Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors wish to acknowledge the previous contributions of Drs. Eugene Braunwald, Joseph Perloff, Robert O’Rourke, and James A. Shaver, which laid the foundation for this chapter.
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
Evaluation of the patient with known or suspected cardiovascular disease begins with a directed history and targeted physical examination, the scope and duration of which depend on the clinical context of the patient encounter. Elective, ambulatory investigations allow comparatively more time for the development of a comprehensive assessment, whereas emergency department visits and urgent bedside consultations necessitate a more focused strategy. The elicitation of the history, with its emphasis on major cardiovascular symptoms and their change over time, demands a direct interaction between the clinician and patient, and should not be delegated to another nor inferred from information gleaned from a cursory chart review. The history also affords a unique opportunity to assess the patient’s personal attitudes, intelligence, comprehension, acceptance or denial, motivation, fear, and prejudices. Such insights allow a more informed understanding of the patient’s preferences and values regarding shared decision making. The interview also can reveal genetic or familial influences and the impact of other medical conditions on the manifesting illness. Although time constraints have limited the emphasis on careful history taking, the information gathered from the patient interview remains essential to inform the design of an efficient diagnostic and treatment plan.
Physical examination skills have declined. Only a minority of internal medicine and family practice residents recognizes classic cardiac findings in relevant diseases. Performance does not predictably improve with experience. Residency work hours and health care system efficiency standards have severely restricted the time devoted to the mentored cardiovascular examination. In 2020, the SARS-CoV-2 virus pandemic drastically limited in-person interactions, catalyzed a movement to virtual visits (VV) and challenged clinicians to develop alternative means for patient assessment through real-time video observations. It is anticipated that VV will become an established feature of ambulatory patient follow-up. Less attention to bedside skills and declining confidence in the powers of observation have led to increasing use of noninvasive imaging, including the use of handheld ultrasound. Educational efforts, which utilize repetition, patient-centered teaching conferences, simulation, and visual display feedback of auscultatory and Doppler echocardiographic findings, can improve physical examination performance.
The evidence base that links the findings from the history and physical examination to cardiovascular disease severity and prognosis is more robust for heart failure, valvular heart disease, and coronary artery disease than for other conditions. For example, vital signs and the presence of pulmonary congestion and mitral regurgitation (MR) contribute importantly to bedside risk assessment in patients with acute coronary syndromes (ACSs). The diagnosis of heart failure is fundamentally made at the bedside from symptoms and signs that reflect congestion and/or inadequate end-organ perfusion; these findings have been correlated with invasive hemodynamic measurements as well as with outcomes : irregularly irregular pulse, a heart murmur suggestive of MR, a heart rate greater than 60 beats/min, and an elevated jugular venous pressure (JVP). Accurate auscultation provides important insight into many valvular and congenital heart lesions. This chapter reviews the fundamentals of the cardiovascular history and physical examination and the evidence to support their utility. A diagnostic test is considered reasonably reliable if the kappa statistic is at least 0.4. A positive likelihood ratio (LR) (sensitivity/[1 − specificity]) increases the likelihood of the condition; a negative LR (1 − sensitivity)/specificity) decreases the likelihood of the condition.
The major signs and symptoms associated with cardiac disease include chest discomfort (see Chapter 35 ), dyspnea, fatigue, edema, palpitations (see Chapter 61 ), and syncope (see Chapter 71 ). In most cases, careful attention to the specific characteristics of chest discomfort—quality, location, radiation, triggers, mode of onset, and duration—along with alleviating factors and associated symptoms can narrow the differential diagnosis (see Chapter 35 ). Angina pectoris can usually be differentiated from the pain associated with pulmonary embolism, pericarditis, aortic dissection, esophageal reflux, or costochondritis. Cough, hemoptysis, and cyanosis may provide additional clues as to the cause of chest pain. Claudication, limb pain, edema, and skin discoloration usually indicate a vascular disorder. The cardiovascular clinician also should be familiar with common manifestations of acute stroke and transient ischemic attack, such as sudden weakness, sensory loss, incoordination, and visual disturbance. The sudden onset of symptoms and associated diaphoresis should always elicit concern that a cardiovascular cause underlies the patient’s complaints.
Typical angina should satisfy three characteristics: (1) substernal discomfort, (2) initiated by exertion or stress, and (3) relieved with rest or sublingual nitroglycerin. Chest discomfort with two of these three criteria is considered atypical angina; pain with one or none of these features is considered nonanginal. When age and sex are considered, the diagnostic accuracy for CAD using these criteria is reasonable (receiver operator curve [ROC] area under the curve [AUC] 0.713). Incorporating a history of diabetes, hypertension, smoking, and dyslipidemia improves the diagnostic accuracy (ROC AUC 0.791).
Several aspects of the presenting symptom of chest pain increase or decrease the likelihood of ACS. For example, pain that is sharp (LR, 0.3; 95% CI, 0.2 to 0.5), pleuritic (LR, 0.2; 95% CI, 0.1 to 0.3), positional (LR, 0.3; 95% CI, 0.2 to 0.5), or reproducible with palpation (LR, 0.3; 95% CI, 0.2 to 0.4) usually is noncardiac, whereas discomfort that radiates to both arms or shoulders (LR, 4.1; 95% CI, 2.5 to 6.5) or is precipitated by exertion (LR, 2.4; 95% CI, 1.5 to 3.8) has a much higher likelihood of reflecting myocardial ischemia. Less classic symptoms (i.e., anginal equivalents) such as indigestion, belching, and dyspnea, also should command the clinician’s attention when other features of the presentation suggest ACS, even in the absence of chest discomfort.
Women, elderly persons, and patients with diabetes more commonly present with a less typical clinical picture. A history of a prior abnormal stress test (LR, 3.1; 95% CI, 2.0 to 4.7), known CAD (LR, 2.0; 95% CI, 1.4 to 2.6) or the presence of peripheral arterial disease (PAD) (LR, 2.7; 95% CI, 1.5 to 4.8) increases the likelihood that the pain indicate an ACS. However, the accuracy of traditional risk factors and symptoms for the diagnosis of ACS is weak. Clinical prediction models that incorporate aspects of the history and examination with serum biomarkers of cardiac injury (troponins) and ECG findings provide better diagnostic accuracy, especially when they have been externally validated ( Table 13.1 ).
| Symptom | Positive LR (95% CI) | PPV (%) | Negative LR (95% CI) | NPV (%) |
|---|---|---|---|---|
| Radiation to both arms | 2.6 (1.8–3.7) | 28 | 0.93 (0.89–0.96) | 12 |
| Radiation to left arm | 1.3 (1.2–1.4) | 16 | 0.88 (0.81–0.96) | 12 |
| Typical chest pain | 1.9 (0.94–2.9) | 22 | 0.52 (0.35–0.69) | 7 |
| Increase with exertion | 1.5–1.8 (NA) | 18–21 | 0.66–0.83 (NA) | 9–11 |
| Radiation to neck or jaw | 1.5 (1.3–1.8) | 18 | 0.91 (0.87–0.95) | 12 |
| Associated diaphoresis | 1.3–1.4 (NA) | 15 | 0.91–0.93 (NA) | 12 |
| Exam Findings | ||||
| Systolic BP <100 | 3.9 (0.98–15) | 37 | 0.98 (0.95–1.0) | 13 |
| Tachypnea | 1.9 (0.99–3.5) | 22 | 0.95 (0.89–1.0) | 12 |
| Pain reproduced with palpation | 0.28 (0.14–0.54) | 4.0 | 1.2 (1.0–1.2) | 15 |
Dyspnea may occur with exertion or in recumbency (orthopnea) or even on standing (platypnea). Paroxysmal nocturnal dyspnea of cardiac origin usually occurs 2 to 4 hours after onset of sleep; the dyspnea is sufficiently severe to compel the patient to sit upright or stand and then subsides gradually over several minutes. The patient’s partner should be questioned about any signs of sleep-disordered breathing, such as loud snoring or periods of apnea. Pulmonary embolism often associates with dyspnea of sudden onset.
Patients may use a variety of terms to describe their awareness of the heartbeat (palpitations), such as “flutters,” “skips,” or “pounding.” The likelihood of a cardiac arrhythmia modestly increases with a known history of cardiac disease (LR, 2.03; 95% CI, 1.33 to 3.11) and decreases when symptoms resolve within 5 minutes (LR, 0.38; 95% CI, 0.22 to 0.63) or when associated with panic disorder (LR, 0.26; 95% CI, 0.07 to 1.01). A report of a regular, rapid-pounding sensation in the neck (LR, 177; 95% CI, 25 to 1251) or visible neck pulsations associated with palpitations (LR, 2.68; 95% CI, 1.25 to 5.78) increases the likelihood that atrioventricular nodal reentrant tachycardia (AVNRT) is the responsible arrhythmia. The absence of a regular, rapid-pounding sensation in the neck makes detecting AVNRT much less likely (LR, 0.07; 95% CI, 0.03 to 0.19).
Cardiac syncope occurs suddenly, with rapid restoration of full consciousness thereafter. Patients with neurocardiogenic syncope may experience early warning signs (nausea, yawning), appear ashen and diaphoretic, and revive more slowly, albeit without signs of seizure or a prolonged postictal state. The complete history consists of information pertaining to traditional cardiovascular risk factors, a general medical history, occupation, social habits, activities, medications, drug allergies or intolerance, family history, and systems review. In most instances, the history, examination, and limited testing can establish the cause of syncope ( Table 13.2 ).
|
It is important to obtain a semiquantitative assessment of symptom severity and to document any change over time. The New York Heart Association (NYHA) and the Canadian Cardiovascular Society (CCS) functional classification systems are useful for both patient care and clinical research, despite their inherent limitations. Current technology now allows patients to self-report symptoms directly into the patient record using iterative responsive survey instruments, which can be quantified and may better reflect the patient’s experience with their cardiovascular condition in contrast to the provider’s interpretation of the patient’s symptoms.
The physical examination can help determine the cause of a given symptom, assess disease severity and progression, and evaluate the impact of specific therapies. It also can identify the presence of early-stage disease in patients without signs or symptoms. In general, the physical examination should be undertaken in a hypothesis-driven manner in which the pretest probability of a specific diagnosis is altered by a specific finding. Depending upon the characteristics of this finding, a post-test probability can be established to guide further testing as appropriate.
The examination begins with an appreciation of the general appearance of the patient, including age, posture, demeanor, and general health status. Is the patient in pain, resting quietly, or visibly diaphoretic? Does the patient choose to avoid certain positions to reduce or eliminate pain? The pain of acute pericarditis, for example, often diminishes with sitting up, leaning forward, or breathing shallowly. Pursing of the lips, a breathy quality to the voice, and an increased anteroposterior chest diameter would favor a pulmonary rather than a cardiovascular cause of dyspnea, although disorders in both etiologic categories may contribute in an individual patient. Pallor suggests anemia as a possible underlying disorder in patients with exercise intolerance or dyspnea, independent of cardiovascular disease. Cyanosis and jaundice also bear noting. Specific genetic cardiovascular disorders may be discernible from the patient’s appearance. Emaciation suggests chronic heart failure or another systemic disorder (e.g., malignancy, infection).
The vital signs, including height, weight, temperature, pulse rate, blood pressure (in both arms), respiratory rate, and peripheral oxygen saturation, are used to determine the urgency of the evaluation and provide initial clues as to the presence of a cardiovascular disorder. The height and weight permit calculation of body mass index (BMI) and body surface area (BSA). Waist circumference (measured at the iliac crest) and waist-to-hip ratio (using the widest circumference around the buttocks) powerfully predict long-term cardiovascular risk. In patients with palpitations, a resting heart rate less than 60 beats/min may increase the likelihood of a clinically significant arrhythmia (LR, 3.00; 95% CI, 1.27 to 7.08). Observation of the respiratory pattern may reveal signs of disordered breathing (e.g., Cheyne-Stokes respirations, obstructive sleep apnea), a finding associated with reduced survival in patients with severe systolic heart failure. Mental status should be assessed and is an important gauge of adequate cerebral and systemic perfusion.
Frailty is defined as a state of decreased physiologic reserve and vulnerability to stressors. Several scales are available that incorporate quantifiable criteria such as unintentional weight loss, grip strength, gait speed, serum albumin, and hemoglobin ( Table 13.3 ). Frailty assessment, a common tool in the evaluation of patients with heart failure, is a routine feature of the preprocedural appraisal of elderly patients referred for heart valve intervention.
| Characteristic | Metrics |
|---|---|
| Shrinking (Unintentional weight loss) | >10 pound or >5% of total body weight in past year. |
| Weakness (Reduced hand grip strength) | Maximum isometric contraction in dominant hand over three attempts using hand dynamometer. |
| Exhaustion (Self-reported exhaustion) | Questions from the Center for Epidemiologic Studies—Depression Scale. |
| Slowness (Slow gait speed) | Slowest quintile according to gender/height based on time to walk 15 feet. |
| Inactivity (Low self-reported physical activity) | Lowest quintile of expended kcal/week using activity questionnaire. |
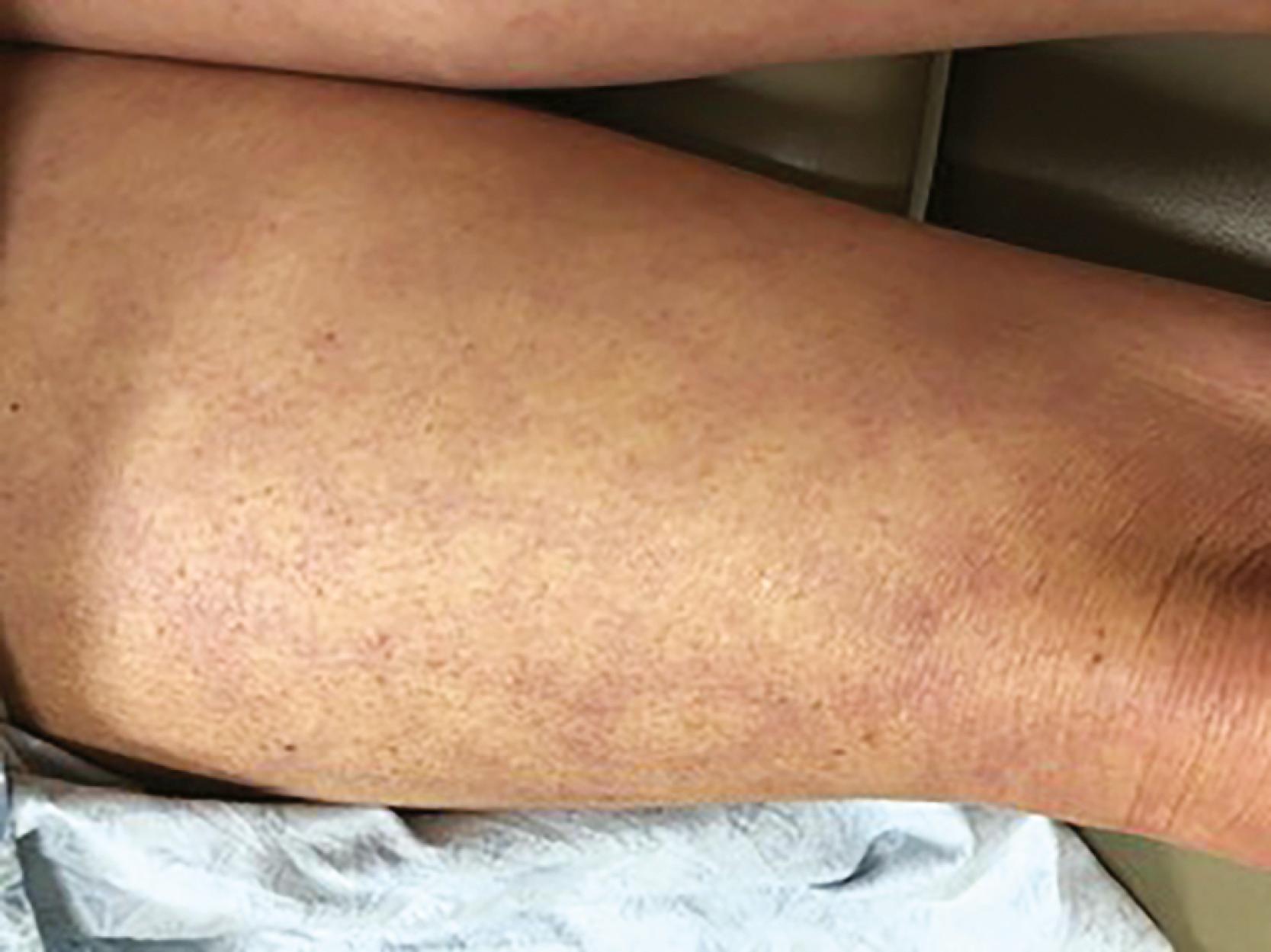
Central cyanosis is present with significant right-to-left shunting at the level of the heart or lungs. It also is a feature of hereditary methemoglobinemia. Peripheral cyanosis or acrocyanosis of the fingers, toes, nose, and ears is characteristic of the reduced blood flow that accompanies small-vessel constriction seen in severe heart failure, shock, or peripheral vascular disease. Differential cyanosis affecting the lower but not the upper extremities occurs with a patent ductus arteriosus (PDA) and pulmonary artery hypertension with right-to-left shunting at the great vessel level. Hereditary telangiectases on the lips, tongue, and mucous membranes (a finding in Osler-Weber-Rendu syndrome) resemble spider nevi; when present in the lungs, they can cause right-to-left shunting and central cyanosis. Telangiectasias also are seen in patients with scleroderma with or without pulmonary hypertension. Livedo reticularis, a lace-like purplish dislocation of the skin that imparts a mottled or reticulated appearance ( Fig. 13.1 ), can occur on exposure to cold in normal individuals, but is also observed in a variety of conditions resulting in sluggish cutaneous blood flow, such as cardiogenic shock or certain autoimmune diseases. Tanned or bronze discoloration of the skin in unexposed areas can suggest iron overload and hemochromatosis. With jaundice, often first appreciated in the sclerae, the differential diagnosis is broad in scope. Ecchymoses often occur with either anticoagulant and/or antiplatelet use, whereas petechiae characterize thrombocytopenia, and purpuric skin lesions can be seen with infective endocarditis and other causes of leukocytoclastic vasculitis. Various lipid disorders can manifest with xanthomas, located subcutaneously, along tendon sheaths, or over the extensor surfaces of the extremities. Xanthomas within the palmar creases are specific for type III hyperlipoproteinemia. The leathery, cobblestone, “plucked chicken” appearance of the skin in the axillae and skinfolds of a young person is characteristic of pseudoxanthoma elasticum, a disease with multiple cardiovascular manifestations, including premature atherosclerosis. Extensive lentiginoses (freckle-like brown macules and café-au-lait spots over the trunk and neck) may be part of developmental delay–associated cardiovascular syndromes (LEOPARD, LAMB, and Carney) with multiple atrial myxomas, atrial septal defect (ASD), hypertrophic cardiomyopathy, and valvular stenoses. In a patient with heart failure or syncope, cardiovascular sarcoid should be suspected in the presence of lupus pernio, erythema nodosum, or granuloma annulare. Certain vascular disorders such as erythromelalgia, chilblains, frostbite, or lymphangitis also may be readily apparent from examination of the skin in the appropriate context.
All patients should undergo assessment of the state of dentition, both as a source of infection and as an index of general health and hygiene. A high-arched palate is a feature of Marfan and other connective tissue disease syndromes. A large protruding tongue with parotid enlargement may suggest amyloidosis. Patients with Loeys-Dietz syndrome characteristically have a bifid uvula. Orange tonsils are typical of Tangier disease. Ptosis and ophthalmoplegia suggest muscular dystrophies, and congenital heart disease often is accompanied by hypertelorism, low-set ears, micrognathia, and a webbed neck, as with Noonan, Turner, and Down syndromes. Proptosis, lid lag, and stare point to Graves hyperthyroidism. Patients with osteogenesis imperfecta may have blue sclerae, mitral or aortic regurgitation (AR), and a history of recurrent nontraumatic skeletal fractures.
Attention to the extraocular movements and the size and symmetry of the pupils may reveal a neurologic disorder. The oft-omitted funduscopic examination can aid in the evaluation of patients with hypertension, atherosclerosis, diabetes, endocarditis, neurologic signs or symptoms, or known carotid or aortic arch disease. Lacrimal gland hyperplasia is sometimes a feature of sarcoidosis. The “mitral facies” of rheumatic mitral stenosis (pink-purplish patches with telangiectasias over the malar eminences) also can accompany other disorders associated with pulmonary hypertension and reduced cardiac output. Relapsing polychondritis is suggested by inflammation of the pinnae and nasal cartilage in association with a saddle-nose deformity.
Inspection and palpation can quickly ascertain the temperature of the extremities and the presence of clubbing, arachnodactyly, and nail changes. Clubbing implies the presence of central shunting. An unopposable “fingerized” thumb and shortened forearm bones occur in Holt-Oram syndrome. Arachnodactyly characterizes the Marfan syndrome. Janeway lesions (nontender, slightly raised areas of hemorrhage on the palms and soles), Osler’s nodes (tender, raised nodules on the pads of the fingers or toes), and splinter hemorrhages (linear petechiae in the mid-nailbed) may be signs of infective endocarditis. Ulcerations and tissue loss of the fingertips may suggest thromboangiitis obliterans in the appropriate context.
Lower extremity or presacral edema with elevated JVP occurs in many volume-overloaded states, including heart failure. With a normal JVP, additional signs of venous disease, such as extensive varicosities, medial ulcers, or brownish pigmentation from hemosiderin deposition, suggest chronic venous insufficiency. A history of lower extremity vein ligation and “stripping” should be recognized. Edema also can occur with dihydropyridine calcium channel blocker therapy. Anasarca seldom occurs in heart failure, unless the condition is long standing, untreated, and accompanied by severe hypoalbuminemia. Asymmetric swelling can reflect local or unilateral venous thrombosis, the sequelae of previous vein graft harvesting, or lymphatic obstruction (lymphedema). Homan sign (calf pain elicited by forceful dorsiflexion of the foot) is neither specific nor sensitive for deep vein thrombosis. Muscular atrophy and the absence of hair in an extremity should suggest chronic arterial insufficiency or a neuromuscular disorder. Redistribution of fat from the extremities to central/abdominal stores (lipodystrophy) in some patients with HIV infection may relate to antiretroviral treatment and is associated with insulin resistance and several features of the metabolic syndrome.
Cutaneous venous collaterals over the anterior chest suggest chronic obstruction of the superior vena cava (SVC) or subclavian vein, especially in the presence of indwelling catheters or leads from cardiac implantable electrical devices (CIEDs). Asymmetric breast enlargement or arm swelling ipsilateral to an implanted device also may be present. Thoracic cage abnormalities, such as pectus carinatum (pigeon chest) or pectus excavatum (funnel chest), may accompany connective tissue disorders; the barrel chest of emphysema or advanced kyphoscoliosis may be associated with cor pulmonale. The severe kyphosis of ankylosing spondylitis should prompt careful auscultation for AR and scrutiny of the electrocardiogram (ECG) for first-degree atrioventricular (AV) block. The “straight back syndrome” (loss of normal kyphosis of the thoracic spine) can accompany mitral valve prolapse (MVP). A thrill may be present over well-developed intercostal artery collaterals in patients with aortic coarctation.
Patients with emphysema may exhibit prominence of the cardiac impulse in the epigastrium. The liver often is enlarged and tender in heart failure; systolic hepatic pulsations signify severe tricuspid regurgitation (TR). Patients with infective endocarditis of long duration may have splenomegaly. Ascites can develop with advanced and chronic right heart failure or constrictive pericarditis. The abdominal aorta normally may be palpated between the epigastrium and the umbilicus in thin patients and in children. The sensitivity of palpation for the detection of abdominal aortic aneurysm (AAA) disease increases as a function of aneurysm diameter and varies inversely with body size. Arterial bruits in the abdomen should be sought.
Careful chest auscultation is an essential component of the cardiovascular exam and is of prime importance when the presenting complaint is dyspnea. Technologic advances have provided important insights into often underappreciated pulmonary auscultatory phenomena ( Fig. 13.2 ) that are commonly encountered in the evaluation of patients with cardiovascular disease. Point of care ultrasound in emergency rooms and intensive care units have assumed increasing importance in the bedside evaluation of dyspnea. ,
The JVP aids in the estimation of volume status. The external (EJV) or internal (IJV) jugular vein may be used, although the IJV is preferred because the EJV is valved and not directly in line with the SVC and right atrium. The EJV is easier to visualize when distended, and its appearance can help to discriminate between low and high central venous pressure (CVP). An elevated left EJV pressure may also signify a persistent left-sided SVC or compression of the innominate vein from an intrathoracic structure. If an elevated CVP is suspected but venous pulsations cannot be appreciated, the patient should be asked to sit upright with the feet dangling. With subsequent pooling of blood in the lower extremities, venous pulsations may be evident. SVC syndrome should be suspected if the venous pressure is elevated, pulsations are still not discernible, the face is swollen, and the skin of the head and neck appears dusky or cyanotic. When hypovolemia is suspected as a cause of hypotension, the patient may need to be lowered to a supine position to assess the waveform in the right supraclavicular fossa.
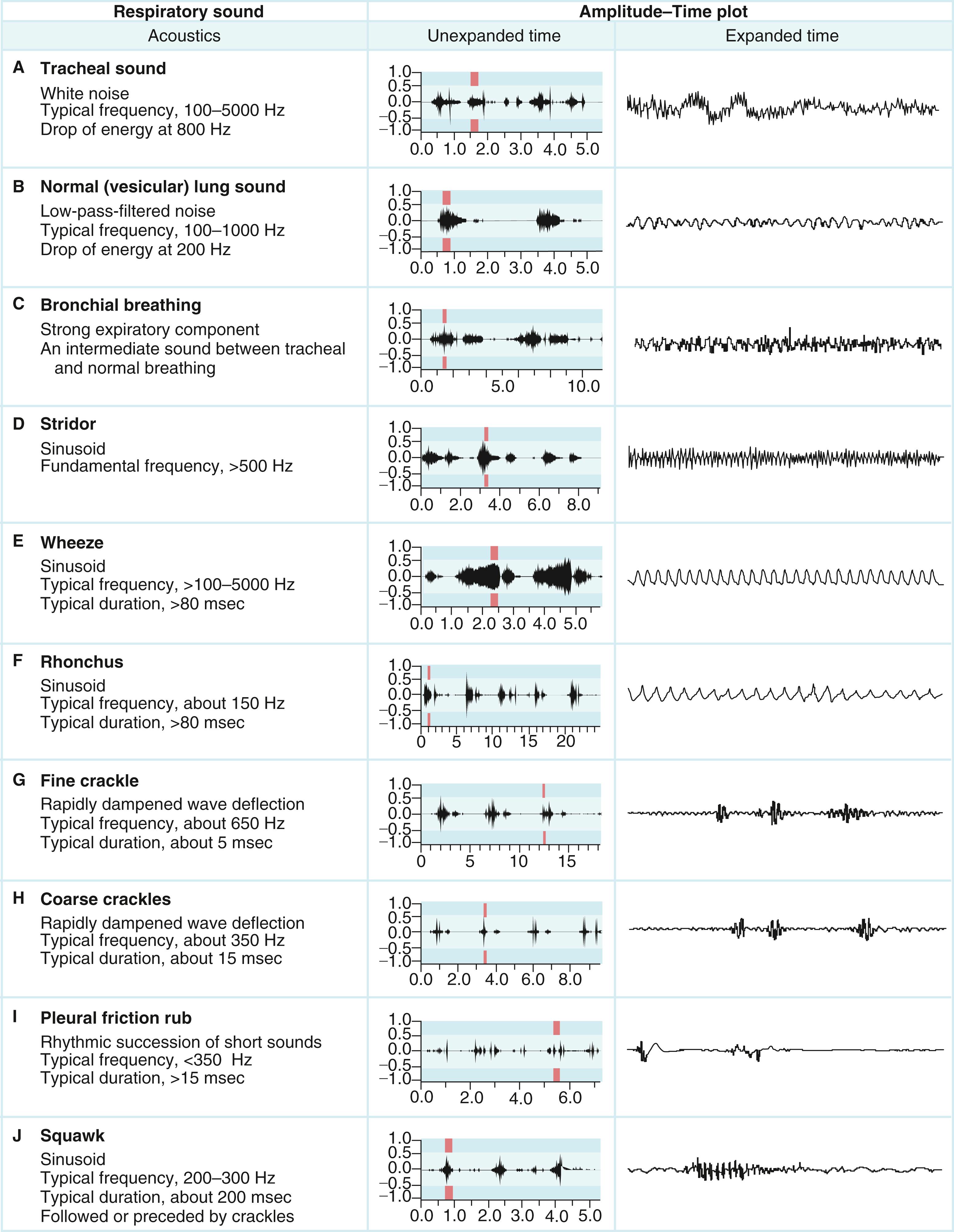
The venous waveform can sometimes be difficult to distinguish from the carotid artery pulse. The venous waveform has several characteristic features ( Fig. 13.3 ; Table 13.4 ) and its individual components can usually be identified. The a and v waves, and x and y descents, are defined by their temporal relation to electrocardiographic events and heart sounds. The estimated height of the venous pressure indicates the CVP or right atrial pressure. Although observers vary widely in their estimates of the CVP, knowledge that the pressure is elevated, and not its specific value, can inform diagnosis and management.
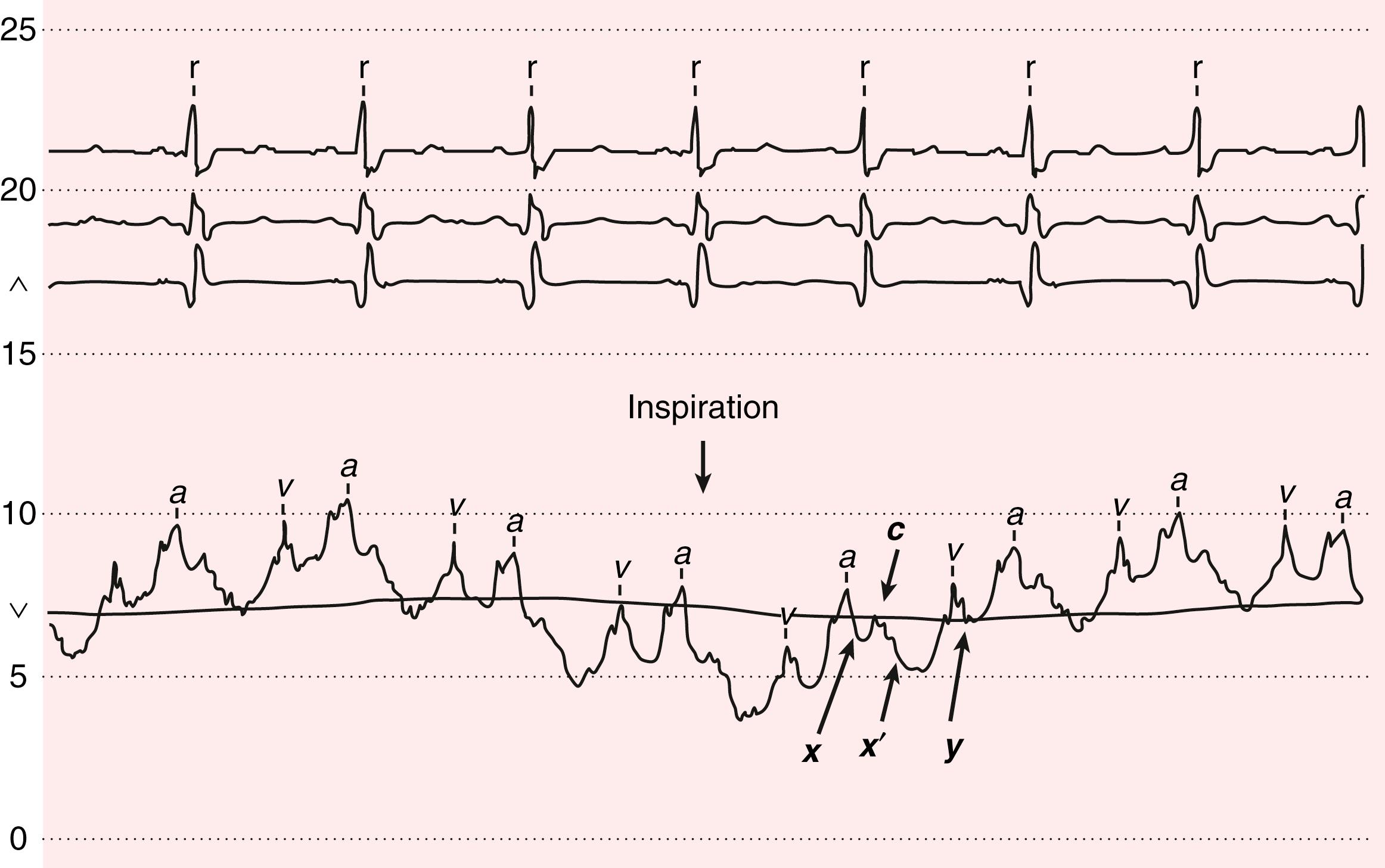
| Feature | Internal Jugular Vein Pulse | Carotid Artery Pulse |
|---|---|---|
| Appearance of pulse | Undulating two troughs and two peaks for every cardiac cycle (biphasic) | Single brisk upstroke (monophasic) |
| Response to inspiration | Height of column falls and troughs become more prominent | No respiratory change to contour |
| Palpability | Generally not palpable (except in severe TR) | Palpable |
| Effect of pressure | Can be obliterated with gentle pressure at base of vein/clavicle | Cannot be obliterated |
The bedside venous pressure is usually estimated by the vertical distance between the top of the venous pulsation and the sternal inflection point, where the manubrium meets the sternum (angle of Louis). A distance of greater than 3 cm is considered abnormal. However, the distance between the angle of Louis and the mid–right atrium varies considerably as a function of body size and position. In general, use of the sternal angle as a reference leads to systematic underestimation of venous pressure. In practice, however, it is difficult to use even relatively simple thoracic landmarks. Measurements obtained by critical care nurses often will vary by several centimeters. Venous pulsations above the clavicle with the patient in the sitting position are clearly abnormal, because the distance from the right atrium is at least 10 cm. Estimated CVP correlates only modestly with direct measurement. Measurements made at the bedside, in units of centimeters of blood or water, require conversion to millimeters of mercury (1.36 cm H 2 O = 1.0 mm Hg), for comparison with values measured with catheterization. Remote assessment of the JVP using video “chat” in patients with heart failure and reduced ejection fraction was demonstrated to be feasible and of comparable accuracy to bedside estimation in a pilot study using invasively measured right atrial pressure as the reference standard. These findings have implications for both remote in-hospital and virtual ambulatory patient assessment (see later).
The venous waveforms include several distinct peaks: a, c, and v (see Fig. 13.3 , Table 13.4 ). The a wave reflects right atrial presystolic contraction, occurs just after the electrocardiographic P wave, and precedes the first heart sound (S 1 ). Patients with reduced right ventricular (RV) compliance from any cause can have a prominent a wave. A cannon a wave occurs with AV dissociation and right atrial contraction against a closed tricuspid valve. The presence of cannon a waves in a patient with wide complex tachycardia identifies the rhythm as ventricular in origin. The a wave is absent with atrial fibrillation (AF). The x descent reflects the fall in right atrial pressure after the a wave peak. The c wave interrupts this descent as ventricular systole pushes the closed valve into the right atrium. In the neck, the carotid pulse also may contribute to the c wave. As depicted in Figure 13.3 , the x descent follows because of atrial diastolic suction created by ventricular systole pulling the tricuspid valve downward. In normal persons, the x descent is the predominant waveform in the jugular venous pulse. The v wave represents atrial filling, occurs at the end of ventricular systole, and follows just after S 2 . Its height is determined by right atrial compliance and by the volume of blood returning to the right atrium from all sources. The v wave is smaller than the a wave because of the normally compliant right atrium. In patients with ASD, the a and v waves may be of equal height; in TR, the v wave is accentuated ![]() ( ). With TR, the v wave will merge with the c wave because retrograde valve flow and antegrade right atrial filling occur simultaneously. The y descent follows the v wave peak and reflects the fall in right atrial pressure after tricuspid valve opening. Resistance to ventricular filling in early diastole blunts the y descent, as is the case with pericardial tamponade or tricuspid stenosis. The y descent will be steep when ventricular diastolic filling occurs early and rapidly, as with pericardial constriction, restrictive cardiomyopathy, or isolated, severe TR.
( ). With TR, the v wave will merge with the c wave because retrograde valve flow and antegrade right atrial filling occur simultaneously. The y descent follows the v wave peak and reflects the fall in right atrial pressure after tricuspid valve opening. Resistance to ventricular filling in early diastole blunts the y descent, as is the case with pericardial tamponade or tricuspid stenosis. The y descent will be steep when ventricular diastolic filling occurs early and rapidly, as with pericardial constriction, restrictive cardiomyopathy, or isolated, severe TR.
The normal venous pressure should fall by at least 3 mm Hg with inspiration. A rise in venous pressure (or its failure to decrease) with inspiration (Kussmaul sign) is associated with constrictive pericarditis, and with restrictive cardiomyopathy, pulmonary embolism, RV infarction, and advanced systolic heart failure. A Kussmaul sign ![]() ( ) is seen with right-sided volume overload and reduced RV compliance. Normally, the inspiratory increase in right-sided venous return is accommodated by increased RV ejection, facilitated by an increase in the capacitance of the pulmonary vascular bed. In states of RV diastolic dysfunction and volume overload, the right ventricle cannot accommodate the enhanced volume, and the pressure rises. Increased pulmonary vascular resistance may also limit RV ejection and contribute to the Kussmaul phenomenon.
( ) is seen with right-sided volume overload and reduced RV compliance. Normally, the inspiratory increase in right-sided venous return is accommodated by increased RV ejection, facilitated by an increase in the capacitance of the pulmonary vascular bed. In states of RV diastolic dysfunction and volume overload, the right ventricle cannot accommodate the enhanced volume, and the pressure rises. Increased pulmonary vascular resistance may also limit RV ejection and contribute to the Kussmaul phenomenon.
The abdominojugular reflux maneuver or passive leg elevation can elicit venous hypertension. The abdominojugular reflux maneuver requires firm and consistent pressure over the upper abdomen, preferably the right upper quadrant, for at least 10 seconds. Classically, a positive abdominojugular reflux sign has been defined as a rise of more than 3 cm in the venous pressure sustained for at least 15 seconds, although in practice a shorter time duration is usually accepted. The patient should be coached to refrain from holding their breath or performing a Valsalva-like maneuver, which can falsely elevate the venous pressure. A positive abdominojugular reflux sign can predict heart failure in patients with dyspnea, as well as a pulmonary artery wedge pressure higher than 15 mm Hg.
Auscultatory measurement of blood pressure yields lower systolic and higher diastolic values than direct intra-arterial recording. Nurse or pharmacist-recorded blood pressure usually is closer to the patient’s average daytime blood pressure than physician measured blood pressure. Blood pressure should be measured with the patient in the seated position, back supported, feet on the floor, with the arm at the level of the heart, using an appropriate-size cuff ( Table 13.5 ), after 5 minutes of rest, repeated 5 minutes later, and the readings averaged. The use of an inappropriately small cuff can result in overestimation of the true blood pressure, an issue of particular relevance in obese patients.
|
On occasion, the Korotkoff sounds may disappear soon after the first sound, only to recur later before finally disappearing as phase 5. This auscultatory gap is more likely to occur in older, hypertensive patients with target organ damage. The systolic pressure should be recorded at the first Korotkoff sound and not when the sound reappears. This finding should be distinguished from pulsus paradoxus (see later). Korotkoff sounds may be heard all the way down to 0 mm Hg with the cuff completely deflated in children, in pregnant patients, in patients with chronic severe AR, or in the presence of a large arteriovenous fistula. In these cases, both the phases 4 and 5 pressures should be noted.
Blood pressure should be measured in both arms either in rapid succession or simultaneously; normally the measurements should differ by less than 10 mm Hg, independent of handedness. As many as 20% of normal subjects, however, exhibit a left-right arm blood pressure differential of more than 10 mm Hg in the absence of symptoms or other examination findings. A blood pressure differential of more than 10 mm Hg can be associated with subclavian artery disease, supravalvular aortic stenosis (SVAS), aortic coarctation, or aortic dissection. Systolic leg pressures may exceed arm pressures by as much as 20 mm Hg; greater leg-arm systolic blood pressure differences are seen in patients with severe AR (Hill sign) and patients with extensive and calcified (noncompressible) lower extremity PAD. Leg blood pressure should be measured using large thigh cuffs with auscultation at the popliteal artery or using a standard large arm cuff on the calf with simultaneous auscultation or palpation at the posterior tibial artery. Measurement of lower extremity blood pressures constitutes the basis of the ankle-brachial index (ABI) (see Chapter 43 ).
Consideration should be given to ambulatory blood pressure monitoring when uncertainty exists about the significance of recordings obtained in the clinic. This approach is especially useful for the patient with suspected “white coat hypertension” (see Chapter 26Chapter 46Chapter 47 ). Measurement of normal or even low blood pressures in the clinic with evidence of hypertensive end organ damage should suggest masked hypertension, which occurs more often than clinicians appreciate and may be present in the absence of obstructive PAD that can lower extremity blood pressure.
Orthostatic hypotension (a fall in blood pressure of more than 20 mm Hg systolic and/or more than 10 mm Hg diastolic in response to moving from the supine to the standing position within 3 minutes) may be accompanied by a lack of compensatory tachycardia, a response suggestive of autonomic insufficiency, as can occur in patients with diabetes or Parkinson disease. The heart rate–blood pressure response to standing also depends on age, hydration, medications, food, conditioning, and ambient temperature and humidity. In patients with postural orthostatic tachycardia syndrome (POTS), blood pressure does not usually fall on standing.
An increase in pulse pressure can represent increased vascular stiffness, usually secondary to aging or atherosclerosis. Aortic stiffness is increased in patients with Marfan syndrome and other connective tissue disorders and may contribute to risk for dissection. Peripheral indices may not correlate well with central aortic stiffness, which is a primary determinant of ventricular-vascular coupling.
The carotid artery pulse wave occurs within 40 milliseconds of the ascending aortic pulse and reflects aortic valve and ascending aortic function. The temporal arteries can be easily palpated to aid in the diagnosis of temporal arteritis. One of the two pedal pulses may not be palpable in a normal subject because of unusual anatomy (posterior tibial, less than 5%; dorsal pedis, less than 10%), but each pair should be symmetric. True congenital absence of a pulse is rare, and in most cases, pulses can be detected with a handheld Doppler device when not palpable. Simultaneous palpation of the brachial or radial pulse with the femoral pulse should be performed in young patients with hypertension to screen for aortic coarctation.
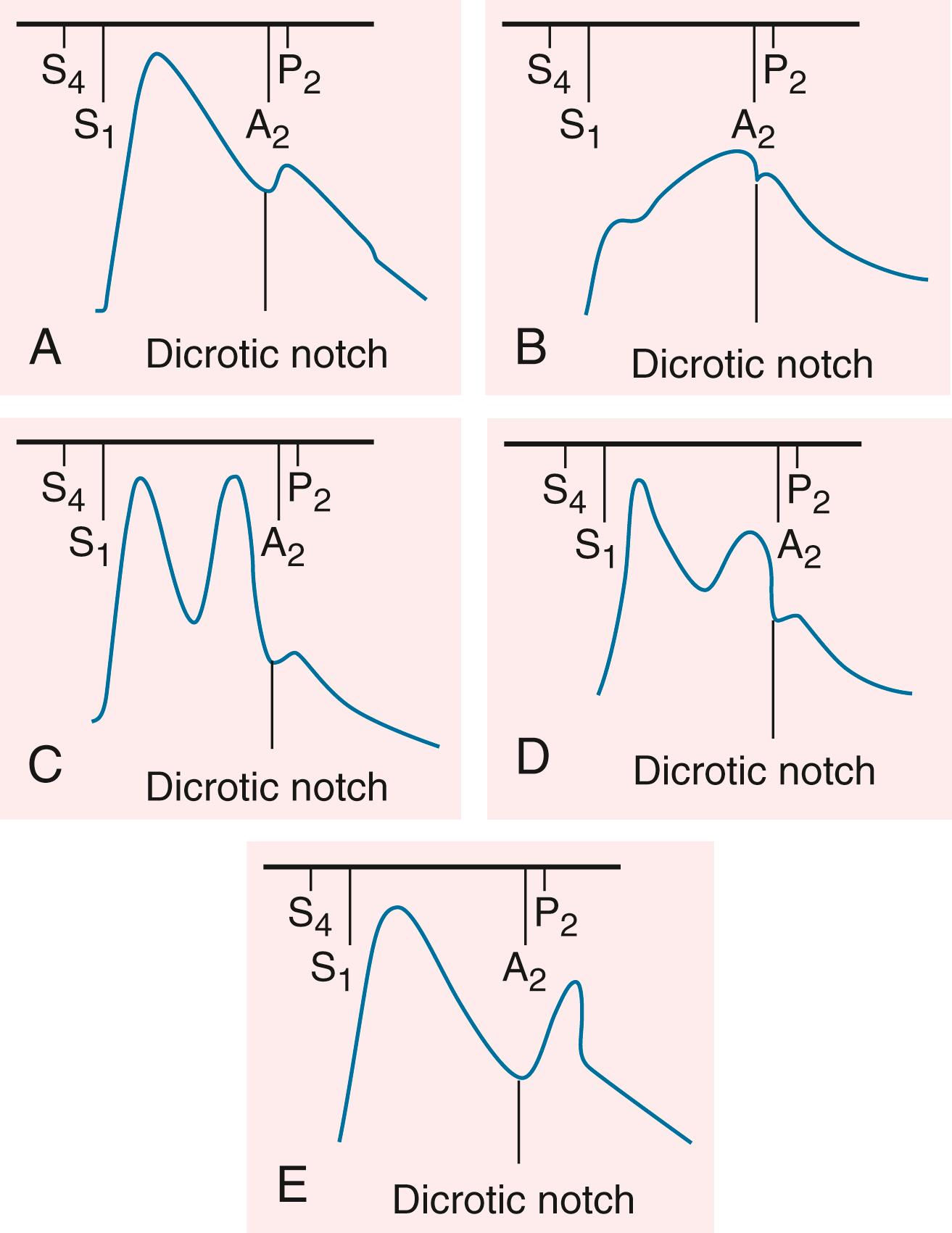
The contour of the pulses depends on the stroke volume, ejection velocity, vascular capacity and compliance, and systemic resistance. The palpable pulse reflects the merging of the antegrade pulsatile flow of blood and reflection of the propagated pulse returning from the periphery. The amplitude of the arterial pulse increases with distance from the heart. Normally, the incident (percussion) wave begins with systolic ejection (just after S 1 ) and is the predominant monophasic pulse appreciated at the bedside ( Fig. 13.4 ). The incisura or dicrotic notch identifies aortic valve closure. A bounding pulse may occur in hyperkinetic states such as fever, anemia, and thyrotoxicosis, or in pathologic states such as severe bradycardia, AR, or arteriovenous fistula. A bifid pulse is created by two distinct pressure peaks. This phenomenon may occur with fever or after exercise in a normal person and is consistent with increased vascular compliance. With chronic severe AR, a large stroke volume ejected rapidly into a noncompliant arterial tree produces a reflected wave of sufficient amplitude to be palpated during systole, rendering the pulse bifid. Hypertrophic cardiomyopathy (HCM) can rarely produce a bifid systolic pulse with percussion and tidal waves (see Fig. 13.4 ). Diastolic augmentation of pressure with an intra-aortic balloon pump also results in a bifid pulse, though with the two components separated by aortic valve closure.
A fall in systolic pressure of more than 10 mm Hg with inspiration ( pulsus paradoxus ) is considered pathologic and a sign of pericardial tamponade or severe pulmonary disease; this phenomenon also can occur in obesity and pregnancy without clinical disease. Pulsus paradoxus is detected by noting the difference between the systolic pressure at which the Korotkoff sounds are first heard (during expiration) and the systolic pressure at which the Korotkoff sounds are heard with each beat, independent of respiratory phase. Between these two pressures, the sounds will be heard only intermittently (during expiration). Appreciation of this finding requires a slow decrease of the cuff pressure. Conditions such as tachycardia, AF, and tachypnea make its assessment difficult. Pulsus paradoxus may be palpable at the brachial artery when the pressure difference exceeds 15 mm Hg (see Chapter 86 ). Pulsus paradoxus is not specific for pericardial tamponade and can accompany massive pulmonary embolus, hemorrhagic shock, severe obstructive lung disease, or tension pneumothorax.
Pulsus alternans is defined by the beat-to-beat variability of the pulse amplitude ( Fig. 13.5 ). It is present when only every other phase 1 Korotkoff sound is audible as the cuff pressure is slowly lowered, in a patient with a regular heart rhythm, independent of the respiratory cycle. Pulsus alternans generally occurs in severe heart failure, severe AR, hypertension, and hypovolemic states. It is attributed to cyclic changes in intracellular calcium and action potential duration. Association with electrocardiographic T wave alternans appears to increase arrhythmic risk.
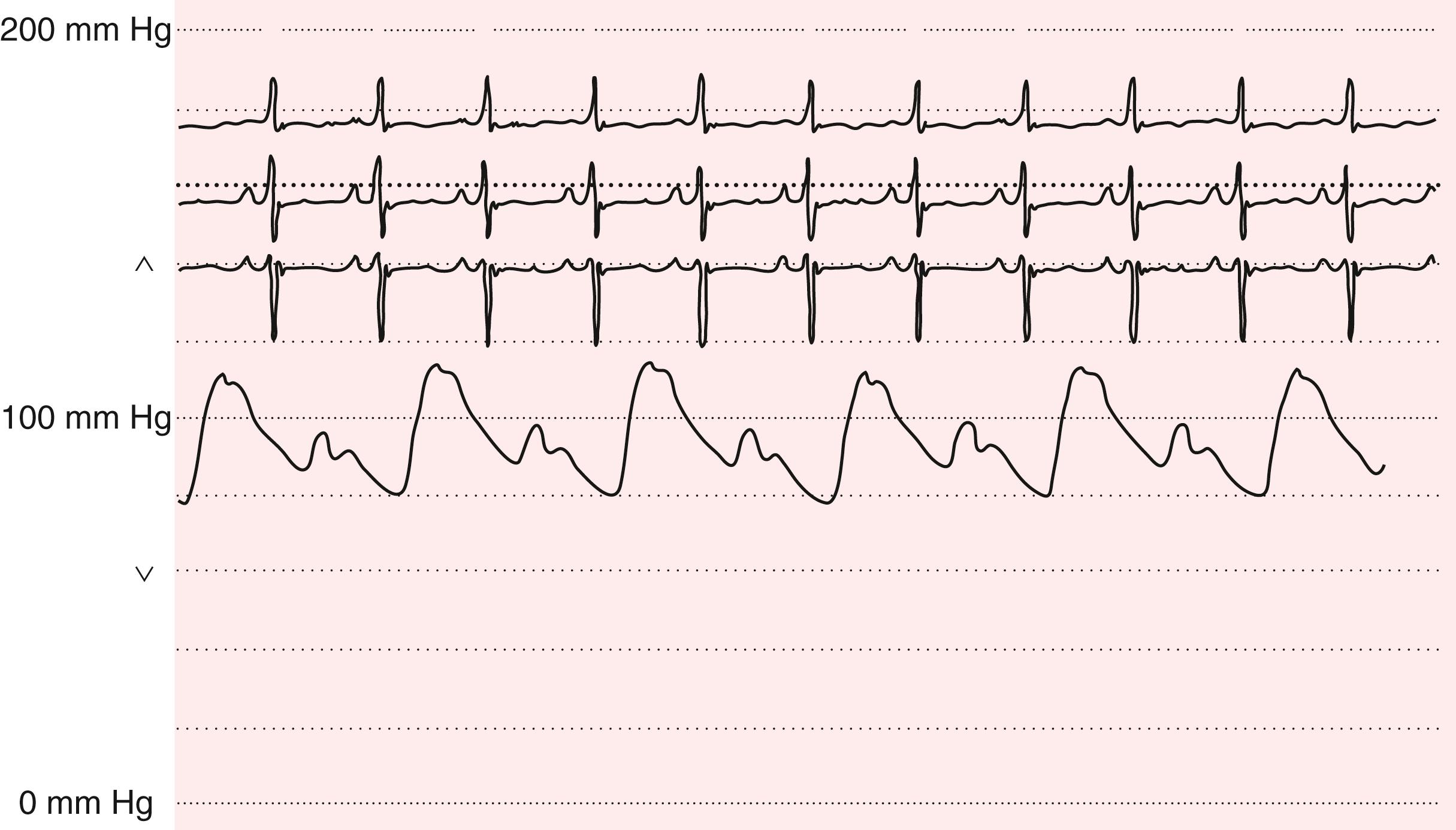
Severe aortic stenosis may be suggested by a weak and delayed pulse ( pulsus parvus et tardus ) and is best appreciated by careful palpation of the carotid arteries (see Fig. 13.4 ; see Chapter 72 ). The delay is assessed during simultaneous auscultation of the heart sounds; the carotid upstroke should coincide with S 1 . This finding is less specific in older, hypertensive patients with reduced vascular compliance and stiffer carotid arteries. An abrupt carotid upstroke with rapid fall-off characterizes the pulse of chronic AR (Corrigan or water-hammer pulse). The carotid upstroke also is rapid in older patients with isolated systolic hypertension and wide pulse pressures.
Pulsation of the abdominal aorta can be appreciated in the epigastric area. Femoral and popliteal artery aneurysms should be sought in patients with AAA disease or underlying connective tissue disease.
The history and physical examination findings can help assess the level of arterial obstruction in patients with lower extremity claudication (see Chapter 43 ). Auscultation for carotid, subclavian, aortic, and femoral artery bruits should be routine. The correlation between the presence of a bruit and the degree of vascular obstruction is weak. Extension of a bruit into diastole or a thrill generally indicates severe obstruction. Other causes of a bruit include arteriovenous fistulas and enhanced flow through normal arteries as, for example, in a young patient with fever.
Integrating the clinical history and presence of atherosclerotic risk factors improves the accuracy of the examination for the identification of lower extremity PAD. In an asymptomatic patient, the presence of a femoral bruit (LR, 4.8; 95% CI, 2.4 to 9.5) or any abnormality of the pulse (LR, 3.1; 95% CI, 3.1 to 6.6) increases the likelihood of PAD. The likelihood of significant PAD increases when there are lower extremity symptoms and cool skin (LR, 5.9; 95% CI, 4.1 to 8.6), pulse abnormalities (LR, 4.7; 95% CI, 2.2 to 9.9), or any bruit (LR, 5.6; 95% CI, 4.7 to 6.7). Abnormal pulse oximetry, defined by a more than 2% difference between finger and toe oxygen saturation, can also indicate lower extremity PAD and is comparable to the ABI (LR, 30.0; 95% CI, 7.6 to 121 versus LR, 24.8; 95% CI, 6.2 to 99.8).
The apical heartbeat may be visible in thin-chested adults. The left anterior chest wall may heave in patients with enlarged and hyperdynamic left ventricles. Right upper parasternal and sternoclavicular pulsations suggest ascending aortic aneurysm disease. A left parasternal lift indicates RV pressure or volume overload. A pulsation in the third intercostal space to the left of the sternum can indicate pulmonary artery hypertension. In very thin, tall patients, or in patients with emphysema and flattened diaphragms, the RV impulse may be visible in the epigastrium and should be distinguished from a pulsatile liver edge.
Palpation of the heart should begin with the patient in the supine position inclined at 30 degrees. If the heart is not palpable in this position, the patient should be examined either in the left lateral decubitus position with the left arm above the head or in the seated position, leaning forward. The point of maximal impulse normally is over the left ventricular (LV) apex beat and should be located in the midclavicular line at the fifth intercostal space. It is smaller than 2 cm in diameter and moves quickly away from the fingers. It is best appreciated at end-expiration, when the heart is closest to the chest wall. The normal impulse may not be palpable in obese or muscular patients or in those with thoracic cage deformities. LV cavity enlargement displaces the apex beat leftward and downward. A sustained apex beat is a sign of LV pressure overload (as in aortic stenosis or hypertension). A palpable, presystolic impulse corresponds to a fourth heart sound (S 4 ) and reflects the atrial contribution to ventricular diastolic filling of a noncompliant left ventricle. A prominent, rapid early filling wave in patients with advanced systolic heart failure may result in a palpable third sound (S 3 ), which may be present when the gallop itself is not audible ![]() ( ). A large ventricular aneurysm may yield a palpable and visible ectopic impulse discrete from the apex beat. HOCM rarely may cause a triple cadence apex beat, with contributions from a palpable S 4 and the two components of the systolic pulse.
( ). A large ventricular aneurysm may yield a palpable and visible ectopic impulse discrete from the apex beat. HOCM rarely may cause a triple cadence apex beat, with contributions from a palpable S 4 and the two components of the systolic pulse.
A parasternal lift occurs with RV pressure or volume overload. Signs of TR (jugular venous cv waves) and/or pulmonary artery hypertension (loud, single, or palpable P 2 ) should be sought. An enlarged RV can give rise to a precordial lift that can extend across the precordium and obscure left-sided findings. Rarely, patients with severe MR will have a prominent left parasternal impulse because of systolic expansion of the left atrium and forward displacement of the heart. Lateral retraction of the chest wall may be present with isolated RV enlargement secondary to posterior displacement of the systolic LV impulse. Systolic and diastolic thrills signify turbulent, high-velocity blood flow. Their locations help to identify the origins of heart murmurs.
The normal first heart sound (S 1 ) comprises mitral (M 1 ) and tricuspid (T 1 ) valve closure. The two components usually are best heard at the lower left sternal border in younger subjects. Normal splitting of S 1 is accentuated with complete right bundle branch block. S 1 intensity increases in the early stages of rheumatic mitral stenosis when the valve leaflets are still pliable, in hyperkinetic states, and with short P-R intervals (less than 160 milliseconds). S 1 becomes softer in the late stages of stenosis, when the leaflets are rigid and calcified, with contractile dysfunction, beta-adrenergic receptor blockers, and long P-R intervals (greater than 200 milliseconds). Other factors that can decrease the intensity of the heart sounds and murmurs include mechanical ventilation, obstructive lung disease, obesity, pendulous breasts, pneumothorax, and pericardial effusion.
The second heart sound (S 2 ) comprises aortic (A 2 ) and pulmonic (P 2 ) valve closure. With normal, or physiologic, splitting, the A 2 –P 2 interval increases during inspiration and narrows with expiration. The individual components are best heard at the second left interspace with the patient in the supine position. The A 2 –P 2 interval widens with complete right bundle branch block because of delayed pulmonic valve closure, and with severe MR because of premature aortic valve closure. Unusually narrow but physiologic splitting of S 2 , with an increase in the intensity of P 2 relative to A 2 , indicates pulmonary artery hypertension. With fixed splitting, the A 2 –P 2 interval is wide and remains unchanged during the respiratory cycle, indicating ostium secundum ASD. Reverse, or paradoxical, splitting occurs as a consequence of a pathologic delay in aortic valve closure, as may occur with complete left bundle branch block, RV apical pacing, severe aortic stenosis, HCM, and myocardial ischemia. A 2 normally is louder than P 2 and can be heard at most sites across the precordium. When both components can be heard at the lower left sternal border or apex, or when P 2 can be palpated at the second left interspace, pulmonary hypertension is present. The intensity of A 2 and P 2 decreases with aortic and pulmonic stenosis, respectively. A single S 2 may result.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here