Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Structural heart disease interventions rely on multimodality imaging, including fluoroscopy and echocardiography (intracardiac, transthoracic, or transesophageal) during the procedure, and computed tomography and cardiac magnetic resonance for procedural planning.
These modalities are complimentary. During the procedure, fluoroscopy allows real-time imaging of radio opaque devices, while intraprocedural ultrasound is ideal for soft tissue visualization including valvular structures.
Fluoroscopy projects three-dimensional anatomy in two dimensions. Therefore, multiple projections (commonly biplane imaging) are needed to precisely locate devices and structures in three-dimensional space.
Three-dimensional and biplane echocardiography are critical for procedural guidance, allowing optimal visualization of valvular structures, and catheter and device guidance.
Ultrasound-based imaging using intracardiac (ICE) or transesophageal echocardiography (TEE) are typically used to guide septal interventions (trans-septal puncture, patent foramen ovale, and atrial septal defect closures), left atrial appendage closure, and mitral valve interventions. They are also routinely used during transcatheter valve implantations and for paravalvular leak closure.
Computed tomography provides three-dimensional data that is important for candidate selection, procedural planning, and device sizing. The advent of has the potential to revolutionize procedural imaging.
Integration of fluoroscopic, computed tomographic, and echocardiographic images in the catheterization laboratory is critical for the success of structural heart interventions.
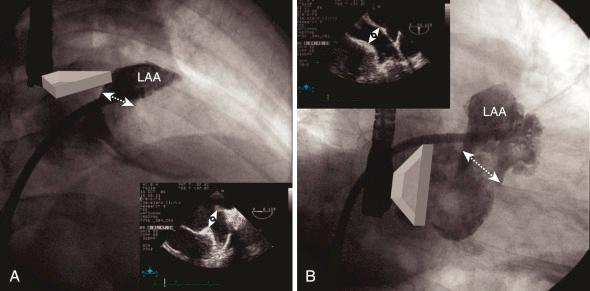
Left atrial (LA) angiogram is rarely performed by direct injection, but the LA is frequently seen on the levophase of the right-sided angiogram or ventriculogram. Direct injection in the left atrial appendage (LAA) is performed to evaluate the anatomy prior to percutaneous closer ( Fig. 48.1 ). Typically the right anterior oblique (RAO) cranial view shows the LAA opening in the medial-lateral diameter, and RAO caudal view delineates the superior-inferior opening diameter (see Fig. 48.1 ). These views are also important to study the shape of the LAA for percutaneous closure planning, as certain shapes are more amenable to percutaneous closure.
The left ventricle is typically divided into inflow and outflow segments. The inflow consists of the mitral valve apparatus, and the outflow portion includes the apex, left ventricular outflow tract (LVOT), and the aortic valve, as seen in Fig. 48.2 . The angle between the inflow and outflow tract is around 30 degrees in young patients and increases with “unfolding of aorta.” Since the interventricular septum maintains its position while the aorta is transposed anteriorly and more horizontally, there can be “bulging” of the septum.
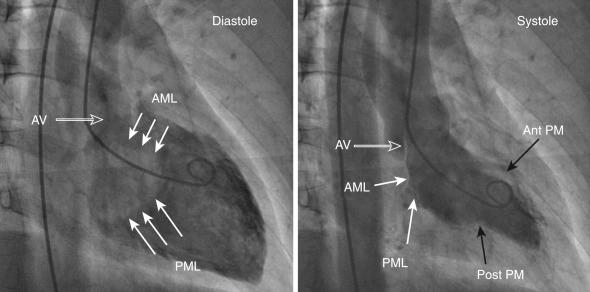
Left ventriculogram is typically performed in the RAO projection 30 degrees; however, different views should be considered, depending on the purpose of the procedure. The RAO projection delineates the separation of atria from ventricles. Various structures seen on the RAO projection are delineated in Fig. 48.2 . Anterior and inferior walls as well as the apex are seen without overlap in this view. The lateral wall and septum are overlapped, and their motion is perpendicular to the x-ray beam. Anterior and posterior mitral valve leaflets are seen from the side in a longitudinal plane, along with the inflow portion of the ventricle. This relationship is critical to recognize when performing mitral valve intervention when devices have to be advanced coaxially in the inflow (e.g., Inoue balloon or MitraClip). In this view, the anterior and posterior leaflets of the mitral valve are foreshortened. The anterolateral and posteromedial commissures are superiorly and inferiorly positioned, as shown in Fig. 48.2 . The aortic valve and the coronary sinuses can be appreciated in this view. The right coronary sinus and the noncoronary sinus are located on the right and left side of the aortic root. The left coronary sinus is located between the right and the noncoronary sinus. In this view, the ostia of the right and left main coronary arteries appear close to one another, and this view is useful for anterior-posterior (AP) localization of either coronary artery within the aortic root.
The anatomical structures seen in the left anterior oblique (LAO) view are outlined in Fig. 48.3 . The LAO projection delineates the interventricular groove and separates the right ventricle from the left ventricle. In order to align the mitral inflow to the apex of the ventricle, some caudal angulation can be added to the LAO projection. This allows one to see the left ventricle in end-on projection, where papillary muscles as well as anterior and posterior leaflets can be clearly identified. Note that the inflow and outflow of the ventricle are typically well separated in this view. The left and the right coronary sinuses and coronary ostia are separated clearly, and the noncoronary sinus is overlapped with the right coronary cusp (RCC; albeit located lower in the aortic root).
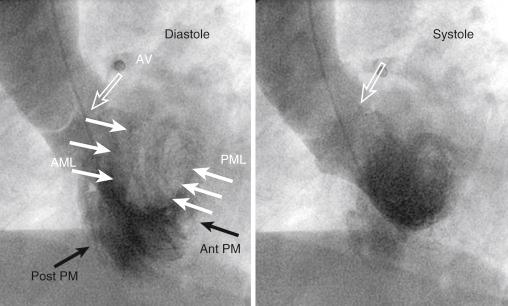
Unconventional views allow better delineation of certain parts of the left ventricle. The LAO cranial projection is typically better to assess LVOT. The purpose of this projection is to see the anterior leaflet of mitral valve in a longitudinal view when it does not overlap with the interventricular septum. It is also the view of choice to see the interventricular septum in the muscular portion for assessment of ventricular septal defect (VSD). The LAO caudal projection allows one to see the mitral valve end-on, and can place the anterolateral and posteromedial commissures in the context of the bifurcation of left main into the left anterior descending artery and left circumflex artery, respectively.
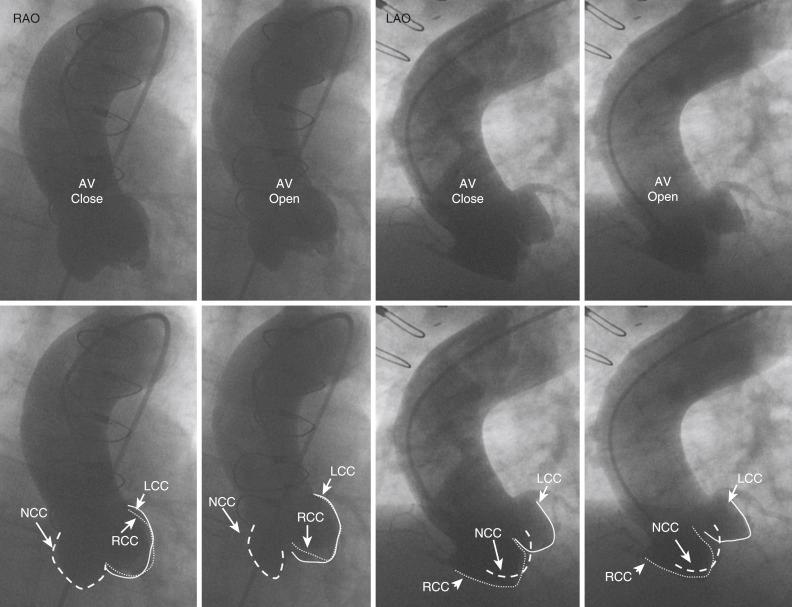
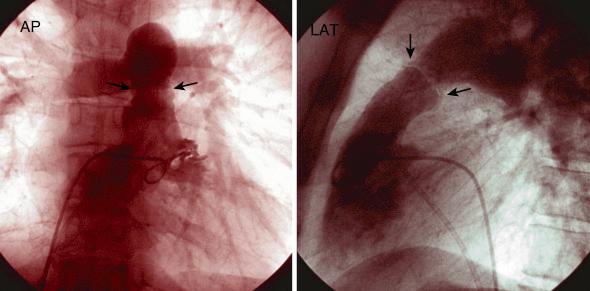
Ascending aortogram is used to examine the aortic valve and root and can be of diagnostic utility with aortic aneurysms, aortic valve insufficiency, and rarely aortic dissection. Aortogram is performed in the RAO (30 degrees) and LAO (40 degrees) projections. Rapid injection (20 mL/s for 2 to 3 seconds) using an assist device and proper catheter positioning allows for the assessment of different aortic cusps. Aortogram is usually performed with a multi-side-hole pigtail catheter positioned just 2 to 3 cm above the sinus of Valsalva ( Fig. 48.4 ). The grading of aortic regurgitation can be made by comparing the degree of opacification of the left ventricle to the aorta two cardiac cycles after contrast injection, as well as evaluating for delayed contrast clearance within the ventricle. Rapid, dense opacification of the ventricle to a greater density than the aorta and delayed contrast clearance indicate significant severe aortic insufficiency. It is critical to assess the aortogram in both the LAO and RAO projections because opacification in the LAO projection is exaggerated due to left ventricle (LV) foreshortening. Information regarding the severity of aortic regurgitation from aortography should be correlated with hemodynamic tracings of LV end systolic pressure, rate of rise of LV diastolic pressures, and the LV end diastolic pressure value itself to increase diagnostic accuracy.
Right atrial angiogram is typically performed to look at the interatrial septum (IAS). A pigtail catheter, Berman angiographic catheter, or National Institutes of Health (NIH) catheter with rapid injection rate is used. Various anatomical relationships are important to recognize on fluoroscopy, including relation of noncoronary cusp (NCC) to IAS for transseptal puncture, superior vena cava (SVC) for intracardiac echocardiography (ICE) imaging, and anterior leaflet of the mitral valve for antegrade interventions. These relationships are well demonstrated in a anteroposterior (AP) view on right atrial angiogram ( Fig. 48.5 ). This procedure can be used to guide transseptal puncture, patent foreman ovale (PFO), and atrial septal defect (ASD) closures.
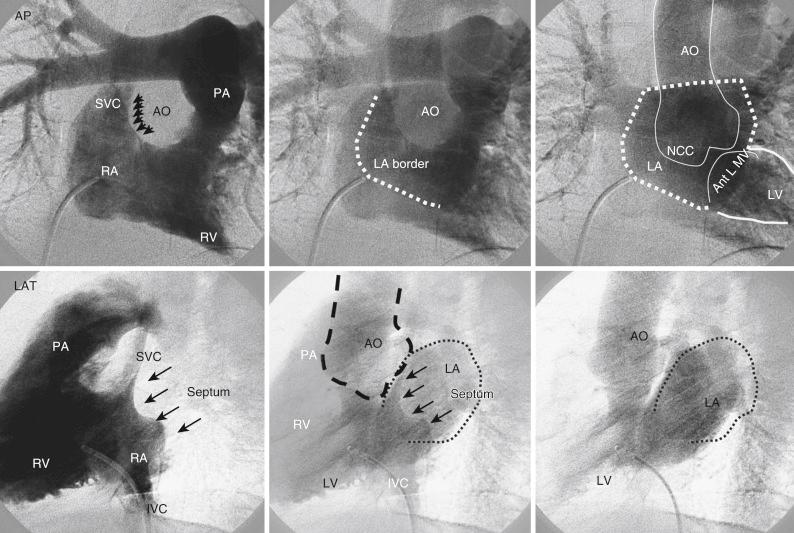
Right ventricle is usually heavily trabeculated with the inflow and outflow tract at the right angles to each other. Most typically, right ventriculogram is performed in AP and lateral projection with the catheter positioned in midcavity to prevent ventricular ectopy. A pigtail catheter, Berman angiographic catheter, or NIH catheter can be used to perform a right ventriculogram. Rate of injection (>25 mL/s) and the volume of contrast (60 mL) administered has to be higher in order to delineate the components of the right ventricular cavity. Right ventriculogram can be used to assess the tricuspid valve, right ventricular outflow tract, pulmonary valve, pulmonary arteries, pulmonary veins, and left atrium (see Fig. 48.5 ).
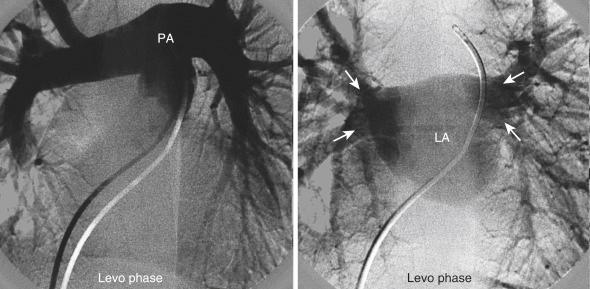
Pulmonary angiography is the gold standard technique for diagnosing pulmonary embolism. In addition, it is used to assess a variety of other conditions, such as pulmonary valve stenosis, pulmonary artery stenosis, anomalous pulmonary venous return, and pulmonary arteriovenous malformation. Frequently, pulmonary artery angiogram is performed in the AP and lateral views to visualize the LA and assess pulmonary vein drainage prior to ASD closure ( Fig. 48.6 ). Most commonly, multi-side-hole pigtail or NIH catheters are used with high injection rates of 40 mL/s to visualize pulmonary veins. The dextro and levo phases of the injections are shown in Fig. 48.7 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here