Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The gastrointestinal (GI) tract has evolved specific mechanisms to allow ingestion of nutrients, their transport for digestion and absorption, and finally the expulsion of unused portions. This aboral propulsion of gastrointestinal contents is orchestrated by the complex interaction between the gastrointestinal muscle and the enteric, peripheral, and central nervous systems and is discussed elsewhere in the text. Each area of the gastrointestinal tract has a specific motility pattern that allows it to perform its necessary function. In children, these patterns have been well characterized for the esophagus, gastric antrum, duodenum, jejunum, ileum, colon, and anorectum.
Gastrointestinal transit can be evaluated by many different techniques (markers, radionuclide testing, etc.). However, the evaluation of gastrointestinal motility can only be performed with the use of manometric studies, which are designed to show the contractile events of the organ that is being studied. This allows the understanding of gastrointestinal physiology and the pathophysiology of motility disorders. The main focus of this chapter is on those manometry tests that have been designed to study gastrointestinal motility. ,
Gastrointestinal motility is assessed by identifying and characterizing intraluminal pressure changes detected by the introduction of specially designed catheters in the lumen of the organ that is being studied. The intraluminal pressure is then transmitted and recorded. In general, intraluminal pressure can be evaluated with water-perfused or solid-state systems. Recent years have seen a shift to most studies being performed using high resolution manometry (HRM) with the use of solid-state transducers, although it is possible to use water-perfused systems, particularly for antroduodenal and colonic manometry.
When perfused manometry is used, the catheter is connected indirectly to a computer by a series of transducers. The catheters have predetermined openings that allow the recording of different segments of the area being studied. The catheters are perfused with a pneumohydraulic pump at a predetermined rate, and pressure changes in the lumens are transmitted to pressure transducers with the use of low-compliance capillary tubing. On the other hand, solid-state catheters have strain-gauge pressure transducers; the information is captured by digital recording systems and is downloaded into computers for analysis. The main advantage of the solid-state catheter is that it does not involve perfusion and allows the performance of prolonged ambulatory studies.
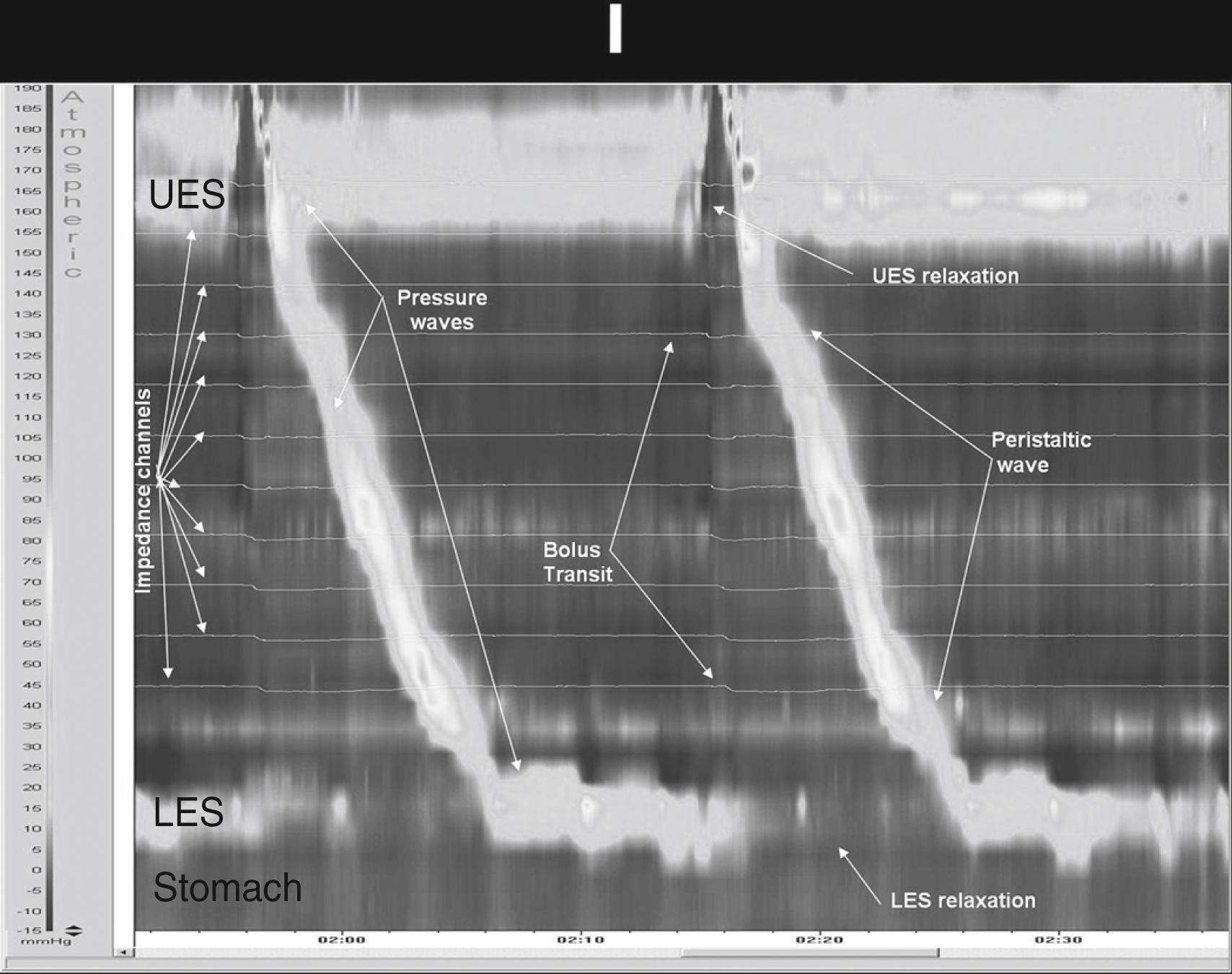
A major technical advance in recent years was the advent of HRM ( Fig. 64.1 ). This is achieved by increasing the number of recording sites and decreasing the spacing between sites, which allows a better definition of the intraluminal pressure environment without spatial gaps and with minimal movement-related artifacts. , The software to analyze the data has also been greatly improved, making it appear as a space-time continuum that can be displayed as isobaric contour plots. The advantages of isobaric contour plots are multiple, but the most evident is that they provide a seamless, dynamic representation of peristalsis at every axial position within and across the organ being studied. ,
The number of pressure ports in HRM varies. It is usually 36 in esophageal, colonic, and antroduodenal manometry (ADM) (but depending on the catheter it may be 24) but is less in anorectal manometry. HRM can be performed with both solid-state and perfused catheters, although most laboratories use solid-state catheters. Advances in the technology have also allowed the building of catheters as small as 6Fr, providing the opportunity to do HRM in small infants.
HRM has largely replaced the traditional line studies previously used for esophageal and anorectal manometry and is replacing the standard manometry in antroduodenal and colonic studies. HRM has an improved sensitivity, has been found to be more reproducible, and has already been shown to change management, particularly in esophageal studies. In children the main impact of the technology has been in the simplification of the performance of the test, as there is no need for a pull-through maneuver. In our experience the tolerance of the procedure has also increased dramatically.
There are important differences in the performance of manometric studies in children when compared to adults. These include the different ages of the studied patients, as there may be developmental changes; a paucity of studies in normal controls; and technical difficulties inherent to studying children. , , The lack of normal controls has been the most important limiting factor for the establishment of normal motility patterns in children, although in recent years more studies performed in children without a motility disorder have been reported. This lack of control information can make the interpretation difficult and may potentially lead to overinterpretation of the findings. Performing studies in children requires a certain level of cooperation, because motility patterns are difficult to interpret when there is significant motion artifact. Young and uncooperative patients may require sedation ; there are studies that report no effect of midazolam on esophageal motility and others that show that chloral hydrate, midazolam, and certain agents used for general anesthesia have no major effect on the rectoanal inhibitory reflex (RAIR).
Recently, a pediatric task force and a group of experts from the American Neurogastroenterology and Motility Society (ANMS) updated the guidelines for the performance of manometric studies in children. , , , ,
Esophageal manometry is the gold standard for the diagnosis of primary motor disorders of the esophagus. , , , The most common indication for esophageal manometry in children is dysphagia without evidence of anatomic obstruction. , ,
There are three functional regions of the esophagus: the upper esophageal sphincter (UES), the esophageal body, and the lower esophageal sphincter (LES). The UES is composed of striated muscle that relaxes in response to swallows and remains closed in between swallows by tonic stimulation of somatic nerves. It is asymmetric, with greater resting pressure values in the anteroposterior axis. The UES measures 0.5 to 1 cm at birth and increases in length to 3 cm in the adult. UES resting pressure in adults varies from 40 to 193 mm Hg, and we have reported a mean UES pressure of 116 ± 9.6 mm Hg in healthy children.
The esophageal body is composed of both striated (in the upper third) and smooth muscle. The resting pressure is usually lower than intragastric pressure and varies with respiration. Primary peristalsis occurs after swallowing, , normally progresses at a speed of 2 to 4 cm/s, and has a typical duration of 4 seconds (see Fig. 64.1 ). The typical contraction pressures of peristaltic waves are between 35 and 180 mm Hg. Limited information is available in healthy children; in our studies, the mean contraction amplitude in the upper esophagus was 60.7 ± 9.5 mm Hg and in the lower esophagus 94 ± 3.3 mm Hg, with a mean duration of contractions of 3.5 ± 0.1 seconds. Peristaltic characteristics should be evaluated during wet swallows. Secondary peristalsis occurs in response to luminal distension, and tertiary contractions consist of spontaneous and usually simultaneous nonperistaltic contractions.
The LES is tonically contracted and its normal resting pressure varies based on series. Some authors have reported a value of 22 ± 5 mm Hg ; others have reported 15 ± 2 mm Hg and 29 ± 2 mm Hg ; and we have reported a mean LES pressure of 24 ± 2 mm Hg. In adults, LES pressure varies from 10 to 45 mm Hg. The measurement of LES pressure is always performed relative to intragastric pressure.
LES relaxation usually occurs with swallowing, but it has also been reported to occur transiently and not associated with swallowing, which is known as transient lower esophageal sphincter relaxations (TLESRs). TLESRs are thought to be the main pathophysiologic mechanism in the development of gastroesophageal reflux disease (GERD). , Before the advent of HRM the most accurate way to measure LES pressure and function was with the use of a sleeve, which straddles the LES. However, HRM has become the gold standard (see Fig. 64.1 ). , , The presence of numerous pressure ports allows the accurate measurement of the LES pressure while also easily detecting movement, which can produce artifacts. With the use of HRM, new parameters to measure LES function have been designated. The Integrated Relaxation Pressure (IRP) is being proposed as a new parameter. , , The IRP applies an electronic-sleeve measurement within a 10-second time box after UES relaxation, and then finds the 4 seconds during which the e-sleeve value was least. , , The IRP is the mean pressure during those 4 seconds and is influenced not only by LES relaxation but also by crural diaphragm contraction and intra-bolus pressure, i.e. outflow obstruction in the postdeglutitive period. , To date, it has not been validated in children, but it is widely used.
HRM has also shown in both children and adults that esophageal peristalsis is comprised of a specific chain of sequential pressure segments. , , , , These segments appear as concentrated loci separated from each other by lower amplitude pressure troughs. The upper one represents the striated muscle, and the other 2 smooth muscle. , HRM predicts the presence of abnormal bolus transport more accurately than conventional manometry, and it identifies clinically important motor dysfunction not previously detected by either manometry or radiography. ,
Medications known to affect motility (e.g., prokinetics, anticholinergics, narcotics) are held for at least 48 hours before the procedure, and children fast for 4 to 6 hours, depending on their age and the need for sedation.
The manometry catheter is usually placed nasally, although it can be placed orally as well, particularly in premature infants. In older children, nasal topical anesthesia with topical cocaine or viscous lidocaine is frequently used.
There is no standardized protocol to perform esophageal manometry in children. The ANMS-NASPGHAN guidelines recommend the following.
Guidelines for NPO status vary depending on whether sedation is used for the placement of the esophageal catheter. The length of NPO may vary depending on the sedation (or lack thereof) used and the suspected underlying problem. In patients at higher risk for aspiration (e.g., gastroparesis, EGJ obstruction), NPO times may need to be longer.
The HRM catheter is introduced nasally and advanced into the stomach, which is identified by specific landmarks (see below). Occasionally fluoroscopy guidance (or even rarer, endoscopic guidance) is needed for patients with complex anatomy. In very small babies, or patients with certain craniofacial malformations, the catheter may be introduced orally. A topical anesthetic (1 to 2 mL of viscous lidocaine, 2% lidocaine jelly, or 4% cocaine) may be applied into the nares and may obviate the need for sedation.
If needed, however, oral, intranasal, or IV sedation can be used. Because the test requires some degree of cooperation and repeated crying and swallowing may make interpretation difficult, sedation choices in pediatrics are important. Care should be taken to avoid deep sedation, as oral intake is critical to assess for intact swallowing and peristalsis. If necessary, oral midazolam has been shown to be an effective anxiolytic that does not change esophageal function. However, in patients with esophageal obstruction, oral sedation may be ineffective as the medication may not reach the stomach for absorption. In those patients, intranasal or IV midazolam can be used. If the patient is still unable to tolerate catheter placement, placement of the catheter using ketamine, pentobarbital, propofol, or general anesthesia can be considered but the study cannot be started until the patient is fully awake (typically 2 to 4 hours after placement) and the medication effects on motility have worn off. With placement under anesthesia, patients with significant dysmotility and/or obstruction will need airway protection with an endotracheal tube to avoid aspiration of retained food or liquids in the esophagus. Care should be taken not to give opiates during the anesthesia, as they can affect motility.
Pediatric esophageal motility testing is usually performed in the semi-upright position typically at angles of 45 to 90 degrees. , A minimum of 10 liquid swallows are observed. Whenever possible, additional viscous and solid food swallows are also performed, because subtle motility abnormalities can be uncovered with different textures. If high-resolution esophageal manometry is being paired with impedance, all liquids and solids need to have ions to detect impedance changes, so added salt or salt water may be required.
To assess swallow function, the patient is given the liquid/viscous/solid swallows, spaced by a minimum of 30 seconds to allow for esophageal recovery and avoid deglutitive inhibition. This can be challenging in young or uncooperative patients and care needs to be taken to ensure abnormal motility patterns are not diagnosed when closely repeated swallows are present. For each swallow, the patient is given 5 mL of liquid with some modification for infants or aspirating patients. Because of high rate of false positive testing when only dry swallows are used, liquid swallows should be attempted with every manometry, even in patients with aspiration or those with food aversion.
At the end of the single swallows, deglutitive inhibition is measured with the use of repetitive swallows. Finally, when rumination is being considered, patients should also consume a symptom-evocative meal after which the patient is observed for 30 minutes to an hour for the development of symptoms as 40% of rumination episodes are only seen after a meal. There is no routine indication for provocative medications during esophageal manometry in children. a
a References 1, 13, 17, 21, 28, 29.
As indicated above, HRM has shown that there are distinctive pressure segments in the esophagus and that esophageal peristalsis is composed of a specific chain of sequential pressure segments. , , , In children Staiano et al. have also found three distinctive segments of peristalsis, similar to those found in adult studies. , ,
LES pressure varies from 10 to 45 mm Hg in adults. LES relaxation needs to be coordinated. Normal peristalsis is considered present when at least 70% of wet swallows are normal. Based on simultaneous manometric, videofluoroscopic, and impedance studies, an esophageal contraction less than 30 mm Hg in amplitude is now considered hypotensive and is used to distinguish effective from ineffective peristalsis , , ; any contraction greater than 180 mm Hg in amplitude is considered hypertensive. ,
The method of interpretation of esophageal manometry has changed with the advent of HRM. Three functional regions of the esophagus are evaluated: the UES, the esophageal body, and the LES. New parameters to define motor abnormalities using HRM have resulted in the Chicago Classification (CC), which consists of a hierarchical approach to HRM interpretation, focusing first on disorders of the esophagogastric junction (EGJ) outflow, then the diagnosis of major and minor peristaltic disorders.
While some of the key HRM measurements have changed over time, the integrated relaxation pressure (IRP; defined as the mean pressure during the 4 seconds of maximal deglutitive relaxation in the 10-second window beginning at UES relaxation), the distal contractile interval (DCI: a composite measurement quantifying the contractile pressures exceeding 20 mm Hg), and the distal latency (DL: interval between UES relaxation and the contraction deceleration point) have endured. The IRP, a more complex version of the residual LES pressures after swallowing measured using standard manometry, is used in pediatrics, but the normal range of IRPs may vary based on age and size, so the adult standards for elevations in the IRP (>15 mm Hg) may be too high. Similarly, small pediatric studies have shown that there may be age and height differences in DCI, distal latencies, and esophageal break size though further studies are needed to determine the clinical significance of some of these findings. , Because of the differences in pediatric measurements, the use of CC diagnostic criteria should be used with caution to avoid incorrect diagnoses. In the only pediatric study available, 66% of children undergoing HRM had a definable motility disorder using the adult CC diagnostic criteria. When measurements were adjusted for age and size, the percentage of patients with a definable motility disorder dropped to 50% and 53%, respectively. The largest reduction was in the reclassification of IRP- and DL-dependent disorders, EGJ outflow obstruction, and diffuse esophageal spasm (13% to 7% and 5% and 14% to 1% and 5%, respectively). This potential for incorrect diagnosis when applying the adult CC criteria to pediatric patients might strongly influence treatment choice. Studies have also shown that the interpretation of HRM is reproducible, but there are still some specific areas that may need more validation.
Esophageal manometry is indicated to assess esophageal function in children and adolescents with dysphagia, odynophagia, and chest pain of noncardiac origin. , Esophageal motility is the gold standard for diagnosing primary motility disorders and there is a new classification of esophageal motor disorders proposed with the use of HRM ( Table 64.1 ), known as the CC. , The CC system is hierarchical. EGJ dysfunction needs to be excluded first. , The system also tries to highlight dysmotility and dysfunction that are never seen in normal individuals and require treatment, in distinction from findings that are merely outside the normal range, in which case symptoms are likely to be associated with both esophageal motor dysfunction and visceral hyperalgesia or hypervigilance.
| Achalasia | |
| Achalasia type I | Classic achalasia: mean IRP > upper limit of normal (usually 15 mm Hg), 100% failed peristalsis |
| Achalasia type II | Achalasia with esophageal compression: mean IRP > upper limit of normal, no normal peristalsis, pan-esophageal pressurization ≥20% of swallows |
| Achalasia type III | Mean IRP > upper limit of normal, no normal peristalsis, preserved fragments of distal peristalsis or premature (spastic) contractions (DCI 450 mm Hg.s.cm ≥20% of swallows |
| EGJ outflow obstruction | Mean IRP > upper limit of normal, some instances of intact peristalsis such that the criteria for achalasia are not met |
| Major Disorders of Peristalsis | |
| Distal esophageal spasm | Normal mean IRP ≥20% premature contractions (DCI > 450 mm Hg.s.cm) |
| Hypercontractile esophagus | At least two swallows with DCI > 8000 mm Hg.s.cm |
| Absent contractility | Normal mean IRP, 100% of swallows with failed peristalsis |
| Minor Disorders of Peristalsis | |
| Ineffective esophageal motility | ≥50% ineffective swallow |
| Fragmented peristalsis | ≥50% fragmented contractions with DCI > 450 mm Hg.s.cm |
The diagnosis of esophageal obstruction is based on the IRP and is the first manometric parameter measured when analyzing HRM tracings. The differential diagnosis for pediatric patients with an elevated IRP includes achalasia or an obstructing fundoplication. As with adults, all three subtypes of achalasia (types I, II, and III) have been observed in pediatrics ( Fig. 64.2 ). Type I achalasia, also known as classic achalasia: mean IRP > upper limit of normal, 100% failed peristalsis (see Fig. 64.2 ); type II achalasia, also known as achalasia with esophageal compression: mean IRP > upper limit of normal, no normal peristalsis, panesophageal pressurization with ≥ 20% of swallows; and type III achalasia: mean IRP > upper limit of normal, no normal peristalsis, preserved fragments of distal peristalsis or premature (spastic) contractions with ≥ 20% of swallows. All of these three subtypes have been seen in children.
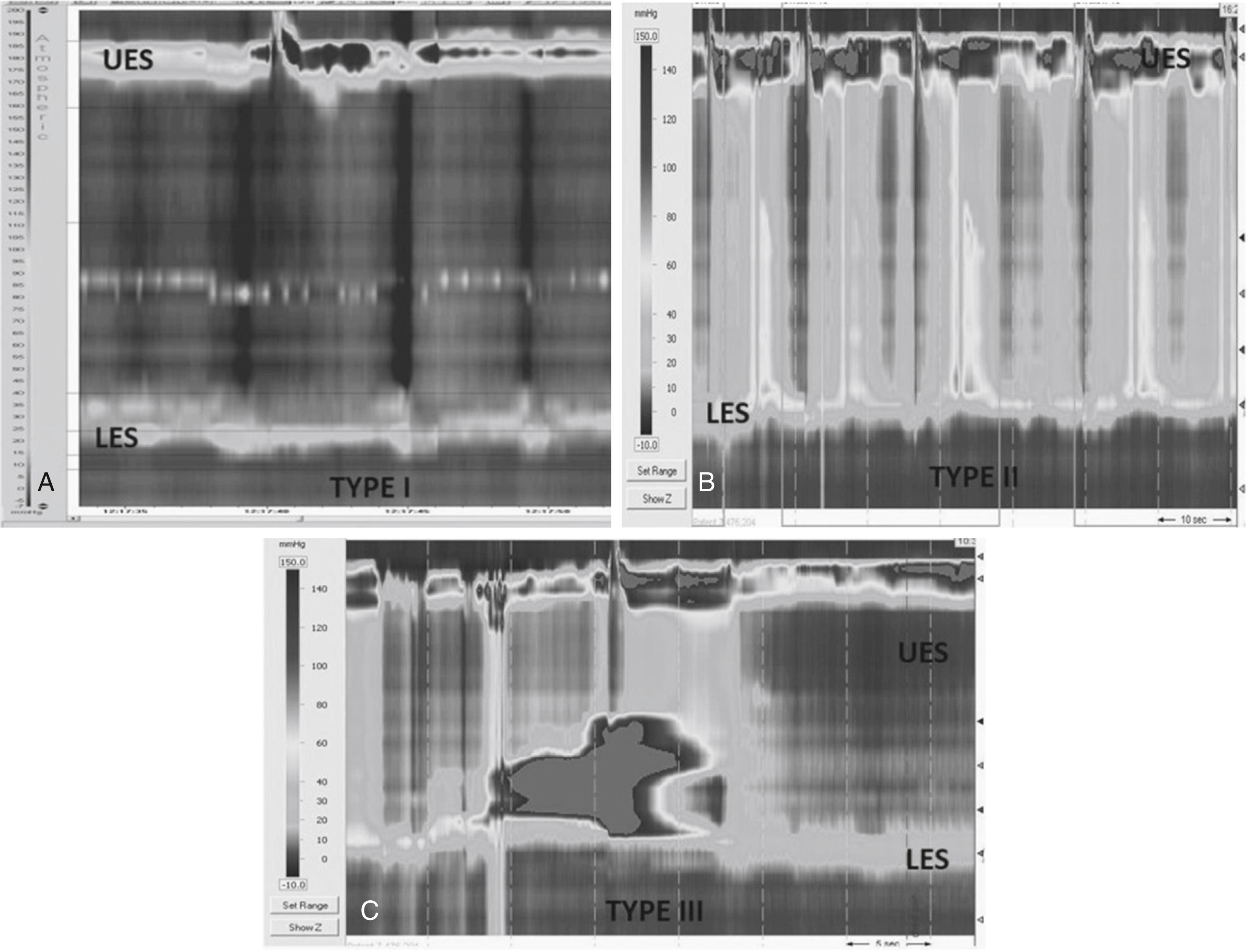
Because of a lack of a validated normal IRP in pediatrics and because of variations in IRP values between vendors, the cut-off value for the diagnosis of EGJ obstruction is not clear; in pediatric achalasia, IRPs as low as 10 mm Hg have been reported. , The significance of an isolated elevated IRP in pediatric patients, in the absence of symptoms or in a patient in whom the bolus, by impedance, passes easily through the area of increased pressure, is unknown.
The next step includes the assessment of the presence and quality of peristalsis, according to the CC which includes an assessment of the strength and the patterns of contractions. , , Assessment of peristalsis in children includes: (1) the presence or absence of antegrade peristaltic contractions; (2) the presence or absence of esophageal pressurization with or without intact peristalsis; (3) the presence or absence of long esophageal breaks; (4) the presence or absence of high-pressure distal esophageal contractions, represented by an elevated DCI; and (5) the ability of the peristaltic wave to clear a bolus, as measured by impedance. Whether the adult definitions of major motor disorders (absent contractions, distal esophageal spasm, and hypercontractile esophagus) , apply in children is not known. Therefore, the most critical questions to address in the assessment of major disorders of peristalsis in pediatrics are (1) is achalasia present? (2) do the motor findings explain the patient’s symptoms? and (3) do the motor patterns result in impaired bolus transit? Additional studies are needed to determine the cut-off values for abnormal DCI, abnormal length of esophageal breaks, abnormal cut-offs for esophageal pressurization, and values for an abnormal DL.
The significance of minor motor disorders (ineffective esophageal motility and fragmented peristalsis) , in pediatrics is even less clear than the major motor disorders. The key with these minor disturbances of pressure is to determine if there is incomplete bolus transit as determined by pairing impedance with pressure sensors. Abnormalities in esophageal peristalsis can be mimicked with poorly spaced swallows, dry swallows, double swallowing, or even by change in consistency of the ingested liquids or position of patient. There are no pediatric data on the frequency or the significance of these minor motor disturbances. Finally, any other abnormality seen during HRM that does not fulfill any of the above criteria is considered normal esophageal motility in adults, and overinterpretation of these findings in children needs to be avoided. , ,
Very little has been published on UES dysfunction using HRM. UES abnormalities are not included in the CC. The main goal in the assessment of UES function is to assess for coordination of pharyngeal contractions with simultaneous UES relaxation and their relationship to bolus transit. In pediatrics, the interrelationship between the UES and bolus transit may predict aspiration risk, and this may be of particular importance in children with respiratory symptoms or oropharyngeal dysphagia.
The addition of impedance provides the ability to establish if the peristaltic abnormalities seen are significantly impairing the bolus transit (see Fig. 64.1 ). , , This is particularly important to evaluate the significance of minor peristaltic abnormalities. While impedance is still not part of the CC, new objective parameters utilizing impedance have been proposed including the incorporation of pressure flow analysis (PFA). , , PFA has been used to assess for UES dysfunction as well as to discriminate among patients with subtle motility abnormalities and to determine the degree of LES obstruction. , In addition the pairing of impedance and manometry into mathematical models has yielded the automated impedance manometry (AIM) analysis to predict dysphagia risk and the swallow risk index (SRI) to predict aspiration in adults and children. , Other parameters have been proposed such as the duration of bolus presence within the EGJ (BPT, or bolus presence time) and the trans-EGJ-bolus flow time (BFT, or bolus flow time). , , This ratio of BFT and BPT (BFT/BPT) can be used to define the effectiveness of trans-EGJ emptying relative to the period of bolus presence. , , It has been shown that in patients these measurements also correlate with dysphagia severity, type of achalasia, and efficacies of therapies in treating EGJ obstruction. In pediatrics, the AIM analysis, however, has been able to discriminate causes of dysphagia in children, , , where it was shown to differentiate patients with dysphagia due to weak peristalsis and resultant poor bolus clearance from abnormal bolus flow resistance due to esophageal outflow obstruction. It has also been applied to children who underwent fundoplication to discriminate those children with and without postoperative dysphagia.
The following are other special pediatric conditions in which HRM, and high-resolution manometry with impedance (HRIM), have significantly changed clinical management:
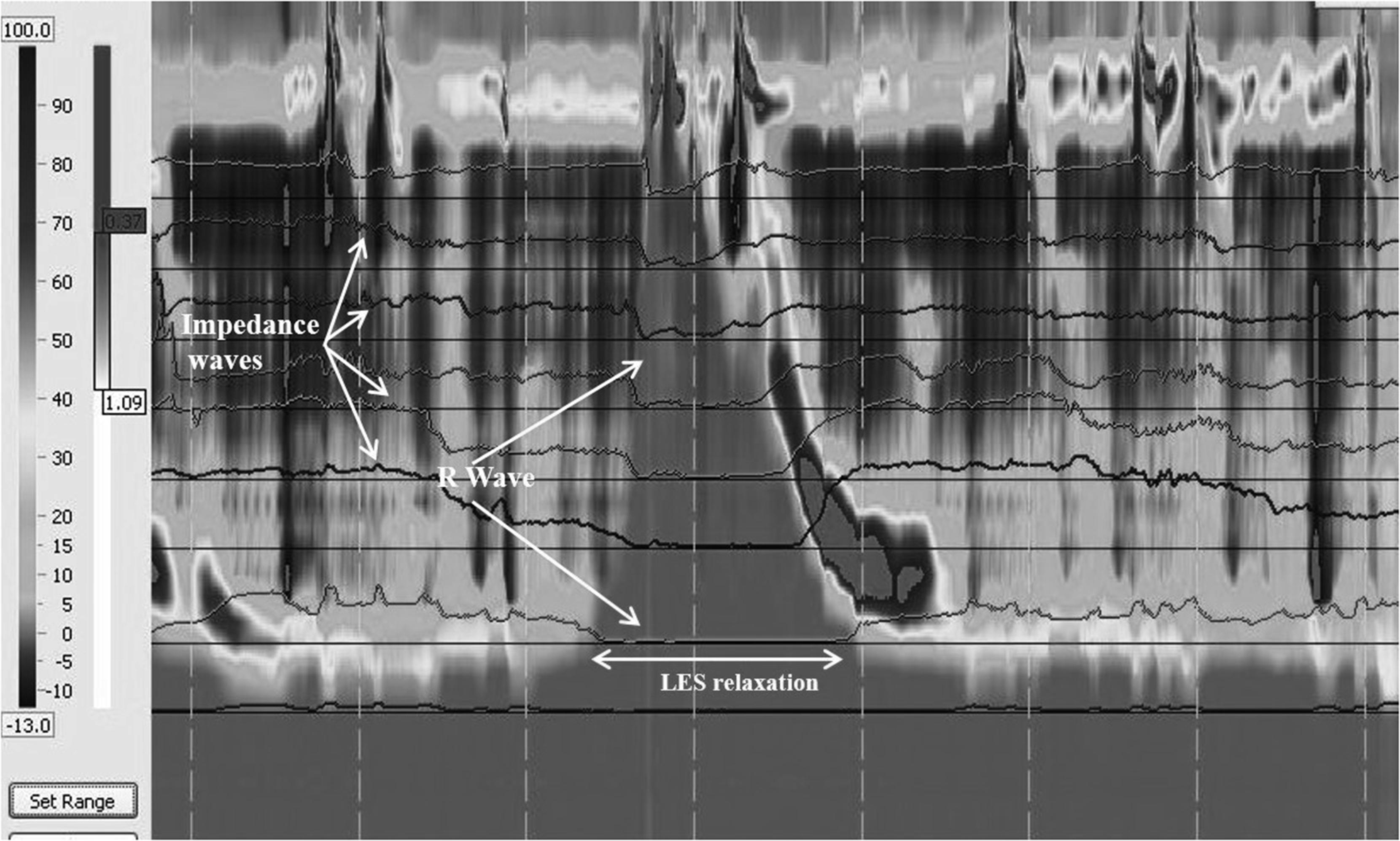
Rumination: HRIM has been effectively used to diagnose rumination and to differentiate subtypes of rumination. , Performance of HRM to diagnose rumination syndrome offers significant advantages over the traditional ADM including (1) the ability to perform the test without sedation; (2) the ability to assess for bolus flow following R waves; (3) the short test duration; and (4) the ability of the patient to choose a meal that triggers symptoms ( Fig. 64.3 ). ,
Esophageal Atresia : The performance in this population may be particularly helpful in patients with recurrent dysphagia with or without a fundoplication and in patients with recurrent respiratory infections. In patients with EA, performance of HRIM is critical in order to assess not only the strength of peristalsis but whether the peristalsis is able to clear the esophageal bolus. This latter point is critical in patients with recurrent respiratory symptoms as esophageal stasis can increase the risk for aspiration of esophageal contents. While several pediatric studies have documented near universal esophageal dysmotility in patients with EA, there are no published studies pairing HRM with impedance to document the physiologic consequences of dysmotility in these children. Current consensus guidelines recommend HRIM in children with EA with persistent symptoms including dysphagia. , ,
Fundoplication: Studies comparing high-resolution esophageal manometry in preoperative patients with or without esophageal dysmotility have shown no difference in the prevalence of postoperative dysphagia. However, in select patients at highest risk for postoperative dysphagia, preoperative HRM testing may be of value, particularly to determine if there is baseline bolus stasis even before the fundoplication; this may be particularly true of patients with esophageal atresia or scleroderma as severity of preoperative dysmotility may be helpful to determine if a fundoplication is warranted or if a partial or complete wrap is warranted. Pediatric literature suggests that while standard HRM measurements can be normal, EGJ outflow obstruction, bolus stasis, and elevated intrabolus pressures can be seen in postfundoplication patients, and these results may be helpful to determine which patients may benefit from postoperative botulinum toxin injection or dilation. b
b References 1, 5, 13, 17, 28, 30, 34, 38, 42–60.
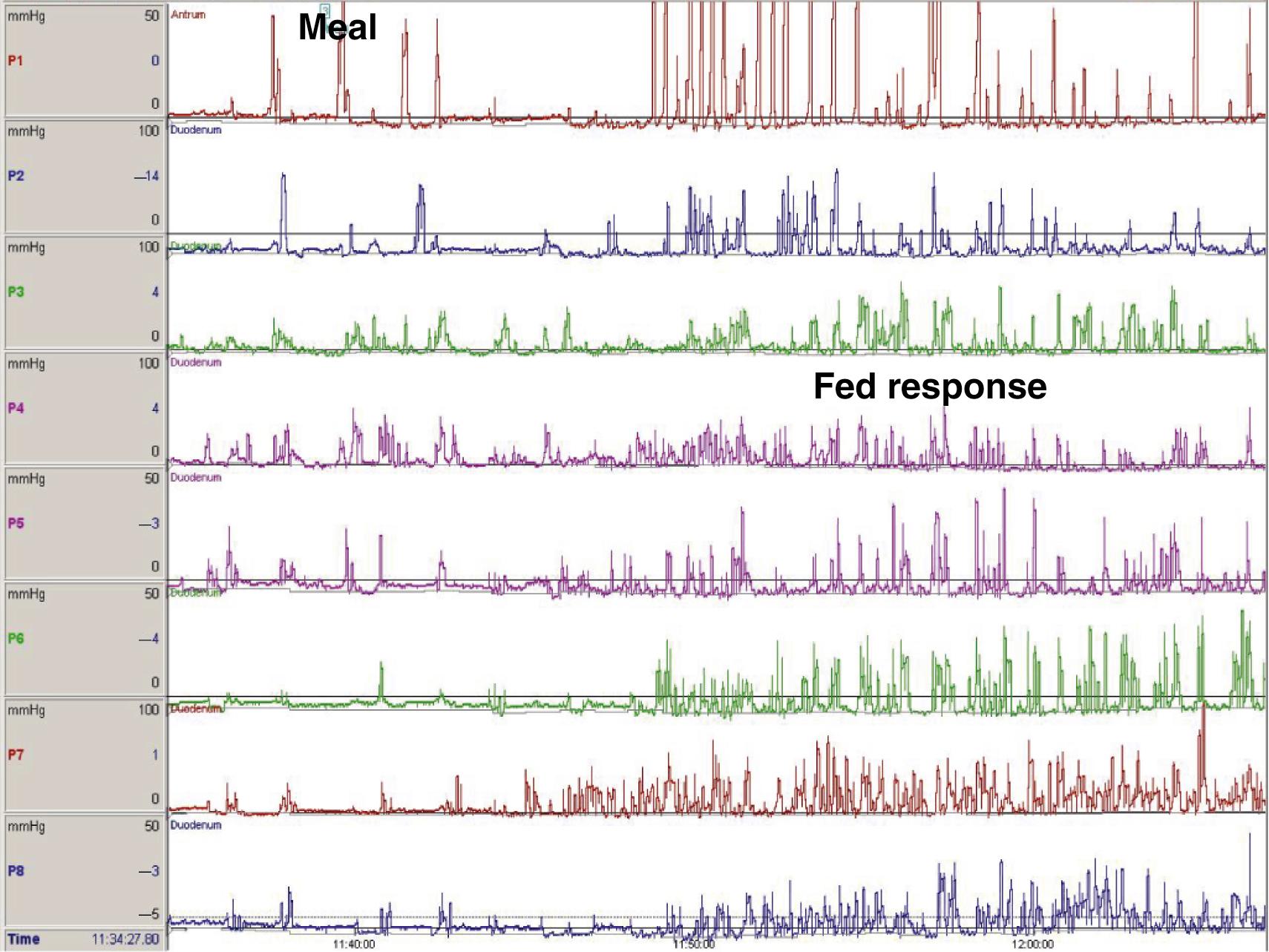
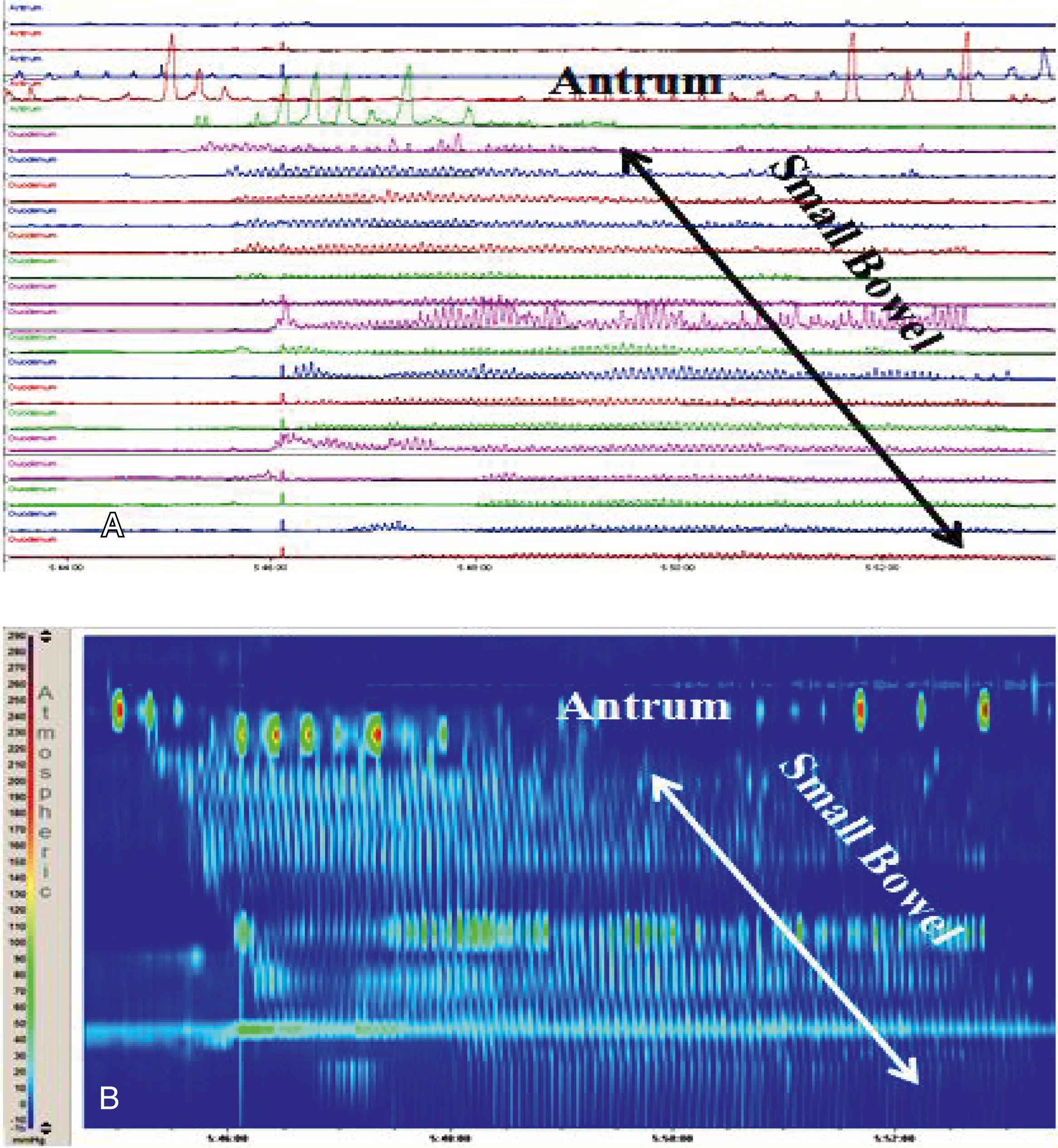
ADM measures the intraluminal pressure of the antrum and duodenum, , , , providing information of the contraction patterns of the upper gastrointestinal tract. It has contributed to the understanding of the pathophysiology of neuromuscular disorders of the stomach and small bowel by assessing pressure amplitude and coordination. , , , In most institutions, ADM is still performed using traditional perfused catheters ( Figs. 64.4 and 64.5 ) though high-resolution catheters are now available ( Fig. 64.6 ). ,
Manometry tests may not be useful in patient management when there is a known underlying cause of dysmotility, , , but they may be important in the study of selected children and adults with unexplained, severe upper gastrointestinal dysfunction or when there is a discrepancy between the clinical impression and the severity of the patient’s condition. As is true of most tests in children, the utility of ADM has been limited by the lack of normal data in healthy controls, and extrapolation of normal values has been derived from the results in patients referred for antroduodenal motility who later were determined to be normal. Some studies in adults report no difference in the frequency of abnormal manometric patterns between healthy and symptomatic patients, resulting in a debate about what constitutes a significant abnormality, so clinicians must pay close attention during interpretation of the study to avoid overinterpretation of the findings. ,
Antroduodenal motility has very characteristic patterns in both the fasting state and after a meal.
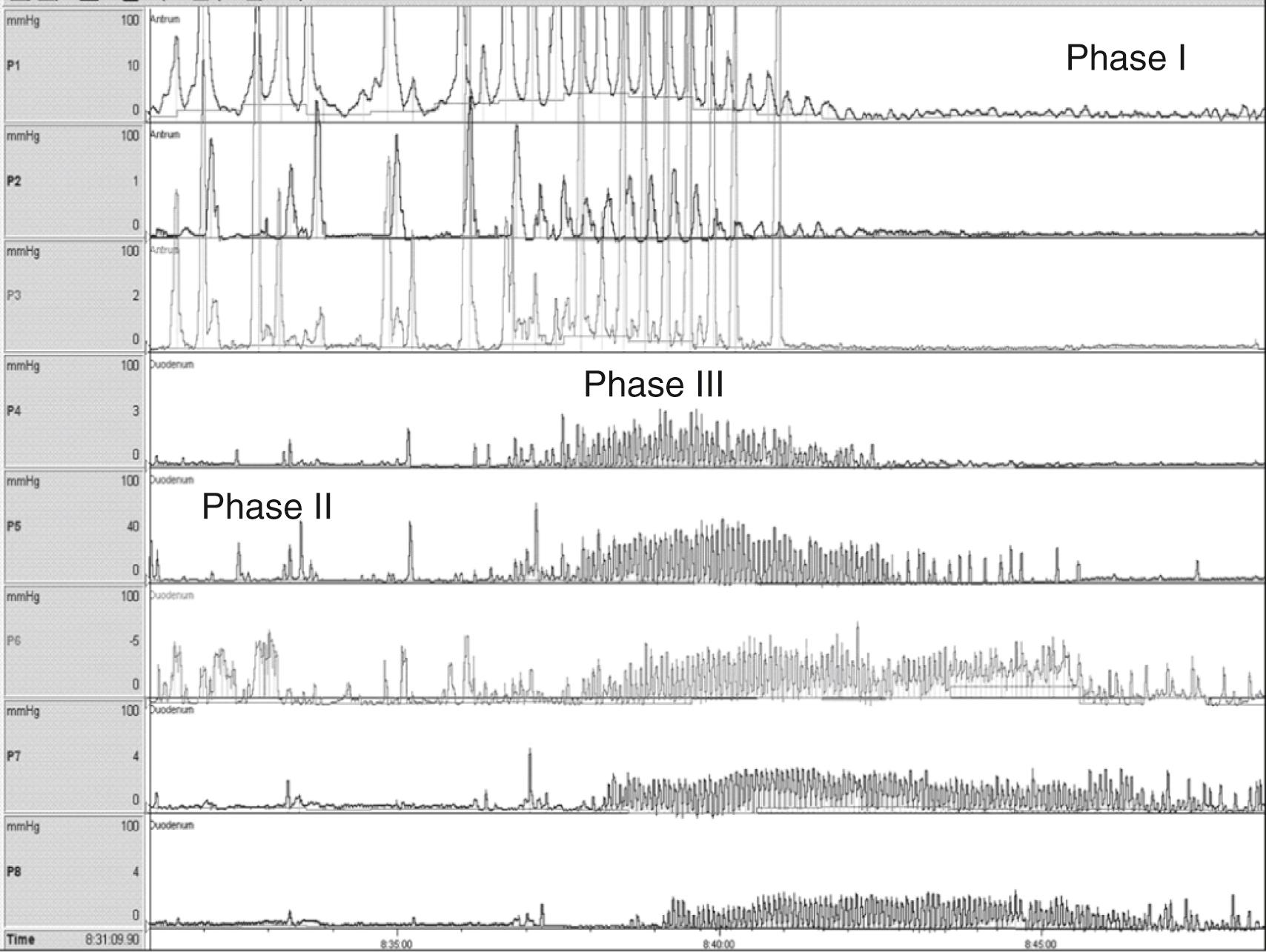
During fasting, the stomach and small bowel show a cyclic pattern, known as the migrating motor complex (MMC) (see Figs. 64.4 and 64.6 ). This cyclic activity is divided into three phases. Phase I is characterized by motor quiescence and seems to be predominant in the antrum. Phase II has irregular contractions with varied amplitude, is the longest in duration, and is predominant in the duodenum and jejunum. Phase III consists of regular rhythmic peristaltic contractions that start proximally and migrate down to the ileum with decreasing velocity of propagation and increased duration. As a cycle is fading upon reaching the ileum, a new one starts in the antrum. Phase III contractions occur at a rate of 3 per minute in the antrum and 9 to 12 per minute in the small bowel. In adults, phase I lasts from 12 to 20 minutes, phase II lasts from 30 to 130 minutes, and phase III lasts from 3 to 15 minutes, , with a large variation in cycle duration between individuals and in the same individual. There are no established standards for normal duration of the cycle in children, but the duration of the MMC seems to be shorter than in adults, , with the phase III propagation velocity increasing with age. The overall cycle length does not show an age-dependent variation. In children without upper gastrointestinal symptoms, the phase III contractions are present in most patients during fasting periods and induced in the remainder with erythromycin administration. Phase III occupies around 3%, phase I about 10%, and phase II roughly 87% of recording time. The presence of phase III activity is a marker of neuroenteric integrity, , , , and its absence is abnormal. , , , Because one third to one half of the activity front may commence distal to the stomach, the absence of antral phase III activity is not necessarily abnormal. , Some normal adult subjects may have no phase III activity during stationary studies, and therefore absent antral phase III activity is not diagnostic of a neuropathic disorder. , Data from 24-hour ambulatory ADM studies show that adult volunteers have at least one or more MMCs per 24 hours, therefore the presence of MMCs provides more definitive evidence of normal enteric nervous system function.
Following a meal, the fasting pattern is interrupted by the fed pattern characterized by irregular contractions of various amplitudes, which are strong and repetitive in the antrum and similar to the phase II of the MMC in the duodenum (see Fig. 64.5 ). An antral motility index has been used to evaluate both the frequency and the amplitude of these contractions, usually over a period of 2 hours, and the calculation is derived from measurements in the prepyloric area. It is usually calculated automatically by the equipment software or manually utilizing this formula: (amplitude × number of contractions + 1), with a normal value being 13.67 to 15.65 (5th to 95th percentile). The characteristics of the fed pattern vary with the type, composition, and amount of nutrients. Liquid nutrients decrease the amplitude of antral contractions and generate an irregular movement in the small bowel, whereas solid foods produce high-amplitude contractions in the antrum and a pattern similar to that of liquids in the small bowel.
The patient should be fasting overnight and stop medications that can affect motility for at least 48 hours before the test.
Small bowel motility tests can be performed with solid-state pressure transducers, impedance sensors, or perfused catheters. , ,
The configuration of the catheters can be customized, but the minimum recommended recording ports include one in the antrum and three in the small bowel. , ,
The distance between the duodenal and jejunal ports varies according to the age of the patient, with a range from 3 to 10 cm between ports. In children a distance of 3 cm and in adolescents a distance of 5 cm between ports is sufficient. Continuous monitoring is important in patients undergoing studies using perfused systems to avoid fluid overload, particularly in infants and small children. The perfusion rates usually vary from 0.1 to 0.4 mL/min per port. The perfusion rates for the study of premature and young infants should be decreased, and some units have reported perfusion rates as low as 0.01 to 0.02 mL/min. , Most adult laboratories use distilled water, but most pediatric centers use 0.2 to half-normal saline or oral hydration solutions , to avoid hyponatremia. We recommend saline solutions over oral hydration solutions to avoid clogging of the system from glucose residues and bacterial growth from the carbohydrate content of the oral hydration solutions. Recently HRM has been used for the study of antroduodenal motility with good results (see Fig. 64.6 ). Perfused systems can be used with 36 pressure ports, usually 2 to 3 cm apart, but the distal port can be spaced as necessary to span more of the small intestine. However, solid-state transducers are now used for most HRM studies. They include 36 pressure ports spaced by 2 to 3 cm in pediatrics, but the distal ports can be separated by longer segments, usually 5 cm. Most catheters also have a central lumen and diameters comparable to that of the water-perfused manometric catheters and can produce pressure topography plots of gastric and small intestine pressure activity (see Fig. 64.6 ). While these catheters avoid the issues of electrolyte imbalances, their utility is limited by their high cost.
It is not clear if the new information provided by the high-resolution catheters will change the diagnosis or abnormalities that have been described. ,
The catheter is introduced nasally or through an existing gastrostomy, jejunostomy, or ileostomy and advanced with endoscopic or fluoroscopic assistance into the small bowel, ideally beyond the angle of Treitz but most importantly across the antroduodenal junction. The position of the catheter needs to be checked during the performance of the test to ensure the correct position across the antroduodenal junction. This can be achieved by looking at the manometric patterns, but at times radiography or fluoroscopy may be needed. One adult study reported that on average up to five adjustments of tube location may be needed, particularly in the postprandial period, to ensure accurate antral recordings. , It is preferable to avoid anesthesia and sedation for placement, as the effects of most anesthetics and sedatives on antroduodenal motility recordings have not been evaluated. It has been suggested that sedation with midazolam (2 to 5 mg, or 0.05 to 0.2 mg/kg) followed by reversal with intravenous flumazenil (0.2 to 0.4 mg) does not result in appreciable change in motility recordings. In children the use of either sedation or general anesthesia is frequently necessary, particularly when catheters are being placed endoscopically. To avoid possible confounding effects of the sedation or anesthesia, in most centers the study is performed the day after the catheter has been placed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here