Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The cardiovascular system can be impacted by a number of different genetic diseases (inner ring). There are numerous types of alterations to the human genome associated with these diseases (outer ring). The techniques to identify genetic variation associated with disease in a given patient continue to grow, but often rely on fundamental principles of molecular genetics (middle ring). Please note that the alignment of the rings does not correlate with each other, and each item on each ring may be associated with multiple members of other rings. SNV , single-nucleotide variant; CNV , copy number variant.
chromogenic in situ hybridization catecholaminergic polymorphic ventricular tachycardia deoxyribonucleic acid formalin fixed paraffin embedded fluorescent in situ hybridization genome-wide association study hypertrophic cardiomyopathy in situ hybridization megabase multiplex ligation-dependent probe amplification next generation sequencing polymerase chain reaction quantitative PCR ribonucleic acid reverse transcription-PCR velocardiofacial syndrome whole exome sequencing whole genome sequencing CISH
CPVT
DNA
FFPE
FISH
GWAS
HCM
ISH
Mb
MLPA
NGS
PCR
qPCR
RNA
RT-PCR
VCFS
WES
WGS
The discipline of molecular biology has seen impressive growth in just a generation. The full spectrum of material relating to this field is far too massive to be adequately covered here; indeed, it can only be superficially discussed even in entire textbooks dedicated to the subject. However, given the importance of understanding the genetic underpinnings of cardiovascular disease, an overview of the types of variation is warranted; the material presented here is intended to provide a foundation to facilitate further learning and understanding in the field.
The vast majority of the topics covered here will focus on germline genetic variation, that is, the genetic changes present in the genome at birth. In contrast, somatic genetic variation typically occurs in only cells capable of division, with those acquired mutations underlying the majority of human malignancies. While many of the techniques described herein were developed to identify somatic variation, they are equally applicable in characterizing the germline variants relevant to cardiovascular disease.
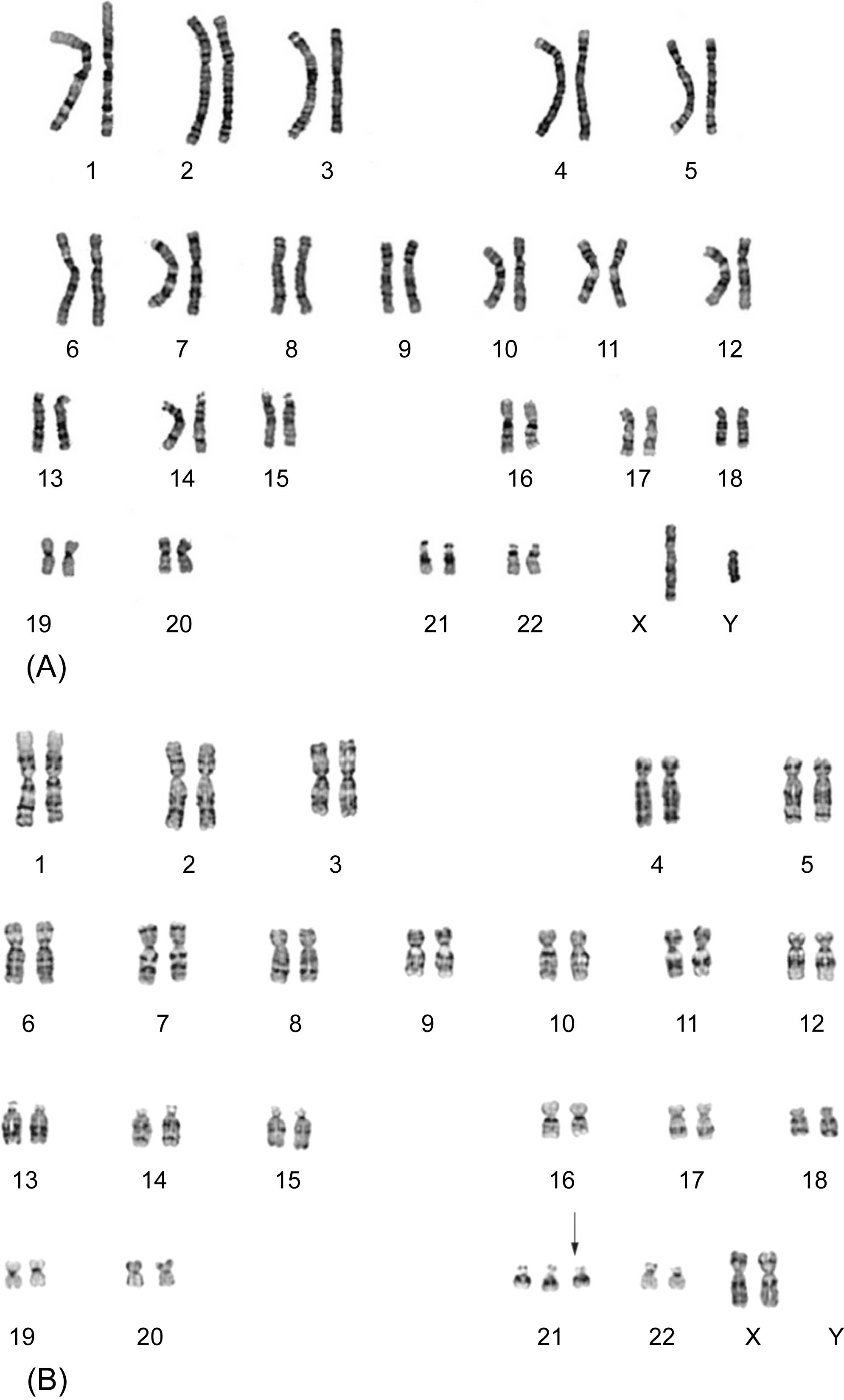
The human genome typically has paired copies of each gene, one derived from each parent, distributed on 22 pairs of autosomes plus 2 sex chromosomes in each cell. In the event that too few or too many of a particular chromosome are present, large numbers of regulatory processes become unbalanced. Some of these effects represent dose effects from having too many or too few copies of the gene, while others result from the ways in which chromosomes interact with each other to regulate cellular processes. Many aneuploidies are incompatible with life, but a few are associated with viable genetic syndromes, most notably the trisomies (three copies of a particular chromosome) of chromosomes 13, 18, and 21 (the latter being Down syndrome, Fig. 4.1B ).
Due to errors in the replication process, material from one chromosome can be swapped ( translocate) with material from another chromosome. The result is two so-called “derivative chromosomes,” each containing genetic material from each of the original chromosomes ( Fig. 4.2A ). The point on each original chromosome where the sequence separated to allow this recombination event is called the break point . If an essential gene was present at the point of translocation, the resulting rearrangement can result in a new recombinant gene, often with abnormal expression patterns and resulting pathophysiology. This is commonly seen in oncology, but is also seen occasionally in cardiovascular pathology.
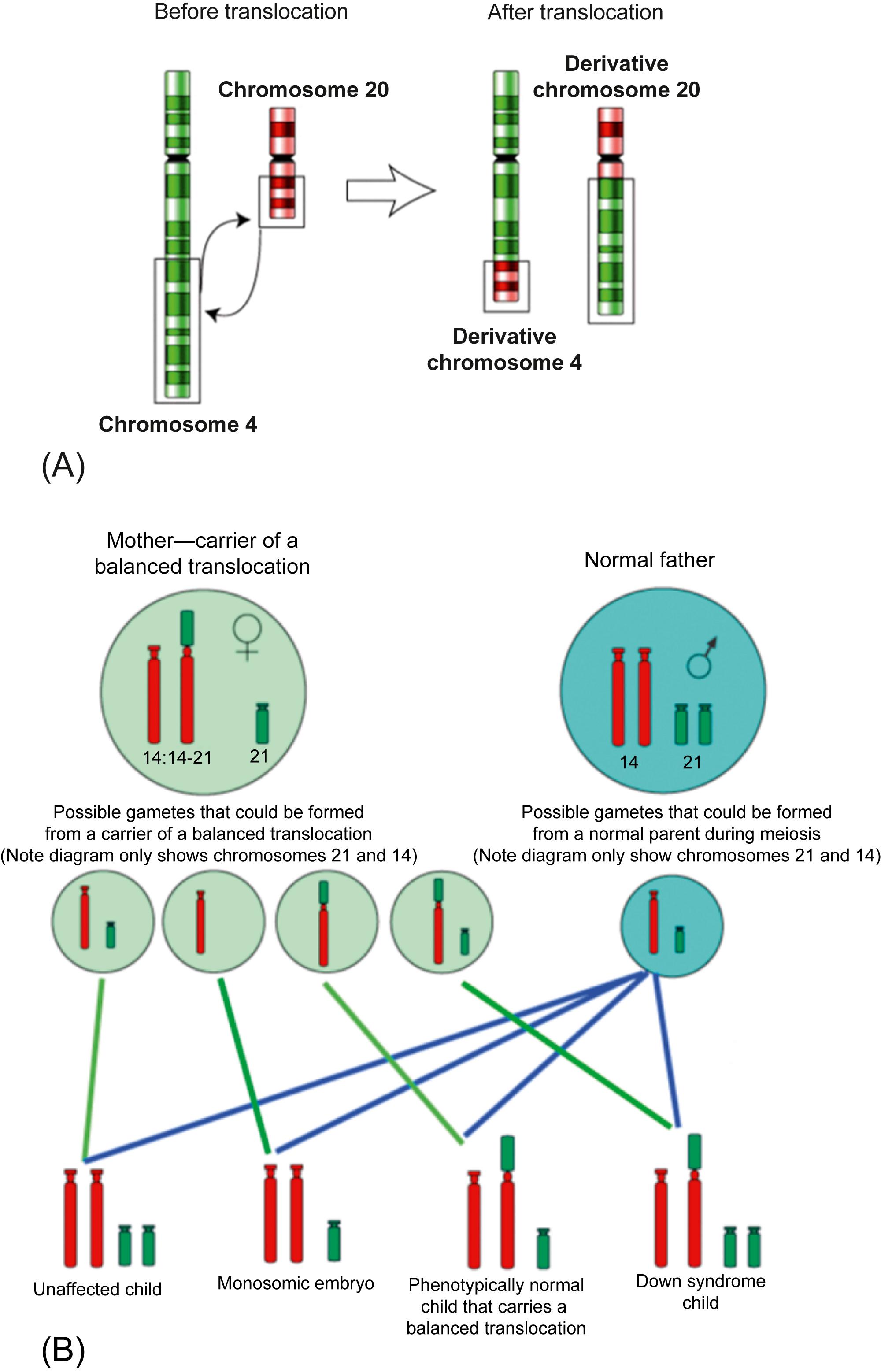
Typically, such translocations cause no significant problem for the original host. However, if a “balanced translocation” is sorted randomly into sperm or oocytes in the germline, the resulting gametes occasionally have one member of the balanced translocation and not the other ( Fig. 4.2B ). This results in too many copies of some genes and not enough of others, with pathology that can be as dramatic as that seen in aneuploidy. Such translocations likely underlie a significant fraction of unexplained infertility—the zygotes are frequently nonviable in the face of an unbalanced chromosomal assortment.
A number of other forms of variant chromosomes can arise, for example, inversions , large scale duplications, and other such events. The pathologies resulting from such perturbations will again relate to the amount of material resulting in partial aneuploidy, that is, a part of the genome with too few or too many copies.
The DNA sequence can be modified by insertion or deletion of sequences of any length. This broad class of variation is called indels , short for insertions and deletions . Insertions might occur through events such as viral infection, with insertion of part or all of the viral genome ( Fig. 4.3A ). A special class of insertion is duplication ( Fig. 4.3B ), in which an existing sequence copies itself into another spot in the genome, either adjacent ( cis duplication, depicted) or distant ( trans duplication). Deletions ( Fig. 4.3C ) occur most commonly through errors in transcription or directly mutagenic events.
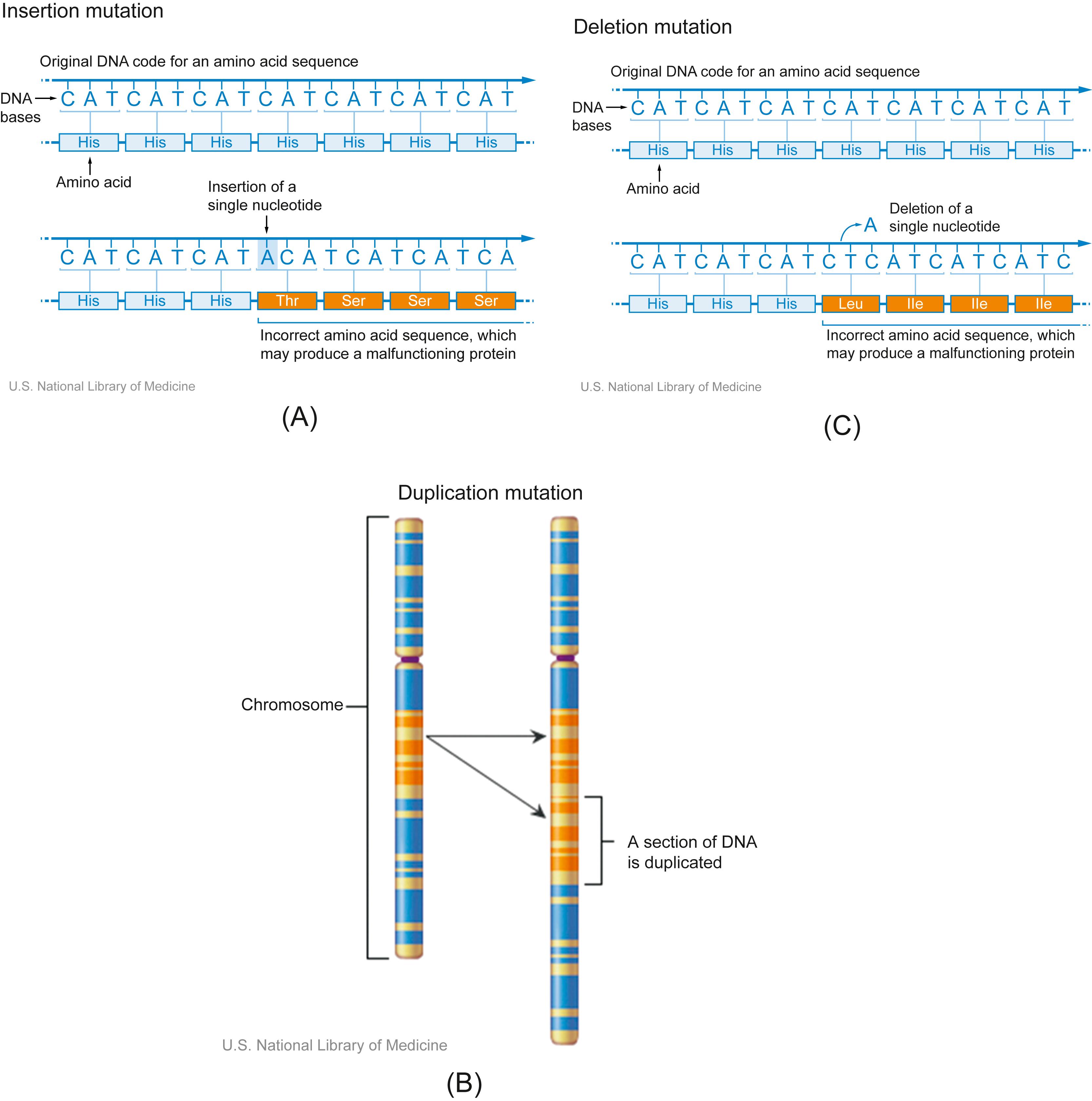
Short indels are responsible for so-called frameshift mutations, where the reading frame of a coding sequence is altered ( Fig. 4.3A and C ). This occurs when a number of nucleotides not divisible by three is inserted or deleted in the middle of a coding sequence, causing all downstream bases to be read-out of frame. Often, this results in grossly abnormal proteins and/or premature strand termination with resulting pathophysiology.
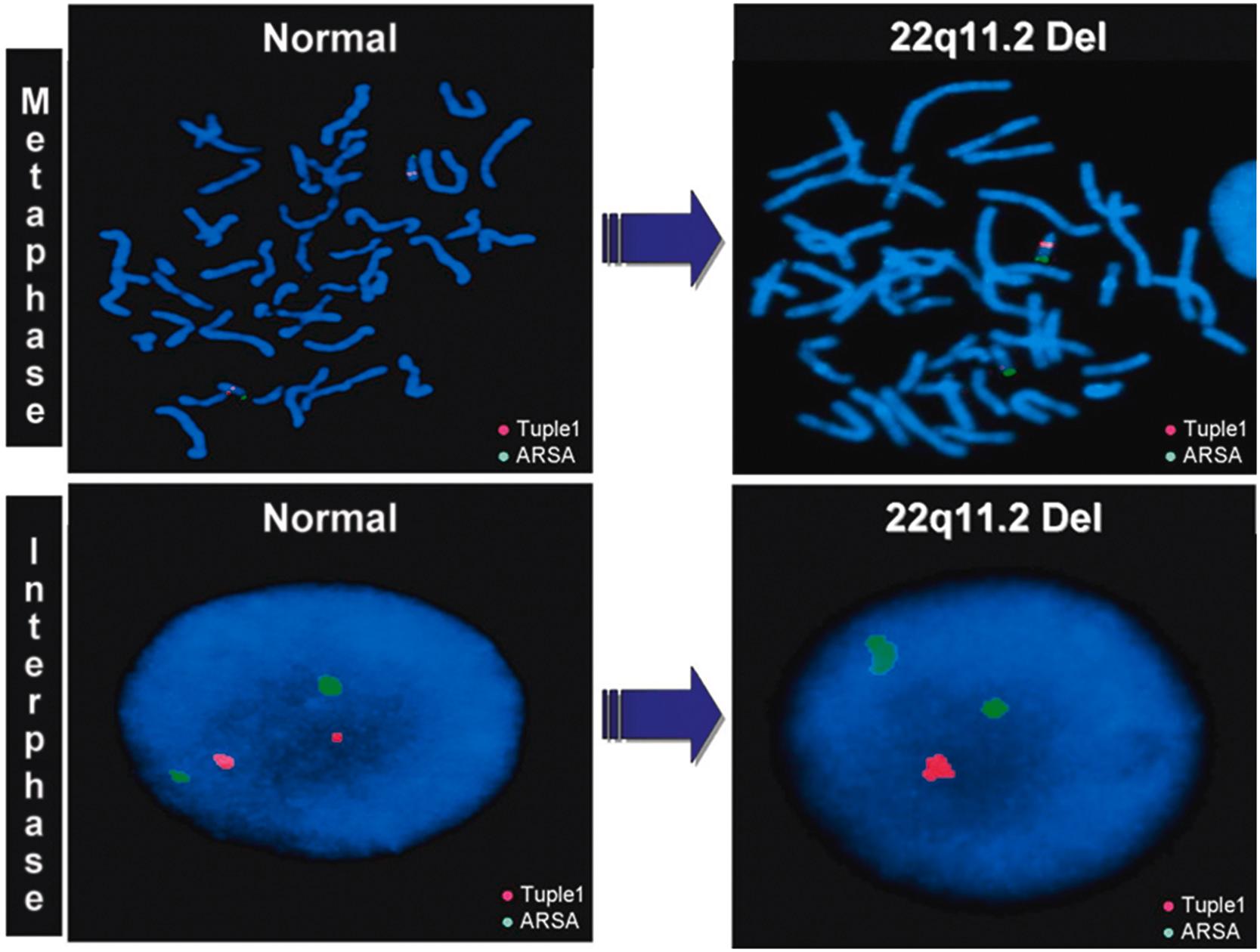
One notable example of a very large deletion is the 22q11.2 deletion syndrome ( Fig. 4.4 ), previously known as DiGeorge syndrome and velocardiofacial syndrome (VCFS), and it is less well-known correlate, 22q11.2 duplication syndrome.
Of minor note, insertions and deletions may coexist with each other and with other types of variation, most notably translocations. At the break points of such events, there is often loss of some genetic material and insertion of other material. As such, sequencing of breakpoints is a common technique for identifying and understanding some complex phenotypes .
The terms insertion and deletion are often applied relative to a reference sequence, and as such it is important to note that some of these represent benign variation in the human genome (see copy number variants below). Further, the exact terminology applied to each of these follows complex rules, outlined at the time of this chapter by the Human Genome Variation Society (HGVS, http://varnomen.hgvs.org/ ).
Copy number variations (CNVs) refer to any variation in the number of times a particular sequence is represented. Many assays designed to look for CNVs simply count the number of times a particular sequence is present without consideration for where it falls in the genome; however, it is worth noting that some of these are in cis , some in trans , and some are a combination of both. In effect, these are just the result of repeated duplications (also called amplification ) and/or deletions over the course of many generations, occurring through multiple mechanisms . CNVs exist as a major source of routine genetic variation , and a number of publically available databases catalog such changes; they are often included in the broad category of structural variation. CNVs range from extremely short sequences, such as dinucleotide and trinucleotide repeats, to multiple megabases (Mb) of sequence.
Very short sequences (two to five bases) repeated numerous times are often called microsatellites . Longer sequences of 10–60 repeated bases can be termed minisatellites . Both of these are sometimes referred to as variable number of tandem repeats , and are noteworthy for their role in “DNA fingerprinting,” that is, establishing a molecular identity. Large homologous regions within the genome, so-called segmental duplications or low-copy repeats, are also part of this family of variation.
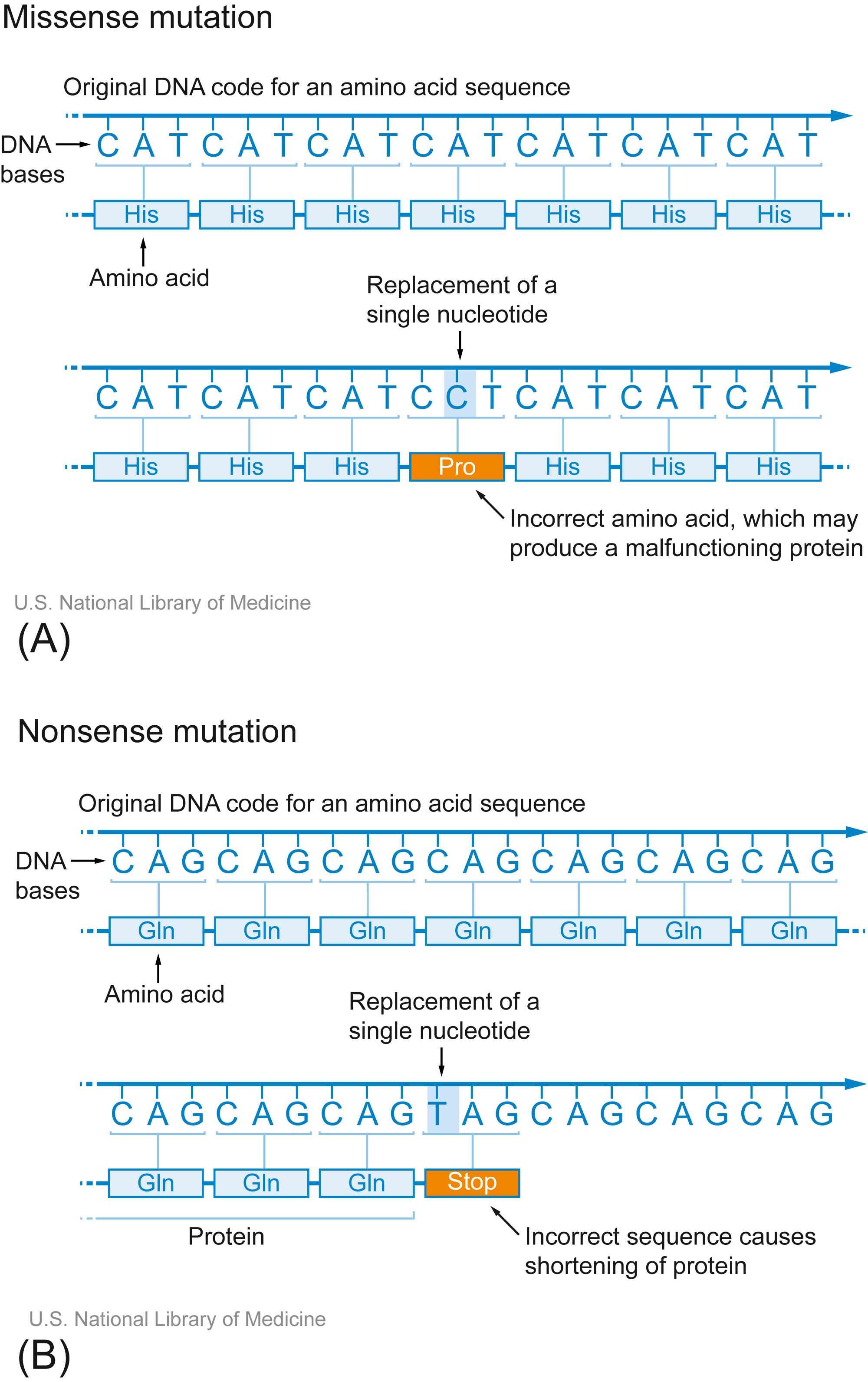
Single-nucleotide substitutions represent the most common source of genetic variation between individuals. However, since most occur in noncoding segments of the genome, they are usually benign or have only minimal impact. Nevertheless, because single-nucleotide variants (SNVs) are readily identified through sequencing, they can be linked to a host of genetic conditions, and such single-nucleotide polymorphisms (SNPs) can be useful markers for certain diseases. When SNVs occur in the coding sequence of a protein ( Fig. 4.5 ), either due to missense mutations (the wrong amino acid is encoded) or nonsense mutations (a premature stop codon is introduced), they can cause significant pathology.
Molecular diagnostics are a critical and increasingly commonplace component of medical practice across all disciplines. The full scope of these analyses is too involved to adequately cover here—in fact, new subspecialties have evolved specifically to handle the complexity of modern genetic testing. However, particular aspects of cardiovascular disease lend themselves to a subset of molecular assays, and it is important for contemporary cardiovascular pathologists to understand these in choosing the appropriate approach and interpreting the results.
Molecular assays can be parsed in a number of ways. One rubric considers the primary diagnostic technique, for example, karyotyping (direct visualization of chromosomes), hybridization , amplification , or sequencing . An alternate strategy highlights the particular molecular change, including genotype, specific sequence, CNV, or broader systematic surveys of genome level alterations.
The discussion below will focus on the technological approaches that are relevant to understanding and characterizing cardiovascular diseases considered in detail elsewhere in this text.
Perhaps the oldest molecular method still in common use, karyotyping is the broad class of techniques used in classical cytogenetics. These approaches directly visualize chromosomes, allowing the quantification and structure of each to be characterized ( Fig. 4.1 ). The greatest advantage of this technique is that it is relatively unbiased, that is, any large chromosomal abnormality is likely to be identified. Nevertheless, cytogenetic analyses are time consuming, and lack the sensitivity to identify many of the genetic variants that underlie cardiovascular disease.
Karyotyping typically requires cell culture to obtain proliferating cells from the individual (or specific tissue) being tested. Since cardiomyocytes are terminally differentiated and nonproliferative, peripheral blood lymphocytes are typically used as surrogates for germline karyotypes. These cells are then treated to arrest chromosome division during mitosis, so that the individual chromosomes can be separated and characterized. Chromosomal banding patterns are visualized using specific dyes that rely on the natural chemistry of the DNA for differential uptake in regions relatively enriched or depleted of specific nucleotide base pairs. The most commonly utilized technique, G banding , uses Giemsa dye to stain areas rich in adenine and thymine dark while areas rich in guanine and cytosine are paler (see Fig. 4.1 ). Since the gross general nucleotide sequence is similar across all individuals, each human chromosome has a characteristic banding pattern. By visualizing the isolated, stained chromosomes microscopically, it is possible to compare them to reference images and determine chromosome identity, how many copies are present, and whether any particular gross abnormalities are present. These techniques are particularly useful for large genome level changes, such as translocations, aneuploidy, and large deletions; current techniques cannot detect changes in DNA smaller than 3–5 Mb.
Karyotyping in cardiovascular pathology may be helpful for the diagnosis of syndromic conditions that manifest as heart or vessel findings, for example, Down syndrome (trisomy 21, Fig. 4.1B ). More commonly, karyotyping can be applied in the setting of unexpected fetal loss where there are complex congenital cardiovascular features, suggestive of an unbalanced translocation in the fetus (see above).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here