Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cricoarytenoid arthritis can lead to airway difficulties in patients with rheumatoid arthritis or systemic lupus erythematosus.
To diagnose vocal cord dysfunction, it is necessary to examine the position of the vocal cords during inspiration and phonation.
The recurrent laryngeal and superior laryngeal nerves may be injured during thyroid surgery, leading to severe vocal cord dysfunction.
Bilateral partial recurrent nerve palsy is more dangerous than complete palsy.
Neck movement during anesthesia can result in movement of the tip of the endotracheal tube (ETT); neck flexion causes the ETT to advance, while neck extension causes withdrawal.
New imaging techniques such as virtual endoscopy and multidetector computed tomography (MDCT) are providing added insight into the structure and function of the airways in health and disease.
Upper airway obstruction in sedated patients occurs at the level of soft palate rather than at the level of the tongue.
Infections, such as COVID-19, may lead to prolonged anatomic changes in the lung parenchyma.
The “airway” is not merely a passive conduit of air; it serves a dynamic and important physiologic role in the human body. The air passages, starting from the nose and ending at the bronchioles, are necessary for the delivery of humidified and filtered respiratory gas to and from the alveoli. During clinical anesthesia, an anesthesiologist uses these air passages to deliver anesthetic gases to the alveoli, while maintaining vital respiratory gas transport. Anesthesiologists and other medical practitioners often gain access to the airway by means of an endotracheal tube (ETT) or other airway devices that are introduced directly into the patient’s upper or lower air passages. An understanding of the airway structures is critical for establishing and maintaining the airway.
For the purpose of anatomic description, the airway is divided into the upper airway, which extends from the nose to the glottis or thoracic inlet, and the lower airway, which includes the trachea, the bronchi, and the subdivisions of the bronchi. The upper airway also serves other important functions, such as olfaction, deglutition, and phonation in addition to humidification and filtering of inspired gases. A detailed anatomic description of all these structures is beyond the scope of this chapter. Structural details as they relate to function in health and disease and some important anesthetic implications are explained here. In addition, some advances in imaging techniques that give insight to functional anatomy are described.
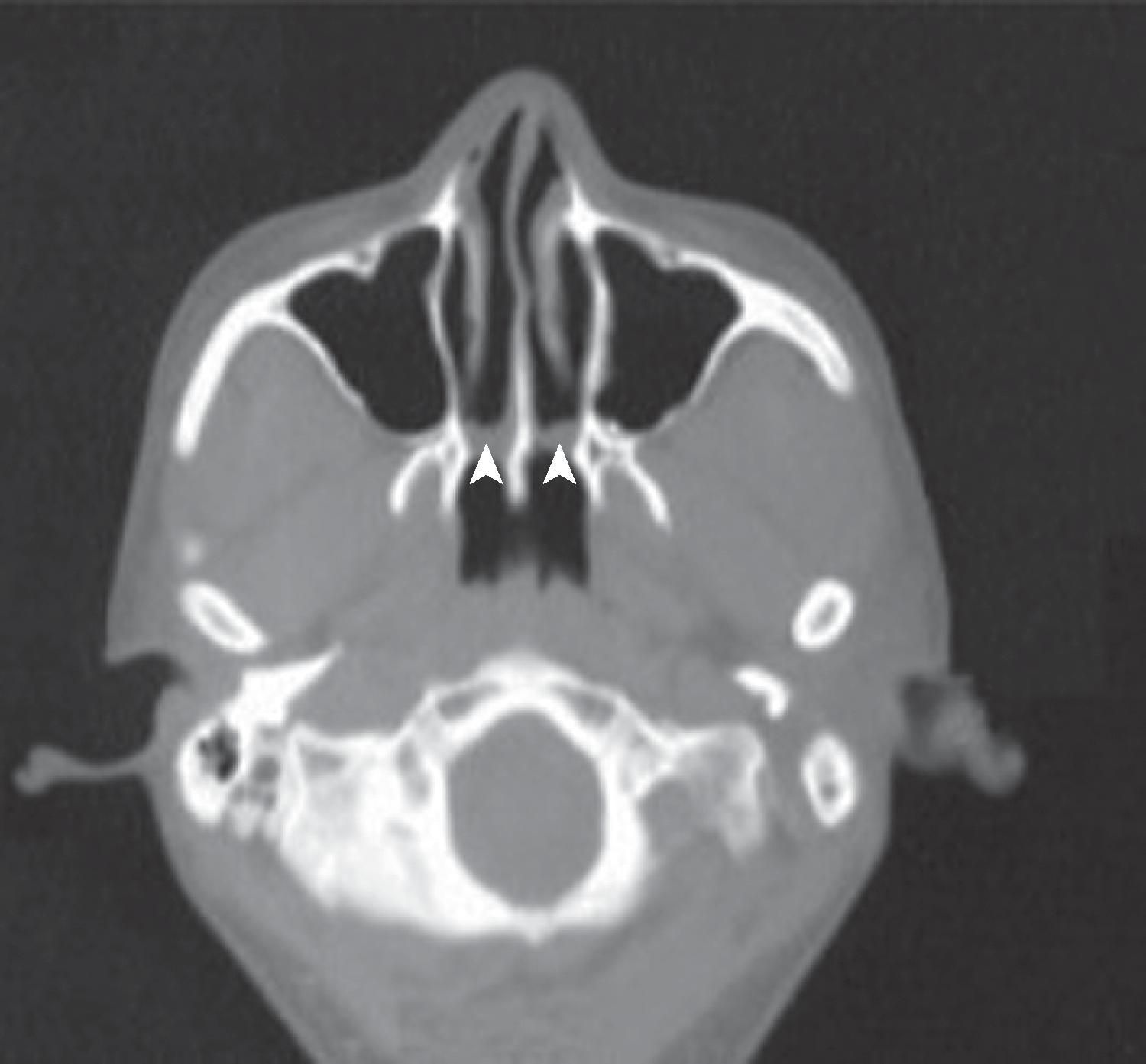
The airway functionally begins at the nares and the mouth, where air first enters the body. In the adult human, the two nasal fossae extend 10 to 14 cm from the nostrils to the nasopharynx. The two fossae are divided mainly by a midline quadrilateral cartilaginous septum together with the two extreme medial portions of the lateral cartilages. The nasal septum is composed mainly of the perpendicular plate of the ethmoid bone descending from the cribriform plate, the septal cartilage, and the vomer ( Fig. 1.1 ). It is normally a midline structure but can be deviated to one side. Disruption of the cribriform plate secondary to facial trauma or head injury may allow direct communication with the anterior cranial fossa. The use of positive-pressure mask ventilation in this scenario may lead to the intracranial entry of air, bacteria, or foreign material, resulting in meningitis or sepsis. In addition, nasal airways, nasotracheal tubes, and nasogastric tubes may be inadvertently introduced into the subarachnoid space in patients with facial trauma. The posterior portion of the septum is usually midline, but trauma-associated septal deviations and congenital choanal atresia can cause posterior obstruction ( Fig. 1.2 ).
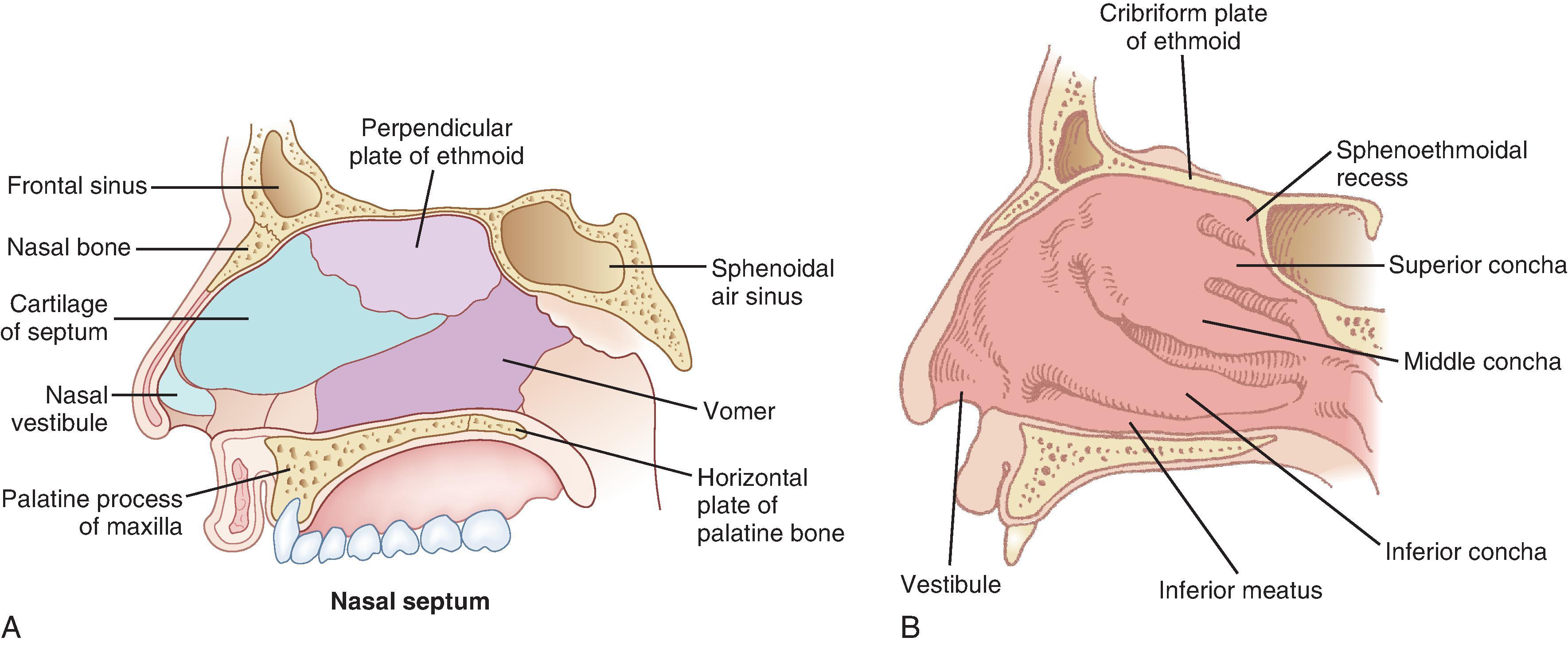
Each nasal fossa is convoluted and provides approximately 60 cm 2 of surface area on each side for warming and humidifying inspired air. The nasal fossa is bound laterally by inferior, middle, and superior turbinate bones (conchae), which divide the fossa into scroll-like spaces called the inferior, middle, and superior meatuses (see Fig. 1.1 ). , , The inferior turbinate usually limits the size of the nasotracheal tube that can be passed through the nose, and damage to the lateral wall may occur as a result of vigorous attempts during nasotracheal intubation. The arterial supply to the nasal cavity is mainly from the ethmoid branches of the ophthalmic artery, the sphenopalatine and greater palatine branches of the maxillary artery, and the superior labial and lateral nasal branches of the facial artery. A confluence of these blood vessels, known as Kiesselbach’s plexus , is situated in Little’s area on the anterior-inferior portion of the nasal septum. This is a common source of clinically significant epistaxis. The turbinates have a rich vascular supply that, depending upon ambient air temperature, affords the nasal airway the ability to expand or contract according to the degree of vascular engorgement. The vascular mucous membrane overlying the turbinates can be easily damaged, leading to hemorrhage, which can be profuse. The paired paranasal sinuses—sphenoid, ethmoid, maxillary, and frontal—drain through apertures termed ostia into the lateral wall of the nose. Prolonged nasotracheal intubation may lead to infection of the maxillary sinus due to obstruction of and lack of drainage through the ostia.
The olfactory area is located in the upper third of the nasal fossa and includes the middle and upper septum and the superior turbinate bone. The respiratory portion is located in the lower third of the nasal fossa. The respiratory mucous membrane consists of both ciliated columnar epithelium containing goblet cells and nonciliated columnar epithelium with microvilli and basal cells. The olfactory cells have specialized hairlike processes, called olfactory hairs , which are innervated by tendrils of the olfactory nerve.
The nonolfactory sensory nerve supply to the nasal mucosa is derived from the first two divisions of the trigeminal nerve—the anterior ethmoidal and maxillary nerves. Airborne chemical irritants cause firing of the trigeminal nerves, which are presumably responsible for reflexes such as sneezing and apnea. The afferent pathway for the sneezing reflex originates at the histamine-activated type C neurons of the trigeminal nerve, and the efferent pathway consists of several somatic motor nerves. The act of sneezing is associated with an increased intrathoracic pressure of up to 100 mm Hg and may produce airflow speeds up to 100 mph.
The nose serves a number of functions: respiration, olfaction, humidification, filtration, and phonation. Phylogenetically, breathing was intended to occur through the nose. This arrangement not only enables an animal to smell dangers but also permits uninterrupted conditioning of inspired air while feeding. Resistance to airflow through the nasal passages is double that of the mouth; therefore, during exercise or respiratory distress, mouth breathing occurs to facilitate a reduction in airway resistance and increased airflow. The nose is also able to prewarm and condition inspired air to a temperature of 32°C to 34°C over a wide range of ambient temperatures, from 8°C to 40°C.
Approximately 10,000 L of ambient air pass through the nasal airway per day, and 1 L of moisture is added to this air in the process. The moisture is derived partly from transudation of fluid through the mucosal epithelium and partly from secretions produced by glands and goblet cells. These secretions have significant bactericidal properties. Foreign body invasion is further minimized by the actions of the stiff hairs (vibrissae), the ciliated epithelium, and the extensive lymphatic drainage of the area.
The parasympathetic autonomic nerves reach the nasal mucosa from the facial nerve after relay through the sphenopalatine ganglion, and sympathetic fibers are derived from the plexus surrounding the internal carotid artery via the vidian nerve. A series of complex autonomic reflexes controls the blood supply to the nasal mucosa and allows it to shrink and swell quickly. Reflex arcs also connect this area with other parts of the body. For example, the Kratschmer reflex leads to bronchiolar constriction on stimulation of the anterior nasal septum in animals. A demonstration of this reflex may be seen in the postoperative period as a patient becomes agitated when the nasal passage is packed.
The pharynx provides a common pathway for food and respiratory gases, extending 12 to 15 cm from the base of the skull to the level of the cricoid cartilage anteriorly and the inferior border of the sixth cervical vertebra posteriorly. The widest level occurs at the hyoid bone (5 cm), while the narrowest occurs at the level of the esophagus (1.5 cm), which is the most common site for obstruction with foreign body aspiration. The pharynx is subdivided into the nasopharynx, oropharynx, and laryngopharynx. The nasopharynx, which primarily serves a respiratory function, lies posterior to the termination of the turbinates and nasal septum and extends to the soft palate. The oropharynx has primarily a digestive function and starts below the soft palate, extending to the superior edge of the epiglottis. The laryngopharynx (hypopharynx) lies between the fourth and sixth cervical vertebrae, starts at the superior border of the epiglottis, and extends to the inferior border of the cricoid cartilage, where it narrows and becomes continuous with the esophagus ( Fig. 1.3 ). The eustachian tubes open into the lateral walls of the nasopharynx.
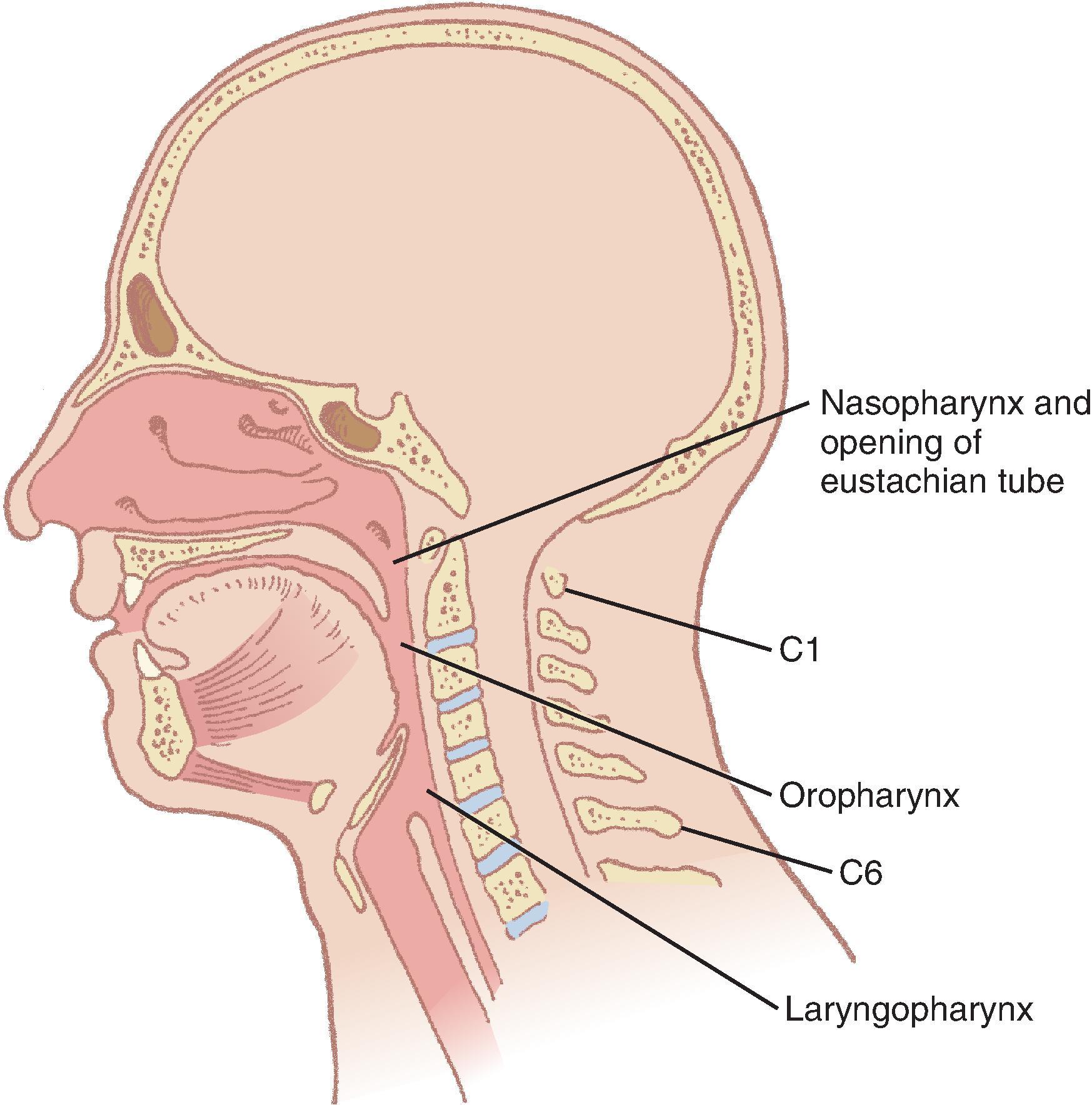
The lateral walls of the oropharynx contain the tonsillar pillars in the fauces. The anterior pillar contains the glossopharyngeus muscle, and the posterior pillar contains the palatoglossus muscle. The wall of the pharynx consists of two layers of muscles, an external circular layer and an internal longitudinal layer. Each layer is composed of three paired muscles. The stylopharyngeus, salpingopharyngeus, and palatopharyngeus muscles form the internal layer; they elevate the pharynx and shorten the larynx during deglutition. The superior, middle, and inferior constrictors form the external layer; they advance food in coordinated contractions from the oropharynx into the esophagus.
The constrictors of the external layer are innervated by filaments arising from the pharyngeal plexus (formed by motor and sensory branches from the vagus, the glossopharyngeal, and the external branch of the superior laryngeal nerve). The inferior constrictor is additionally innervated by branches of the recurrent laryngeal and external laryngeal nerves. The internal layer is innervated by the glossopharyngeal nerve. The glossopharyngeal nerve may easily be blocked by local anesthetic to the level of the posterior tonsillar pillars to facilitate awake airway management (see Chapter 13 ).
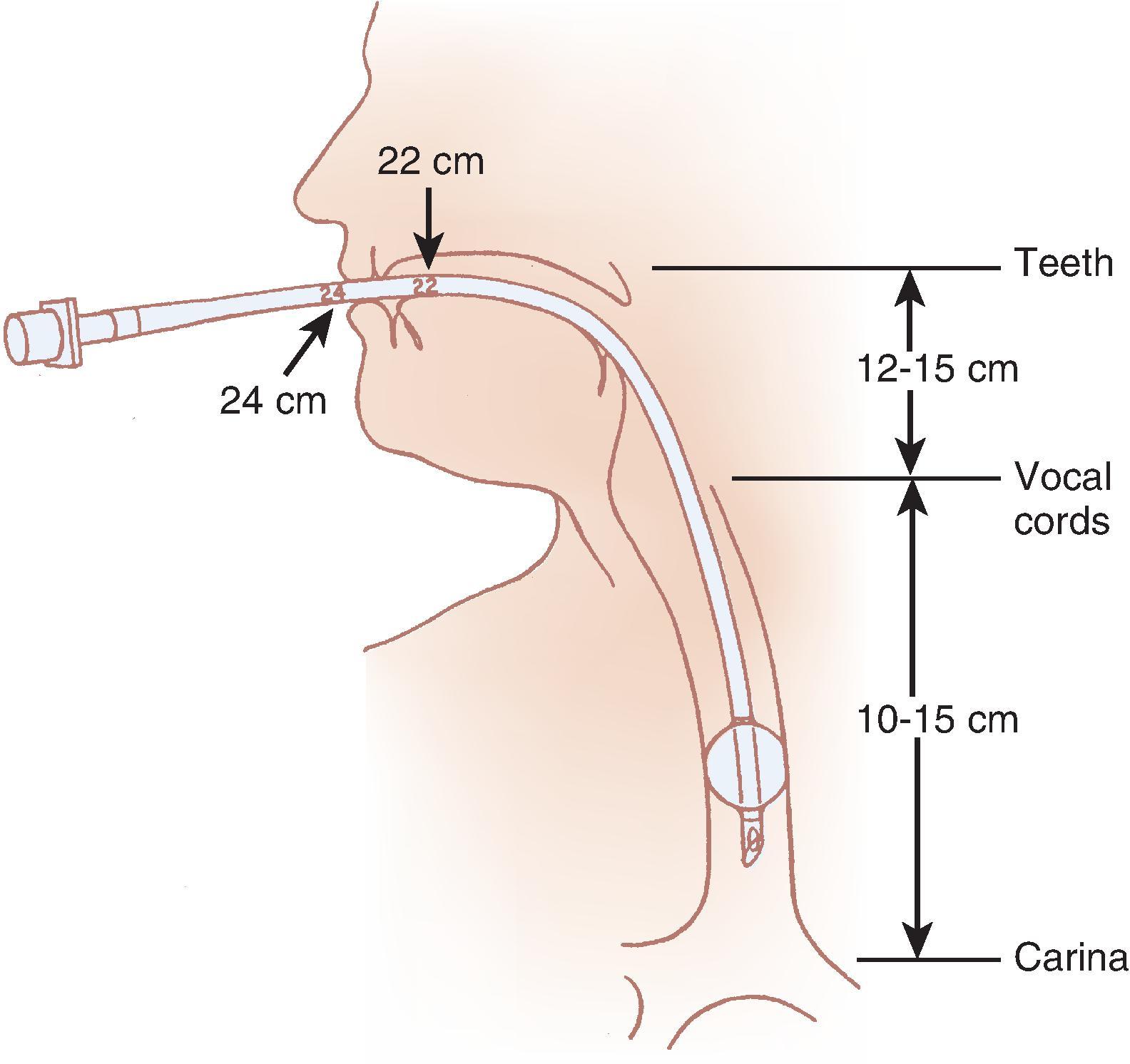
Patency of the pharynx is vital to the patency of the airway and proper gas exchange in nonintubated patients. Proper placement of an ETT requires an understanding of the distance relationships from the oropharynx to the vocal cords and carina. Complications, such as an ETT cuff leak at the level of the vocal cords or endobronchial intubation, may thus be avoided ( Fig. 1.4 ).
Inhaled particles greater than 10 µm are removed by inertial impaction on the posterior nasopharynx. In addition, smaller particles lose momentum and become suspended as a result of the sharp directional change of the inhaled airstream (90 degrees) at the nasopharynx. Being unable to remain suspended, the particles subsequently impact and become trapped by the pharyngeal walls. The body defends against the trapped impacted particles, bacteria, and viruses by the circularly arrayed lymphoid tissue located at the entrance to the respiratory and alimentary tracts, known as the ring of Waldeyer ( Fig. 1.5 ). The ring includes masses of lymphoid tissue or tonsils, including the two large palatine, lingual, eustachian tubal, and nasopharyngeal tonsils.
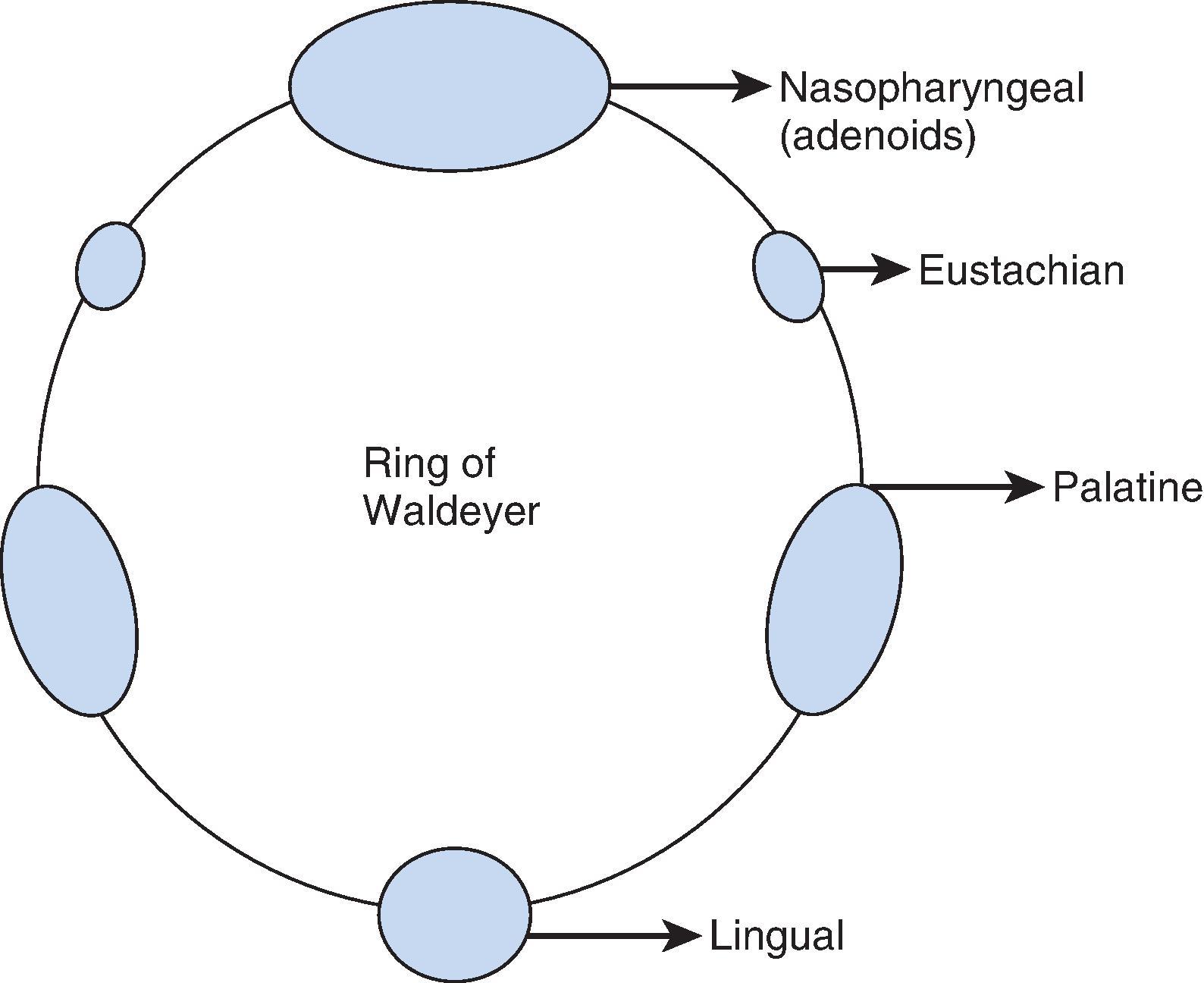
The nasopharyngeal tonsils are also called the adenoids . , These structures occasionally impede the passage of ETTs, especially if they are infected or enlarged. Specifically, enlarged adenoid tissue may impede passage of a nasotracheal tube or nasal airway or may simply obstruct the nasal airway passages. The lingual tonsils are located between the base of the tongue and the epiglottis. During routine anesthetic evaluation of the oropharynx, the lingual tonsils are typically not visible. Lingual tonsillar hypertrophy, which is usually asymptomatic, has been reported as a cause of unanticipated difficult intubation and fatal upper airway obstruction. In addition, sepsis originating from one of the numerous lymphoid aggregates may lead to a retropharyngeal or peritonsillar abscess, which poses anesthetic challenges.
Ciliary activity also works to clear trapped nonsoluble particles that are held in an outer mucous layer within the nares. This function is influenced by temperature, viscosity of the mucus, and the osmotic properties of the discharge. The ciliary movement can be negatively affected by many factors, such as viral infections or environmental agents, including air pollution and cigarette smoke. The loss or decrease of ciliary function leads to chronic and recurrent infections and can gradually severely injure the respiratory tract, leading to conditions such as chronic bronchitis, sinusitis, and otitis.
Traditionally, upper airway obstruction in patients who are sedated or anesthetized (without an ETT) or who have altered levels of consciousness for other reasons has been understood to be the result of the tongue falling back onto the posterior pharyngeal wall. Specifically, a reduction in genioglossus muscle activity leads to posterior displacement of the tongue with subsequent obstruction.
However, a number of publications offer a different explanation. The velopharyngeal segment of the upper airway adjacent to the soft palate has more recently become the primary focus. This area is particularly prone to collapse and has been found to be the predominant flow-limiting site during sedation and anesthesia, speech disorders, and obstructive sleep apnea (OSA) ( Fig. 1.6 ). Nandi and colleagues, using lateral radiographs in patients under general inhalational anesthesia, showed that obstructive changes in the airway occurred at the level of the soft palate and epiglottis. A magnetic resonance imaging (MRI) study found that patients receiving intravenous sedation for anxiolysis with midazolam had anterior-posterior dimensional changes in the upper airway at the level of the soft palate and epiglottis while sparing the tongue (see Fig. 1.6 ). In addition, Mathru and colleagues, using MRI to evaluate volunteers receiving propofol anesthesia, found that obstruction occurs at the level of the soft palate and not the tongue. Therefore, it appears that the soft palate and epiglottis may play a more significant role than the tongue in pharyngeal upper airway obstruction.
Reduction in the luminal size of the pharynx also serves as a factor in the development of respiratory obstruction in patients with OSA. This problem has been studied with the use of imaging techniques including computed tomography (CT) and MRI, nasopharyngoscopy, fluoroscopy, and acoustic reflection. Structural changes that include tonsillar hypertrophy, retrognathia, and variations in craniofacial structures have been linked to an increased risk of OSA, presumably by increasing upper airway collapsibility. CT and MRI studies in awake subjects have shown increased fatty tissue deposition and submucosal edema in the lateral walls of the pharynx, both of which can narrow the pharyngeal lumen and predispose to obstruction during sleep, when protective neuromuscular mechanisms wane. Obesity, the most common risk factor for OSA, has been shown to increase pharyngeal collapsibility through reductions in lung volumes, particularly decreases in functional residual capacity (FRC), which are accentuated with the onset of sleep. A decrease in FRC may increase pharyngeal collapsibility by reduction of tracheal traction on the pharyngeal segment. ,
The subatmospheric intraairway pressure created by contraction of the diaphragm against the resistance of the nose can lead to a reduction in size of the pharyngeal airway. The collapsible segments of the pharynx are divided into three areas: retropalatal, retroglossal, and retroepiglottic. In awake male patients with OSA, CT revealed a reduced airway caliber at all levels of the pharynx when compared with normal patients, with the narrowest portion posterior to the soft palate. Patency depends on the contractile function of pharyngeal dilator muscles in these segments. The muscles involved are the tensor veli palatini, which retracts the soft palate away from the posterior pharyngeal wall; the genioglossus, which moves the tongue anteriorly; and the muscles that move the hyoid bone forward, including the geniohyoid, sternohyoid, and thyrohyoid muscles. , In the awake state, genioglossal and tensor veli palatini muscle activity has been observed to be elevated in OSA patients when compared to normal subjects. Observations such as these suggest that increased upper airway dilator muscle activity compensates for a more anatomically narrow upper airway in OSA. Consequently, the reduction in upper airway muscle activity during sleep has been implicated in an increased likelihood of upper airway obstruction in patients with OSA compared with healthy subjects. ,
Studies also show that the configuration of the airway may differ in patients with OSA. Normally, the longer axis of the pharyngeal airway is transverse; however, in OSA patients the anterior-posterior axis is predominant. This orientation may be less efficient and affect upper airway muscle function. Continuous positive airway pressure (CPAP) has been found to be effective in treating airway obstruction in these patients. The application of CPAP appears to increase the volume and cross-sectional area of the oropharynx, especially in the lateral axis. Upper body elevation can improve respiratory mechanics and reduce the risk of OSA.
The velopharynx, an area of the pharynx adjacent to the soft palate, has assumed an increased importance in the understanding of OSA, speech disorders, and airway obstruction under anesthesia (see Fig. 1.6 B). Flexible nasoendoscopy and MRI are recommended for studying velopharyngeal dysfunction. Six skeletal muscles—the tensor veli palatini, levator veli palatini, musculus uvulae, palatoglossus, palatopharyngeus, and superior pharyngeal constrictor—help form the so-called velopharyngeal sphincter. The proper function of the sphincter is vital to opening and closing of the nasal passages to airflow during deglutition and normal breathing. Recent air pressure measurement studies during sleep have confirmed the velopharynx as the site of significant obstruction in OSA patients.
The same anatomic characteristics associated with OSA can make direct laryngoscopy and intubation more difficult. Patients with a history of severe OSA have a 16% incidence of difficult intubation compared with a 3% risk in the control group.
The larynx, which lies in the adult neck opposite the third through sixth cervical vertebrae, sits at the crossroads between the food and air passages (or conduits) and consists of cartilages forming the skeletal framework, along with ligaments, membranes, and muscles. The larynx may be located somewhat higher in females and children. Until puberty, no differences in laryngeal size exist between males and females. At puberty, the larynx develops more rapidly in males than in females, almost doubling in the anteroposterior diameter. The female larynx is smaller and more cephalad. The average measurements of the length, transverse diameter, and sagittal diameter of the adult larynx are 44, 36, and 43 mm, respectively, in the male and 41, 36, and 26 mm, respectively, in the female. Most larynxes develop somewhat asymmetrically. The inlet to the larynx is bounded anteriorly by the upper edge of the epiglottis, posteriorly by a fold of mucous membrane stretched between the two arytenoid cartilages, and laterally by the aryepiglottic folds.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here