Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In 55% to 75% of gastrointestinal (GI) lymphomas, the stomach is the primary site. Lymphoma accounts for 1% to 7% of all gastric malignancies. , Diffuse large B-cell lymphoma and extranodal marginal-zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) are the most common types, with some series having more of the former, and others more of the latter. Other types of lymphoma are quite uncommon, but occasional cases of follicular lymphoma, Burkitt’s lymphoma, mantle cell lymphoma, and peripheral T-cell lymphoma may arise in the stomach ( Table 31.1 ). The proportion of gastric T-cell lymphomas appears to be higher in Asian countries than in Western countries, although diffuse large B-cell lymphoma is still more common than T-cell lymphomas.
| Stomach | Small Intestine | Colon | Anus | Appendix | Liver | Gallbladder | Pancreas |
|---|---|---|---|---|---|---|---|
| MZL | DLBCL ∗ | DLBCL | DLBCL | DLBCL | DLBCL | DLBCL | DLBCL |
| DLBCL | MZL (including IPSID) | MZL | Burkitt | Burkitt | MZL | Burkitt | |
| Burkitt | Burkitt | Mantle cell | Follicular | MZL | Follicular | ALCL | |
| Mantle cell | Mantle cell | Follicular | MZL | HSTCL | MZL | ||
| Follicular | Follicular | Burkitt | |||||
| PTCLs | EATL | MEITL | |||||
| MEITL | Other PTCLs | ||||||
| Other PTCLs | |||||||
| Extranodal NK/T-cell, nasal type |
In 15% to 35% of GI lymphomas, the small intestine or the ileocecal region is the presenting site. , , Within the intestines the ileocecal region is the most common site for lymphoma, accounting for about 40% of cases. Lymphoma accounts for approximately 25% of small intestinal neoplasms. The proportions of different types of lymphomas vary from one series to another, depending on the age and ethnic background of patients studied, and the part of the world in which the study was performed. The most common type is diffuse large B-cell lymphoma, followed by extranodal marginal-zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma, including immunoproliferative small intestinal disease), Burkitt’s lymphoma, T-cell lymphomas, mantle cell lymphoma, and follicular lymphoma. , , The ileum is more commonly affected than the duodenum or jejunum. Lymphomas that arise in the ileocecal region are mostly diffuse high-grade B-cell lymphomas (diffuse large B-cell lymphoma or Burkitt’s lymphoma) (see Table 31.1 ). , ,
The large intestine is the primary site in 3% to 20% of GI lymphomas , , and in 21% to 35% of all intestinal lymphomas. Lymphoma accounts for only about 0.5% of malignant neoplasms of this site. , The most common type of lymphoma is diffuse large B-cell lymphoma, followed by extranodal marginal-zone lymphoma, mantle cell lymphoma, and rare cases of follicular lymphoma, Burkitt’s lymphoma, and peripheral T-cell lymphoma. The cecum is the most common site of involvement in the large intestine, followed by the rectum; other portions of the colon are only rarely affected. , , Anal lymphomas are very rare; they are usually diffuse large B-cell lymphomas (see Table 31.1 ).
A variety of lymphomas may present with multifocal GI involvement, including mantle cell lymphoma, follicular lymphoma, enteropathy-associated T-cell lymphoma, marginal-zone lymphoma, and diffuse large B-cell lymphoma.
The most common presenting findings associated with GI lymphomas are abdominal pain, anorexia or weight loss, obstruction, palpable mass, diarrhea, nausea or vomiting, fever, perforation, and bleeding. Intussusception may be seen with bulky lymphomas in the ileocecal region. In a few cases, the lymphoma may be discovered as an incidental finding. , , , , , Symptoms distinctive for different types of lymphoma are noted in the corresponding sections. The more common types of lymphoma, based on pathological subclassification, will be discussed individually. Lymphoma of the appendix is also discussed separately.
The stomach is the most common site for the development of extranodal marginal-zone lymphoma, accounting for about one-third of all cases. , Gastric marginal-zone lymphoma (extranodal marginal-zone lymphoma of mucosa-associated lymphoid tissue [MALT lymphoma]) affects similar numbers of men and women, with a slight male preponderance in some series. Most patients are older adults, with a median age in the sixth or seventh decade. Infrequently, young adults and even adolescents are affected. Patients present with epigastric pain or dyspepsia; nausea and vomiting, bleeding, and weight loss may occur but are unusual. The symptoms often suggest gastritis or peptic ulcer disease rather than lymphoma.
On endoscopy, or on gross examination of a gastrectomy specimen, this type of lymphoma often consists of erosions, shallow ulcers, mucosal granularity, or thickened mucosal folds, or it appears as a diffusely infiltrative, ill-defined lesion. The appearance may mimic gastritis. , , Lymphomas usually involve one portion of the stomach, but tumors may be multifocal and widespread. , Multifocal, widespread, or ill-defined lymphomas may be associated with positive resection margins in gastrectomy specimens. , Superficial lesions are more common than large masses. A discrete, localized, polypoid tumor, or an exophytic lesion mimicking carcinoma may be found, but this is much less common. Most lymphomas are confined to the mucosa or submucosa. Lymph node involvement is unusual with superficially invasive lymphomas, but it is common when there is invasion into the muscularis propria.
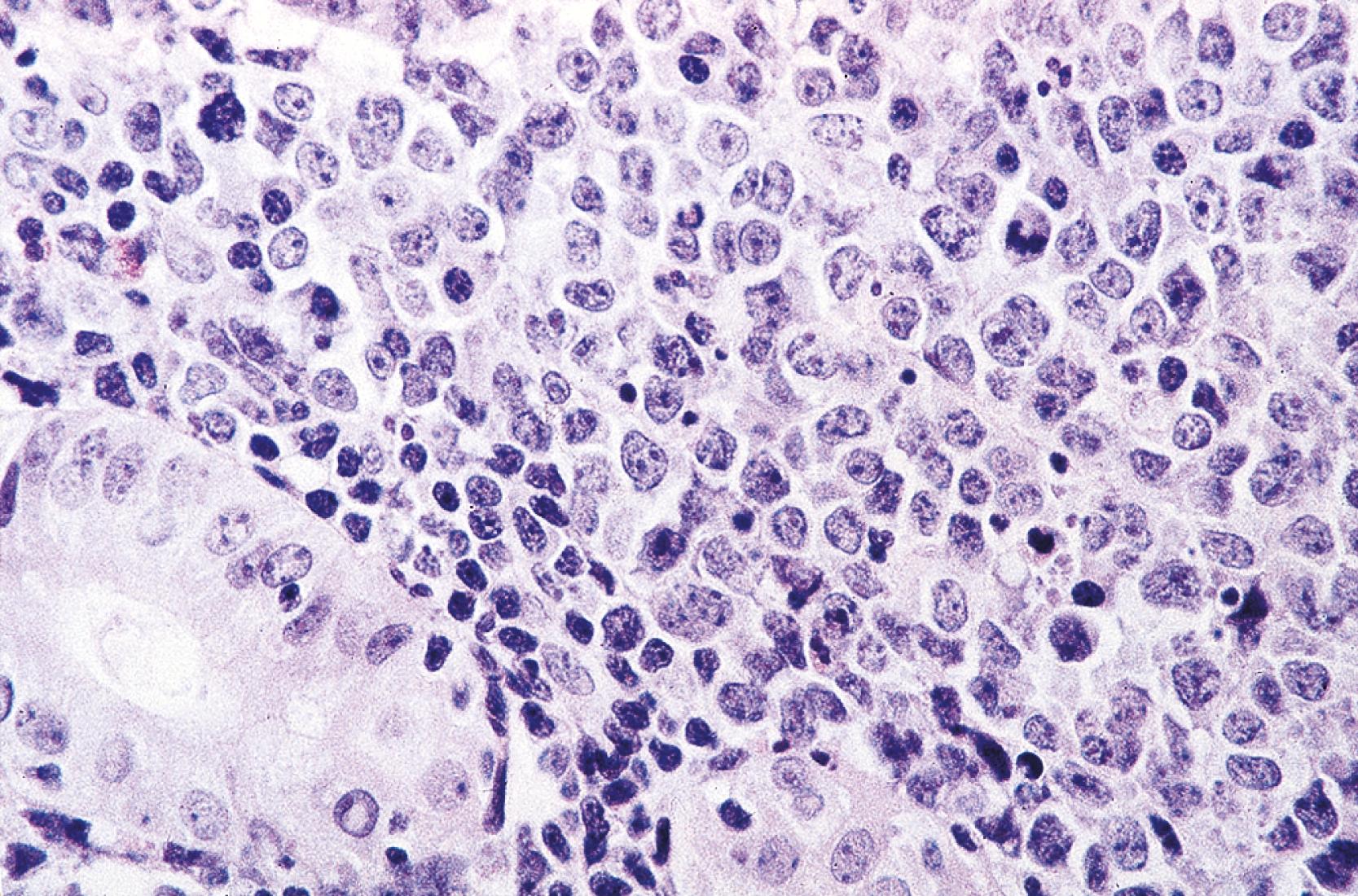
Microscopic examination reveals a diffuse or vaguely nodular infiltrate of marginal-zone cells, small- or medium-sized cells with oval to slightly irregular nuclei, and a distinct rim of clear cytoplasm. In about a third of cases, there is prominent plasmacytic differentiation, often in the form of a bandlike infiltrate of plasma cells in the most superficial portion of the lymphoma. The plasma cells may have the appearance of normal, mature plasma cells or may have cytoplasm with crystalline inclusions, or nuclei with Dutcher bodies. Small clusters of neoplastic cells often infiltrate and disrupt gastric glands to form lymphoepithelial lesions. It is usually possible to identify reactive lymphoid follicles in the lymphoma, often with infiltration and replacement by neoplastic cells (follicular colonization). The cells that colonize the follicles are most commonly marginal-zone–type cells, but plasma cells or large lymphoid cells may also be found. Even when follicles cannot be seen on routinely stained sections, evidence of preexisting follicles can generally be found using antibodies to follicular dendritic cells, such as CD21 or CD23. A few large cells can often be found scattered among the marginal-zone cells ( Fig. 31.1 ). ,
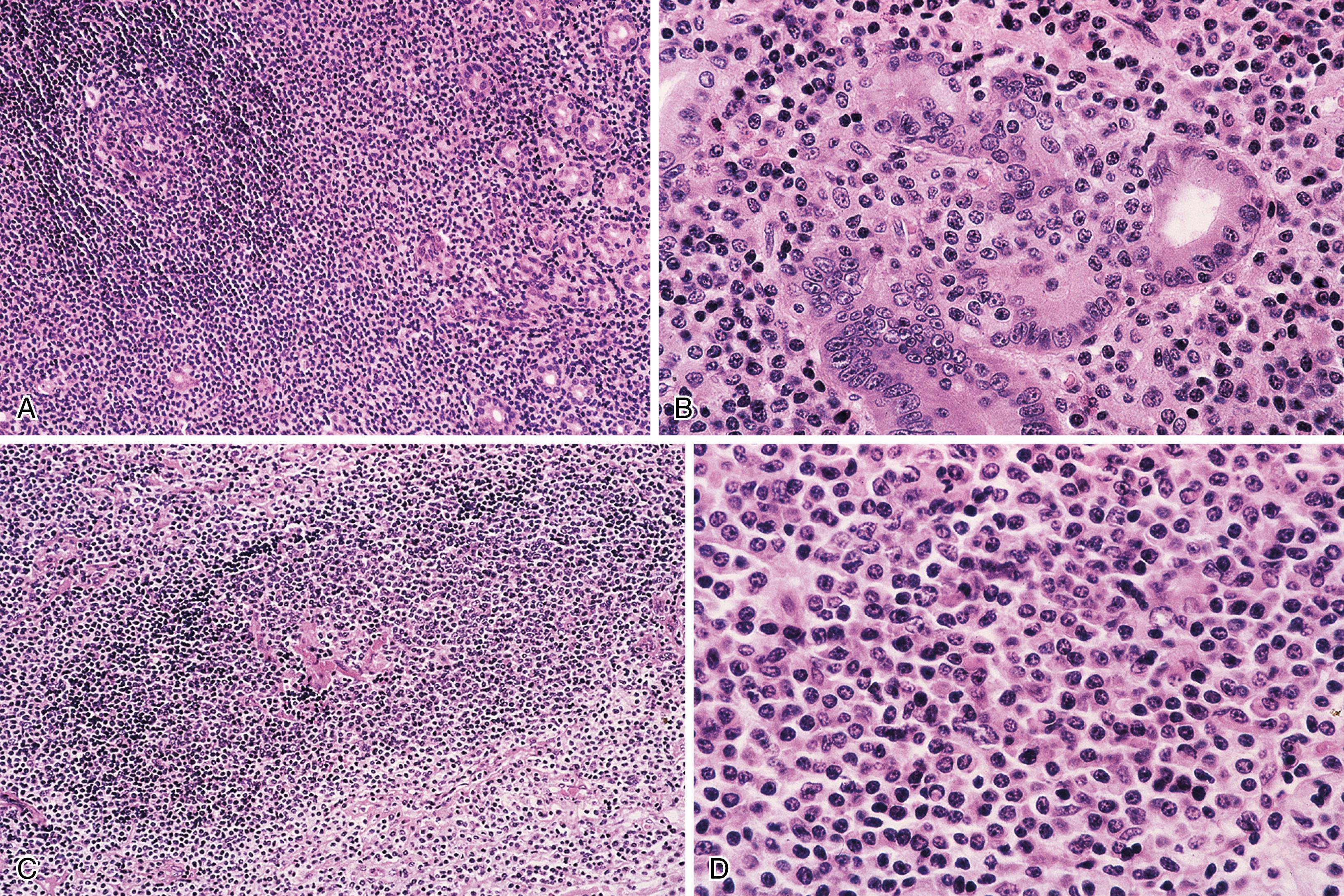
Biopsies of the gastric mucosa also frequently show evidence of infection with Helicobacter pylori , detectable on routinely stained sections in some cases, or with special stains such as the thiazine or Steiner stain, or by immunohistochemistry. Alternatively, evidence of H. pylori infection can be detected using the rapid urease test (CLO test), stool antigen test, or serology. The likelihood of finding H. pylori is greater with lymphomas that are superficial (confined to the mucosa or submucosa) and those that are entirely low grade (rather than having areas of progression to diffuse large B-cell lymphoma). In addition, the frequency of H. pylori positivity varies among studies. Although some series report that 80% to 100% of patients harbor H. pylori , , in other studies it is only a minority.
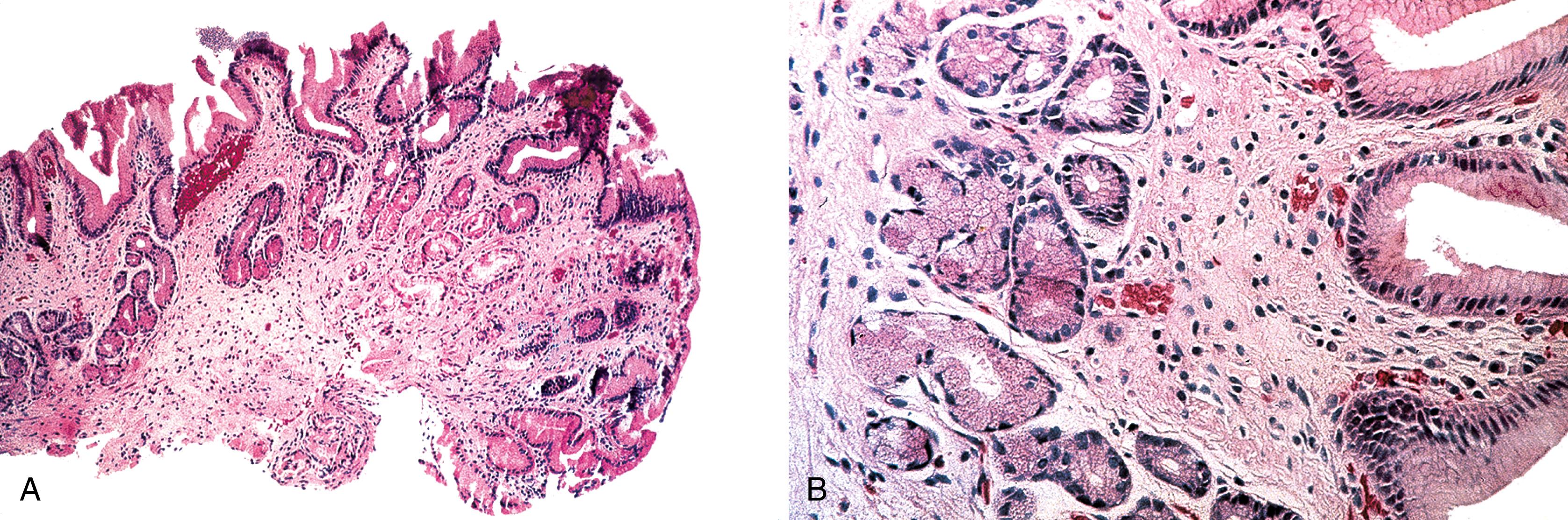
After therapy to eradicate H. pylori , complete histological regression of lymphoma is characterized by a lamina propria with a distinctive empty appearance, basal aggregates of small lymphocytes, and scattered plasma cells ( Fig. 31.2 ). Partial regression shows areas of empty lamina propria with foci of atypical lymphoid cells or lymphoepithelial lesions, or both.
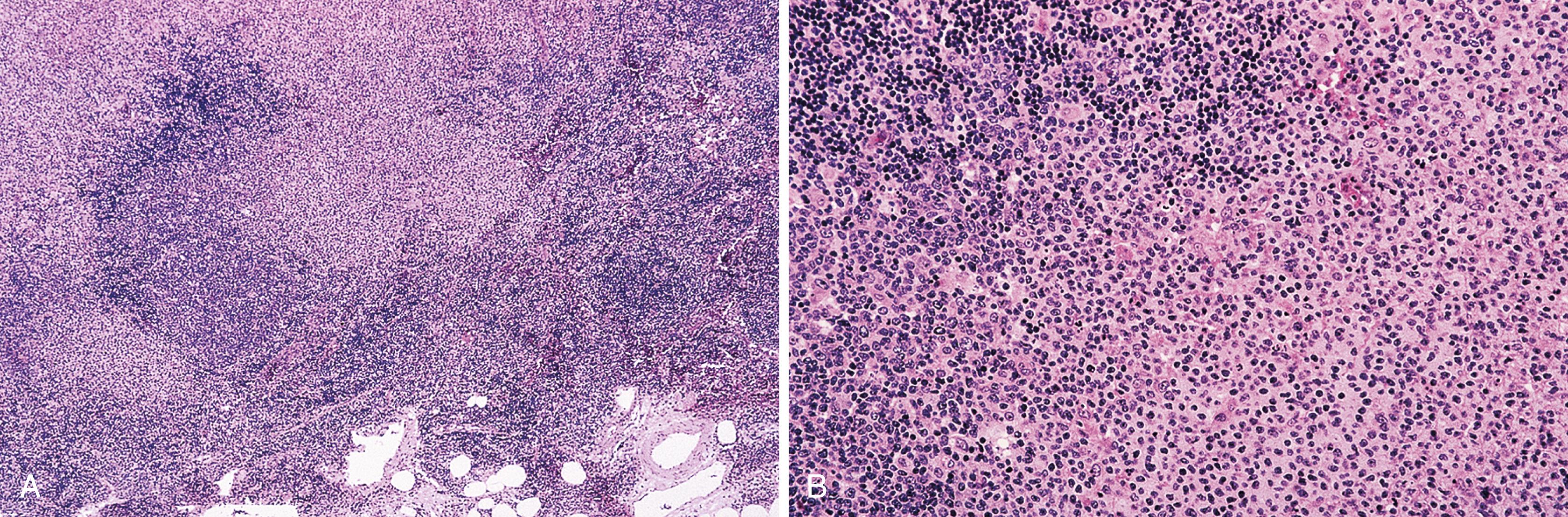
Early involvement of lymph nodes often takes the form of bands of marginal-zone cells along sinuses, sometimes with parafollicular aggregates with a marginal-zone pattern, progressing to confluent sheets of marginal-zone cells either with or without monocytoid B cells and follicular colonization. , The appearance is indistinguishable from that of nodal marginal-zone lymphoma ( Fig. 31.3 ).
Immunophenotyping shows CD20+, CD5−, CD10−, BCL6−, cyclin D1−, and monotypic surface immunoglobulin (sIg)+ B cells, with plasma cells expressing polytypic or monotypic cytoplasmic immunoglobulin (cIg). The immunoglobulin expressed by neoplastic cells is most often IgM, but in some cases it is IgA or IgG. The neoplastic cells are BCL2+, although BCL2 may be lost in colonized follicles. CD43 is coexpressed in up to one-third of cases. An immunostain for cytokeratin can be helpful for highlighting lymphoepithelial lesions. The proliferation fraction, as measured with Ki67, is low, although it is characteristically high in residual reactive germinal centers. , , ,
Molecular genetic studies show clonally rearranged immunoglobulin heavy- and light-chain genes. Analysis of heavy-chain genes shows somatic hypermutation, consistent with neoplastic cells at a postgerminal center stage of development. MALT lymphomas often show biased usage of certain IGHV segments; together with the presence of ongoing somatic mutation, these findings suggest the proliferation of clonal lymphoid cells could be antigen driven. Translocation t(11;18)(q21;q21) is the most common cytogenetic abnormality in gastric marginal-zone lymphoma, although its frequency varies widely, from 5% to 50% of cases in different series. This translocation involves BIRC3 on chromosome 11 and MALT1 on chromosome 18; it results in a chimeric transcript that can be detected by reverse transcriptase polymerase chain reaction (RT-PCR). The fusion is believed to confer a survival advantage to the neoplastic cells via activation of nuclear factor–kappa B (NFκB). This translocation is found nearly exclusively in extranodal marginal-zone lymphoma. The t(11;18) is associated with a number of clinical and pathological correlates. Marginal-zone lymphomas that fail to regress with H. pylori therapy may harbor the t(11;18), whereas t(11;18) is consistently absent in cases that do regress with such therapy. This translocation tends to be associated with higher-stage disease. , A higher proportion of H. pylori –negative gastric marginal-zone lymphomas is also associated with t(11;18)(q21;q21); this could be because of a different pathogenesis or related to the tendency of lymphomas with this translocation to present at an advanced stage, when the lymphoma is able to grow independent of the presence of the bacteria. The t(11;18) is only very rarely found in cases of diffuse large B-cell lymphoma, suggesting that marginal-zone lymphomas with this translocation are very unlikely to undergo large-cell transformation. In general, the proportion of European gastric marginal-zone lymphomas that are positive for t(11;18) has been higher than the proportion of Asian or North American cases. The reason for this variation is uncertain, but possibilities include host factors related to genetic makeup; prevalence of different strains of H. pylori ; study of resection specimens as opposed to biopsies, because larger, more deeply invasive tumors more often harbor the t(11;18); inclusion of marginal-zone lymphomas in some series secondarily involving stomach; and possibly techniques used (RT-PCR vs. fluorescence in situ hybridization) ( Table 31.2 ).
| Type of Lymphoma | Composition | Usual Immunophenotype | Genetic Features |
|---|---|---|---|
| B-Cell Lymphomas | |||
| Extranodal marginal-zone lymphoma, MALT type | Small lymphocytes, marginal-zone B cells, plasma cells, reactive follicles, lymphoepithelial lesions | Monotypic sIg+, cIg+/− (IgM > IgG or IgA), CD20+, CD5−, CD10−, BCL6−, BCL2+, CD43−/+, cyclin D1− | IGH clonally rearranged; t(11;18) ( BIRC3-MALT1 ) in some gastric and intestinal cases; trisomy 18 or trisomy 3 in some cases; t(14;18) (IGH -MALT1 ) in some hepatic cases |
| Diffuse large B-cell lymphoma | Large centrocytes, centroblasts, immunoblasts and/or anaplastic large B cells | Monotypic sIg+, CD20+, BCL6+/−, CD10−/+, BCL2+/−, MUM1+/−, CD43+/−; can be GCB or non-GCB type | IGH clonally rearranged; BCL6 abnormalities (translocations or amplifications) are common; t(8;14) ( MYC- IGH) sometimes found; t(14;18) (IGH -BCL2 ) uncommon |
| Burkitt’s lymphoma | Medium-sized atypical lymphoid cells with round nuclei, basophilic cytoplasm, tingible body macrophages | Monotypic sIgM+, CD20+, CD10+, BCL6+, BCL2−, cMYC+, Ki67 ~100% | IGH clonally rearranged; t(8;14), t(2;8) or (8;22) ( MYC ); rearrangements of BCL2 and BCL6 are absent; ∼all endemic cases and minority of sporadic and immunodeficiency-associated cases are EBV+ |
| Mantle cell lymphoma | Small to medium-sized, slightly irregular cells with scant cytoplasm | Monotypic sIgMD+, CD20+, CD5+, CD10−, CD43+/−, cyclin D1+, usually SOX11+ | IGH clonally rearranged; t(11;14) ( CCND1- IGH) |
| Follicular lymphoma | Mixture of centrocytes and centroblasts, follicular dendritic cells | Monotypic sIg+, CD20+, CD10+, BCL6+, BCL2+, CD5−, CD43−, cyclin D1−; hollow FDC meshworks (CD21+, CD23+) | IGH clonally rearranged, t(14;18) (IGH -BCL2 ) |
| Plasmablastic lymphoma | Plasmablasts, sometimes with more mature plasmacytoid cells, high mitotic rate | CD20−, MUM1+, CD79a−/+, CD138+/−, cIg+/−, Ki67 high, HHV8− | IGH clonally rearranged; MYC commonly rearranged or amplified; EBV+, most cases |
| T/NK Cell Lymphomas | |||
| Enteropathy-associated T-cell lymphoma | Medium-sized and/ or large, sometimes bizarre cells, many admixed reactive cells | CD3+, CD5−; CD4−/CD8− >CD8+; granzyme B+, perforin+, CD56−, CD30+/−, SYK−, Alk1−, CD103+; TCRab−/TCRgd−> TCRab+> TCRgd+ | T-cell receptor genes clonally rearranged; EBV−; gains of 9q34 ( NOTCH1 , ABL , and VAV2 ) OR deletions of 16q are common; gains of 5q (APC) are common; mutations of JAK-STAT pathway genes and of chromatin modifiers such as SETD2 are common |
| Monomorphic epitheliotropic intestinal T-cell lymphoma | Small- to medium-sized cells with pale cytoplasm; admixed reactive cells are sparse | CD3+, CD5−, CD4−, CD8+, CD56+, CD30−, granzyme+, perforin+; MATK+, TCRgd+ > TCRab+ | T-cell receptor genes clonally rearranged; EBV−; gains of 9q34 ( NOTCH1 , ABL , and VAV2 ) OR deletions of 16q are common; mutations of JAK-STAT pathway genes and of chromatin modifiers such as SETD2, MYC amplification and upregulated MAPK signaling are common |
| Extranodal NK/T-cell lymphoma, nasal type | Small, medium-sized, and/or large atypical lymphoid cells, necrosis, vascular damage | cCD3+, CD2+, CD4−, CD8−, CD5−, CD56+, granzyme+, perforin+ | T-cell receptor genes germline; EBV+; mutations of JAK-STAT pathway genes are common |
| Hepatosplenic T-cell lymphoma | Medium-sized cells with oval nuclei, fine to condensed chromatin, small to absent nucleoli, pale cytoplasm; can become larger and more atypical over time | CD2+, CD3+, CD5−/+, CD4−, CD8−/+, CD7+/−, CD16+/−, CD56+/−, CD57−/+, TIA1+, granzyme B−, perforin−, TCRgd+ > TCRab+ | T-cell receptor genes clonally rearranged; mutations of JAK-STAT pathway genes and of chromatin modifiers such as SETD2 are common; isochromosome 7q and other changes of chromosome 7 are common |
Another cytogenetic abnormality associated with gastric marginal-zone lymphoma, although less commonly, is t(1;14) (p22;q32), involving the genes for BCL10 and immunoglobulin heavy chain (IGH). This change results in deregulation of the BCL10 gene, with resultant loss of its normal proapoptotic activity and acquisition of oncogenic potential. The t(1;14) is also associated with failure of lymphoma to regress with antibiotics. The t(1;14) is associated with strong nuclear expression of BCL10 protein; the t(11;18) is associated with moderate nuclear expression of BCL10. Some cases lacking both translocations demonstrate moderate BCL10 expression, so that the protein expression is not specific for a certain cytogenetic change. Rare cases show a t(14;18) involving the genes IGH and MALT1 . , The three translocations—t(11;18), t(1;14), and t(14;18)—are mutually exclusive. All three are believed to contribute to lymphomagenesis via activation of NFκB. Trisomy 3 and trisomy 18 are found in a subset of cases. , , The BCL1 (cyclin D1) and BCL2 genes are germline. ,
Genetic features of H. pylori may also have an impact on lymphomagenesis. In patients with H. pylori infection, t(11;18) shows a tendency to be more frequent when the H. pylori strain is positive for cytotoxin-associated gene A ( cagA ). CagA+ strains are associated with a more prominent neutrophilic infiltrate and thus with more reactive oxygen species capable of causing DNA damage, predisposing to the acquisition of translocations. In one study H. pylori with multiple HopQ ( H. pylori outer membrane Q) types, truncated cagPAI (pathogenicity activity island) with increased expression of cagT, LEF1 (left end of cagA gene 1), and vacAs1 alleles were more often associated with B-cell lymphoma (MALT lymphoma or diffuse large B-cell lymphoma) than nonneoplastic gastric mucosa.
Staging reveals disease confined to the stomach in an estimated 63% to 88% of cases , , , ; regional lymph nodes are involved in the remaining cases, and in a few, there is more widespread disease. , , Patients with widespread disease may have involvement of another MALT site, especially the small intestine or colon, but also the lung, kidney, salivary gland, thyroid, ocular adnexa, and others; a few have bone marrow involvement. , , , , The lymphoma is indolent and may remain localized for years, even without specific therapy.
In 1993, Wotherspoon and colleagues described the remarkable observation of regression of gastric marginal-zone lymphoma after eradication of H. pylori with antibiotics. Since then, many other medical centers have reported the same phenomenon, with response rates typically ranging from 60% to 90%. , , , The interval to histological regression may be prolonged, with a range of 1 to 35 months and a median of 3 to 10 months. , , , Features associated with failure of regression include invasion beyond the submucosa, spread beyond the stomach, absence of H. pylori , and presence of certain chromosomal abnormalities: t(11;18) and t(1;14). , , , , A component of diffuse large B-cell lymphoma also decreases the chance of regression. Remarkably, however, complete regression of some cases of diffuse large B-cell lymphoma arising in association with marginal-zone lymphoma confined to the stomach with antibiotic therapy alone has been described ; regression appears more likely to occur when the lymphoma is superficial (confined to the mucosa or submucosa) on endoscopic ultrasound. ,
A minority of patients experience relapse of lymphoma; in some cases this is precipitated by H. pylori reinfection. In patients who are histologically negative for lymphoma after H. pylori eradication, clonal B cells can be detected by polymerase chain reaction (PCR) for months to years later. , , , Microdissection studies suggest that the clonal cells reside in basal lymphoid aggregates. Clonal B cells can be detected by PCR at presentation in endoscopically normal mucosa in a subset of cases; this finding may predict a longer time to achieve histological remission.
When the lymphoma does not respond, or relapses, after H. pylori eradication, other modalities (e.g., surgery, radiation, chemotherapy) may be used, , , , , and cure can usually be obtained. The 5-year survival rate of patients with gastric marginal-zone lymphoma is at least 90%, , , , and the 10-year survival rate is between 80% and 90%. Gastric marginal-zone lymphoma patients have better progression-free survival than extranodal marginal-zone lymphomas arising in most other sites. Of note patients with H. pylori infection with or without MALT lymphoma appear to be at increased risk for gastric carcinoma. Follow-up of patients with MALT lymphoma reveals development of gastric carcinoma in occasional cases. The carcinomas may occur in a background of atrophy with intestinal metaplasia, in the same site previously involved by lymphoma.
The majority of gastric marginal-zone lymphomas are believed to arise from a background of gastritis with a component of acquired MALT induced by H. pylori infection. Persistent infection with chronic antigenic stimulation leads to the appearance of a clonal population of B cells. Surprisingly, the B cells do not have specificity for H. pylori ; instead, they produce antibodies that may be reactive with a variety of autoantigens. It is the T cells in the infiltrate that have strain-specific reactivity for H. pylori . In the early phase of the disease, the B cells require H. pylori and the T cells to proliferate. Accordingly, in this phase, the lymphoma remains localized and may respond to antibiotic therapy directed against H. pylori . With time, the clonal B cells acquire genetic abnormalities associated with autonomous growth, leading to a low-grade lymphoma that does not regress with H. pylori eradication and that may spread beyond the stomach. Additional genetic abnormalities, such as TP53 inactivation and allelic loss, CDKN2A (gene for p16 Ink4A ) deletion, and MYC translocation , , may occur and lead to large-cell transformation.
Important pathogenetic questions remain. Only a small proportion of individuals with H. pylori gastritis develop marginal-zone lymphoma. It is possible that host factors and environmental factors play a role in the pathogenesis of this type of lymphoma. One study suggests that certain polymorphisms in the T-cell regulatory gene CTLA4 (cytotoxic T-lymphocyte antigen 4) may be associated with an increased or decreased risk for marginal-zone lymphoma. Others describe an increased risk of gastric marginal-zone lymphoma with certain polymorphisms of genes involved in inflammatory response and antioxidative capacity, with those individuals with a stronger inflammatory response to H. pylori and diminished antioxidative capacity more likely to develop gastric marginal-zone lymphoma. Also, in some cases, there is no evidence of H. pylori infection, even in patients studied by histology, immunohistochemistry, urease test, serology, or a combination of these methods. In such cases, it is not clear whether infection was present previously and resolved at a stage after the lymphoma attained autonomous growth, or whether there are other, as yet unrecognized, etiological agents for gastric marginal-zone lymphoma.
Factors other than H. pylori infection occasionally contribute to lymphomagenesis. A few H. pylori –negative marginal-zone lymphomas (as well as a small proportion of cases of chronic gastritis) may be caused by non– H. pylori species, such as Helicobacter heilmannii . H. heilmannii is detectable on histological examination as long, thin, spiral bacilli adjacent to the surface epithelium. , A case of an Epstein-Barr virus (EBV)+ gastric marginal-zone lymphoma has been reported in a severely immunosuppressed allogeneic stem cell recipient, thus representing an EBV+ posttransplant lymphoproliferative disorder.
Gastric marginal-zone lymphoma versus gastritis: In favor of a diagnosis of lymphoma, on routinely stained sections are the presence of an expansile, destructive infiltrate with loss of glands, cytological atypia of the infiltrating cells (having the appearance of marginal-zone rather than normal small lymphocytes), frequent lymphoepithelial lesions, and Dutcher bodies. Immunohistochemical studies are often of assistance. Demonstration of monotypic Ig light-chain expression (detectable mainly in cases with plasmacytic differentiation) confirms a diagnosis of lymphoma. A diffuse infiltrate of B cells, and coexpression of CD43 by B cells, both favor a diagnosis of lymphoma. In selected cases, gene rearrangement studies to detect clonal B-cell populations can be useful to distinguish marked chronic gastritis from early involvement by marginal-zone lymphoma.
Gastric marginal-zone lymphoma versus other low-grade lymphomas: In the stomach, marginal-zone lymphoma is much more common than either follicular lymphoma or mantle cell lymphoma. However, either of these may involve the stomach and mimic marginal-zone lymphoma. Marginal-zone lymphoma is composed of CD5−, CD10− B cells with relatively abundant clear cytoplasm, and may show plasmacytic differentiation and an admixture of a few large cells. Mantle cell lymphoma typically is composed of a monotonous population of small- to medium-sized CD5+, cyclin D1+ B cells with scant cytoplasm, without a large-cell or plasmacytic component. However, in a small proportion of mantle cell lymphomas the neoplastic cells may have pale cytoplasm and resemble marginal-zone cells, so that relying on morphology alone may occasionally be misleading. Follicular lymphoma generally has a more distinct follicular architecture and is typically composed of CD10+, BCL6+ B cells with nuclei that are usually more irregular, and cytoplasm that is usually more scant, than marginal-zone cells (see Table 31.2 ).
Histological progression to diffuse large B-cell lymphoma versus gastric marginal-zone lymphoma: When there are sheets or confluent clusters of large, transformed cells found outside of follicles in a background of marginal-zone lymphoma, a diagnosis of diffuse large B-cell lymphoma, arising in association with marginal-zone lymphoma should be made. “High-grade MALT lymphoma” is not the preferred nomenclature and should not be used. Before diagnosing focal large-cell transformation, it is important to exclude the possibility that the large cells are residual reactive germinal center cells, or neoplastic large cells colonizing follicles. Immunohistochemical stains to demonstrate a follicular dendritic network can be helpful in resolving these uncertainties. The diffuse large B-cell lymphomas that arise in association with gastric marginal-zone lymphomas are often positive for BCL6, and immunostaining for BCL6 may be helpful in highlighting possible areas of large-cell transformation. However, reactive germinal centers are also positive for BCL6, so, as noted earlier, stains for follicular dendritic cells can be performed to investigate this as a possibility.
Poorly preserved high-grade lymphoma versus gastric marginal-zone lymphoma: In a small biopsy with artifactual degenerative change in diffuse large B-cell lymphoma, or Burkitt’s lymphoma, there may be cellular shrinkage and distortion leading to a false impression of low-grade lymphoma. The presence of apoptotic debris or mitotic figures suggests a higher-grade tumor. Staining with the proliferation marker Ki67 can be quite helpful in distinguishing low-grade from high-grade lymphomas. Clinical information, such as the endoscopic appearance of the tumor, can also provide a clue to the correct diagnosis.
Plasmacytoma versus marginal-zone lymphoma: Convincing cases of GI plasmacytoma are rare. Most reported cases are more likely marginal-zone lymphomas with prominent plasmacytic differentiation. In favor of a diagnosis of lymphoma are the presence of a component of B lymphocytes, lymphoepithelial lesions, and IgM+ neoplastic cells (marginal-zone lymphomas may express other heavy chains, but IgM expression by a plasma cell neoplasm would be very rare).
Intestinal marginal-zone lymphoma is the second most common type of lymphoma to arise in the intestines (after diffuse large B-cell lymphoma), accounting for approximately 10% to 18% of all primary intestinal lymphomas. Almost all patients are middle-aged or older adults. Both men and women are affected. , , , Patients occasionally have concurrent gastric marginal-zone lymphoma. Rare patients have inflammatory bowel disease (IBD) or are status post solid organ transplantation, suggesting a role for an immunological abnormality in lymphomagenesis. Many patients are asymptomatic, and the lymphomas may be detected during screening colonoscopy. This type of lymphoma may be located in any portion of the small or large intestine, but a disproportionately large number arise in the rectum, , , , , with the cecal area next most often affected. In the majority of cases, disease is localized to the bowel, either with or without regional lymph node involvement. , , A few patients have serum M components.
The prognosis is favorable. Intestinal marginal-zone lymphoma has a better prognosis than any other type of intestinal lymphoma. , , In one study, 80% of patients with marginal-zone lymphoma were alive and well at last follow-up. The 5-year overall survival for intestinal marginal-zone lymphoma in one large series was 88%. In another, 5-year progression-free survival, overall survival, and disease-specific survival were 92%, 94%, and 98% respectively.
Rare patients with intestinal marginal-zone lymphoma respond to antibiotic therapy, , suggesting that H. pylori , or some other organism, plays a role in the pathogenesis of a subset of these lymphomas.
On gross examination, these lymphomas form raised or polypoid masses, sometimes with ulceration, , , , or infrequently they have the appearance of multiple, small, slightly raised lesions with erosion and erythema, , akin to early gastric marginal-zone lymphoma. Most are single lesions, but occasionally they are multiple. , The lymphomas can be superficially , or transmurally invasive. , In a few cases, the lymphoma may have the appearance of multiple lymphomatous polyposis (see Mantle Cell Lymphoma, later). In one series, the lesions were subclassified into subepithelial tumor type (most common), polyposis type, epithelial mass type, and ileitis type (least common).
The histological, immunophenotypic, and genetic features of marginal-zone lymphoma of the intestine are similar to those seen in the stomach. , , Rarely there is associated amyloid deposition, without evidence of systemic amyloidosis. The t(11;18) resulting in the BIRC-MALT1 fusion is found in 12% to 42% of cases in different series. , , , In one series, the presence of t(11;18) was associated with marginal-zone lymphomas that were larger and higher stage, and they more often affected men. As for gastric marginal-zone lymphoma, trisomy 3 and trisomy 18 are relatively common, and t(1;14) (involving the BCL10 and IGH genes) and t(14;18) (involving the IGH and MALT1 genes) are rare or absent (see Table 31.2 ). ,
A substantial number of marginal-zone lymphomas involving the intestine are secondary; in these cases, the stomach is the most common primary site. Secondary intestinal marginal-zone lymphomas have an even higher proportion of cases with t(11;18), correlating with the tendency of this translocation to be associated with higher-stage disease.
The frequency of small intestinal lymphoma is higher in the Middle East than in Western countries. The small intestine is the most common primary site for extranodal lymphoma, accounting for 50% of such cases, and for 75% of GI lymphomas in adults in the Middle East. Approximately half of these small intestinal lymphomas have been of the distinctive immunoproliferative small intestinal disease (IPSID) type. Over the past several decades, however, the incidence of IPSID appears to have declined. Although IPSID is found mainly in the Middle East and in countries around the Mediterranean Sea, occasional cases have been described in South Africa, the Far East, Europe, and the United States. Because of its geographic distribution, IPSID has also been called Mediterranean lymphoma. IPSID is now considered to be a distinct subtype of extranodal marginal-zone lymphoma. In an association similar to that between H. pylori and gastric marginal-zone lymphoma, Campylobacter jejuni is hypothesized to be an important factor in the development of IPSID. , A unique case of IPSID that developed in a Pacific Islander with multiple enteric infections ( C. jejuni, Vibrio fluvialis , whipworm) as well as Escherichia coli lymphadenitis and H. pylori gastritis has been reported.
Most patients are young adults (median age, 25 years), ranging from adolescence to middle age. Males and females are equally affected. IPSID tends to be associated with lower socioeconomic status. Patients present with abdominal pain, malabsorption, diarrhea, and weight loss of months’ to years’ duration. Many patients also have digital clubbing. , , Obstruction, bleeding, and perforation are uncommon, in contrast to other types of small intestinal lymphoma. At laparotomy, patients may have one or more recognizable intestinal masses, diffuse mural thickening and/or luminal dilation, or normal-appearing bowel. The abnormalities involve the proximal small intestine, the entire small intestine, or, rarely, just the ileum, or the small intestine in combination with either the stomach or colon. Mesenteric lymph nodes are often enlarged. , , , As with gastric marginal-zone lymphoma, in the early phase of the disease the lymphoma may respond to broad-spectrum antibiotics. Nonresponders may be treated with chemotherapy. Later in the course of disease, when muscle invasion or transformation to a high-grade lymphoma occurs, the lymphoma behaves in an aggressive manner. For resectable stage Ie or IIe1 disease, the 5-year survival rate is 40% to 47%. For higher-stage, unresectable disease, the 5-year survival rate has been reported to be from 0% to 25%. However, patients treated with anthracycline-containing combination chemotherapy have a better outlook, with a complete remission rate of approximately 60%. ,
In about half of cases, there is a highly characteristic laboratory abnormality: the serum contains free α heavy chains without associated light chains. Free α heavy chain may also be found in body fluids, such as intestinal fluid, urine, and saliva. Secreted α heavy chain is more likely to be found in early stages of IPSID. Cases with this abnormality have been called α heavy-chain disease. Closer analysis of this paraprotein reveals that it is a truncated α 1 heavy chain lacking the variable region and the first constant region, and unable to bind light chain. The corresponding mRNA shows an internal deletion of V H and C H 1. It has been suggested that IPSID develops in patients with recurrent, or persistent, intestinal infections, leading to chronic antigenic stimulation of IgA-secreting lymphoid tissue in this site, with a resultant clonal population that acquires mutations, leading to the production of α heavy chain with the internal deletion noted earlier.
IPSID is typically characterized by a dense, continuous, bandlike mucosal lymphoid or lymphoplasmacytic infiltrate that is uninterrupted along the length of the small intestine. The mucosa also shows broad villi, although the intestinal epithelial cells usually remain intact. , The extent of the infiltrate explains the pathogenesis of the malabsorption that affected patients experience. The histological features are similar to those of marginal-zone lymphomas in other extranodal sites, except that in IPSID there consistently is marked plasmacytic differentiation. The following staging system has been proposed to subclassify the infiltrate in IPSID.
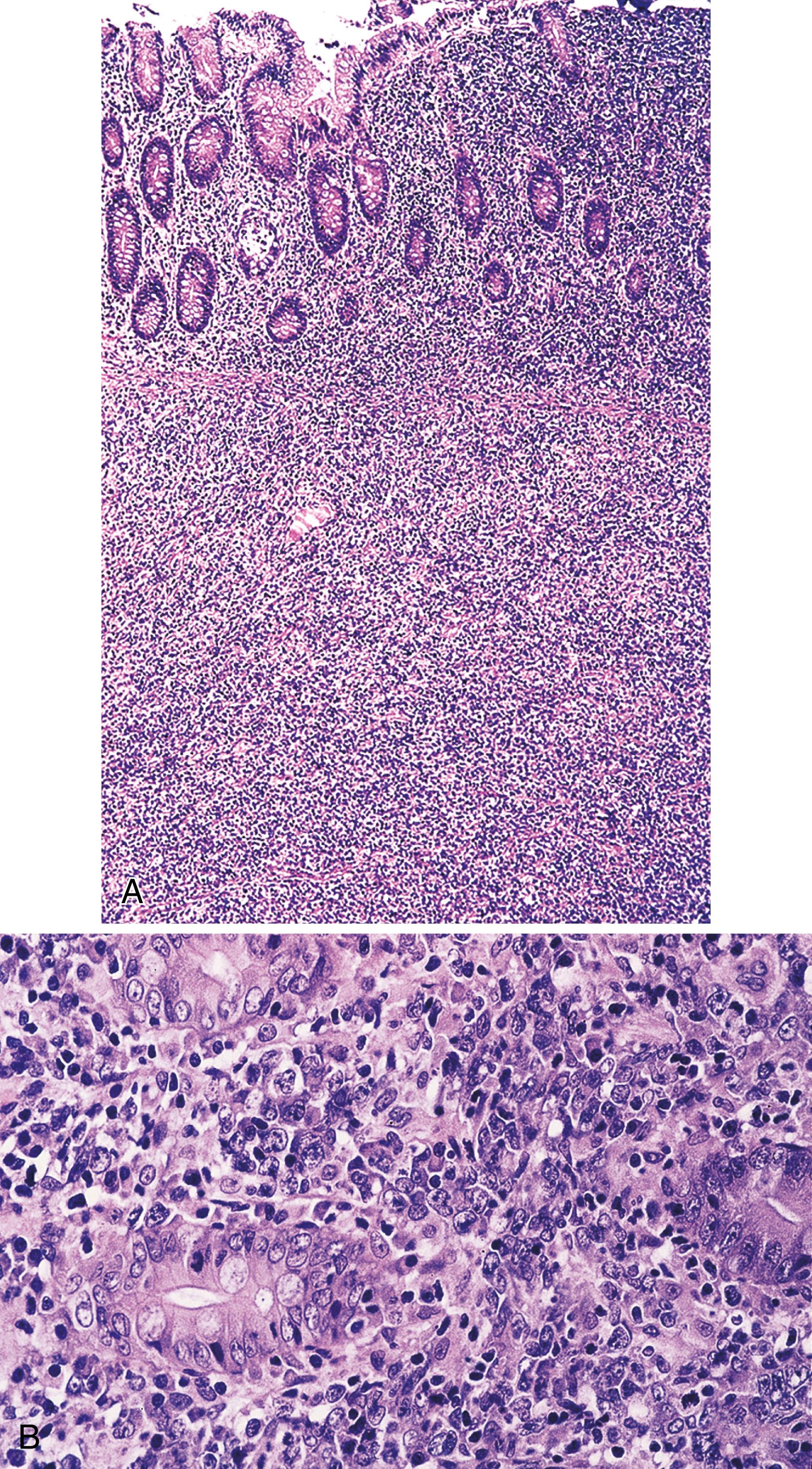
Stage A: Lymphoma is confined to the small intestinal mucosa and mesenteric lymph nodes. The infiltrate consists predominantly of plasma cells, with smaller numbers of marginal-zone cells. The B cells may be inconspicuous, and an immunostain for CD20 can help with recognition of B cells and also of lymphoepithelial lesions. There is variable villous atrophy ( Fig. 31.4 ).
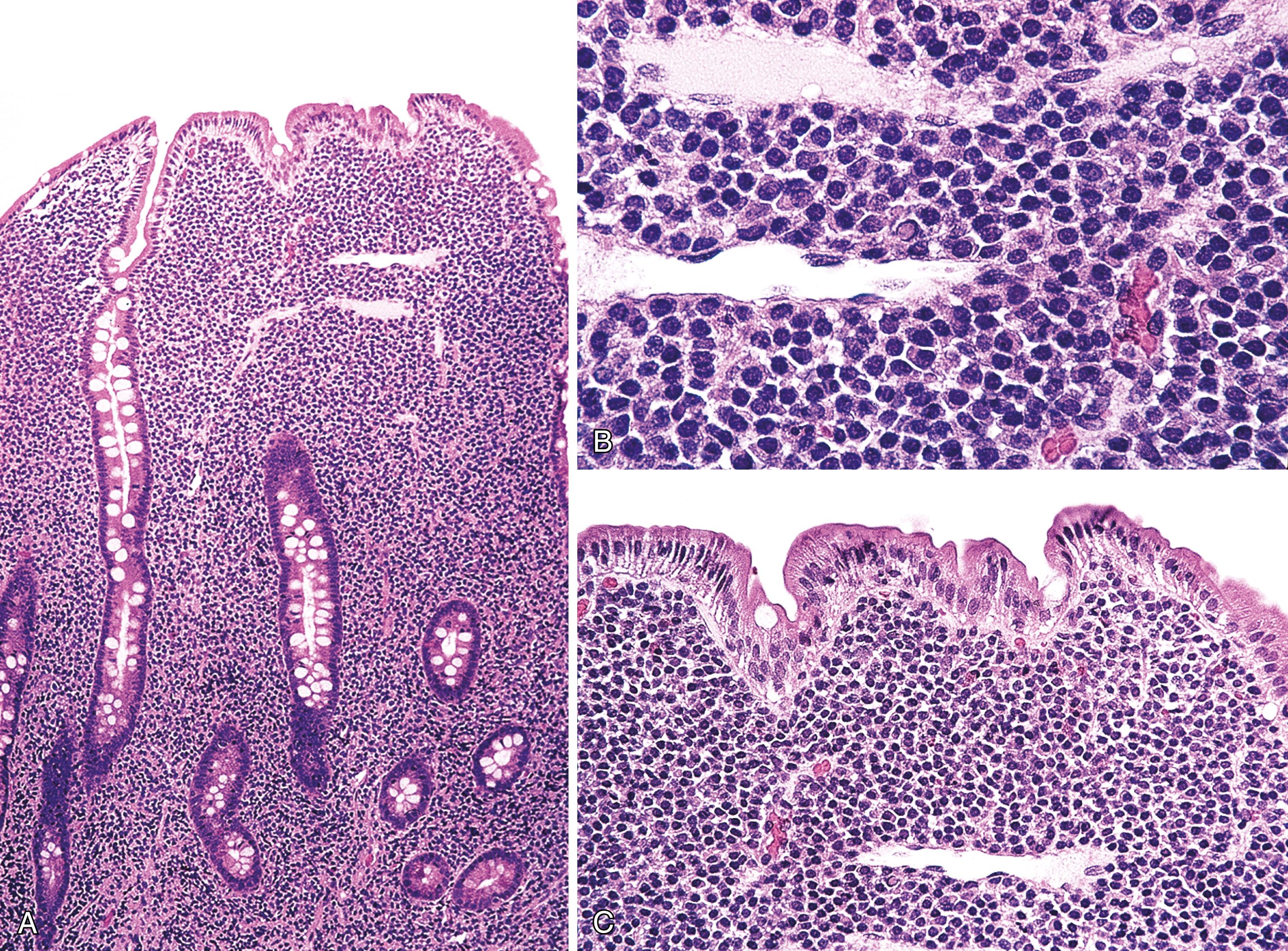
Stage B: In addition to the findings characteristic of stage A, the infiltrate has areas of nodularity that correspond to reactive lymphoid follicles colonized by neoplastic B cells. There are also occasional atypical, immunoblast-like cells. The infiltrate invades beyond the muscularis mucosae.
Stage C: Stage C is characterized by high-grade B-cell lymphoma, typically diffuse large B-cell lymphoma, with formation of one or more large masses. The lymphoma is composed of large cells, sometimes with the appearance of immunoblasts or plasmacytoid immunoblasts. In other cases, the neoplastic cells are pleomorphic and bizarre. The high-grade lymphoma is almost always in the bowel, but in one case, the high-grade lymphoma presented with tonsillar involvement.
Immunohistochemical analysis usually shows expression of α heavy chain without light chain, correlating with the serum paraprotein. In a minority of cases, monotypic light chain is expressed. Molecular genetic analysis has shown clonal rearrangement of Ig heavy and light chains, even in early cases responsive to antibiotic therapy. Previously, early phases of IPSID were thought to be inflammatory, but this information indicates that the infiltrate is neoplastic despite response to antibiotics. ,
IPSID and celiac sprue are both characterized by lymphoplasmacytic infiltrates and villous atrophy. Patient demographic data should provide a strong clue to the diagnosis, because patients with celiac disease are predominantly of northwestern European descent and they improve on a gluten-free diet, in contrast to IPSID patients. Histological features in favor of a diagnosis of celiac disease include total villous atrophy (in contrast to the villous broadening seen in early-stage IPSID), hyperplastic, elongated crypts, intraepithelial lymphocytosis, and surface epithelial damage. High-grade lymphomas may be found in association with either, but the lymphoma that complicates celiac disease is a T-cell lymphoma prone to cause multifocal perforation.
It may be difficult to distinguish nonspecific chronic inflammation from early-stage IPSID, particularly on a small biopsy sample. In favor of a diagnosis of IPSID is a dense, predominantly plasmacytic infiltrate distorting the mucosal architecture in a patient with clinical features compatible with IPSID. Immunohistochemical stains showing plasma cells expressing only α heavy chain help establish the diagnosis.
Gastric diffuse large B-cell lymphoma (DLBCL) is mainly a disease of older adults, with a median age in the seventh decade; younger adults are affected only occasionally. There is a slight male preponderance overall. , , , , Patients may have a palpable mass on physical examination. Some arise in association with marginal-zone lymphoma, consistent with large-cell transformation of the low-grade lymphoma, whereas other large B-cell lymphomas appear to arise de novo; the proportion of cases in the two categories varies among series. , , , , , Anemia is common, likely related to bleeding from the lesion. Constitutional symptoms are uncommon. Most patients have normal lactate dehydrogenase (LDH). ,
On gross examination, the lymphomas are usually single, but occasionally, one may encounter multiple large ulcerated or exophytic lesions. The DLBCLs are usually transmurally invasive and may invade adjacent viscera. , The corpus and antrum are affected more often than the fundus or cardia. Microscopic examination reveals a diffuse proliferation of large cells with round, oval, irregular, or lobated nuclei, distinct nucleoli, and a narrow but distinct rim of cytoplasm ( Fig. 31.5 ). Immunophenotyping reveals CD20+ B cells coexpressing CD43 in about half of cases. In some cases, expression of monotypic immunoglobulin is demonstrable. The large-cell lymphomas that arise from extranodal marginal-zone lymphoma are CD5−, CD10− (nearly all), BCL6+/−, MUM1−/+, BCL2−/+, with a high proliferation index. Gastric DLBCL includes both germinal center B-cell (GCB, CD10+ or CD10−, BCL6+, MUM1−), and nongerminal center B-cell (non-GCB, CD10−, BCL6+, MUM1+, or CD10−, BCL6−) types (see Table 31.2 ). A minority are MYC+. , , , ,
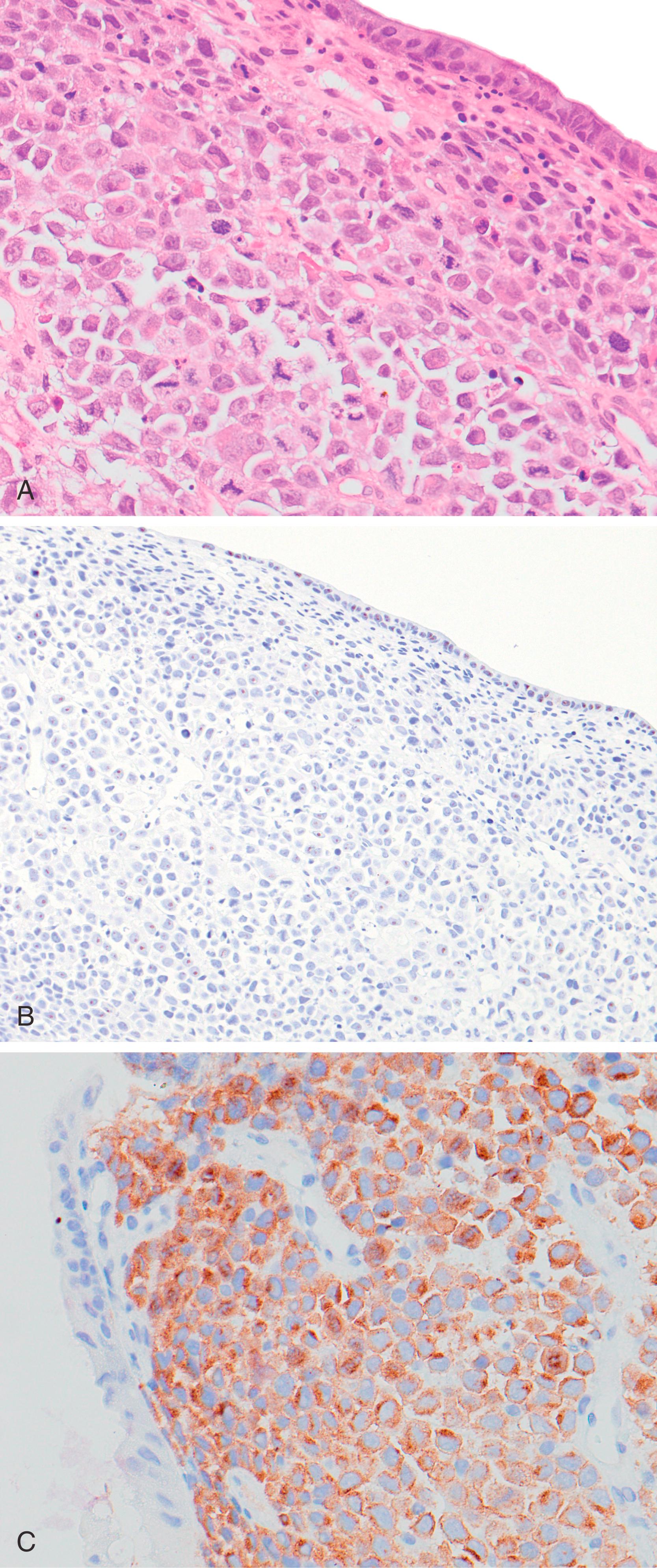
Most DLBCLs arising in the GI tract would be subclassified as DLBCL, not otherwise specified (NOS) according to the World Health Organization (WHO) Classification. The WHO has defined a number of other types of lymphomas of large B cells, some of which may involve the GI tract. One of these is EBV+ diffuse large B-cell lymphoma, NOS, which is defined as an EBV+ clonal B-cell lymphoproliferation occurring in patients with no known immunodeficiency or prior lymphoma. The pathogenesis is thought to be related to the deterioration of immunity that may occur with advancing age. This lymphoma can involve a variety of extranodal sites (as well as lymph nodes), but the GI tract is among the more common. The composition may be monomorphous, with a predominance of large, atypical lymphoid cells, which may have the appearance of immunoblasts or Reed-Sternberg–like cells and variants. In some cases, the composition may be more polymorphous, with B cells of a range of stages of maturation including large atypical cells and also reactive cells, including histiocytes, small lymphocytes, and plasma cells. Necrosis is common. Tumor cells usually express pan B-cell antigens (CD20, Pax5, and/or CD79a), often CD30, and occasionally express CD15. They have a non-GCB phenotype, with expression of MUM1. CD10 and frequently BCL6 are negative. By definition, EBV is present in tumor cells.
Anaplastic lymphoma kinase (ALK) + DLBCL is a rare type of large B-cell lymphoma ( Fig. 31.6 ); a case of this type of lymphoma arising in the stomach of a 21-year-old man has been reported. Other lymphomas of large B cells are plasmablastic lymphoma and HHV8+ large B-cell lymphoma. Both of these rare types of lymphoma may be encountered in the GI tract, but as they occur most often in HIV+ individuals, they are discussed below, under GI Lymphoproliferative Disorders in Abnormal Immune States.
Immunoglobulin heavy and light chains are clonally rearranged. Complex cytogenetic abnormalities are common. In contrast to nodal large B-cell lymphomas, BCL2 rearrangement is rare and MYC rearrangement is common. Abnormalities of the BCL6 gene are more common in gastric than nodal diffuse large B-cell lymphoma. BCL6 abnormalities include both translocation and somatic hypermutation. One study documented frequent (42% of cases) loss of heterozygosity (LOH) on chromosome 6q in sites of putative tumor suppressor genes, as well as a smaller number of cases with LOH of tumor suppressor genes, including TP53 and APC . Amplification of genomic material in the BCL6 locus, the KMT2A (formerly MLL/mixed-lineage leukemia) gene, and others were also found. Homozygous deletion of CDKN2A , encoding p16 Ink4A , has also been described in high-grade gastric lymphoma.
Activation of the NFκB pathway is important in the pathogenesis of lymphomas of a variety of types. Recently mutations in A20 , CARD11 , and ABIN-1 and ABIN-2 (A20 binding inhibitor of NFκB) likely to contribute to activation of the NFκB pathway have been documented in DLBCL of the GI tract. Mutations of A20 were associated more often with DLBCLs with the non-GCB phenotype (according to the Hans algorithm, earlier) and with significantly worse overall survival and event-free survival. A small minority (14% in one series ) harbor the MYD88 L265P mutation.
Unless a component of low-grade lymphoma is identified, it may not be possible to distinguish between de novo DLBCL and DLBCL arising from histological progression of marginal-zone lymphoma (or other low-grade lymphoma). Genetic and cytogenetic features may be helpful. Despite the high frequency of t(11;18) and trisomy 3 in gastric marginal-zone B-cell lymphoma, they are uncommon in gastric DLBCL, suggesting they are not important in transformation of marginal-zone lymphoma to large B-cell lymphoma. , One report suggests that trisomies (most often involving chromosomes 12 and 18) are more common in large-cell lymphomas that have arisen through transformation of marginal-zone lymphoma than in de novo large B-cell lymphomas.
A variety of mechanisms may lead to transformation of MALT lymphoma to DLBCL, including TP53 mutation, alterations in BCL6 , aberrant DNA hypermethylation, and others. , Translocations involving BCL6 and MYC may play a role in progression of gastric marginal-zone lymphoma to DLBCL, although these alterations may be involved in the pathogenesis of de novo DLBCL as well. Recently, overexpression of Myc was shown to alter microRNA signature in gastric lymphomas. In the setting of Myc-repressed miRNAs normally showing tumor suppressive activity, FoxP1 was overexpressed, theoretically promoting lymphomagenesis and large-cell transformation. Accordingly, MALT lymphomas are typically negative for Myc and FoxP1 by immunohistochemistry while DLBCLs are often positive.
In the majority of cases, patients present with stage I or II disease. , , , , Patients may be treated with surgery, radiation, chemotherapy, or a combination of these modalities. In a study of patients treated with R-CHOP +/− radiation or surgery, 5-year overall and event-free survival were 85.8% and 89.6%, respectively. Some studies have documented pathological features that have an impact on prognosis. In one study, large B-cell lymphoma associated with a component of marginal-zone lymphoma, or with lymphoepithelial lesions formed by small cells, had a 5-year cause-specific survival rate of 84%, compared with 64% for de novo large B-cell lymphoma. Lymphoepithelial lesions formed by large cells were not associated with a favorable prognosis. In one study using immunophenotyping to subclassify gastric diffuse large B-cell lymphoma as germinal center or non–germinal center type, patients with germinal center type had a significantly better outcome.
Stage is also prognostically important. Patients with stage Ie or IIe1 have a better outcome than those with IIe2 or higher. , Older age, male sex, Black race, high LDH, and ascites have been associated with a poor outcome. , Other factors reported to have an impact on prognosis are International Prognostic Index (IPI) score, B symptoms, and Eastern Cooperative Oncology Group (ECOG) performance score.
Poorly differentiated carcinoma may be composed of discohesive-appearing cells that form few or no glands, and thus may be difficult to distinguish from diffuse large B-cell lymphoma. Lymphoid cells may show artifactual vacuolar change and mimic signet ring cells, but mucin stains and immunohistochemical stains are helpful in distinguishing between lymphoma and carcinoma.
Diffuse large B-cell lymphoma (DLBCL) is the most common type of intestinal lymphoma, accounting for up to two-thirds of cases. Most patients with intestinal DLBCL are middle-aged to older adults. Few cases occur in younger adults or children. There is a slight male preponderance among adults, whereas affected children are almost exclusively male. DLBCL in children is virtually found only in the ileocecal area. , , , , In the majority of cases, the lymphoma is confined to the bowel except for regional lymph node involvement. , , In the remainder, disease is more widespread. , Anemia is common but constitutional symptoms are usually absent.
This type of lymphoma is relatively aggressive but potentially curable. , , , In one report, de novo DLBCL had a less favorable outcome than DLBCL that arose in association with marginal-zone lymphoma.
On gross examination, the tumors are similar to, or larger than, low-grade lymphomas. , They are elevated or infiltrative, ulcerated lesions that are usually transmurally invasive. A subset shows perforation. , Most are composed of centroblasts, often with an admixture of immunoblasts, or multilobated large lymphoid cells, whereas a minority are composed almost exclusively of immunoblasts ( Fig. 31.7 ). , A case of intravascular large B-cell lymphoma involving the colon and mimicking ulcerative colitis has been reported.
In some cases, a component of marginal-zone lymphoma is found, which is consistent with large-cell transformation of a low-grade lymphoma. The proportion of cases with an underlying low-grade lymphoma varies greatly in different series, from 10% to more than 50% of large B-cell lymphomas. , , , The immunophenotypic features overlap with those found in gastric DLBCL. De novo intestinal DLBCLs can have a germinal center or a non–germinal center B-cell immunophenotype. ,
Intestinal DLBCL is genetically heterogeneous. In one study of 14 cases, for example, 3 had a t(14;18) translocation involving genes IGH and BCL2 , 1 had a t(8;14) translocation involving IGH and MYC , 5 had a BCL6 rearrangement, and 1 had a t(11;18) translocation involving BIRC3 and MALT1 . Although the t(11;18) characteristic of a subset of marginal-zone lymphomas is generally considered to prevent histological progression to DLBCL, rare cases of intestinal DLBCL harbor t(11;18), which suggests large-cell transformation of an underlying marginal-zone lymphoma with this translocation may occur (see Table 31.2 ). , The MYD88 L265P mutation, which is common in DLBCLs arising in certain other extranodal sites, is rare in the GI tract.
The differential diagnosis for intestinal DLBCL is similar to that for gastric DLBCL. In addition, floridly reactive lymphoid tissue in the intestine occasionally raises the question of lymphoma. The bowel normally harbors hyperplastic lymphoid tissue in the terminal ileum (Peyer’s patches), but hyperplastic lymphoid tissue in other portions of the intestine, when prominent, may suggest lymphoma. This problem may arise with lymphoid polyps, polypoid lesions composed of reactive lymphoid tissue that may be found in the colon. Large collections of hyperplastic lymphoid tissue may develop in the rectum; these have been referred to as rectal tonsils , which are large, discrete nodules of organized lymphoid tissue with reactive follicles, often showing florid hyperplasia, located in the lamina propria or submucosa. Rectal tonsils may be accompanied by overlying cryptitis, mild architectural distortion, intraepithelial lymphocytes, and lymphoepithelial lesions, typically without crypt obliteration. In lymphoid follicular proctitis, a condition characterized by congested, nodular mucosa and often associated with rectal bleeding, reactive lymphoid tissue, including lymphoid follicles, is found in the distal portion of the GI tract. Familiarity with these entities and appreciation of the follicular architecture, with enlarged B cells mostly confined to follicles, can avoid overdiagnosis as lymphoma.
Mantle cell lymphoma affects middle-aged or older adults with a male preponderance. Almost all patients are 50 years of age or older. , , , , Mantle cell lymphoma usually presents with widespread disease with involvement of lymph nodes and a variety of extranodal sites; GI involvement is common. Patients may present initially with GI tract involvement. Any portion of the GI tract may be affected, , and frequently the lymphoma affects multiple sites (stomach, small intestine, colon, and, on occasion, the esophagus). , GI tract involvement can result in abdominal pain, diarrhea, bloody stool, and weight loss. Mantle cell lymphoma often takes the form of innumerable polyps and then is termed multiple lymphomatous polyposis ; this pattern is particularly prevalent in the intestines. The ileocecal region tends to contain the largest polyps. , In some cases, particularly when mantle cell lymphoma involves the stomach, it takes the form of a smaller number of protruded, ulcerated or fold-thickening lesions. Mesenteric lymph nodes are usually involved by lymphoma. Staging frequently reveals widespread disease away from the GI tract. Although there is usually a good response to chemotherapy, relapses are common and survival is usually only 3 to 5 years. Predisposing factors for the development of mantle cell lymphoma are unknown.
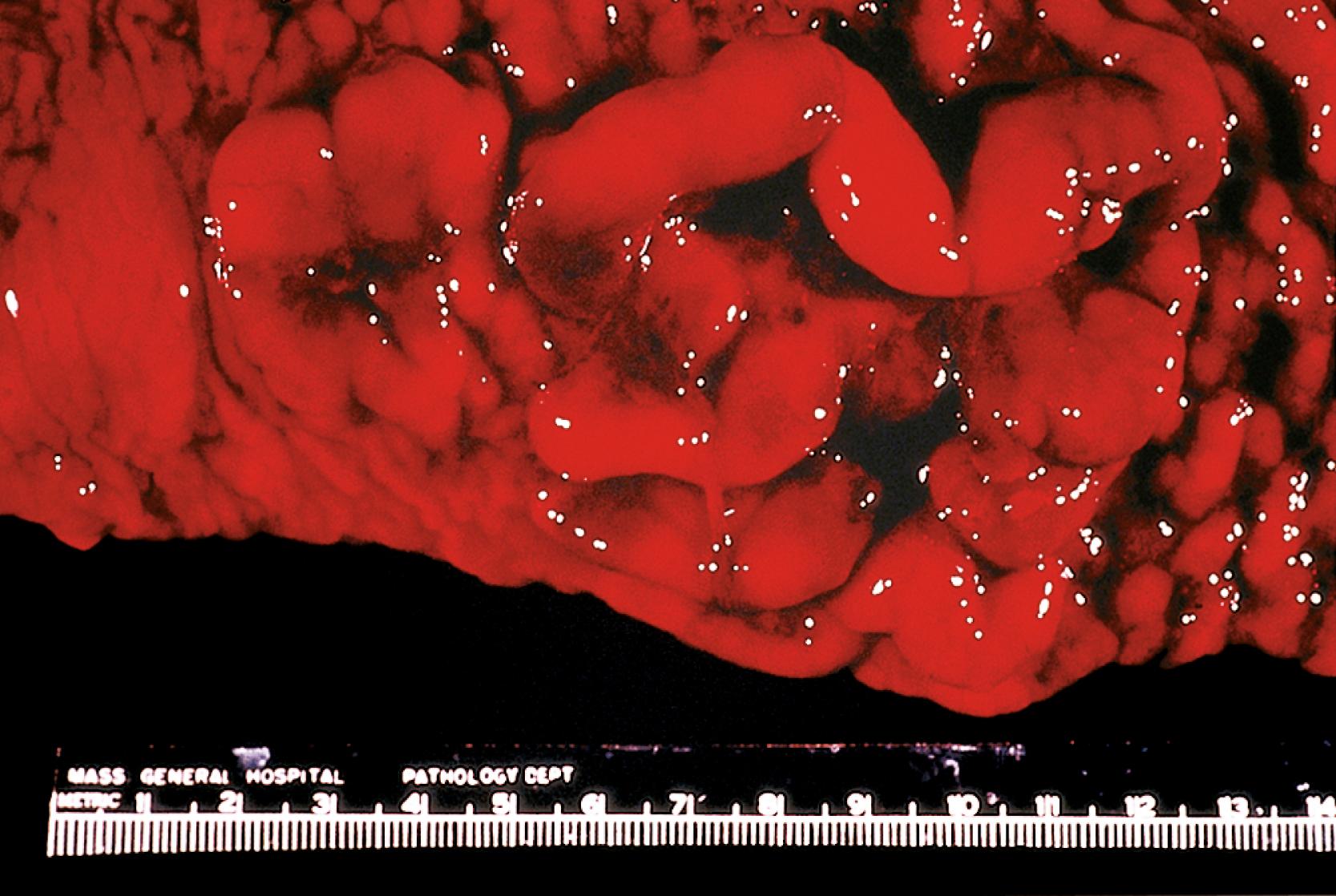
On gross examination, multiple lymphomatous polyposis has the appearance of multiple fleshy white nodules, 0.5 to 2 cm in greatest dimension, involving the mucosa, but sometimes with superficial submucosal involvement ( Fig. 31.8 ). Less commonly, mantle cell lymphoma takes the form of a discrete mass or an ulcerated lesion.
Microscopic examination shows a bandlike infiltrate, or multiple ill-defined nodules, of atypical, monotonous lymphoid cells that are slightly larger and more irregular than normal lymphocytes, with scant cytoplasm and without conspicuous nucleoli. Single epithelioid histiocytes may be scattered among the neoplastic cells. Remnants of reactive follicle centers may be identified in some nodules. The lymphoma tends to displace and obliterate intestinal glands, but formation of true lymphoepithelial lesions is not a feature ( Fig. 31.9 ). , ,

A minority of cases of mantle cell lymphoma are characterized by histological features suggesting more aggressive behavior; these “aggressive variants” include blastoid and pleomorphic variants. In the blastoid variant, neoplastic cells have finely dispersed chromatin, and the mitotic rate is high (usually >20 mitoses/10 high-power fields [hpf]) so that the appearance is reminiscent of lymphoblastic lymphoma. In the pleomorphic variant, neoplastic cells are larger and more pleomorphic than in the usual mantle cell lymphoma, and in contrast to typical mantle cell lymphoma, nucleoli are often prominent in at least some of the neoplastic cells. The appearance may mimic diffuse large B-cell lymphoma. Occasional cases of mantle cell lymphoma are composed of small, uniform, dark cells (small cell variant), or have a moderate amount of pale cytoplasm (marginal-zone–like variant); these may mimic small lymphocytic lymphoma/chronic lymphocytic leukemia or extranodal marginal-zone lymphoma, respectively.
Immunophenotyping typically shows CD20+, CD5+, CD43+, CD10−, CD23−, cyclin D1+ B cells expressing monotypic immunoglobulin, IgMD type, with λ being more frequent than the κ light chain. With antibodies to follicular dendritic cells such as CD21, a loose, expanded dendritic network is seen. This lymphoma is associated with t(11;14), a translocation that involves CCND1 and IGH (see Table 31.2 ). Rare cases of mantle cell lymphoma are negative for cyclin D1. Nuclear expression of SOX11 has been demonstrated in >90% of cases of mantle cell lymphoma ; expression of SOX11 can be helpful in identifying the rare cyclin D1− cases. Of note, however, nonnodal mantle cell lymphoma, which often behaves in a more indolent manner than typical mantle cell lymphoma involving lymph nodes +/− extranodal sites, is often negative for SOX11. Expression of α4β7, the mucosal homing receptor, by mantle cell lymphoma is highly associated with GI involvement. , ,
On occasion, other types of lymphoma have the endoscopic or gross appearance of multiple lymphomatous polyposis. In addition, as noted earlier, not all mantle cell lymphomas take the form of lymphomatous polyposis. Follicular lymphoma, , , extranodal marginal-zone lymphoma, and even T-cell lymphomas can have the appearance of lymphomatous polyposis. Mantle cell lymphoma without the classic polyposis appearance may be mistaken for other small B-cell lymphomas, particularly if the cytomorphology of the neoplastic cells is that of the small cell or marginal-zone–like variant. Small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL), another CD5+ B-cell neoplasm, can secondarily involve the GI tract; the case of a patient with severe diarrhea due to SLL/CLL infiltrating the bowel has been reported. SLL/CLL and marginal-zone lymphoma are negative for cyclin D1 and SOX11, in contrast to mantle cell lymphoma. A diagnosis can be readily established in most cases with careful study of hematoxylin and eosin (H&E)-stained slides augmented by immunohistochemistry. The distinction is important, because mantle cell lymphomas have a poor outlook compared with other low-grade B-cell lymphomas.
In cases of the blastoid variant of mantle cell lymphoma, occurrence in an older adult is a clue that lymphoblastic lymphoma is unlikely, and the immunophenotype (CD20+, CD5+, cyclin D1+, monotypic surface immunoglobulin+) confirms a diagnosis of mantle cell lymphoma and excludes lymphoblastic lymphoma. In cases of the pleomorphic variant, even though there are many large pleomorphic neoplastic cells, there is often an admixture of medium-sized cells more closely resembling those more typically seen in mantle cell lymphoma. Cyclin D1 expression by neoplastic cells confirms a diagnosis of mantle cell lymphoma.
Follicular lymphoma of the GI tract is rare, accounting for less than 4% of all primary GI lymphomas. The 2017 WHO Classification recognizes a distinct variant of follicular lymphoma arising in the GI tract, designated “duodenal-type follicular lymphoma.” Affected patients are mostly middle-aged adults with a mean or median age in the 50s. There is a slight to moderate female preponderance in some series , , while others report men and women equally affected. , Precise evaluation of distribution of disease is evolving with the availability of techniques allowing more complete evaluation of the small bowel, including double balloon enteroscopy and capsule endoscopy. The duodenum is the site most often involved, most commonly the second portion. , , , Duodenal involvement is frequently accompanied by jejunal or ileal involvement. , The stomach and colorectum are affected less often. Only 3% of gastric B-cell lymphomas were follicular lymphomas in one large series. Esophageal involvement is rare. , Patients present with a variety of symptoms, but abdominal pain is the most common. Others have mild or vague GI symptoms. A few have diarrhea, nausea, vomiting, or bleeding. Bleeding is more common with colorectal lesions. In centers with active screening for GI cancers, it is common for the lymphoma to be an incidental finding. , , , Elevated LDH is distinctly unusual, and the Follicular Lymphoma International Prognostic Index (FLIPI) is typically low.
On endoscopy, nodularity of the mucosa is most common, sometimes with the picture of multiple lymphomatous polyposis, which may involve part or all of the GI tract. , , The nodules are typically 1 to 2 mm, white or yellow-white, and scattered or confluent. The presence of multiple small nodules is more common with follicular lymphomas that involve the duodenal second portion. GI follicular lymphoma may also take the form of a large, discrete, polypoid or ulcerated mass, , although such cases may not represent “duodenal-type” follicular lymphoma. The finding of single lesions, or of small numbers of lesions, may be more common in the stomach and colorectum. The lymphomas may be confined to the bowel wall or show regional nodal involvement. More distant spread suggests origin outside the GI tract.
The lymphomas consist of follicles, usually lacking mantles, that are composed of a monotonous population of centrocytes with few to scattered centroblasts distorting the normal glandular architecture. Very often there are increased numbers of lymphoid cells outside follicles, including the stroma of small intestinal villi. Lymphoepithelial lesions are not characteristic. The vast majority are low-grade (grade 1 to 2 of 3), with only rare grade 3 follicular lymphomas. ,
GI follicular lymphomas typically have an immunophenotype similar to that found in nodal follicular lymphomas (CD20+, CD10+, BCL2+, BCL6+) ( Fig. 31.10 ). , , , , Often some of the lymphoid cells outside follicles are B cells with a similar immunophenotype, although CD10 and BCL6 may be more dimly expressed. Proliferation index with Ki67 is low. Follicular dendritic meshworks are hollow rather than intact.

Molecular genetic and cytogenetic studies show clonally rearranged Ig heavy- and light-chain genes and BCL2 rearrangement, , although rare cases negative for BCL2 protein and for BCL2 rearrangement have been reported. The pathological features of GI follicular lymphoma are thus similar to lymph nodal follicular lymphoma. In contrast to follicular lymphoma arising in lymph nodes, however, expression of α4β7 integrin, a mucosal homing receptor, by GI follicular lymphomas is described. Investigators have noted ongoing somatic hypermutation in the absence of activation-induced cytidine deaminase expression and restricted usage of variable regions of the immunoglobulin heavy chain gene, which together with the hollow dendritic meshworks, are features reminiscent of extranodal marginal-zone lymphoma that suggest response to antigen is important in the pathogenesis of these lymphomas. Gene expression profiling studies have shown that duodenal-type follicular lymphoma has features more in common with gastric marginal-zone lymphoma than with nodal follicular lymphoma.
Treatment has varied widely, and the best therapy has yet to be determined. Watchful waiting may be appropriate in many cases. Patients generally do well, although some have persistent lymphoma, and some experience relapse. Relapses may involve the GI tract, lymph nodes, or other sites. Rare instances of response to antibiotics are reported, although in general antibiotics are ineffective. Some authorities recommend a “watch and wait” approach, with careful monitoring of disease for asymptomatic patients with low tumor burden. A superior progression-free survival has been associated with female gender, lack of symptoms at diagnosis, and involvement of the second portion of the duodenum. Histological transformation to diffuse large B-cell lymphoma has been described, but is uncommon. In one case the patient progressed on watch-and-wait surveillance from follicular lymphoma, grade 1 to follicular lymphoma, grade 3A at 5.5 years, to diffuse large B-cell lymphoma at 6 years of follow-up. Death due to lymphoma is very uncommon. , , ,
The differential diagnosis of duodenal-type follicular lymphoma includes follicular lymphoma arising in the GI tract that is not of duodenal type, and secondary involvement of the GI tract by follicular lymphoma arising in lymph nodes. Staging studies are helpful in excluding a primary site outside the GI tract. Follicular lymphomas that are not of duodenal type are more likely to show transmural invasion of the bowel wall, to be less consistently low grade, to be more likely to have intact (rather than hollow) dendritic meshworks, and to behave in a less indolent manner. ,
Burkitt’s lymphoma is a highly aggressive, rapidly growing B-cell lymphoma with distinctive pathological features, including the presence of a translocation involving MYC . In the WHO classification of tumors of the hematopoietic and lymphoid tissues, three clinical variants of Burkitt’s lymphoma are described: endemic, sporadic, and immunodeficiency associated. Endemic Burkitt’s lymphoma occurs mainly in young children in sub-Saharan Africa. A complex interplay of environmental and host-related factors—climate, malaria, EBV, age and developmental stage of the patient—appear to play a role in the development of this subset of Burkitt’s lymphoma. Most patients with immunodeficiency-associated Burkitt’s lymphoma are HIV+, and a few have an underlying iatrogenic or congenital immunodeficiency. Patients with sporadic Burkitt’s lymphoma are those who are neither immunodeficient nor fit the epidemiology for endemic Burkitt’s lymphoma. Sporadic Burkitt’s lymphoma is rare, although among children who develop lymphoma, it accounts for 30% to 50% of all cases of lymphoma.
Burkitt’s lymphoma accounts for about 5% of all intestinal lymphomas. Involvement of the ileocecal region is the most common manifestation of sporadic Burkitt’s lymphoma. Ileocecal disease is occasionally seen with endemic and immunodeficiency-associated Burkitt’s lymphoma, although presentation with disease outside of the GI tract is more common. Infrequently, sites in the GI tract other than the ileocecal area, including the stomach and more distal portions of the colon, are involved. , , Only 1% of gastric B-cell lymphomas were Burkitt’s lymphomas in one large series. GI Burkitt’s lymphoma affects children and young adults, with a marked male preponderance. , In some cases, staging reveals disease beyond the GI tract. Immunocompetent patients with Burkitt’s lymphoma who are treated with aggressive, high-intensity, short-duration chemotherapy have an excellent prognosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here