eye coordination and the extrusion reflex abates ( Table 85.5 ).
| Newborn | Head Up | Supported Sitter | Independent Sitter | Crawler | Beginning to Walk | Independent Toddler | |
|---|---|---|---|---|---|---|---|
| Physical skills | Needs head support | More skillful head control with support emerging | Sits with help or support On tummy, pushes up on arms with straight elbows |
Sits independently Can pick up and hold small object in hand Leans toward food or spoon |
Learns to crawl May pull self to stand |
Pulls self to stand Stands alone Takes early steps |
Walks well alone Runs |
| Eating skills | Baby establishes a suck–swallow–breathe pattern during breast- or bottle-feeding | Breast- or bottle-feeds Tongue moves forward and back to suck |
May push food out of mouth with tongue; this gradually decreases with age Moves puréed food forward and backward in mouth with tongue to swallow Recognizes spoon and holds mouth open as spoon approaches |
Learns to keep thick purées in mouth Pulls head downward and presses upper lip to draw food from spoon Tries to rake foods toward self into fist Can transfer food from one hand to the other Can drink from a cup held by feeder |
Learns to move tongue from side to side to transfer food around mouth and push food to the side of the mouth so food can be mashed Begins to use jaw and tongue to mash food Plays with spoon at mealtime, may bring it to mouth, but does not use it for self-feeding yet Can feed self-finger foods Holds cup independently Holds small foods between thumb and first finger |
Feeds self easily with fingers Can drink from a straw Can hold cup with two hands and take swallows More skillful at chewing Dips spoon in food rather than scooping Demands to spoon-feed self Bites through a variety of textures |
Chews and swallows firmer foods skillfully Learns to use a fork for spearing Uses spoon with less spilling Can hold cup in one hand and set it down skillfully |
| Baby’s hunger and fullness cues | Cries or fusses to show hunger Gazes at caregiver; opens mouth during feeding indicating desire to continue Spits out nipple or falls asleep when full Stops sucking when full |
Cries or fusses to show hunger Smiles, gazes at caregiver, or coos during feeding to indicate desire to continue Spits put nipple or falls asleep when full Stops sucking when full |
Moves head forward to reach spoon when hungry May swipe the food toward the mouth when hungry Turns head away from spoon when full May be distracted or notice surroundings more when full |
Reaches for spoon or food when hungry Points to food when hungry Slows down in eating when full Clenches mouth shut or pushes food away when full |
Reaches for food when hungry Points to food when hungry Shows excitement when food is presented when hungry Pushes food away when full Slows down in eating when full |
Expresses desire for specific foods with words or sounds Shakes head to say “no more” when full |
Combines phrases with gestures, such as “want that” and pointing Can lead parent to refrigerator and point to a desired food or drink Uses words like “all done” and “get down” Plays with food or throws food when full |
| Appropriate foods and textures | Breast milk or infant formula | Breast milk or infant formula | Breast milk or infant formula Infant cereals Thin puréed foods |
Breast milk or infant formula Infant cereals Thin puréed baby foods Thicker puréed baby foods Soft mashed foods without lumps 100% juice |
Breast milk or infant formula 100% juice Infant cereals Puréed foods Ground or soft mashed foods with tiny soft noticeable lumps Foods with soft texture Crunchy foods that dissolve (such as baby biscuits or crackers) Increase variety of flavors offered |
Breast milk, infant formula, or whole milk 100% juice Coarsely chopped foods, including foods with noticeable pieces Foods with soft to moderate texture Toddler foods Bite-sized pieces of foods Bites through a variety of textures |
Whole milk 100% juice Coarsely chopped foods Toddler foods Bite-sized pieces of foods Becomes efficient at eating foods of varying textures and taking controlled bites of soft solids, hard solids, or crunchy foods by 2 years |
As breast-fed infants are at risk of developing iron and zinc deficiency, complementary foods high in these minerals, such as meats, should be introduced as the first food. Iron-fortified infant cereal is often recommended as the initial solid food; however, the absorption and bioavailability of iron from fortified cereals is low, , and they do not provide zinc.
Juice is sometimes recommended in the first year of life and parents often provide it without consulting their doctors. Historically, pediatricians recommended fruit juice as a source of vitamin C when scurvy was a serious concern for infants. Fruit juice is marketed as a healthy, natural source of vitamins and, in some instances, calcium. Because juice tastes good, children readily accept it. There is no nutritional indication to feed juice to infants younger than 6 months of age. Offering juice before solid foods are introduced into the diet could risk having juice replace breast milk or infant formula in the diet. This can result in reduced intake of protein, fat, vitamins, and minerals such as iron, calcium, and zinc. Malnutrition has been associated with excessive consumption of juice.
Because foods high in iron are recommended as weaning foods, beverages that contain vitamin C do not offer a nutritional advantage for iron-sufficient individuals. The AAP recommends that juice not be offered to infants younger than 6 months and for those older than 1 year it be provided in limited amounts.
As noted above, exclusively breast-fed infants require supplementation with vitamin D to prevent rickets and iron to prevent iron deficiency. Exclusively breast-fed infants of vegan mothers require supplementation with vitamin B 12 . Healthy, term, formula-fed infants do not require vitamin or mineral supplements as all of the formulas, except the low-iron formulas, are complete foods. Infants aged 6 months or more may benefit from fluoride supplementation if their water is not fluoridated. No information is available on the fluoride content of bottled water.
There is no widely accepted definition of “toddler.” The term is taken from the wide-based gait seen in children who are just learning to walk. It is generally agreed that “toddlerhood” begins at the age of 12 months; the upper boundary of this age bracket is poorly defined. In this discussion it is assumed that toddlerhood ends at 36 months. Thus, a child aged less than 12 months is an infant and after 36 months a toddler becomes a “preschooler.” Because many of the data in the literature are given for children aged 2 to 5 years, “preschoolers” are often included in this discussion.
Growth and therefore nutritional requirements peak during the first year of life. Growth rate during the second 12 months of life continues to be high. The second year of life is one of transition from an infant diet to a modified adult diet, yet there is a paucity of studies and guidelines on toddlers’ nutrition. There are a number of studies that describe what toddlers eat. There have been studies documenting change in eating patterns of toddlers , and reports on the psychologic and behavioral aspects of toddler nutrition. However, few studies have been performed to determine the nutritional requirements of toddlers. , There are also few published guidelines, and those that are available often use data extrapolated from other age groups.
In 1995 Fall et al. suggested that “a baby’s nourishment before birth and during infancy” programs the development of raised blood pressure, fibrinogen concentration, factor VIII concentration, and glucose tolerance, and so is an important determinant of future coronary artery disease. This concept, known as the Barker hypothesis, as originally stated, was limited to fetal and infant nutrition and the subsequent development of coronary artery disease. The hypothesis has been broadened to include any adult ailment that may have its beginning during childhood, such as osteoporosis and renal disease. The Barker hypothesis continues to be debated, but to the extent that it proves true, early nutrition gains tremendous importance. It is during toddler years that dietary patterns are established for life.
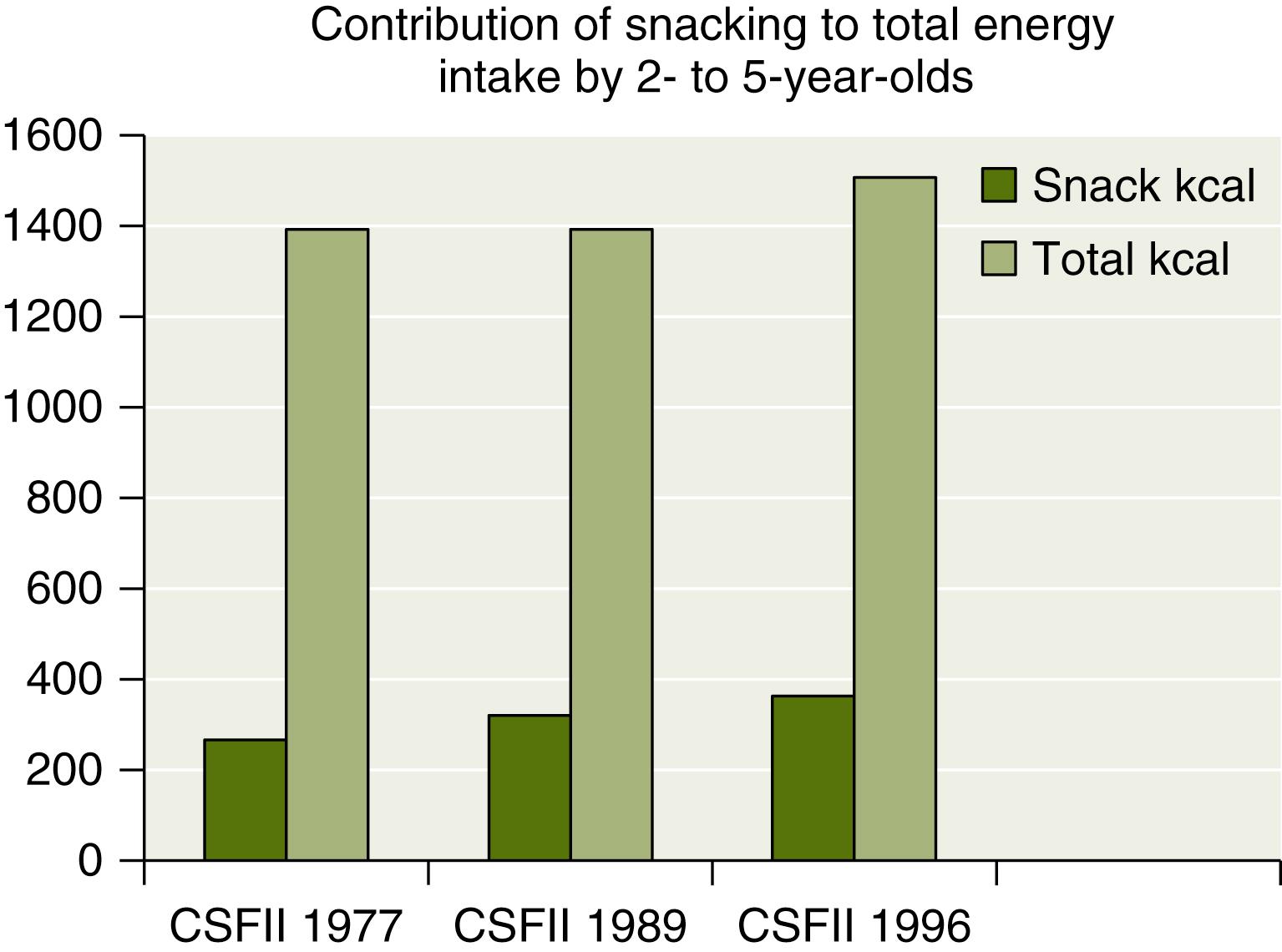
A number of cross-sectional national studies as well as some longitudinal studies are presently available for analysis. The major national cross-sectional studies are the National Health and Nutrition Examination Survey (NHANES I, II, and III). Since 1999, NHANES data are collected continuously and reported in 2-year cycles ( https://www.cdc.gov/nhanes/ ). There are four Continuing Survey of Food Intake by Individuals studies (CSFII, 1998; 1994–1996; 1989–1991; 1985–1986). Data on toddlers are also available from the National Food Consumption Survey (1977–1978) and the National Food Consumption Survey (1987–1988). In addition, there is an industry-sponsored survey addressing toddler nutrition, the Feeding Infants and Toddlers Study (FITS), conducted in 2002 and supported by Gerber Products. The FITS data include 3022 infants and toddlers aged from 4 to 24 months. FITS does not provide information on the older toddler. In 2008 there was a follow-up of the FITS study that included older toddlers and preschoolers. All of these studies point to consistency in energy intake and in the relative proportions of macronutrients across the country, across racial and ethnic groups, and over the years. This consistency lends support to the long-held notion that toddlers, when presented with adequate nutrition, self-regulate their intake to a remarkable extent. In counter-distinction to this consistency, a number of trends in toddler nutrition have been documented over the past 30 years. As with other segments of the population, in children less than 5 years of age there has been an increased prevalence of obesity from 7.2% in the NHANES II to 10.3% in the NHANES continuous survey in 2010. Between 2000 and 2010, there was little change in percentage of overweight toddlers, suggesting that the obesity epidemic may have peaked ( Table 85.6 ). Snacking has always been an important source of nutrition for toddlers. Surveys have shown an increase in the importance of snacking as a component of toddlers’ diets. Snacks now represent 24% of total caloric intake by 2- to 5-year-olds ( Fig. 85.1 ). The change is attributed to an increase in the number of snacks (rather than larger portion size) and a shift to higher-calorie, higher-fat snacks.
| Time Period | Percent Obese |
|---|---|
| 1971–1974 | 5.0 |
| 1976–1980 | 5.0 |
| 1988–1994 | 7.2 |
| 1999–2000 | 10.3 |
| 2001–2002 | 10.6 |
| 2003–2004 | 13.9 |
| 2005–2006 | 10.7 |
| 2007–2008 | 10.1 |
| 2009–2010 | 12.1 |
| 2011–2012 | 8.4 |
| 2013–2014 | 9.4 |
| 2015–2016 | 13.9 |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here