Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pretransfusion testing includes ABO and Rhesus (Rh) type, and antibody screening to determine whether a patient has an unexpected red blood cell (RBC) antibody. If the antibody screen is positive, an identification panel is performed to identify the specificity. Unexpected antibodies can be clinically significant causing hemolysis (i.e., acute or delayed hemolytic reaction) after transfusion of RBCs carrying the reciprocal antigen, or can be insignificant. The clinical significance of an antibody is assessed by correlating the serologic information with clinical experiences reported in the literature and with the patient’s medical history. Notably, the majority of clinically significant antibodies (outside the ABO system) are in response to RBC antigen exposure either through transfusion or pregnancy. Other antibody characteristics that are used to predict clinical significance include immunoglobulin (Ig) class and in vitro characteristics such as strength of reactivity and titer; however, no foolproof method exists to predict the clinical significance. For antibodies with well-known clinical significance, antigen-negative blood is selected for transfusion. Predicting clinical significance is more difficult when a patient has an antibody to a novel or rare high-prevalence antigen, and requires a transfusion but antigen-negative blood is not available.
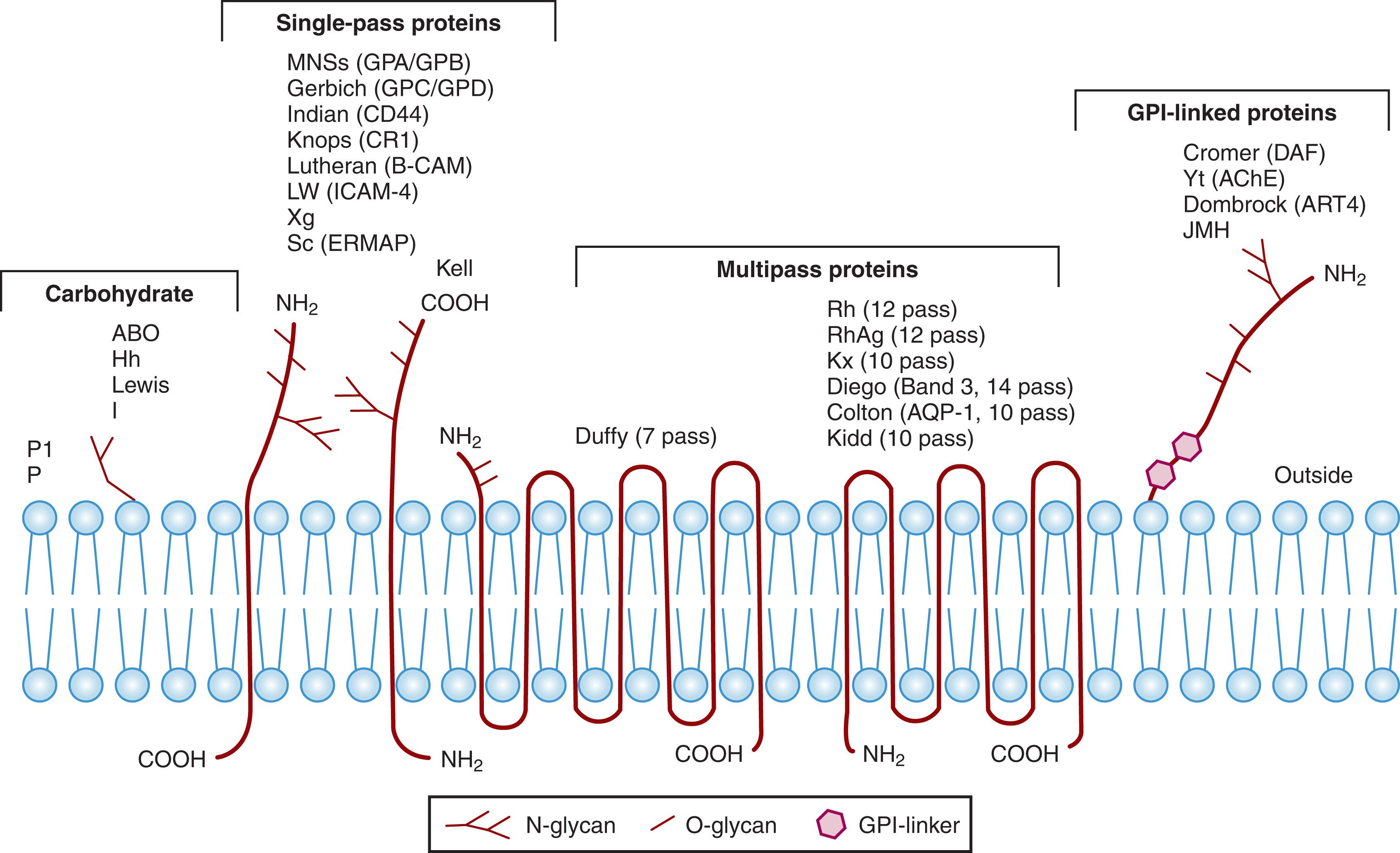
Erythrocyte blood group antigens are polymorphic, inherited, carbohydrate, or protein structures located on the surface of the RBC membrane. There are more than 300 blood group antigens, most of which are included in 38 different blood group systems ( Table 111.1 ). The protein antigens are primarily located on integral transmembrane proteins, but a few are on glycosylphosphatidylinositol (GPI)–linked proteins ( Fig. 111.1 ). Some antigens are carbohydrates attached to proteins or lipids, some require a combination of a specific portion of protein and carbohydrate, and a few antigens are carried on proteins that are adsorbed from the plasma. Many of the proteins carrying blood group antigens reside in the erythrocyte membrane as complexes with other proteins.
| ISBT System Name (Number) | Gene Name | Predicted Topology (Number of Amino Acids [AA]) | Component Name | Principal Associated Blood Group Antigens (Null Phenotype) | Present in Other Tissue | Disease Association | Function |
|---|---|---|---|---|---|---|---|
| ABO (001) | ABO |
|
Carbohydrate |
|
Secretions, platelets, broad tissue distribution | Altered in some hematologic disorders, leukemia | Glycosylation |
| MNS (002) |
|
|
|
M, N, S, s, U, Vw; M k M k lack GPA and GPB; En(a−) lack GPA; S−s−U− lack GPB | Renal endothelium and epithelium |
|
Negative charge on sialic acid; receptor for microbes |
| P1PK (003) | A4GALT |
|
Carbohydrate | P1, Pk (P1−, PP1Pk−) | Lymphocytes, granulocytes, monocytes, platelets |
|
Glycosylation |
| Rh (004) |
|
Multipass—12 spans (417 AA) |
|
D, C, E, c, e, G, V/VS (Rh null syndrome) | RBC-specific |
|
Structural link to underlying cytoskeleton |
| Lutheran (005) |
|
Type I IgSF (597 AA) (557 AA) | Lutheran glycoprotein B-CAM | Lu a , Lu b , Lu3, Au a , Au b (recessive Lu(a−b−)) |
|
Increased expression possibly involved in vasoocclusion in sickle cell disease |
|
| Kell (006) | KEL | Type II (732 AA) | Kell glycoprotein | K, k, Kp a , Kp b , Ku, Js a , Js b (K 0 or K null ) |
|
Depressed in McLeod syndrome (see XK) | Cleaves big endothelin 3 to ET-3 (a potent vasoconstrictor) |
| Lewis (007) | FUT3 (LE) |
|
|
Le a , Le b , Le ab , Le bh , ALe b , BLe b (Le(a−b−)) |
|
Increased expression in fucosidosis | Fucosyl transferase |
| Duffy (008) | ACKR1 (DARC, FY) | Multipass—7 spans (338 AA) | Fy glycoprotein | Fy a , Fy b , Fy3, Fy6 (Fy(a−b−)) | Broad tissue distribution Endothelial and epithelial cells, Purkinje cells, colon, lung, spleen, thyroid, thymus, kidney |
|
Chemokine receptor |
| Kidd (009) | SLC14A1 (JK) | Multipass—10 spans (389 AA) | Kidd glycoprotein | Jk a , Jk b , Jk3 (Jk(a−b−) or Jk null ) |
|
Urine concentrating defect | Urea transport |
| Diego (010) | SLC4A1 (DI) | Multipass—14 spans (911 AA) | Band 3, AE1 | Di a , Di b , Wr a , Wr b , (1 reported—transfusion dependent; predicted to be incompatible with life) | Kidney: intercalated cells of distal and collecting tubules | Southeast Asian ovalocytosis, hereditary spherocytosis, renal tubular acidosis |
|
| Yt (011) | ACHE (YT) | GPI-linked (557 AA) | Acetyl-cholinesterase | Yt a , Yt b | Brain, muscle, nerves | Absent from PNH III RBCs | Enzymatic |
| Xg (012) | XG | Type I (180 AA) (163 AA) | Xg a glycoprotein | Xg a | The antigen may be restricted to RBC, but CD99 has broad tissue distribution | Adhesion molecule | |
| Scianna (013) | ERMAP (SC) | Type I (475 AA) | ERMAP | Sc1, Sc2, Sc3, Rd (Sc −1, −2, −3) | Possible adhesion | ||
| Dombrock (014) | ART4 (DO) | GPI-linked (314 AA) | Do glycoprotein; ART 4 | Do a , Do b , Gy a , Hy, Jo a (Gy(a−)) | Lymphocytes, spleen, lymph nodes, GI, ovary, testes, heart, liver | Absent from PNH III RBCs | Enzymatic |
| Colton (015) | AQP1 (CO) | Multipass—6 spans (269 AA) | Aquaporin | Co a , Co b , Co3 (Co(a−b−)) |
|
Monosomy 7, congenital dyserythropoietic anemia | Water transport |
| Landsteiner-Wiener (016) | ICAM4 (LW) | Type I IgSF (241 AA) | LW glycoproteinICAM-4 | LW a , LW b , LW ab (LW(a−b−)) | Depressed in some malignant diseases; decreased in Rh null syndrome | Ligand for integrins | |
| Chido/ Rodgers (017) | C4A, C4B ( CH/RG ) | Not endogenous to RBC (1191 AA) | C4A; C4B | Ch1, Ch2, Rg1 | Adsorbed from plasma |
|
Part of the complement cascade |
| H (018) | FUT1(H) |
|
Carbohydrate | H (Bombay O h ) | Broad distribution Soluble—all fluids except CSF in secretors | Decreased in some tumor cells Increased in hematopoietic stress | Glycosylation |
| Kx (019) | XK (XK) | Multipass—10 spans (444 AA) | XK glycoprotein | Kx (McLeod) | Fetal liver, adult skeletal muscle, brain, pancreas, heart | X-linked midlife onset neuropathy, elevated CPK, muscular dystrophy, acanthocytosis; sometimes associated with CGD | Transport; possible neurotransmitter |
| Gerbich (020) | GYPC (GE) | Type I (128 AA) (107 AA) | GPC GPD | Ge2, Ge3, Ge4 (Leach phenotype) | Fetal liver, renal endothelium | Hereditary elliptocytosis, hemolytic anemia, receptor P. falciparum | Structural Interacts with protein 4.1 and p55 |
| Cromer (021) | CD55 (CROM) | GPI-linked (347 AA) | DAF | Cr a , Tc a , Tc b , Tc c , Dr a , Es a , IFC (Inab) |
|
|
Complement regulation; binds C3b; disassembles C3/C5 convertase |
| Knops (022) | CR1 (KN) | Type I (1998 AA) | CR1 | Kn a , Kn b , McC a , Sl a , Yk a , KCAM (no nulls reported) | Blood cells, glomerular podocytes, follicular dendritic cells | Antigens depressed in certain autoimmune and malignant conditions | Complement regulation; binds C3b and C4b; mediates phagocytosis |
| Indian (023) | CD44 (IN) | Type I (341 AA) | Hermes antigen | In a , In b | Wide tissue distribution | 1 case—congenital dyserythropoietic anemia | Binds hyaluronic acid; mediates adhesion of leukocytes |
| Ok (024) | BSG (OK) | Type I IgSF (248 AA) | Basigin | Ok a | All cells tested | Receptor P. falciparum | Possible adhesion |
| RAPH (025) | MER2 | Multipass—4 spans (253 AA) | CD151 | MER2 (Raph−) | Fibroblasts | Absence associated with renal disease and kidney failure | |
| JMH (026) | SEMA7A(JMH) | GPI-linked (656 AA) | Semaphorin 7A | JMH | Absent from PNHIII RBCs | Adhesion molecule | |
| I (027) | GCNT2 | N -acetyl-glucosaminyl-transferase type II (400 AA) | Carbohydrate | I (I− or i adult) | Broad tissue distribution | Cataracts in Asians | Glycosylation |
| Globoside (028) | B3GALT1 | N -acetyl-galactosaminyl-transferase type II (331 AA) | Carbohydrate (Gb 4 , globoside) |
|
|
|
Glycosylation |
| GIL (029) | AQP3 (GIL) |
|
AQP3 | GIL (GIL−) |
|
Glycerol/ water/ urea transport | |
| RHAG (030) | RHAG | Multipass—12 spans (409 AA) | Rh-associated glycoprotein |
|
|
Hereditary overhydrated stomatocytosis | Ammonia transport |
| FORS (031) | GBGT1 | Type II (347 AA) | Carbohydrate | FORS1 | Broad tissue distribution | Glycosyltransferase | |
| JR (032) | ABCG2 | Type III—6 spans (655 AA) | ATP-binding cassette sub-family G member 2 | Jr a (Jr(a−)) | Broad tissue distribution High in placenta, seminal vesicles | Gout; multidrug resistance in cancer | Urate exporter; porphyrin hemostasis |
| LAN (033) | ABCB6 | Type III—6 spans (842 AA) | ATP-binding cassette sub-family B member 6, mitochondrial | Lan (Lan−) | Broad tissue distribution | Dyschromatosis universalis hereditaria; Microphthalmia | Binds heme and porphyrins. Important in heme synthesis |
| VEL (034) | SMIM1 | Type 1 (78 AA) | SMIM1 | Vel (Vel−) | RBCs, salivary glands, testis | Not known | |
| CD59 (035) | CD59 | GPI-linked (128 AA) | CD59 glycoprotein | CD59.1 (CD59.1−) | Broad tissue distribution; soluble form in plasma | Hemolytic anemia, with or without polyneuropathy | Complement regulation; inhibits MAC complex |
|
SLC29A1 | Type III—11 spans (456 AA) | Equilibrative nucleoside transporter 1 | At a (At(a−) | Broad tissue distribution | Pseudo gout in the null phenotype | Nucleoside transmembrane transporter |
|
PRNP | (253 AA) | Prion | KANNO | Broad tissue distribution | Absence of KANNO possibly protective for Creutzfeldt-Jakob disease | Possible role in neuronal development and synaptic plasticity |
|
B4GALNT2 |
|
Carbohydrate |
|
Kidney, colon, stomach | Extra strong expression of Sd a Sd(a++) makes cells resistant to malarial parasite P. falciparum | Glycosylation |
Recognition of a new blood group antigen begins with discovery of an antibody. When an individual whose RBCs lack an antigen is exposed to RBCs that possess the antigen, they may mount an immune response and produce antibodies that react with the antigen. Depending on the characteristics of the antibody and the number and topology of antigens in the RBC membrane, the interaction in vivo between antibody and antigen may result in removal of antibody-coated RBCs by the reticuloendothelial system or in hemolysis if complement is activated.
In blood group testing, most assays are designed to detect antibody-antigen binding with clumping of the RBCs (“agglutination”) as the detectable endpoint. The ability to detect and identify blood group antigens and antibodies has contributed significantly to current safe supportive blood transfusion practice, to the appropriate management of pregnancies at risk for hemolytic disease of the fetus and newborn (HDFN), and to management of hematopoietic progenitor cell and solid organ transplantation.
Some blood group systems bear the family surname in which the antibody was first discovered (Kell, Kidd, Duffy, etc.), with abbreviations to indicate antigens (K/k, Jk a /Jk b , Fy a /Fy b , etc.). Others have been given letter designations (A, B, D, M, N, etc.). A committee for terminology of RBC surface antigens and alleles, organized by the International Society of Blood Transfusion (ISBT), works to standardize terminology of new blood group antigens and the coding alleles.
The majority of genes encoding blood group antigens have been identified and cloned, and the molecular basis of most blood group antigens has been determined. Details concerning the alleles associated with blood group antigens are found on the ISBT Red Cell Immunogenetics and Blood Group Terminology working party webpage, and the Blood Antigens and Erythrogene websites ( www.isbtweb.org/working-parties/red-cell-immunogenetics-and-blood-group-terminology/; bloodantigens.com; erythrogene.com ). Knowledge of the genes has advanced understanding of the structure and function of the components carrying antigens, and resulted in an appreciation of diseases associated with loss of expression of some blood groups, for example, null phenotypes (see Table 111.1 ). Of importance, knowledge of the gene has made it possible to perform DNA analyses to predict the serologic phenotype, to determine gene dosage (zygosity), to perform noninvasive fetal typing, and to type for numerous blood group antigens in a single assay.
Although the simple hemagglutination test remains the principal assay for RBC antigen typing for ABO and Rh, antibody screen, and compatibility testing; DNA-based typing (genotyping), especially for the minor blood group antigens, has become commonplace in some clinical situations ( Table 111.2 ). These include determination of the extended blood group phenotype in patients who are multiply transfused, which avoids false typing because of contaminating donor RBCs and aids determination of antibody specificity. This approach is also preferred in patients with strongly direct antiglobulin test (DAT)-positive RBCs, as well as for typing for antigens when no serologic reagents are available and for fetal typing from amniocytes or from free DNA present in the maternal plasma. In these instances and others (see Table 111.2 ), hemagglutination is not helpful, and genomic analysis is a useful adjunct to routine testing. More recently, genotyping is useful in patients treated with daratumumab (anti-CD38) because treatment results in panreactivity on antibody screening (all cells being reactive). High-throughput genotyping systems have enabled blood centers to screen donors for a large number of antigens in a single assay. RHD genotyping is also well suited for the precise determination of known weak D and partial D variations.
|
Polymerase chain reaction (PCR) -based genotyping assays currently dominate the market. However, next generation sequencing (NGS) has proven to be a naive, but promising approach. NGS allows for the sequencing of large genomic regions, making it ideal for novel antigen discovery, and has been used to determine the genetic basis of several recent blood group systems. Interpretation of NGS based whole genome and whole exome data can be used to genotype for essentially all antigens with a known genetic basis. Furthermore, the development of automated interpretive software has allowed for genotyping from large scale genomic sequencing projects. In addition, targeted NGS of either one or multiple blood group genes is possible, including the ABO and Rh blood group systems. Targeted NGS based ABO genotyping particularly has value alongside NGS based human leukocyte antigen (HLA) typing of buccal swabs when screening stem cell transplant donors. In addition, NGS, especially whole genome sequencing (WGS), is well suited for the evaluation of structural variations including large deletions (e.g. MNS U− GYPB deletion) and gene recombinations (e.g., Rh partial D DIIIa-CE(4-7)-D).
The common causes of immunization against blood group antigens are transfusion, pregnancy, transplantation, or occasionally, practices such as sharing needles. “Naturally occurring” antibodies are not a result of RBC exposure; rather, a response to microbes encountered by way of the digestive tract and other mucosal surfaces regularly (e.g., anti-A, anti-B,) or sometimes (e.g., anti-M, -P, -P k , -P1, -Le a , -Le b , -I, -IH) which result in production of antibodies with these specificities. These are the most common antibodies present in children and nontransfused male patients, and are primarily IgM. These multivalent IgM antibodies directed to carbohydrate antigens optimally bind and directly agglutinate to RBCs at temperatures below 37°C. Most are not clinically significant (outside of ABO). Exceptions occur if the antibody is reactive at 37°C and/or has an IgG component. Antibodies that are considered not to be clinically significant unless the antibody reacts in tests performed at 37°C include those to A1, P1, M, N, Lu a , Le a , Le b , I, IH, and Sd a antigens.
In contrast, antibodies occurring following immunization to protein antigens such as those in the Rh, Kell, Kidd, and Duffy blood group systems are primarily of the IgG isotype that react at 37°C and are detected by the indirect antiglobulin test (IAT). These bivalent antibodies optimally bind to, but do not directly agglutinate, RBCs at 37°C. The addition of an antiglobulin reagent (i.e., antihuman globulin [AHG], also known as Coombs serum) is required to induce RBC agglutination. Most of these are clinically significant antibodies, with the exception of antibodies to Knops (Kn), Chido/Rodgers (Ch/Rg) and John Milton Hagen (JMH) systems ( Table 111.3 summarizes the Ig class and clinical significance associated with alloantibodies; see also box on Indirect Antiglobulin Test and Direct Antiglobulin Test).
| Antibody Specificity | IgM | IgG | Clinical | |
|---|---|---|---|---|
| Transfusion Reaction | HDFN | |||
| ABO | Most | Some | Immediate; mild to severe | Common; mild to moderate |
| H in Bombay | Most | Some | Immediate; mild to severe | Rare; mild |
| Rh | Some | Most | Immediate/delayed; mild to severe | Common; mild to severe |
| RhAG | Rare | Most | Immediate/delayed; mild to severe | Mild to severe |
| Kell | Some | Most | Immediate/delayed; mild to severe | Mild to severe |
| Kidd | Few | Most | Immediate/delayed; mild to severe | Rare; mild |
| Duffy | Rare | Most | Immediate/delayed; mild to severe | Rare; mild |
| S | Some | Most | Delayed/mild | Rare; mild to severe |
| s | Rare | Most | Delayed/mild | Rare; mild to severe |
| U | Rare | Most | Immediate/delayed; mild to severe | Rare; severe |
| PP1P k | Most a | Most a | Immediate; mild to severe | Mild to severe b |
| Vel | Most a | Most a | Immediate/delayed; mild to severe | Mild to severe |
| Diego | Some | Most | Delayed; none to severe | Mild to severe |
| Colton | Rare | Most | Delayed; mild | Rare; mild to severe |
| Lutheran | Some | Most | Delayed | Rare; mild |
| Dombrock | Rare | Most | Immediate/delayed; mild to severe | Rare; mild |
| M | Some | Most | Delayed (rare) | Rare; mild to severe |
| N | Most | Rare | None | None |
| LW a | Rare | Most | Delayed; none to mild | Rare; mild |
| Yt a | Rare | Most | Delayed (rare); none to mild | None |
| Ch/Rg | Rare | Most | Anaphylactic (rare) | None |
| JMH | Rare | Most | Delayed (rare in genetic variants); none to mild | None |
| P1 | Most | Rare | None (rare) | None |
| Le a | Most | Few | Immediate (rare) | None |
| Le b | Most | Few | None | None |
| I | Most | Rare | None to mild in I adults | None |
| Knops | Rare | Most | None | None |
| Xg a | Rare | Most | None | None |
a Most examples of these antibodies are both IgM and IgG.
b Seldom hemolysis of fetal cells but high incidence of recurrent spontaneous abortions.
Antibodies recognizing antigens in the ABO system are by far the most clinically significant and are present in nearly all individuals who lack the antigen (they typically appear by 4 months of age). Other clinically significant antibodies occur in the following approximate order, from the most to the least commonly encountered in transfusion practice: anti-D, anti-K, anti-E, anti-c, anti-Fy a , anti-C, anti-Jk a , anti-S, and anti-Jk b . Clinically significant antibodies occur in approximately 3% of transfused patients but have a higher incidence of 35% to 55% in patients undergoing chronic transfusion. The frequency of antibody production depends on the antigen immunogenicity and prevalence of the antigen in a population.
Manual tube, solid phase, or gel-column methods based on agglutination of RBCs are the most common serologic assays performed in transfusion medicine laboratories.
The indirect antiglobulin test (IAT) is used to detect alloantibodies in patient sera in vitro following incubation at 37°C and includes antibody screening and identification and crossmatching with donor red blood cells (RBCs). IAT is also sometimes used for antigen typing to detect RBCs coated with antibody following incubation with reagent antisera. After incubation, unbound antibodies are removed by washing with saline and an antiglobulin reagent containing either antihuman IgG (AHG) or a mixture of AHG and monoclonal antihuman complement is added. Differential agglutination suggests the presence of antibodies to one or more specific RBC antigens while agglutination of all cells suggests the presence of an antibody to a high-prevalence antigen or the presence of an autoantibody.
The direct antiglobulin test (DAT) is used to detect the presence of antibody or complement (or both) on the surface of RBCs in vivo such as autoantibodies coating the patient’s cells in warm autoimmune hemolytic anemia, cold hemagglutinin disease, or alloantibodies coating the patient’s cells in immediate or delayed transfusion reactions, or hemolytic disease of the fetus and newborn. Patient RBCs obtained in ethylenediaminetetraacetic acid, to prevent in vitro complement deposition on the RBCs, are washed with saline and then incubated with a commercial antiglobulin reagent containing AHG or antihuman complement or a mixture of the two. Antiglobulin reagents containing anti-IgM or anti-IgA are available in specialized centers to detect coating of RBCs by antibodies of these isotypes.
Rh immune globulin (RhIG) is a human plasma-derived hyperimmunoglobulin product consisting of IgG antibodies to D antigen that is administered to D-negative pregnant women who are at risk for D sensitization. RhIG is administered (1) at 28 weeks gestational age, (2) when there is a risk for fetal maternal hemorrhage through amniocentesis, trauma, or other procedures, and (3) postpartum in the case of a known or potential D-positive newborn or fetus. If the Rh(D) typing of a pregnant woman is discrepant with prior results, or typing reactions are weaker than expected, or if variable reactivity is seen, RHD genotyping should be considered to guide RhIG prophylaxis and selection of blood for transfusion.
RhIG is sometimes administered outside of pregnancy to D-negative patients who receive D-positive blood products, most commonly whole blood derived platelet products. This is primarily considered for females of childbearing potential when the formation of anti-D has serious consequences. Because the risk for D alloimmunization from whole blood derived platelet transfusion is less than 4%, and even less for apheresis platelets, the majority of D-incompatible platelets are given without RhIG administration. RhIG in significantly higher doses is used to treat immune thrombocytopenic purpura (ITP) in patients who are D-positive and have not been splenectomized.
For prevention of D-sensitization in the United States, 300 μg are routinely administered, but dosing is increased if there is evidence of large fetal-maternal hemorrhage (300 μg for every 15 mL of RBC exposure or 30 mL of whole blood exposure). The dose is calculated based on the estimated volume of D-positive fetal RBCs from Kleihauer-Betke or flow cytometry, which is more precise. RhIG dosing calculators are available. RhIG should be given within 72 hours, which was the time period for the original studies, but should not be withheld if not administered within this time period. Adverse events to low doses used to prevent D immunization include fever, chills, and pain at the injection site. Rarely, hypersensitivity reactions are noted. RhIG doses used to treat ITP are substantial: 50 μg/kg for hemoglobin values ≥10 g/dL and 25–40 μg/kg when hemoglobin is 8–10 g/dL. Adverse events include possible anemia, hemolysis, disseminated intravascular coagulopathy, and rarely, death ( Chapter 131 ).
Commercially available mouse monoclonal anti-A and anti-B are used to determine ABO type, and these reagents directly agglutinate RBCs at room temperature. To confirm the RBC ABO reactivity, the plasma is tested for the presence of the corresponding agglutinins by testing with commercially available group A1 and group B RBCs. In both tests, agglutination is macroscopically visible.
Patient and donor RBCs are routinely tested for the presence of the D antigen in the Rh system. Reagents containing monoclonal anti-D that directly agglutinate D-positive (Rh-positive) RBCs suspended in saline at room temperature are commonly used for testing. Testing by a method that detects expression of a weak D antigen on RBCs is required for donors, but additional testing for weak D is optional when testing patient samples. Exceptions include typing the RBCs of a newborn when the mother is D-negative to determine whether she is a candidate for Rh immune globulin (RhIG) (see box on Rh Immune Globulin ).
Patient plasma is incubated at 37°C with commercially available reagent RBCs of known antigen type. After incubation, unbound antibodies are removed by washing with saline, and an antiglobulin reagent containing either AHG, or a mixture of AHG and antihuman complement is added. Column agglutination technology is now widely used and eliminates the requirement to wash unbound IgG. If the antibody screen is positive, the specificity of the antibody is determined by testing the plasma against a panel of different group O reagent RBCs (usually 10) varying in antigen phenotype (termed antibody identification).
Once a patient is actively immunized to an RBC antigen and produces a clinically significant alloantibody, the patient is considered immunized for life and should be transfused with antigen-negative RBCs, even if the antibody is no longer detectable. Patients with passively acquired antibody (e.g., neonates with maternal antibody; recipients of plasma and platelet products or RhIG) need to be transfused with antigen-negative RBCs only while the passive antibody is present. Selection of blood for transfusion to patients with alloantibodies requires typing of donor units for the corresponding antigen to identify antigen-negative units and crossmatch of the selected units with the patient’s plasma. Antigen-negative units are provided by, or can be located by, most donor centers. Provision of antigen-negative blood will to some extent depend on the prevalence of the target antigen(s) in the donor population. Transfusion service staff are vital for communication between the patient’s physician and/or consultant transfusion medicine specialist to determine the immediate and ongoing transfusion needs of the patient and to ensure that antigen-negative blood is available. Understanding the risks and benefits of transfusion are important, as well as understanding the potential clinical significance of the antibody and the urgency of transfusion. When a patient’s antibody is directed at a high-prevalence antigen, it is important to test siblings in the quest for compatible blood and to urge the patient to donate blood for long-term storage when clinical status permits. In hemolytic anemia because of warm-reactive autoantibodies, compatibility may be difficult to demonstrate. In this scenario, the important issue is to be sure that there are no clinically significant alloantibodies underlying the warm reactive autoantibodies. Donor RBCs antigen-matched with the patient for clinically significant blood group antigens should be considered in lieu of transfusion with “least incompatible” blood to minimize alloimmunization.
Transfusion management of patients who require chronic transfusion therapy, in particular patients with sickle cell disease can be challenging. Many programs attempt to reduce or prevent alloimmunization by transfusion of RBCs that are prophylactically antigen-matched, typically for C, E, and K, and some match for additional antigens. Some centers match for extended antigens once the patient makes an antibody ( Table 111.4 ). The goal is to prevent hemolytic or delayed transfusion reactions, which are known to be underreported because they can manifest as a sickle cell crisis and may result in decreasing the transfusion interval.
|
The absence of some blood group antigens and their carrier molecules can result in disease. For example, an absence of the Rh and Rh-associated glycoprotein (RhAG) proteins causes stomatocytosis and anemia, termed Rh null syndrome . The absence of Xk protein is associated with the McLeod syndrome , which is associated with myopathy and neurodegeneration. RBCs and white blood cells, such as T cells, from patients with leukocyte adhesion deficiency II (also known as congenital disorder of glycosylation type II ) lack antigens that are dependent on fucose. The RBCs have the Le(a−b−) Bombay phenotype and the white blood cells lack sialyl-Le x , which explains the high white blood cell count and infections in these patients. Hemagglutination is a simple test that can be used to diagnose these syndromes. In patients with paroxysmal nocturnal hemoglobinuria, a proportion of the RBCs will lack antigens carried on GPI-linked proteins. Other associations between blood group antigens and diseases are summarized in Table 111.1 .
Diseases associated with antibodies to blood group antigens include hemolytic disease of the newborn, warm autoimmune hemolytic anemia, cold hemagglutinin disease, and paroxysmal cold hemoglobinuria. Hemagglutination is a valuable aid in diagnosis of these conditions.
Presented here is a brief description of the most clinically relevant blood group systems in approximate order of clinical significance. For further information and prevalence of blood group antigens in different populations, refer to specialized texts such as Human Blood Groups , The Blood Group Antigen Facts Book , and the AABB Technical Manual .
The ABO blood group system is by far the most clinically significant, because of the presence of naturally occurring IgM antibodies (and sometimes IgG). The original observation by Landsteiner that certain human erythrocyte suspensions were agglutinated by other human sera led to the recognition of ABO polymorphism. This initial observation is still the cornerstone of modern transfusion practice more than a century later.
H antigens (ABH) occur on glycoproteins and glycolipids and are synthesized in a stepwise fashion by glycosyltransferases that sequentially add specific monosaccharides in specific linkages to a growing oligosaccharide precursor chain (reviewed in Clausen and Hakomori ). The terminal sugar determines antigen specificity ( Fig. 111.2 ). Group O individuals have H antigen only, the terminal sugar of which is fucose, and this is the precursor substrate for A and B antigens. Group O individuals have defective A or B transferases. The A and B transferase enzymes differ only by the nature of the monosaccharide added to the chain. N -acetyl- d -galactosamine is added by A-transferase, and d -galactose is added by B-transferase. In clinical practice, four ABO phenotypes (A, B, O, and AB) are discriminated. In addition, two common variations of group A (A 1 and A 2 ) can be distinguished. The differences between A 1 and A 2 phenotypes are quantitative and qualitative. Not only is the A 1 transferase more efficient in converting H to A antigen (approximately five times more A sites per RBC on A 1 RBCs than on A 2 RBCs), it also has the capacity to make A 1 antigen on the repetitive A epitope. Quantitatively normal ABH expression also requires the branching of carbohydrate chains, which is performed by the blood group I enzyme. Some H antigen precursor remains on A and B RBCs in this order: A 2 > B > A 2 B > A 1 > A 1 B.
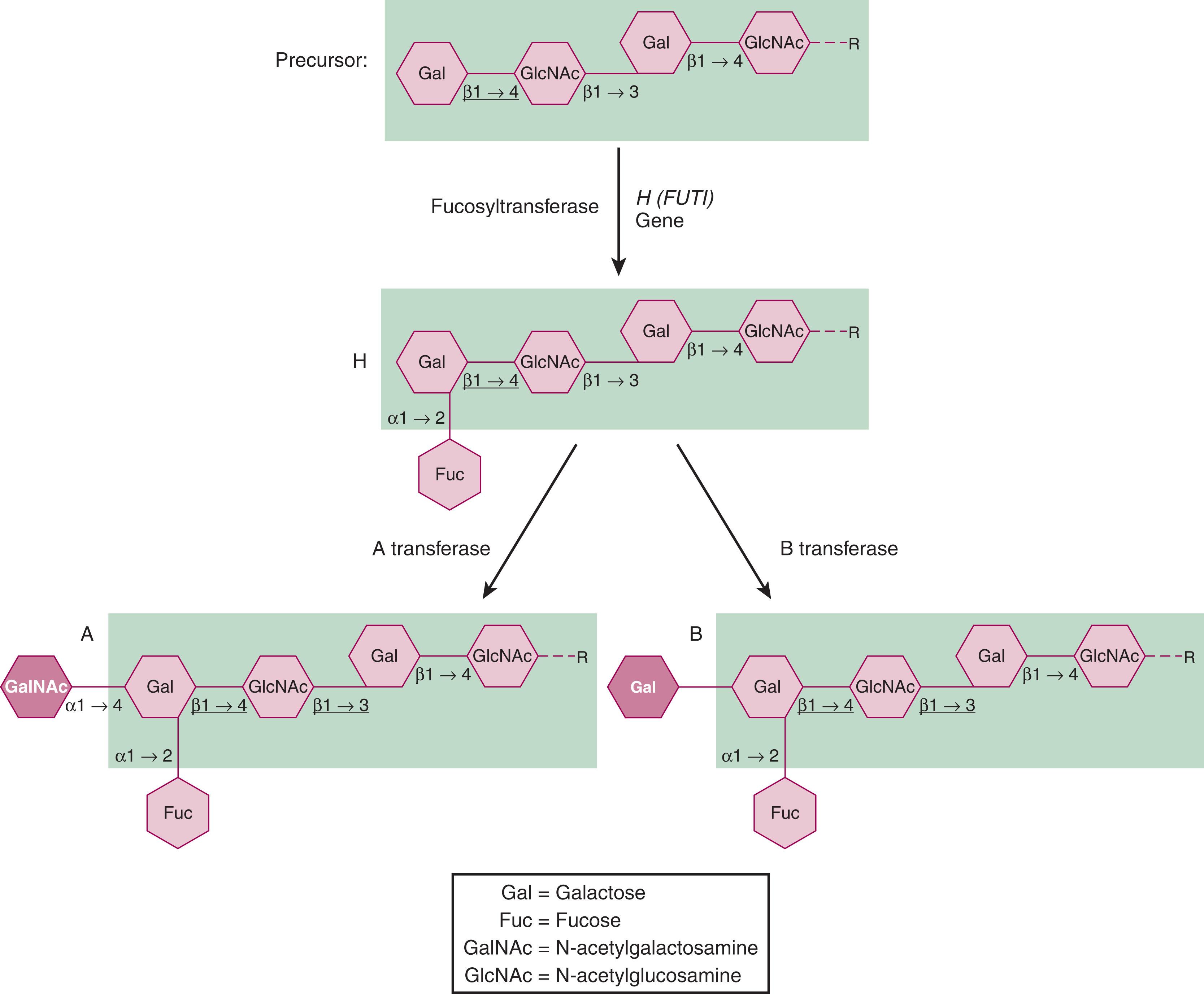
In addition to the main ABO types, there are many other inherited phenotypes with a weaker expression of the specified antigen, for example, A 3 , A x , A el , B 3 , B(A), and cis-AB. This can cause problems in determining the ABO blood group, but for patients needing immediate transfusion, the selection of group O red cells and AB plasma products is an option. In blood donors, if a very weak expression of A or B antigens on the RBCs is not detected, the major risk is that they may be transfused to a patient whose antibodies may cause accelerated destruction of the transfused cells.
Rare Bombay (O h ) phenotype RBCs, first reported in Bombay (Mumbai), India, lack H antigen and, consequently, A and B antigens. Other variants with weak H expression on RBCs, with or without H in secretions, also occur (para-Bombay) and have been reviewed. Of clinical relevance, potent anti-H with the same hemolytic potential as anti-A and anti-B can be produced by Bombay individuals. Anti-H is often found in para-Bombay individuals but is generally not a potent antibody. HDFN caused by anti-H has not been reported.
Acquired B antigen is a rare phenomenon that results from the action of bacterial deacetylase, an enzyme that can remove an acetyl group from the A-terminal sugar, N -acetylgalactosamine. Galactosamine is similar to galactose, the B-specific terminal residue, and anti-B reagents can cross-react with the deacetylated structure. Acquired B can occur in individuals suffering from gram-negative infections of gastrointestinal origin or carcinoma and can be clinically significant if a patient’s blood group is misinterpreted and group AB blood is transfused. Other polyagglutinable states (e.g., T, Tn, Tk) are detected by naturally occurring antibodies found in the serum of most people; these can be identified by a panel of lectins.
A or B antigen expression can weaken in patients with acute leukemia or stress hematopoiesis or, occasionally, during pregnancy. Chromosomal deletions or lesions that involve the ABO locus can result in the loss of transferase expression in the leukemic cell population. A decrease in A or B antigen expression, when found without a hematologic disorder, can be prognostic of a preleukemic state.
The ABO gene was cloned in 1990 following purification of A transferase; since then, over 200 different alleles have been described. There are only four amino acid differences between A and B transferases in the catalytic domain, two of which (Leu266Met and Gly268Ala) are primarily responsible for the substrate specificity. The group O phenotype results from mutations in ABO that cause a loss of glycosyltransferase activity. The most common group O allele (ABO*O1) results from a single nucleotide deletion near the 5’ end of the gene that causes a frameshift and early termination with no active enzyme production. The rare B(A) and cis-AB phenotypes have both A and B enzyme activity from a single allele caused by variant glycosyltransferases that have a combination of A-specific and B-specific residues.
The fucosyltransferases required for H synthesis are encoded by two closely linked genes on chromosome 19, FUT1 (or H) and FUT2 (or Se for secretor), which have different substrate specificity and expression in tissues. Homozygosity for defective FUT2 alleles is responsible for the common nonsecretor phenotype in which A, B, and/or H antigen are not present in secretions. Individuals homozygous for null alleles at both the FUT1 and FUT2 loci have the Bombay phenotype (see earlier section).
As tissue antigens, ABO antigens are important in solid organ transplantation. Recipient antibodies will react with antigens on the transplanted organ and complement activation at the surface of endothelial cells can result in rapid destruction and hyperacute rejection. However, successful transplantation across ABO barriers is possible, particularly with blood group A 2 to O and with current immunosuppressive and pretreatment regiments including removal of ABO antibodies. Allogeneic hematopoietic stem cell transplantations are routinely performed regardless of ABO compatibility, but occasionally initial hemolysis or pure red cell anemia because of persisting anti-A or anti-B titers in the recipient can result.
Anti-A and anti-B are found in the sera of individuals who lack the corresponding antigens. They are produced in response to environmental stimulants, such as bacteria. These antibodies are produced after birth, reaching a peak at 5 to 10 years of age, and declining with increasing age. The antibodies are mostly IgM and can activate complement, which in conjunction with the high density of ABO antigen sites on RBCs, are responsible for the severe, life-threatening transfusion reactions that may result following ABO-incompatible transfusions. In contrast, HDFN caused by ABO antibodies is usually mild because (1) placental transfer is limited to the fraction of IgG anti-A and anti-B found in maternal serum, (2) ABH antigens are not fully developed on fetal RBCs because of a lack of fully branched carbohydrate chains, and (3) tissue ABH antigens provide additional targets for the antibodies.
Platelets have intrinsic A, B, and H antigens; thus, ABO incompatibility can decrease the posttransfusion platelet increment, but this is often not of clinical significance. However, platelets from donors with an A 2 phenotype lack both A and H antigens. Approximately 20% of group A platelets would be from A 2 donors and would be appropriate for “universal” use. Platelets from A 2 donors may also be a superior product for patients undergoing A/O major mismatch allogeneic progenitor cell transplantation.
Potent anti-H (along with anti-A and anti-B) found in O h (Bombay) individuals will destroy transfused RBCs of any ABO group, so these individuals must be transfused only with H−RBCs. In contrast, anti-H identified in individuals with low expression of H antigen, notably A 1 B and A 1 , is usually IgM, reacts only at lower temperatures, and is thus clinically insignificant.
As for all glycoconjugate structures, sequential enzymatic action is required to build other carbohydrate antigenic epitopes, and the genetic background of all these involves different glycosyltransferase loci.
The null p phenotype, P 2 k and P 1 k , are of clinical interest because of potent naturally occurring antibodies that are present in plasma of individuals whose RBCs lack the glycolipid-based antigens P1/P/P k , P1/P/PX2, or P/PX2, respectively. In analogy with the ABO blood group system, antibodies of IgM and IgG class (anti-PP1P k , anti-P1PPX2, or anti-PPX2) are made against the missing antigens. Although the incidence of the null phenotypes is only 5 to 10 per million, they have attracted considerable interest because of their relationship to disease and as receptors for pathogens. Women with p and P k phenotypes suffer a high incidence of spontaneous abortion, a phenomenon most likely caused by destruction of the placenta by anti-P. In addition, anti-P and anti-P k cause hemolytic transfusion reactions if antigen-positive RBCs are transfused. Transient autoanti-P, produced following a viral infection, causes paroxysmal cold hemoglobinuria and lysis of autologous P-positive RBC. P antigen (also known as globoside ) is the cellular receptor for the parvo-B19 virus that causes erythema infectiosum (fifth disease) in children, sometimes complicated by severe aplastic anemia because of lysis of early erythroid precursors. P-fimbriated Escherichia coli expresses both P-binding and P k -binding molecules at the tips of their pili, a finding with implications for uropathogenicity. Individuals lacking P, or P k and P, appear to be naturally resistant to these bacterial and viral infections. In contrast to anti-P and anti-P k , it should be noted that anti-P1 is a cold-reactive agglutinin that seldom has clinical importance. The clinical importance of anti-PX2 made by P k individuals is unclear.
Lewis antigens are fucosylated glycolipids that are synthesized by nonerythroid cells, circulate in plasma, and are passively adsorbed onto RBC. Antibodies to Lewis can be made by individuals with the Le(a−b−) phenotype. These antibodies are of IgM class and seldom cause any clinical problems. Lewis antibodies are commonly found in pregnant women.
The i and I antigens are nonterminal epitopes on linear and branched carbohydrate structures, respectively, carrying ABH antigens at their terminal ends. During the first years of life, linear chains are modified into branched chains, resulting in the appearance of I antigens. The i phenotype is very rare among adults, but it is the normal state on RBCs from fetuses and infants. The gene encoding the I-branching β-1,6-N-acetylglucosaminyltransferase (GCNT2) has three alternative forms of exon 1 with common exons 2 and 3. Mutations in exon 2 or exon 3 silence GCNT2 and give rise to the form of the i phenotype that is associated with cataracts in Asians. Mutations in exon 1 C silence the gene in erythrocytes (but not in other tissues) and lead to the i phenotype without cataracts.
Alloanti-I made by a person with the rare i adult phenotype can be clinically significant and cause destruction of transfused I-positive RBCs. However, the sera of all I-positive individuals contain autoanti-I that is clinically benign and reactive only at or below room temperature. In contrast, cold hemagglutinin disease is characterized by a high titer of complement-fixing monoclonal anti-I, which causes in vivo hemolysis and hemolytic anemia. The titer and thermal range of autoanti-I is often increased following infection with Mycoplasma pneumoniae . If transfusion cannot be avoided, donor RBCs should be transfused through a blood warmer.
The FORS1 blood group antigen is a rare low prevalence antigen that is A-like in that it is defined by a terminal N -acetylgalactosamine and was first recognized as a weak A subgroup. The antigen has been defined as Forssman antigen, commonly found on the RBCs of nonprimate mammals, and arises from a gain-of-function mutation in the GBGT1 pseudogene. The clinical relevance of anti-FORS1 found naturally occurring in the plasma of most individuals is not known.
The blood group system Sid (symbol SID; number 038) was recently assigned to the β-1,4-N-acetylgalactosaminyltransferase (β4GalNAc-T2) encoded by the B4GALNT2 gene. Through a combination of experimental and bioinformatics approaches, it was shown that that Sd(a−) was due to a c.1396 T>C variation in the B4GALNT2 gene leading to a likely deleterious amino acid polymorphism (p.Cys466Arg).
The Rh system is second only to the ABO system in importance in transfusion medicine. Rh antigens, especially D, are highly immunogenic; thus in most countries, blood for transfusion is tested and labeled with the D antigen type (Rh-positive or Rh-negative) and D−recipients are transfused with D−RBC products.
Three systems for naming Rh antigens have been used. Two are shown in Table 111.5 , which indicates the incidence of the common Rh haplotypes present in different ethnic groups. The Fisher-Race nomenclature was based on the premise that there were three closely linked genes (D, C/c, and E/e), whereas the Wiener nomenclature (Rh-Hr) was based on the belief that a single gene encoded multiple factors (antigens). Although it is now well established that two genes, the RHD and RHCE , encode the Rh proteins, the Fisher-Race (D, C/c, and E/e) terminology is often preferred for written communication; for spoken communication, a modified version of the Wiener nomenclature is preferred. Uppercase R indicates that D antigen is present and use of a lowercase r (or “little r”) indicates that it is absent. The C or c and E or e antigens carried with D are represented by subscripts: 1 for Ce (R 1 ), 2 for cE (R 2 ), 0 for ce (R 0 ), and Z for CE (R z ). The presence of these antigens without D is represented by a superscript: prime for Ce (r′), double-prime for cE (r″), and y for CE (r y ). This terminology allows one to convey the common Rh antigens (the phenotype) with a single term. The third system of numeric designations is not widely used in the laboratory, with a few exceptions (Rh17, Rh32, Rh33).
| Fisher-Race Haplotype | Modified Weiner Haplotype | Incidence (%) | ||
|---|---|---|---|---|
| White | African Black | Asian | ||
| Rh-Positive | ||||
| DCe | R 1 | 42 | 17 | 70 |
| DcE | R 2 | 14 | 11 | 21 |
| Dce | R 0 | 4 | 44 | 3 |
| DCE | R Z | <0.01 | <0.01 | 1 |
| Rh-Negative | ||||
| ce | r | 37 | 26 | 3 |
| Ce | r′ | 2 | 2 | 2 |
| cE | r″ | 1 | <0.01 | <0.01 |
| CE | r y | <0.01 | <0.01 | <0.01 |
The Rh proteins are designated RhD (encoded by RHD), which carries the D antigen, and RhCE (encoded by RHCE), which carries the CE antigens (either ce, cE, Ce, or CE). RhD differs from the various forms of RhCE by 32 to 35 amino acids. RhD and RhCE are not glycosylated but form a complex in the RBC membrane with RhAG (Rh-associated glycoprotein). Other proteins present in the Rh-complex are CD47 (an integrin-associated protein), Lansteiner-Wiener (LW) proteins, and glycophorin B. The Rh-complex also associates with band 3 (the anion exchanger) as a macrocomplex in the membrane.
The D-negative (Rh-negative) phenotype is prevalent in Whites (15% to 17%), less common in African Blacks (3% to 5%), and rare in Asians (<0.1%). The absence of D in Europeans is primarily caused by a deletion of the RHD gene. African Blacks, and rare D-negative Whites and Asians carry a RHD gene that is silenced by a variety of molecular events.
RBCs with weak D have D antigen but at lower levels than normal because of one or more amino acid changes that are often predicted to be in the intracellular or transmembrane regions of RhD. The RBCs do not lack, or have altered, epitopes of D. Many individuals with a serologic weak D phenotype have weak D types 1, 2, and 3 by RHD genotyping, and individuals with these genotypes can safely receive D-positive blood and do not make clinically significant anti-D.
Partial D antigens (previously called D categories or D mosaics ) are caused either by point mutations in RHD that encode amino acid changes that alter D epitopes, or by replacement of RHD nucleotides or exons by the equivalent part of RHCE that result in loss of D epitopes. RBCs with a partial D antigen may have strong or weak reactivity with anti-D. Because patients with partial D antigens can make anti-D directed to the D epitopes that are altered or absent, they ideally should receive D-negative blood and women of childbearing potential are candidates for Rh immune globulin. In practice, many type as D-positive and are recognized only after they make anti-D. However, in the United States monoclonal D typing reagents licensed by the Food and Drugs Administration for patient testing classify partial Rhesus antigen D category VI (DVI) phenotypes as D-negative in direct testing, and as D-positive by IAT. RHD genotyping is very useful to distinguish weak D phenotypes from partial D to guide selection of blood for transfusion and prevent D alloimmunization and to avoid unnecessary Rh immune globulin injection (see box on Weak or Variable D Typing). Several phenotypes, including D− −, Dc−, and DC w −, have an enhanced expression of D antigen and no, or variant, CE antigens. They are caused by replacement of portions of RHCE by RHD . The RhD sequences in RhCE, along with a normal RhD, explain the enhanced D and account for the lack, or reduced expression, of CE antigens. Immunized individuals with these CE-depleted phenotypes can make antibodies to high-prevalence Rh antigens.
Clinically significant D-sensitization potentially results in a pregnancy with a fetus at risk for hemolytic disease of the fetus and newborn and hemolytic transfusion reactions if transfused with D-positive red blood cells (RBCs). Individuals with RBCs that express a partial D antigen are at risk for D-sensitization, whereas those with weak D antigen are usually not at risk. These cannot be distinguished by serologic reactivity, because either may present as weak or moderately positive or give variable results with anti-D reagents. Particularly in the prenatal setting, RHD genotyping should be done to distinguish weak D from partial D. Women with weak D expression, particularly weak D types 1, 2, and 3, are not at risk for clinically significant D-sensitization and therefore are not candidates for RhIG prophylaxis. In contrast, individuals with partial D, lack D epitopes and have produced clinically significant anti-D and should receive RhIG prophylaxis. RHD genotyping avoids unnecessary treatment with RhIG and excess use of Rh-negative blood in patients with weak D antigen.
C and c antigens differ by four amino acids, but only residue Ser103Pro is predicted to be extracellular. E and e differ by one amino acid, Pro226Ala. The RhD and various combinations of RhCE proteins (ce, Ce, cE, and CE) are typical for the majority of White transfusion recipients. However, Rh proteins in other ethnic groups often carry additional polymorphisms, particularly in individuals of African descent, and this fact often complicates transfusion in patients with sickle cell disease. For example, the RBCs of more than 30% of Blacks are VS+ because of a Leu245Val substitution in Rhce, and expression of this antigen is associated with variant expression of e antigen. Many other amino acid changes in Rhce, as well as in RhD, are associated with production of Rh antibodies in patients with sickle cell disease. RH genotyping by DNA methods allows enhanced Rh antigen matching of patients and donors and is particularly important in patients who present with Rh antibodies reacting with all, or the majority, of cells tested.
The Rh null phenotype is very rare and occurs on two genetic backgrounds: the “regulator” type, caused by mutations in RHAG , which encodes an Rh-associated glycoprotein, and the “amorph” type, caused by mutations in RHCE on a D− (deleted RHD ) background. Rh null RBCs are stomatocytic, fragile, and associated with anemia. RhAG is involved in maintenance of cation balance in RBCs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here