Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
An understanding of basic wound healing, scalp anatomy, and principles of reconstructive surgery are helpful to achieve consistent, well-healed wounds.
There are three phases of wound healing: inflammatory, proliferative, and remodeling phases.
Closed incisional wounds regain approximately 20% of their preinjury strength at 3 weeks postinjury, 60% at 6 weeks, and ultimately regain 80% at 12 weeks; however, they will never reach 100% of their preinjury strength.
Certain conditions, such as diabetes, radiation exposure, age, and smoking, can negatively affect wound healing.
Understanding the vascular supply of the scalp is critical to planning surgical approaches with maximum healing potential. Over the past three decades, the concept of angiosomes has developed. Angiosomes are composite blocks of tissue that are supplied by identifiable source arteries and represent a defined vascular territory. Understanding the major and minor blood supply to the scalp is critical for planning appropriate flap reconstruction.
Closing a surgical incision involves considering the reconstructive ladder, which is often used by plastic surgeons to reconstruct various tissue defects.
For the medically complicated patient or contaminated wound area, healing by secondary intention may be preferred.
Wounds should be primarily closed with minimal tension whenever possible.
Skin grafts are good options for large areas that must be covered, but they need an intact pericranium, and alopecia will result.
Tissue expansion is indicated for large defects (>9 cm 2 ) and offers many advantages including excellent color and texture match. Tissue expansion increases the amount of hair-bearing scalp, but it is not appropriate for patients with contaminated wounds or those who cannot tolerate the prolonged disfigurement from the expander.
The majority of scalp defects are best treated by replacing like tissue with like, which is accomplished with local flaps from adjacent tissue.
Regional pedicled flaps are good options in certain cases when the local tissue is radiated or for large defects, but they are limited in their ability to reach the scalp as they remain attached to their blood supply.
Free tissue transfer brings distant healthy tissue to large cranial wounds or those with prior radiation and requires microsurgical technique.
Uncomplicated wound healing with minimal scarring and deformity are desired outcomes in any surgical procedure. Quality outcomes are also measured by how unobtrusive the “footprint” of the injury, or the surgical plan is. Chronic wounds, infections, or notable scarring can significantly undermine patient and family satisfaction, even in thje setting of a highly successful neurosurgical procedure. An understanding of basic wound healing, scalp anatomy, and principles of reconstructive surgery are helpful to achieve consistent, well-healed wounds.
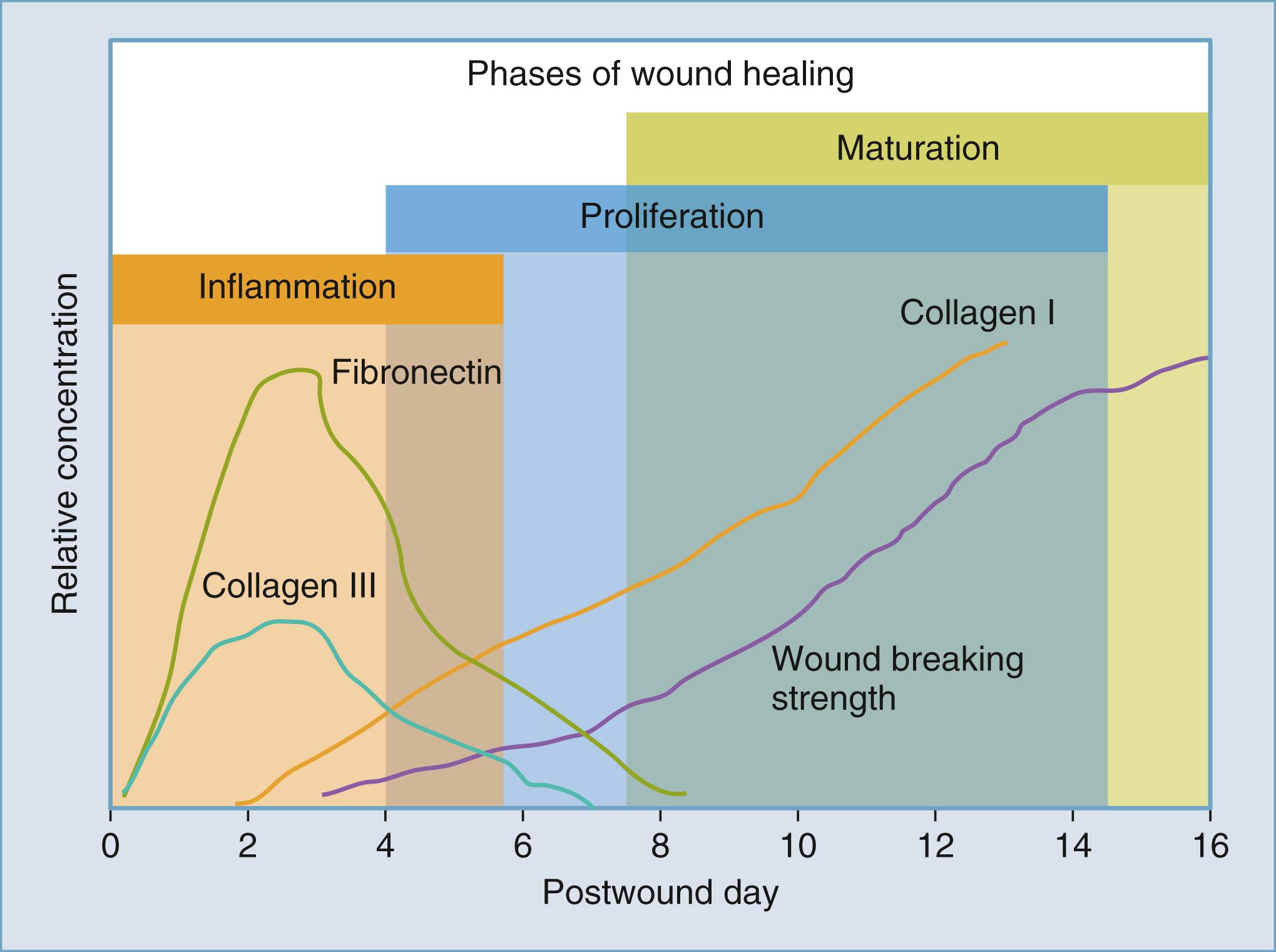
To properly manage wounds, an understanding of the basics of normal wound healing is necessary. Historically, wound healing was divided into three phases: the inflammatory, proliferative, and maturation/remodeling phases, with some authors including hemostasis as the initial phase. In reality, wound-healing phases overlap; however, division of this complex process into phases is better for ease of understanding ( Fig. 23.1 ).
Following initial injury or trauma, there is disruption of the vascular endothelium and exposure of the basal lamina, which result in extravasation of blood constituents and concurrent platelet activation. Aggregation and activation of these products result in subsequent release of growth factors involved in the deposition of extracellular matrix (ECM) (transforming growth factor β [TGF-β]) and chemotaxis (platelet-derived growth factor [PDGF]). Fibroblast growth factor (FGF) and epidermal growth factor (EGF) are involved in epithelialization, while vascular endothelial growth factor (VEGF) promotes angiogenesis. , The end product of the clotting cascade is fibrin, which combines with platelets to create a hemostatic blood clot. This biodynamic clot provides a link between hemostasis and the initial inflammatory phase of wound healing through its interaction with various proteins such as integrins, FGF-2, VEGF, and thrombin. Thrombin is at the center of this activation cascade, promoting vasodilation, attraction, and adhesion of neutrophils, monocytes, and other inflammatory cells through inflammatory cytokines such as interleukin-6 (IL-6), IL-8, interferon-γ, and tumor necrosis factor-α (TNF-α). The ultimate degradation of the clot and release of platelet granules further amplify this chemoattractant cascade. Current understanding of this blood clot structure is complex. It represents a dynamic structure composed of cross-linked fibrin, cells such as platelets and erythrocytes, and functionally active proteins, which further activate the inflammatory response.
Activation of platelets is then followed by an influx of inflammatory cells typically within the first 1 to 2 days, initiating the inflammatory phase. Neutrophils are first to arrive and are the predominant cell type in this phase ( Fig. 23.2 ). Once they reach the site of injury, they phagocytose invading bacteria and serve to stabilize the wound bed. The neutrophil response is more significant in contaminated wounds compared with clean, surgical incisions. Although they are first to arrive to the wound, neutrophils are not integral to this aspect of the healing process. Several studies have shown that the absence of neutrophils did not affect wound healing in clean wounds and that the chemokines released by neutrophils are not required for healing. ,
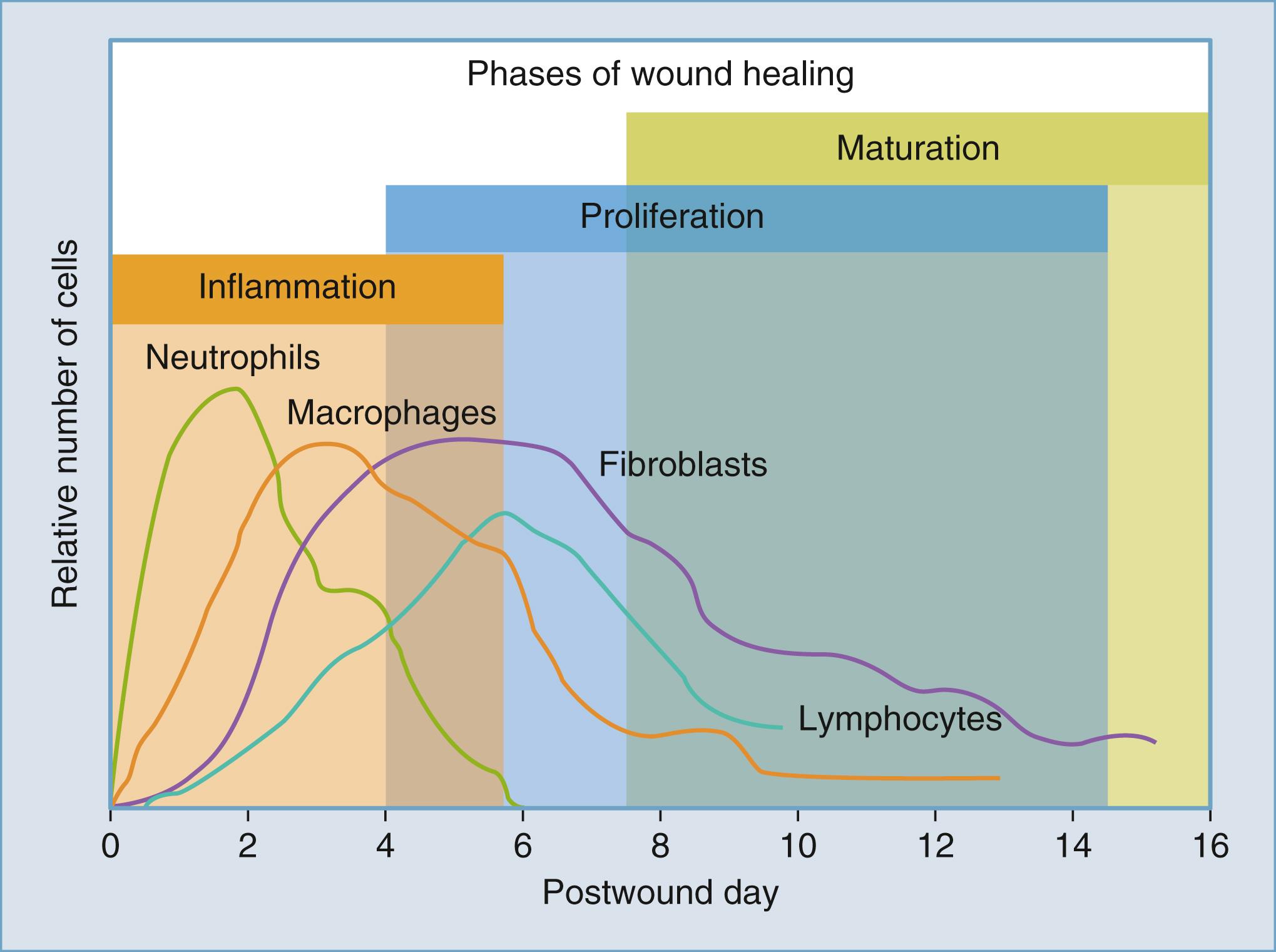
Monocytes follow the arrival of the neutrophils typically 2 to 3 days after initial tissue damage and are activated into tissue macrophages, which are responsible for further phagocytosis. Monocytes also secrete additional cytokines and growth factors that promote fibroblast proliferation, angiogenesis, and keratinocyte migration ( Table 23.1 ). While noncontaminated wounds can heal without neutrophils, no healing is possible with macrophage activation. As monocytes (the precursor cell to macrophages) leave the circulation to enter the wound bed, they begin their differentiation into macrophages, which is facilitated by a number of Toll-like receptors (TLRs), complement, and Fc receptors. Once macrophages enter the wound, they function primarily as exuberant phagocytic cells (scavenging their predecessors, the now-expended neutrophils, along with other bacteria and debris) and release a host of proteins and growth factors. These include PDGF, TGF-β, TGF-α, and VEGF, which further activate cell proliferation, angiogenesis, and production of ECM. Monocytes are considered integral for the wound-healing process.
| Cytokine | Cells of Origin | Role in Inflammatory Response | Clinical Application |
|---|---|---|---|
| EGF | Platelets, macrophages, fibroblasts | Angiogenesis, reepithelialization | Decreased in chronic wounds |
| FGF | Macrophages, mast cells, T lymphocytes | Fibroblast recruitment | Decreased in chronic wounds |
| IFNα | Monocytes, macrophages | Decreases collagen production | Used to treat hypertrophic/ keloid scars |
| PDGF | Platelets, macrophages, fibroblasts | Fibroblast recruitment, myofibroblast stimulation | Recombinant form used to treat diabetic ulcers |
| TNFα | Macrophages, T lymphocytes, keratinocytes | Leukocyte chemoattraction | Elevated levels linked to deficient healing |
| TGFβ | Platelets, fibroblasts, macrophages | Reepithelialization, wound fibroplasia | Elevated in hypertrophic and keloid scars |
| IL-1 | Keratinocytes, neutrophils, macrophages | Leukocyte chemoattraction, wound fibroplasia | Increased in chronic wounds |
| IL-8 | Macrophages, endothelial cells | Reepithelialization, angiogenesis | Increased in delayed-healing wounds |
| IL-10 | Monocytes, lymphocytes | Downregulation of other cytokines, limits fibroblast proliferation | Necessary for scarless fetal wound healing |
The next stage of wound healing is proliferation, which starts by day 3 to 5 after initial tissue injury. Timing of the proliferative phase is marked by the presence of local fibroblasts, which proliferate in the wound and become the dominant cell type by this time period. Fibroblasts are responsible for elastin production and organization of ECM proteins. Fibronectin and hyaluronic acid (a glycosaminoglycan) make up the initial ECM, which encourages further fibroblast migration into the wound. This mix of fibrous connective tissue connected by a vascular network is also known as granulation tissue. Subsequently, this is partially eroded by the fibroblast’s hyaluronidase, with collagen deposited haphazardly onto this provisional matrix in a disorganized fashion, which is recognizable as a scar. Type I and type III collagen are the major ECM components of both scar and mature skin. While there is initially a slightly more robust type III production in an immature scar, there is always more type I than type III collagen in any wound. In a mature scar or normal skin, the ratio of type I to type III collagen is approximately 4:1.
Angiogenesis is another feature of the proliferative phase, which involves the formation of endothelium from preexisting vessels with the help of VEGF. This is distinguished from vasculogenesis, which relates to de novo vessel formation.
Wound reepithelialization is accomplished by the actions of intact basal keratinocytes, found at either the edges of the wound or along deeper epidermal appendages (such as hair follicles, sweat, and sebaceous glands). Initially, these basal keratinocytes dissociate from their surrounding cells and migrate along the fibroblastic ECM into the open portion of the wound. A subset of keratinocytes, along the periphery of the wound, undergo mitosis to create a new supply of keratinocytes; they undergo differentiation and layering to restore functional epidermal layers. In closed incisional wounds, this process is usually completed within 48 hours. This biologic phenomenon underlies the common clinical practice of uncovering wounds and allowing them to be exposed to air and water 2 days after surgery. However, in a larger, open wound, this may take longer depending upon the width/area of the wound that the keratinocytes must migrate across.
The next phase of wound healing, the maturation or remodeling phase, involves the reorganization of collagen fibers over time, a process that can continue for weeks to months, and sometimes even years. Initially, fibroblasts multiply and increase collagen production per cell. This early collagen is thinner than mature collagen and is oriented parallel to the skin. Type III collagen is initially predominant and composes 30 percent of the granulation tissue matrix in injured skin. After some time, the ratio of type I collagen increases, while the rate of type III collagen decreases. This remodeling gradually increases the strength of the wound closure parallel to the increase in type I collagen formation. An overall increase in collagen formation is seen for 4 to 5 weeks after wounding as wound strength increases. Closed incisional wounds regain approximately 20% of their preinjury strength at 3 weeks postinjury, 60% at 6 weeks, and ultimately regain 80% at 12 weeks; however, they will never reach 100% of their preinjury strength.
Certain conditions can impair wound healing. In general, a wound can be considered chronic if it has not healed within 4 weeks. Perhaps the most common of these encountered in neurosurgical patients is previous radiation to the area of surgical interest. Radiation not only damages DNA, but it also creates free radicals that damage proteins and cell membranes. Radiation acutely produces stasis and occlusion of vasculature, endothelial edema, and thrombosis. The ability to heal wounds is therefore decreased as a result of impaired fibroblast proliferation, migration, and wound contraction. , As a result, wounds are unable to heal well because of slower epithelialization, decreased tensile strength, and higher infection and dehiscence rates. Radiation also results in hyperpigmented, ischemic, and sometimes ulcerated wounds that are vulnerable to infection, which may lead to wound breakdown ( Fig. 23.3 ). There may be some healing potential with time. In the plastic surgery literature, some studies show fewer complications with free flaps done over 12 months after radiation compared with those done within 12 months, while other studies are inconclusive. , Limiting the radiation field or using brachytherapy to target the radiation dose to the tumor is effective in limiting the extent of radiation damage to surrounding tissue. Cytoprotective agents, such as amifostine, epoetin-α, and pentoxifylline have also demonstrated a protective effect for healthy tissues against the damaging effects of radiation therapy. There is also some evidence of therapeutic promise for adipose-derived stem cells in treating the negative effects of radiation therapy, , but large randomized controlled trials have yet to be performed.
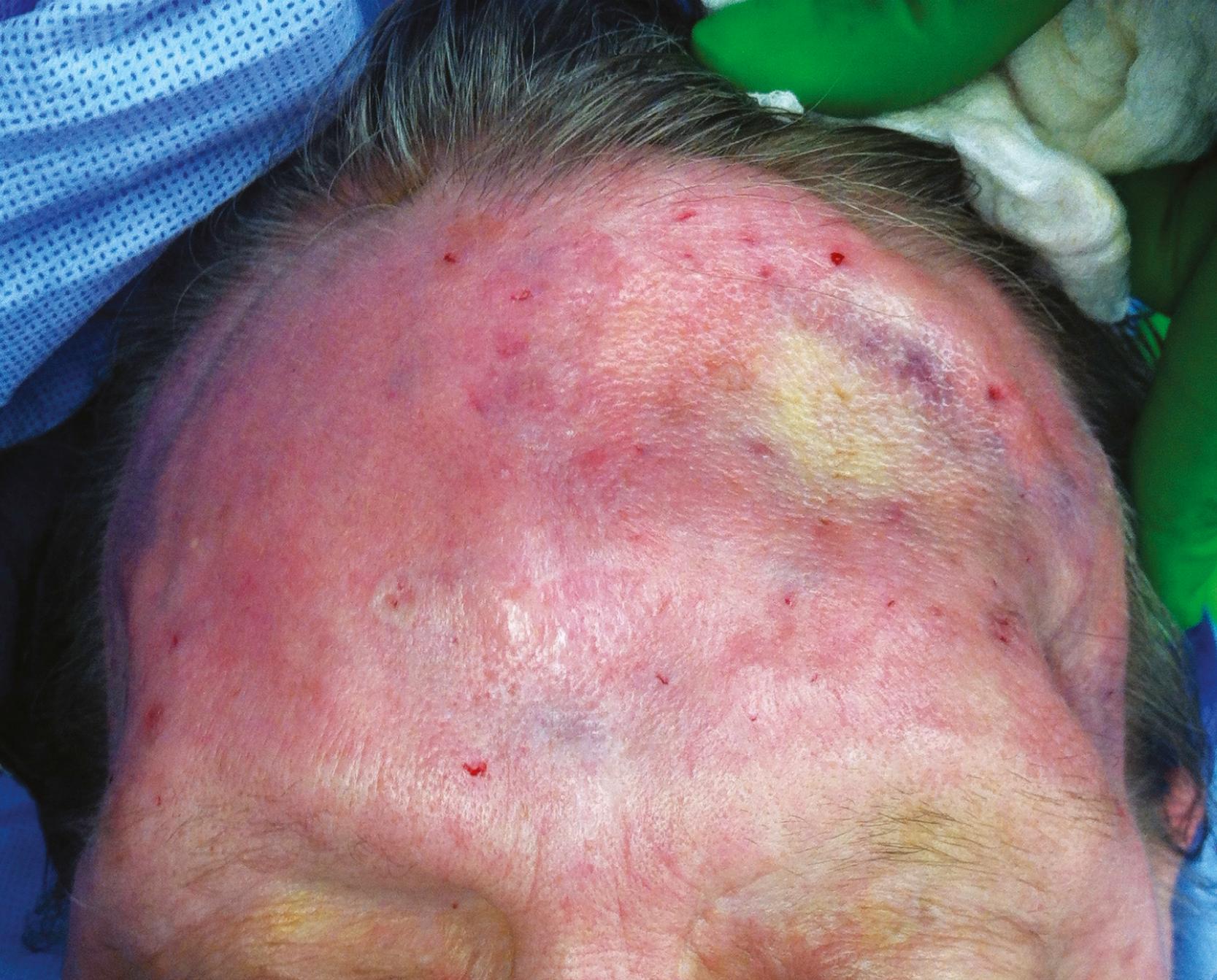
Skin in aging patients, particularly those older than 65 years of age, also tends to have a thinner dermis. Their skin is more delicate, prone to tearing with lesser shear forces, and heals more slowly than skin in younger patients.
Smoking is well known to impair wound healing. Cigarette smoke contains a number of substances that negatively affect wound healing, namely nicotine, carbon monoxide, and hydrogen cyanide. Nicotine acts as a vasoconstrictor and results in local tissue ischemia. Additionally, it increases platelet adhesiveness, which promotes thrombus formation and further contributes to decreased blood flow. It also inhibits proliferation of erythrocytes, fibroblasts, and macrophages. Carbon monoxide binds to hemoglobin with 200 times greater affinity than oxygen, producing methemoglobin and thereby reducing oxygen delivery to the wound bed. Hydrogen cyanide inhibits oxidative metabolism and oxygen transport as well. ,
Diabetes affects the body on a systemic level and ultimately impairs the ability of wounds to heal through multiple mechanisms. Diabetes increases serum glucose and is associated with decreased neutrophil concentration and effectiveness. Diabetic patients are also found to have impaired blood flow caused by microvascular and macrovascular disease, which results in insufficient oxygen delivery to tissues. Reduced blood flow, hyperglycemia, and derangement of the immune system make patients with diabetes more prone to postoperative infection. , It has been shown that glucose levels greater than 200 mg/dL are associated with poorer outcomes in terms of wound healing. Current guidelines recommend maintaining a postoperative blood glucose level of 180 mg/dL or lower.
Patient nutritional status is critical for wound healing. A baseline prealbumin and albumin level should be obtained preoperatively, and nutritional optimization should be obtained. The surgeon should also consider consultation with a nutrition specialist for patients with low prealbumin or albumin. Protein supplementation, in particular, should be encouraged. Prior study has shown a relationship between protein malnutrition and wound dehiscence in dogs.
Vitamin C is the vitamin most commonly associated with wound healing. Vitamin C is a cosubstrate for hydroxylase enzymes required for collagen formation. The recommended dietary allowance of vitamin C is 60 mg/day. Vitamin A is another vitamin that is important for wound healing. It has been shown to promote epithelialization and collagen synthesis for patients on steroids; however, it does not reverse the negative effects of steroids on wound healing. The recommended dose of vitamin A is 25,000 IU daily preoperatively and 4 days postoperatively.
Zinc and magnesium are important micronutrients in wound healing. Zinc functions as a cofactor for RNA and DNA polymerase. Zinc deficiency results in decreased wound strength and epithelialization. Magnesium also functions as a cofactor in enzymes required for protein and collagen synthesis. For both zinc and magnesium, there is no added benefit for patients who are not deficient.
Certain drugs are known to have a negative effect on wound healing. Steroids in particular are shown to decrease inflammation, inhibit epithelialization, and decrease collagen production. They demonstrate decreased TGF-β and insulin-like growth factor 1 (IGF-1), decreased hydroxyproline content in tissue, and, consequently, reduced collagen deposition in wounds. Chemotherapeutic drugs are commonly thought to have a deleterious effect on wound healing through their effects on DNA and RNA production, protein synthesis, and cell division. Interestingly, in a review of National Surgical Quality Improvement Program data of chemotherapeutic drugs, there was no increase in wound complications after breast surgery in patients receiving neoadjuvant chemotherapy.
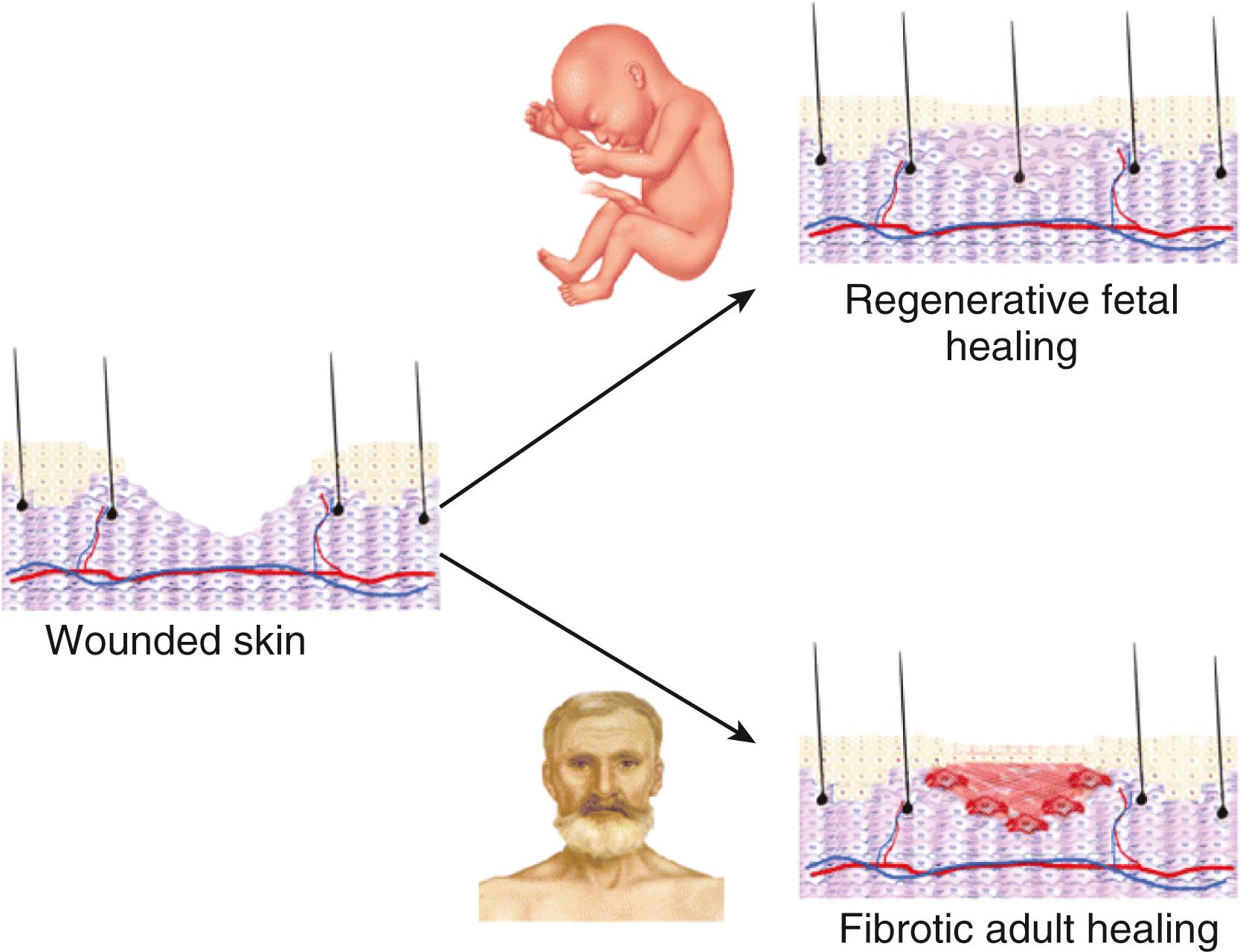
While adult healing always involves scar formation, fetuses can regenerate lost or damaged tissue before 24 weeks of gestational life. Once injured, fetal dermis can replicate the original collagen matrix, which is indistinguishable from the original tissue ( Fig. 23.4 ). Dermal structures, such as hair follicles and sebaceous glands, also continue to develop normally in this tissue. The mechanism behind this unique scarless healing remains unknown, but it appears to be related to differences in fetal inflammatory response, ECM, gene expression, and stem cell function between fetal and adult healing processes. Fully elucidating these differences and one day achieving regenerative healing in adults remain active research foci.
Much attention has recently been paid to stem cells and their role in wound healing, particularly with the increased understanding of the regenerative potential of adipose-derived stem cells (ADSCs). Widely available in stromal adipose tissue, ADSCs provide ready sources of new cells that can undergo multilineage differentiation and secrete a number of growth factors integral to the healing process. A number of studies have applied ASDCs to healing wounds and have shown accelerated healing. ASDCs and our understanding of stem cells and wound healing may well lead to a paradigm shift in difficult wound management over the coming decades.
The benefit and use of antimicrobials in preventing wound infection has been a controversial subject for years. Tissue infection has been defined quantitatively as greater than 10 5 bacteria per gram of tissue. However, infectivity can vary depending on the patient’s immune system and the type of organisms present.
In the case of traumatic wounds, the time interval between wounding and intravenous (systemic) administration of antibiotics is influential in preventing infection. Less than 3 hours from the time of injury to the time of antibiotic administration is preferred to reduce the likelihood of infection in contaminated wounds. This is because although antibiotics given systemically reach high levels in serum, they do not reach the wound surface where the bacteria are located. Because of reestablishment of vascular integrity during this 3-hour window, antibiotics given outside this time frame do not reach the wound surface. Traumatic wounds can also benefit from immediate high-pressure irrigation and debridement of nonviable tissue or contamination. Irrigation can be achieved simply with a syringe and splash shield or a small- gauge angiocatheter to increase the pressure of the irrigation. Extensive debridement also can be performed more thoroughly in the operating room with pulse lavage devices, which has been shown to significantly reduce bacterial contamination in traumatic wounds. A greater than 6-hour delay from the time of injury to cleansing and debridement leads to higher rates of infection, and closure without debridement (blunt or sharp) results in higher infection rate. Therefore both antibiotic administration and debridement of the wound should be employed to obtain a higher potention for healing without infection.
For wounds that are not considered contaminated, current guidelines for prevention of surgical site infections recommend administering antibiotics within 1 hour before incision (to maximize concentration) and discontinuing them within 24 hours after surgery. Antibiotic dosing should be adjusted on the basis of patient weight. For example, for patients weighing 80 kg or more, 2 g of cefazolin should be administered.
The anatomic location of the wound has also been shown to have an impact on infection rate. Fortunately, the scalp and face exhibit a low rate of infection related to their rich vascular supply, in contrast with lower extremity wounds, which demonstrate a high rate of infection related to reduced vascularity in the local skin. The brain itself however has a slightly higher risk for infection compared to skin and can be considered infected with a concentration of 10 4 bacteria per gram of tissue. ,
Trauma incurred by repeatedly passing the scalpel through tissue has also been shown to reduce tissue vascularity and thereby increase the incidence of infection. Studies have demonstrated that stellate lacerations or crush injuries have higher rates of infection when compared to linear lacerations with less tissue damage, likely secondary to the relative hypovascularity of traumatic wounds.
Preoperative hair removal and its impact on reducing surgical site infection has previously been the subject of much debate. A recent meta-analysis of over 11,000 patients showed no evidence that shaving decreased the incidence of postoperative wound infections, and repeated microtrauma to the scalp may increase the risk for infection. Surgical site infection prevention guidelines recommend not removing hair at the operative site unless the presence of hair will significantly interfere with the operation. If hair removal is necessary, they do not recommend using razors, but instead removing the hair outside the operating room using clippers or a depilatory agent.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here