Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Arterial and venous thromboses are common problems for all clinicians. Some patients with thrombosis have an underlying hypercoagulable state. These hypercoagulable states can be divided into three categories: inherited disorders, acquired disorders, and those that are mixed in origin.
Inherited hypercoagulable states, also known as thrombophilic disorders, can be due to loss of function of natural anticoagulant pathways or gain of function in procoagulant pathways ( Table 138.1 ). Acquired hypercoagulable states represent a heterogeneous group of disorders in which the risk for thrombosis appears to be higher than that in the general population. These include such diverse risk factors as a prior history of thrombosis, obesity, pregnancy, cancer and its treatment, antiphospholipid antibody syndrome, drug-induced thrombosis such as heparin-induced thrombocytopenia or thrombosis associated with chemotherapeutic agents, or myeloproliferative disorders. The pathogenesis of thrombosis in these situations is likely multifactorial in origin. Mixed disorders are those with both inherited and acquired components, for example, hyperhomocysteinemia. Although severe hyperhomocysteinemia and associated homocystinuria are rare genetic disorders, most cases of mild to moderate hyperhomocysteinemia result from acquired folate and/or vitamin B 12 deficiency superimposed on common genetic mutations in biochemical pathways involved in methionine metabolism.
| Hereditary | Mixed | Acquired |
|---|---|---|
| Loss of Function | ||
| Antithrombin deficiency | Hyperhomocysteinemia | Previous venous thromboembolism |
| Protein C deficiency | Obesity | Pregnancy, puerperium |
| Protein S deficiency | Cancer | Drug-induced: |
| Heparin-induced thrombocytopenia | ||
| Prothrombin complex concentrates | ||
| l -asparaginase | ||
| Hormonal therapy | ||
| Gain of Function | ||
| Factor V Leiden | Postoperative | |
| Prothrombin FII G20210A | Myeloproliferative disorders | |
| Elevated factor VIII, IX, or XI | ||
Genetic hypercoagulable states and acquired risk factors combine to establish an intrinsic risk for thrombosis for each individual. This risk can be modified by extrinsic or environmental factors, such as surgery, immobilization, or hormonal therapy, which also increase the risk for thrombosis. When the intrinsic and extrinsic forces exceed a critical threshold, thrombosis occurs ( Fig. 138.1 ). Appropriate thromboprophylaxis can prevent the thrombotic risk from exceeding this critical threshold, but breakthrough thrombosis can still occur if procoagulant stimuli overwhelm protective mechanisms.
This chapter describes the inherited, acquired, and mixed hypercoagulable states, details their laboratory evaluation, and provides practical advice for the management of these conditions.
Inherited disorders are found in up to half of patients who present with venous thromboembolism before the age of 40, particularly those whose event occurred either in the absence of well-recognized risk factors, such as surgery or immobilization, or with minimal provoking factors, such as minor trauma, long-distance flight, or estrogens. Patients with inherited thrombophilic disorders often have a family history of thrombosis. Of greatest significance is a family history of sudden death due to pulmonary embolism or a history of multiple family members requiring long-term anticoagulation therapy because of recurrent thrombosis. Patients who present with venous thrombosis in unusual sites, such as the cerebral or mesenteric veins, those with recurrent thrombosis, and patients who develop skin necrosis upon initiation of warfarin therapy should also be suspected of having an inherited hypercoagulable state.
From a pathophysiologic perspective, inherited hypercoagulable states fall into two categories. First are those associated with loss of function of endogenous anticoagulant proteins. These include deficiencies of antithrombin, protein C, and protein S. The second category involves gain of function in procoagulant pathways. These disorders include factor V Leiden and the FIIG20210A mutation, as well as increased levels of procoagulant proteins, such as factors VIII, IX, and XI. Each of these conditions will be briefly described.
Antithrombin, a single-chain glycoprotein with a molecular weight of 52,000 Da, is a member of the serine proteinase inhibitor (serpin) superfamily. Antithrombin is synthesized in the liver and endothelial cells and plays a critical part in regulating coagulation by forming a 1:1 covalent complex with thrombin, factor Xa, and other activated clotting factors. Once covalent complexes are generated, they are cleared from the circulation via the liver. The rate of antithrombin interaction with its target proteases is accelerated by heparin by 1000-fold. Heparan sulfate proteoglycan, which coats the vasculature, is the physiologic counterpart of medicinal heparin.
Newborn infants have approximately 50% of normal adult antithrombin levels, and much lower levels are found in preterm infants because of liver immaturity; adult levels are attained at 6 months.
Antithrombin deficiency can be inherited or acquired, and congenital deficiency of antithrombin was the first reported inherited risk factor for venous thromboembolism. Congenital antithrombin deficiency is relatively rare, occurring in about 1 in 2000, and can be one of two types ( Table 138.2 ), both of which are inherited in an autosomal-dominant fashion and affect both sexes equally. Type I deficiency, which represents the classic deficiency state, is the result of reduced synthesis of biologically normal antithrombin. Heterozygotes with this condition have parallel reductions in antithrombin antigen and activity with levels reduced to about 50% of normal. A heterogeneous group of nonsense mutations, small deletions, insertions, or single-base substitutions are the molecular cause of most cases, although gene deletions can also be responsible. An antithrombin mutation database compiled by members of the Plasma Coagulation Inhibitors Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis summarizes the mutations and can be accessed on the Imperial College London website ( https://www.imperial.ac.uk/immunology-inflammation/research/haematology/haemostasis-and-thrombosis/database/#database ) or in the human gene mutation database ( www.hgmd.cf.ac.uk ).
| Type | Antigen | Activity (No Heparin) | Activity (With Heparin) |
|---|---|---|---|
| I | Low | Low | Low |
| II (active site defect) | Normal | Low | Low |
| II (heparin-binding site defect) | Normal | Normal | Low |
Type II antithrombin deficiencies are characterized by normal levels of antithrombin with impaired functional activity due to the presence of a variant protein. This condition is mainly caused by missense mutations that result in single amino acid substitutions. The clinical consequences of type II antithrombin deficiency depend on the location of the mutation, which may involve the reactive center loop or the heparin-binding domain. For example, some mutations in the reactive center loop of antithrombin slow its interaction with target proteases and are characterized by reduced antithrombin activity in the absence or presence of heparin. In contrast, mutations in the heparin-binding domain are associated with reduced antithrombin activity in the presence of heparin but normal activity in its absence. Unlike other inherited forms of antithrombin deficiency, which are embryonic lethal in the homozygous state, mutations in the heparin-binding domain only have clinical consequences in individuals homozygous for these mutations and do not increase the risk for thrombosis in the heterozygous state.
Because of the wide variety of heritable forms of antithrombin deficiency, functional antithrombin assays are the preferred method for screening. Most functional assays use synthetic substrates to monitor the rates at which added thrombin or factor Xa are inhibited in patient plasma. However, the assays differ in terms of whether bovine or human thrombin is used and whether heparin is added. Defects in the heparin-binding domain of antithrombin will be detected only in the presence of heparin. When antithrombin deficiency is identified with functional assays, immunologic assays are performed to distinguish between type I and type II deficiency.
Congenital antithrombin deficiency can be associated with spontaneous venous thromboembolism, but thrombosis often occurs in the setting of pregnancy and the puerperium; with the use of estrogen-containing oral contraceptives; or after major trauma or surgery. Thrombotic events are rare in children, with events typically occurring from mid to late teenage years and into the early twenties. The European Prospective Cohort on Thrombophilia (EPCOT) study compared the risks of a first episode of venous thromboembolism in asymptomatic individuals with antithrombin, protein C, or protein S deficiency with that in subjects with factor V Leiden over a 6-year period. The annual incidence of venous thromboembolism was highest in those with antithrombin deficiency. Likewise, in a cohort of Italian patients, the risk of venous thrombosis was higher with antithrombin deficiency than with other thrombophilic defects. The most common sites for venous thrombosis in patients wit antithrombin deficiency are the deep veins of the leg, but thrombosis can occur in mesenteric veins, as well as the renal and retinal veins. The risk for recurrence is high, particularly in those with lower antithrombin levels, and the risk varies depending on the subtype of antithrombin deficiency.
Acquired antithrombin deficiency can reflect decreased antithrombin synthesis, increased consumption, or enhanced clearance ( Table 138.3 ). Decreased synthesis can occur in patients with severe hepatic disease, particularly cirrhosis, or in those given l -asparaginase, the latter because of drug-induced retention of antithrombin within the endoplasmic reticulum. Increased thrombin generation can result in antithrombin consumption. Disorders associated with excessive thrombin generation include acute thrombosis, disseminated intravascular coagulation (DIC), severe sepsis, polytrauma, disseminated malignancy, extensive burns, or prolonged extracorporeal circulation. Heparin treatment can reduce antithrombin levels up to 20% by enhancing its clearance. Severe antithrombin deficiency can also occur in some patients with nephrotic syndrome because of the loss of protein in the urine.
| Decreased Synthesis | Increased Consumption | Enhanced Clearance |
|---|---|---|
| Hepatic cirrhosis | Major surgery | Heparin |
| Severe liver disease | Acute thrombosis | Nephrotic syndrome |
| l -asparaginase | Disseminated intravascular coagulation | |
| Severe sepsis | ||
| Multiple trauma | ||
| Malignancy | ||
| Prolonged extracorporeal circulation |
Users of oral contraceptive pills or hormone replacement therapy may have moderate reductions in antithrombin levels; during pregnancy, the antithrombin levels do not fall significantly but decreases are found in preeclampsia and pregnancy-induced hypertensive illnesses.
The protein C pathway is an important natural anticoagulant pathway. This on-demand pathway is activated when thrombin is generated ( Fig. 138.2 ). Thrombin binds to thrombomodulin, a transmembrane thrombin receptor found on the surface of endothelial cells. Once bound to thrombomodulin, the substrate specificity of thrombin is altered so that it no longer serves as a procoagulant enzyme, but becomes a potent activator of protein C, a vitamin K–dependent glycoprotein. Thus, thrombin bound to thrombomodulin activates protein C 1000-fold more efficiently than free thrombin. The endothelial cell protein C receptor (EPCR), another transmembrane receptor on the endothelial cell surface, binds protein C and presents it to the thrombin-thrombomodulin complex for activation, thereby producing an additional 20-fold increase in the rate of protein C activation. The physiologic importance of thrombomodulin and EPCR is highlighted by the fact that deficiency of either receptor results in embryonic lethality in mice.
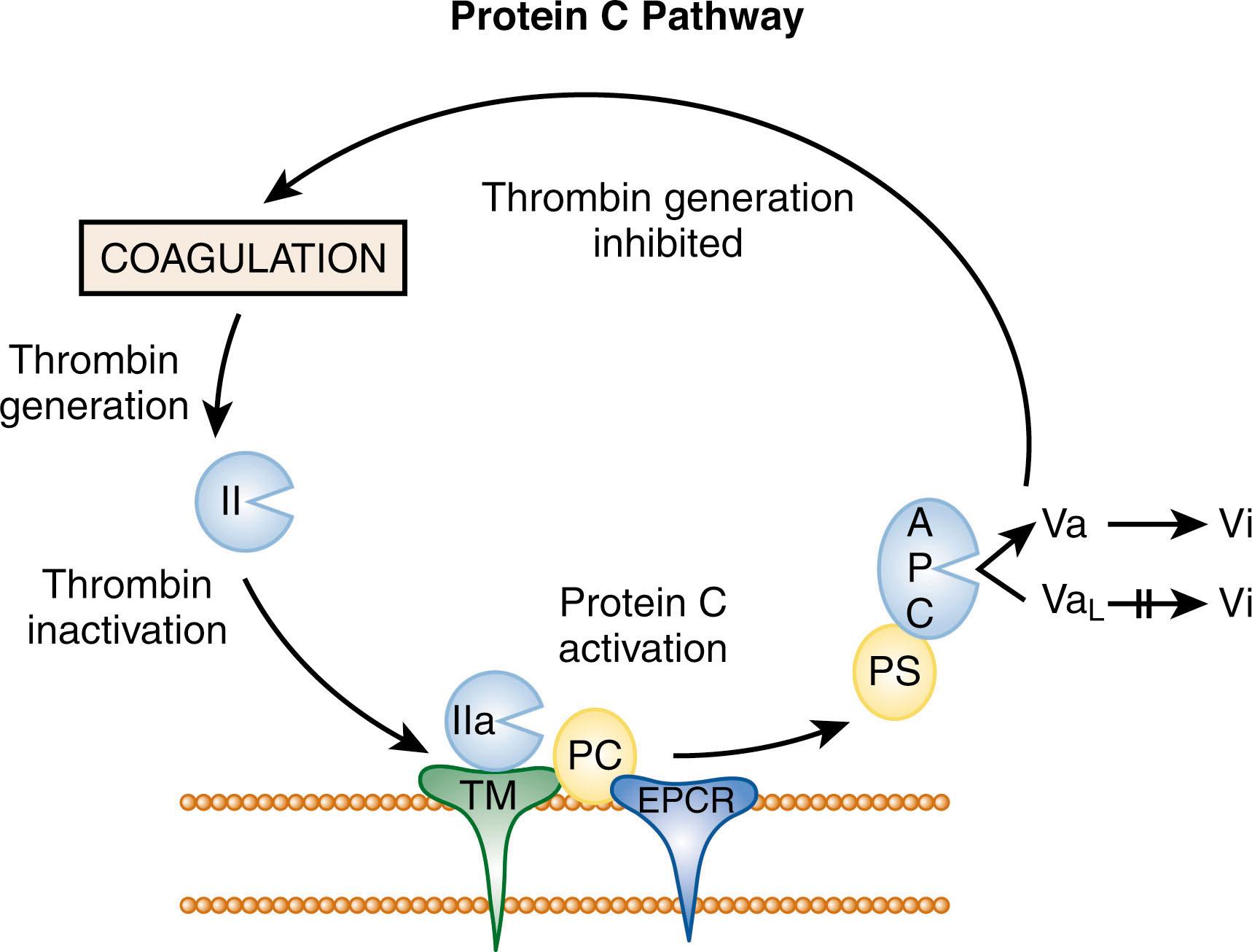
Activated protein C (APC) dissociates from this activation complex and serves as an anticoagulant by proteolytically degrading and inactivating factors Va and VIIIa, thereby attenuating thrombin generation. For efficient inactivation of factors Va and VIIIa, APC must bind to protein S, its cofactor. This interaction facilitates APC binding to activated cell surfaces, particularly the platelet surface, where factors Va and VIIIa are localized.
APC has a half-life in the circulation of about 15 minutes, whereas thrombin has a half-life of about 10 seconds. APC is inhibited by protein C inhibitor and α 1 -proteinase inhibitor (α 1 -antitrypsin), both of which are relatively slow inhibitors. Only the activity of protein C inhibitor is enhanced by heparin, but both inhibitors appear to contribute to APC inhibition in vivo.
Protein C deficiency can be inherited or acquired. Like antithrombin deficiency, protein C deficiency is inherited in an autosomal-dominant fashion and has a proven association with venous thrombosis. Based on studies in healthy blood donors, heterozygous protein C deficiency can be found in 1 in 200 to 500 of the adult population, but many of these individuals do not have a history of thrombosis. Thus, the phenotypic expression of hereditary protein C deficiency is highly variable and may depend on other, as yet unrecognized, modifying factors. In contrast to antithrombin deficiency in which the homozygous state is embryonic lethal, homozygous or doubly heterozygous protein C deficiency can occur. The prevalence of homozygous protein C deficiency is estimated to be 1 in 160,000 to 360,000 births. Newborns with these disorders may present with purpura fulminans characterized by widespread thrombosis.
Individuals with heterozygous protein C deficiency can develop skin necrosis upon initiation of treatment with vitamin K antagonists, such as warfarin. Typically, skin lesions are found on the extremities, breasts, or trunk. Starting as erythematous macules, the central regions of the cutaneous lesions become purpuric and then necrotic over a period of hours unless protein C is administered. Biopsies reveal fibrin thrombi within the vessels of the skin associated with interstitial hemorrhage. The skin lesions are clinically and histologically similar to those seen in infants with purpura fulminans and are attributable to the transient hypercoagulable state that is induced by warfarin, thereby explaining why they occur early during the course of warfarin therapy. The half-life of protein C is short and similar to that of factor VII. Starting warfarin in patients with protein C deficiency causes a further reduction in protein C levels, particularly if loading doses of warfarin are given. Thus, the activity of the natural anticoagulant protein C pathway is compromised before warfarin lowers the levels of vitamin K–dependent procoagulant proteins, particularly prothrombin and factor X, into the range required for its antithrombotic effects. Warfarin-induced skin necrosis has also been reported in association with acquired deficiency of protein C.
Hereditary protein C deficiency can be further delineated into two subtypes using immunologic and functional assays ( Table 138.4 ). Most functional assays use Protac, a protease isolated from the venom of the copperhead snake, to activate protein C in plasma. The enzymatic activity of APC can then be determined directly using an APC-directed synthetic substrate, or it can be indirectly quantified by measuring the extent of prolongation of the activated partial thromboplastin time (aPTT). The most common form of hereditary protein C deficiency is the classic or type I deficiency state. This disorder reflects the reduced synthesis of a normal protein and is characterized by a parallel reduction in protein C antigen and activity, resulting in a quantitative deficiency due to reduced synthesis or stability of protein C. A variety of genetic defects can produce type I protein C deficiency, including promoter mutations, splice-site abnormalities, in-frame deletions, frameshift deletions, in-frame insertions, and frameshift insertions in the protein C gene (PROC) , but missense or nonsense mutations are the most common.
| Type | Antigen | Activity |
|---|---|---|
| I | Low | Low |
| II | Normal | Low |
Type II protein C deficiency reflects synthesis of a dysfunctional protein and is characterized by normal protein C antigen with reduced functional activity, a qualitative deficiency. Most type II protein C deficiency states are caused by point mutations. Mutations in the active site of APC reduce its activity against synthetic substrates and decrease its capacity to prolong the aPTT. In contrast, mutations that affect other protein C domains essential for its activity may reduce its anticoagulant activity but may not affect its capacity to cleave synthetic substrates activity. Therefore, coagulation-based functional assays are preferred when screening patients for protein C deficiency.
Diagnosis of protein C deficiency is complicated. Protein C circulates in human plasma at an average concentration of 4 μg/mL. Plasma protein C antigen levels are widely distributed in healthy adults such that 95% of the values range from 70% to 140%. Furthermore, protein C levels increase with age, particularly in postmenopausal women. The wide range of values renders the establishment of a normal range difficult. Levels less than 55%, however, are likely to reflect deficiency, whereas those between 55% and 70% are considered borderline and may be consistent with a deficiency state or the lower end of the normal distribution. Acquired causes of protein C deficiency must be excluded, and to document the presence of protein C deficiency, it is necessary to repeat the testing. Family studies may also be helpful to highlight the autosomal-dominant pattern of inheritance.
Acquired protein C deficiency can be due to decreased synthesis or increased consumption. Decreased synthesis can occur with liver disease or with warfarin therapy. Warfarin decreases functional activity more than immunologic activity. Newborns have protein C levels 20% to 40% lower than those of adults, and premature infants have even lower levels. Protein C consumption can occur with severe sepsis, with DIC, and after surgery. Reduced protein C levels have also been reported in cancer patients receiving cyclophosphamide, methotrexate, 5-fluorouracil, or l -asparaginase. A particularly severe form of acquired protein C deficiency has been described in association with meningococcal septicemia. In contrast to antithrombin, which is excreted in the urine of patients with nephrotic syndrome, the levels of protein C are normal or elevated in patients with nephrotic syndrome.
Protein S serves as a cofactor for APC and enhances its capacity to inactivate factors Va and VIIIa. In addition, protein S may have direct anticoagulant activity by inhibiting prothrombin activation through its capacity to bind anionic phospholipid, factor Va, or factor Xa, components of the prothrombinase complex. The importance of the direct anticoagulant activity of protein S is uncertain.
In the circulation, about 60% of total protein S is bound to C4b-binding protein, an acute phase complement component. Because only 40% of the protein S that is free is functionally active, only patients with low free protein S levels are prone to venous thrombosis. Therefore, the diagnosis of protein S deficiency requires measurement of both free and bound forms of protein S. Total protein S levels can be measured immunologically under conditions that dissociate protein S from C4b-binding protein. The free fraction can then be quantified with a monoclonal antibody that only recognizes free protein S, while the functional activity of protein
S can be measured using an APC cofactor assay. This assay depends on the prolongation of the aPTT when diluted patient plasma is added to protein S–depleted plasma containing APC and factor Va.
Protein S deficiency can be inherited or acquired. Heterozygous protein S deficiency is inherited in an autosomal-dominant manner; the prevalence varies between 1% and 7% among patients with thrombotic events. There is an association with unprovoked venous thromboembolism. Based on measurements of total and free protein S antigen and protein S activity, three subtypes of inherited protein S deficiency have been identified ( Table 138.5 ). Type I or classic deficiency results from the decreased synthesis of a normal protein and is characterized by reduced levels of total and free protein S antigen together with reduced protein S functional activity. Molecular analysis of protein S deficiency is complicated because there are two homologous protein S genes, one of which is likely a pseudogene. Nonetheless, most cases of type I protein S deficiency are caused by partial gene deletions, missense mutations, base pair insertions or deletions, premature stop codons, or mutations affecting a splice site in the gene encoding protein S (PROS1) . Type II protein S deficiency is characterized by normal levels of total and free protein S associated with reduced protein S activity. This type of deficiency is uncommon, and most of the causative mutations encode protein S domains involved in its interaction with APC.
| Type | Total Protein S | Free Protein S | Protein S Activity |
|---|---|---|---|
| I | Low | Low | Low |
| II | Normal | Normal | Low |
| III | Normal | Low | Low |
Type III protein S deficiency is characterized by normal levels of total protein S, but low levels of free protein S are associated with reduced protein S activity. The molecular basis of this type of deficiency appears to be similar to that of the type I deficiency states. In fact, type I and type III protein S deficiency are likely to be manifestations of the same disease because they often coexist in families. Thus, younger family members present with type I deficiency, whereas older family members have type III deficiency because protein S levels increase with age.
Acquired protein S deficiency can be due to decreased synthesis, increased consumption, loss, or shift of free protein S to the bound form. Decreased synthesis can occur in patients with severe liver disease, in those given l -asparaginase, and in patients given vitamin K antagonists. Increased consumption of protein S occurs in patients with acute thrombosis or in those with DIC. Patients with nephrotic syndrome can lose free protein S in their urine, causing decreased protein S activity. Total protein S levels in these patients are often normal because the levels of C4b-binding protein increase, shifting more protein S to the bound form. C4b-binding protein levels also increase in pregnancy and with the use of oral contraceptives. This shifts more protein S to the bound form and lowers the levels of free protein S and protein S activity. The pathophysiologic consequences of this phenomenon are uncertain. An association between antiphospholipid antibodies and acquired protein S deficiency has been reported in patients with severe forms of varicella zoster virus infection complicated by purpura fulminans. In healthy neonates, the total protein S antigen levels are 15% to 30% of normal, and the C4b-binding protein is significantly reduced to less than 20%, such that the free form of protein S predominates and the functional levels are only slightly reduced compared with normal adult levels.
Gain of function mutations include factor V Leiden , FIIG20210A, elevated levels of procoagulant proteins, and other less well-characterized genetic disorders. Gain of function mutations are more prevalent in the general population than those associated with loss of function.
In 1993, Dahlback and colleagues described three families with a history of venous thromboembolism. Affected family members exhibited limited prolongation of the aPTT when APC was added to their plasma. Accordingly, this phenotype was designated APC resistance (APCR). Bertina and colleagues demonstrated that APCR co-segregated with the factor V gene and was due to a single-base substitution, guanine to adenine at position 1691, that produced an Arg 506 Gln mutation at one of the APC cleavage sites on factor Va. This mutation, which is designated factor V Leiden , endows activated factor V Leiden with a 10-fold longer half-life in the presence of APC than its wild-type counterpart.
The factor V Leiden mutation is responsible for most cases of APCR. Other causes are mutations at Arg 306, another APC cleavage site. Arg 306 is replaced by a Gly residue in factor V Hong Kong and by a Thr residue in factor V Cambridge . Neither of these mutations is strongly associated with thrombosis.
The factor V Leiden mutation is inherited in an autosomal-dominant fashion. The prevalence of the mutation ranges from 2% to 5% in Whites but it is rare in Asians and Africans. This racial difference likely reflects a founder effect with the mutation arising 20,000 to 30,000 years ago, after the divergence of non-African from African and Caucasoid from Mongoloid subpopulations. The prevalence of factor V Leiden homozygosity is about 1 in 2500. The risk for thrombotic complications is lower with factor V Leiden than it is with deficiencies of antithrombin, protein C, or protein S; and in the heterozygous state, factor V Leiden does not appear to be a strong risk factor for recurrent venous thrombosis. The risk for thrombosis is higher in homozygotes than in heterozygotes. Acquired APC resistance may be caused by hormonal changes during pregnancy or by the administration of estrogens, such as oral contraceptive pills or hormone replacement therapy.
A diagnosis of APCR is established using a functional assay based on the ratio of the aPTT after APC addition divided by that determined before APC addition. Second-generation tests, which add dilute patient plasma to factor V-deficient plasma, are more specific for factor V Leiden . A normal functional test excludes factor V Leiden ; a positive functional test for APCR should be confirmed with a genetic test for the factor V Leiden mutation. Some laboratories only use the genetic test for the diagnosis of factor V Leiden .
After extensive screening of 28 families with unexplained venous thromboembolism, Poort and colleagues identified a heterozygous G to A nucleotide transition at position 20210 in the 3′-untranslated region of the prothrombin gene in five of the probands. This mutation, FII G20210A, results in elevated levels of prothrombin. Elevated levels of prothrombin, in turn, may increase the risk for thrombosis by enhancing thrombin generation or by inhibiting factor Va inactivation by APC.
The mechanism by which the FII G20210A mutation causes increased prothrombin levels appears to vary. Enhanced protein synthesis may result from more efficient 3′-end formation, increased messenger RNA stability, increased translation efficiency, or some combination of these mechanisms. An intronic FII gene polymorphism, A19911G, which influences splicing efficiency, may modulate the effect of the FII G20210A mutation such that heterozygous carriers of both mutations have a greater risk for thrombosis than those with only the FII G20210A mutation.
Like the factor V Leiden mutation, the prevalence of the FII G20210A mutation is higher in whites and low in Asians, American Indians, and African Americans. A founder effect likely explains the higher prevalence in whites. The mutation may have provided a survival advantage based on a protective effect with childbirth or severe sepsis.
FII G20210A is found in 1% to 6% of Whites. The mutation is more common in southern Europe than in northern Europe, a gradient opposite to that of factor V Leiden . Rare individuals homozygous for the FII G20210A mutation have been identified. In the Leiden Thrombophilia Study, 6.2% of venous thrombosis patients and 2.3% of healthy matched controls had the FII G20210A mutation. The mutation independently confers a 2.8-fold increased risk for venous thrombosis, with no gender bias; a risk lower than that with antithrombin, protein C, or protein S deficiency. The abnormality confers a weaker increased risk of venous thrombosis than protein C, protein S, or antithrombin deficiencies.
Laboratory diagnosis of FIIG 20210 A depends on genetic screening after PCR amplification of the 3′-untranslated region of the FII gene. Although FII G20210A heterozygotes have 30% higher levels of prothrombin than non-carriers, the wide range of prothrombin levels in healthy individuals precludes the use of this phenotype to identify carriers.
Elevated levels of factor VIII and other coagulation factors, including factors XI, IX, and VII, have been implicated as independent risk factors for thrombosis. Although the molecular bases for the high levels of these coagulation factors have yet to be identified, genetic mechanisms are likely responsible because the hereditability of these quantitative abnormalities is high.
The dysfibrinogenemias represent a heterogeneous group of disorders characterized by abnormal fibrinogen structure and are diagnosed by low functional and/or immunologic levels of fibrinogen, in association with prolonged thrombin and reptilase times. Acquired causes of dysfibrinogenemia, such as liver disease, must be excluded in the diagnostic work-up. Most congenital dysfibrinogenemias are asymptomatic and are often identified as incidental findings when coagulation testing is performed for other reasons. Up to 40% of the known dysfibrinogenemias are associated with a bleeding diathesis. Approximately 15 variant fibrinogens, which represent less than 10% of known dysfibrinogenemias, have been reported to be associated with thrombotic complications, including fibrinogen Marburg, Caracas V, Chapel Hill III, Hannover II, Nijmegen, New York I, Christchurch II and III, and Milano III. The exact mechanism by which these dysfibrinogenemias increase the risk for thrombosis depends on the nature of the fibrinogen defect. Most affect the C-terminal domain of the Aα chains or the thrombin cleavage site on the Bβ chains. Some biochemical defects have been further characterized, such as defects in the release of fibrinopeptides A or B by thrombin, impaired binding of thrombin or tissue plasminogen activator to fibrin, or resistance to lysis by plasmin. It is likely that acquired and/or other hereditary factors contribute to the thrombosis that occurs in patients with dysfibrinogenemia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here