Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors would like to acknowledge and thank Dr. Neil E. Green and Dr. Nathan L. Van Zeeland for their contributions to the previous versions of this chapter.
Fractures about the elbow are extremely common, and injuries about the elbow occur more frequently in the skeletally immature than they do in adults. It is estimated that upper extremity injuries account for 65% of all fractures and dislocations in children and that fractures and dislocations about the elbow are second in frequency only to fractures of the distal end of the forearm.
Ossification of the distal end of the humerus progresses with age. At birth, the distal humeral metaphysis is ossified; however, none of the structures that constitute the epiphysis are ossified. The capitellum is the first structure to ossify and may be seen radiographically as early as 6 months of age, according to Silberstein and colleagues. Haraldsson, in his classic article in 1959, stated that the capitellum may ossify as early as 1 month of age; however, 6 months is probably the youngest age at which this ossification center is seen ( Fig. 17.1 ). Although ossification of the capitellum may not take place until as late as 2 years of age, Silberstein and associates state that it is invariably present by that time.
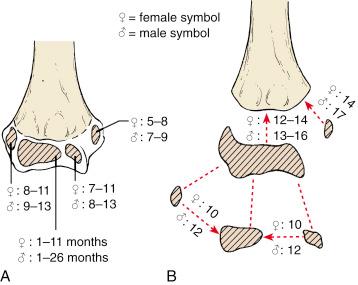
The medial epicondyle is the next ossification center to appear. It may be seen radiographically as early as 5 years of age in some but may not appear until 9 years in others. The medial epicondyle forms its own ossification center in the distal end of the humerus, whereas the capitellum, the trochlea, and the lateral epicondyle fuse to form a single ossification center. The trochlea, which appears next, may become ossified as early as 7 years of age, but more commonly, it begins to ossify between the ages of 9 and 10 years. The lateral epicondyle is the last portion of the distal humeral epiphysis to ossify. It may be identified radiographically as early as 8 to 9 years of age.
The capitellum and trochlea may fuse as early as the age of 10 years, but fusion usually begins by 12 years of age. This combined ossification center fuses to the lateral epicondyle at the same time to form the main body of the distal humeral epiphysis. The epiphysis fuses to the metaphysis of the humerus as early as 12 to 13 years of age, which signals the end of longitudinal growth of the distal humeral physis. Finally, the medial epicondyle fuses to the distal end of the humerus between 14 and 17 years of age.
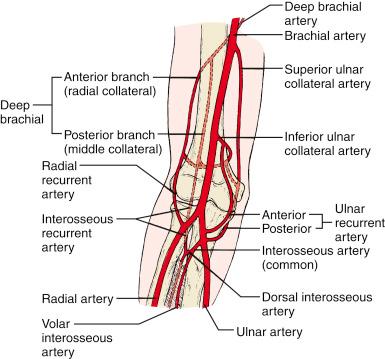
The collateral circulation about the elbow is rich and usually sufficient for maintaining adequate circulation to the forearm and hand, even if the main blood supply from the brachial artery is interrupted ( Fig. 17.2 ). Although interruption of the brachial artery may not result in loss of the limb, it usually produces some signs of ischemia, such as claudication and cold intolerance.
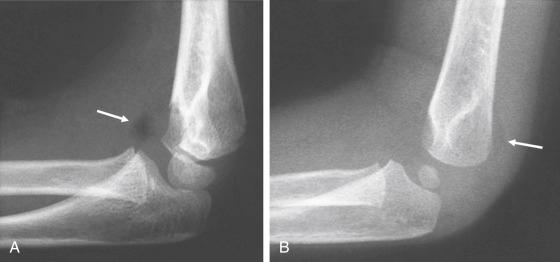
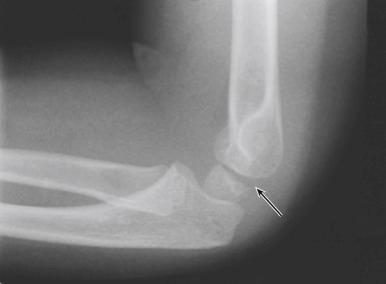
The entire articular surface of the distal end of the humerus is intraarticular; however, the medial and lateral epicondyles are both extraarticular. The elbow capsule attaches to the ulna distal to the olecranon and coronoid process, so these structures are intraarticular. In addition, the entire radial head is located within the capsule, thus making it intraarticular. Two elbow fat pads are located between the capsule and the distal end of the humerus: one anterior and the other posterior. The radiographic appearance of these fat pads may aid in diagnosing injuries about the elbow; with an elbow effusion, one or both may become elevated from the distal humeral surface and can be seen as a lucent area on lateral radiographs ( Fig. 17.3 ). De Beaux and associates analyzed 45 cases of elbow trauma in children with one or two elevated fat pads and no radiographic evidence of a fracture. They found only 6% of those who underwent repeated radiographs 2 weeks later to have a fracture and concluded that routine repeated radiographs are not necessary unless children remain symptomatic. In a study by Skaggs and Mirzayan, 76% of children with a visible posterior fat pad but no fracture on initial radiographs were subsequently found to have a fracture on follow-up radiographs at 2 to 3 weeks, as evidenced by periosteal reaction of the distal humerus, olecranon, or proximal radius.
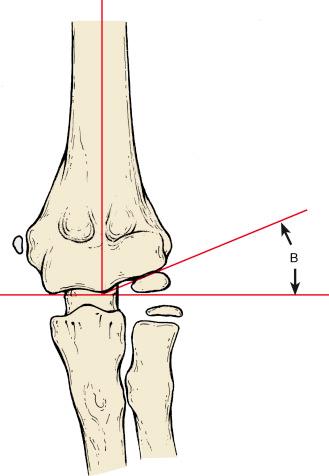
Suspected elbow fractures are best evaluated on high-quality anteroposterior (AP) and lateral radiographs. On the AP view, the Baumann angle and the medial epicondylar epiphyseal angle are important landmarks for assessing supracondylar fractures. The Baumann angle is defined by the intersection of a line drawn along the physis of the capitellum and a line perpendicular to the longitudinal axis of the humerus on radiographs ( Fig. 17.4 ). Williamson and associates found the Baumann angle to be 72 degrees (standard deviation, 4 degrees), and 95% of normal elbows had a Baumann angle of 64 to 81 degrees. The Baumann angle is, however, dependent on the position of the elbow and is particularly affected by rotation. Accuracy of measurement is assured by obtaining a true AP radiograph of the distal end of the humerus ( Fig. 17.5 ). When the elbow cannot be fully extended, the x-ray beam and radiographic plate must be adjusted so that the beam is perpendicular to the distal end of the humerus and the plate parallel to the long axis of the humerus to avoid inadvertently obtaining a tangential view of the distal humerus and any parallax that might adversely affect measurement. Comparison of the Baumann angle of the contralateral uninjured elbow is often the best way to determine the adequacy of reduction of a supracondylar fracture, with the caveat that radiographs of the distal ends of each humerus should be obtained in matched rotation and flexion to attempt to negate any variability in measurement caused by differences in position.
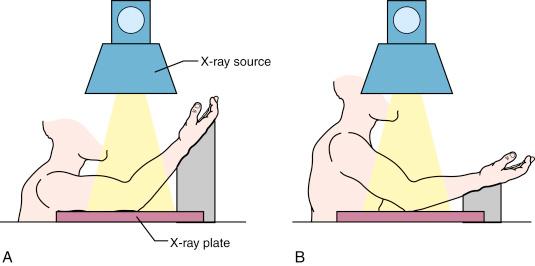
The medial epicondylar epiphyseal angl e may also be useful in determining the accuracy of reduction of supracondylar humeral fractures. This angle is formed by the intersection of lines drawn along the medial epicondylar growth plate and the longitudinal axis of the humerus. In younger children, in whom the medial epicondyle has not yet ossified, one can measure this angle by drawing a line along the medial metaphyseal border of the distal humerus and referencing it to a line drawn along the longitudinal axis of the humerus ( Fig. 17.6 ). As with the Bauman angle, comparison with the contralateral uninjured elbow is the best way to determine reduction.
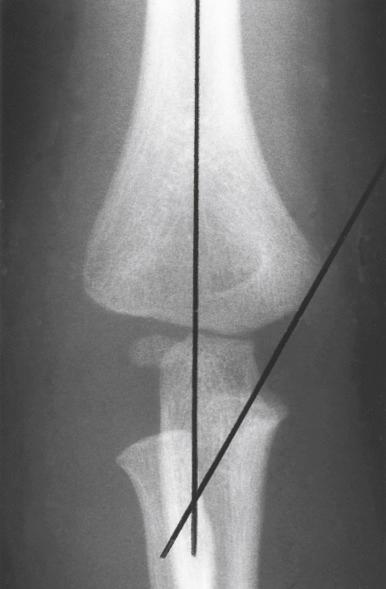
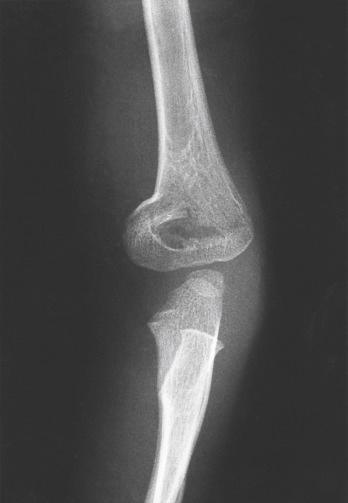
On the lateral view, the lateral capitellar angle is measured by the intersection of a line parallel to the midpoint of the distal humeral shaft and one drawn through the midpoint of the capitellum. The normal inclination is approximately 30 degrees anterior ( Fig. 17.7A ). The anterior humeral line is a radiographic marker that is drawn along the anterior cortex of the humerus. It should pass through the middle of the ossified capitellum. If this line passes anterior to the middle of the capitellum, the distal end of the humerus has been displaced posteriorly. Conversely, if it passes posterior to the middle of the capitellum, the distal end of the humerus has been displaced anteriorly ( Fig. 17.7B ). This measurement is only accurate on a true lateral radiograph of the distal humerus. Skibo and Reed showed that if the humerus is rotated even slightly, the anterior humeral line is not reliable and, in many instances, is falsely positive. More recently, Herman and associates have shown that in children younger than 4 years, the line may lie in the anterior third of the capitellum. However, Murphy-Zane and Pyle have shown high intraobserver reliability in using this measurement to characterize posteriorly hinged supracondylar humerus fractures. The anterior coronoid line is another sagittal radiographic marker. It is defined by a curvilinear line drawn along the coronoid and continued proximally, where it should just touch the anterior aspect of the capitellum. If the distal humerus is angled or displaced posteriorly, the capitellum will lie more posterior ( Fig. 17.7C ). Silberstein and colleagues noted that the physis of the capitellum is wider posteriorly than anteriorly when viewed on a lateral radiograph. This appearance may be mistaken for an injury to the physis if one is not familiar with the normal radiographic anatomy ( Fig. 17.8 ).
Comparison radiographs of the uninjured elbow have long been advocated as a way to assist in the diagnosis of subtle elbow trauma in children. However, in a study by Cheng and Shen, in which orthopedic residents, emergency physicians, and a pediatric radiologist evaluated radiographs of injured and contralateral elbows in 3350 children, the authors found that comparison radiographs did not improve the diagnostic accuracy of elbow trauma in the pediatric emergency department.
Other means of evaluating elbow trauma include magnetic resonance imaging (MRI) and ultrasound. MRI has been shown to be a sensitive and accurate method in the diagnosis of occult fractures about the elbow and more accurate than conventional radiography in defining the fracture pattern and extent of articular disruption in fractures extending into the cartilaginous epiphysis. Access to MRI in the acute setting and need for sedation in uncooperative children are two significant barriers to the use of MRI for the evaluation of elbow trauma. Ultrasonography has also been shown to be a sensitive study for diagnosing fractures of the elbow in children, especially the very young in whom ossification of the distal end of the humerus is minimal. A recent prospective study of 130 patients showed that point-of-care ultrasonography performed by emergency department physicians was also highly sensitive in diagnosing elbow fractures in older children (mean age, 7.5 years).
The carrying angle of the elbow is the clinical measurement of coronal (varus/valgus) angulation of the arm with the elbow fully extended and the forearm fully supinated. The intersection of a line along the midaxis of the upper part of the arm and a line along the midaxis of the forearm defines this angle. Beals has shown that the carrying angle varies widely among individuals. The angle increases with age, and no consistent difference is seen between males and females. The carrying angle of a given elbow is best evaluated by comparison with the contralateral elbow.
Supracondylar fractures occur most often in the first decade of life and account for up to 17% of all pediatric fractures and 30% of all fractures in children younger than 7 years. Extension injuries are the most common type, accounting for more than 95% of cases.
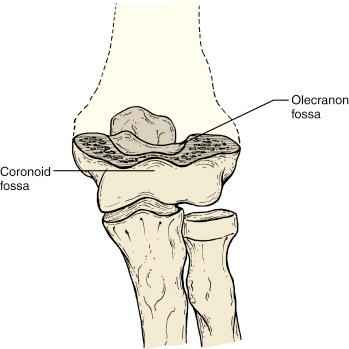
The anatomy of the distal end of the humerus explains the susceptibility to injury in this location and instability of these types of fractures when they do occur. The medial and lateral columns of the distal humerus, which are relatively thick and strong, are connected by a thin wafer of bone that is only 1 mm thick in the central portion, separating the olecranon fossa posteriorly from the coronoid fossa anteriorly ( Fig. 17.9 ).
Supracondylar fractures of the humerus may be produced by either a hyperextension or a flexion mechanism. The extension -type fracture, resulting from a fall on an outstretched hand with the elbow hyperextended, is the more common pattern of injury. In this type of injury, the full force from the fall is directed to the anatomically weak olecranon fossa. Several studies have implicated the occurrence of supracondylar humeral fractures with the presence of ligamentous laxity and elbow hyperextension, both of which are common findings in children in the first decade of life when this injury is most common. Henrikson studied children who had sustained supracondylar humerus fractures and found that their uninjured elbows were capable of more than the average amount of hyperextension. Others have correlated the relationship of ligamentous laxity and the site of upper extremity fractures, suggesting that children whose elbows are capable of hyperextension because of the presence of ligamentous laxity are more likely to sustain a supracondylar fracture as a result of a fall on an outstretched hand, whereas children without hyperextension of the elbow are more likely to fracture the distal forearm. The less common flexion -type supracondylar fracture results from a fall on the olecranon with the elbow bent.
When the elbow is loaded in extension, the distal humerus begins to fail under tension. The anterior cortex of the distal humerus then fractures, and the anterior periosteum stretches over the fracture. If at this point, the hyperextension force ceases, a nondisplaced or minimally angulated fracture occurs. This injury has been termed a stage I fracture by Abraham and colleagues. Radiographically, one may see only soft tissue swelling or possibly a decrease in the normal anterior inclination of the capitellum on a lateral view.
Continued application of an extension force leads to a stage II fracture that is characterized by further extension and angulation of the distal fragment but no translation. In a stage III fracture , the anterior periosteum is completely torn, and the distal fragment is displaced posteriorly, with translation. The periosteum is usually intact on the side to which the fracture is displaced, and can be used as a hinge to assist in reduction of the fracture. If the fracture is displaced posteromedially, which is generally the case, a posteromedial periosteal hinge is usually preserved. If the fracture is displaced posterolaterally, the intact periosteum is on the posterolateral side of the elbow. The location of the bruise in the antecubital fossa represents location of periosteal disruption and is another clue as to the direction of displacement of the fracture.
Ipsilateral fractures of the distal radius (so-called remote floating elbow) are most commonly caused by a similar injury mechanism (fall on an outstretched hand). Concomitant diaphyseal radial and ulnar fractures are less common but, when present, are associated with a higher risk of compartment syndrome.
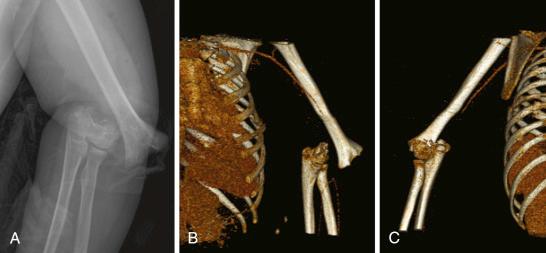
Supracondylar fractures of the humerus are associated with a relatively high risk of nerve injury (between 7% and 15.5%), although Campbell and associates found that in 59 patients with type III supracondylar fractures, 24 (41%) had acute nerve injuries. A recent metaanalysis indicated that nerve injuries occur in 11.3% of patients with supracondylar fractures. Garg and associates reported a 12% incidence of nerve injury in the largest single center study of type III supracondylar humeral fractures ( n = 872) reported to date.
Older reports showed that 45% of nerve injuries involved the radial nerve, and 32% involved the median nerve. Campbell and colleagues found that anterior interosseous and/or median nerve injury was associated with posterolateral displacement 87% of the time. The radial nerve was injured when the fracture became displaced in a posteromedial direction. The ulnar nerve is less commonly involved (23% of the time) and is more often associated with flexion-type supracondylar fractures. Although radial nerve injury was the most commonly reported in the older literature, more recent investigators have found the anterior interosseous branch to be the most commonly injured nerve. As the metaphyseal fragment is displaced anteriorly, the median nerve is stretched. Anatomically, the anterior interosseous nerve (AIN) branch is at risk because it is tethered under the fibrous arch that arises from the deep head of the pronator teres. The AIN is a purely motor nerve; therefore, diagnosis of injury to it requires a specific examination of the flexor pollicis longus and flexor digitorum profundus of the index finger. Concomitant loss of sensation would be suggestive of a median nerve injury, rather than just an AIN palsy.
Although the consequences of vascular injury associated with supracondylar humeral fractures may be significant, permanent vascular compromise of the extremity is rare. In a recent single-center study of 872 type III supracondylar humeral fractures, an absent pulse was found in 54 (6%), of which only five underwent vascular repair. The brachialis muscle protects the brachial artery. With significant posterior displacement, the brachialis muscle may be torn by the metaphyseal fragment, compromising the protection it provides to the brachial artery ( Fig. 17.10 ). The supratrochlear artery can also tether the brachial artery when there is significant displacement of the fracture, causing occlusion. Reducing the fracture usually relieves occlusion of the artery unless it is physically present within the fracture gap. In this instance, the circulation is usually satisfactory until the fracture is manipulated and the artery is compressed by the fracture fragments, which results in loss of the radial pulse and compromised perfusion of the extremity. An entrapped brachial artery may be accompanied by the median nerve, which together may prevent reduction of the fracture. Whenever the inability to reduce a supracondylar humeral fracture perfectly is accompanied by absence/loss of the radial pulse, particularly in the presence of a median/AIN deficit, one should be aware of the strong possibility that the artery and (possibly) nerve are entrapped in the fracture and require open reduction to free the neurovascular structures.
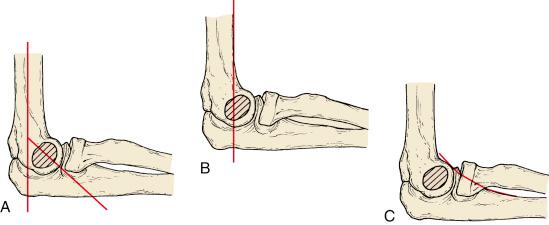
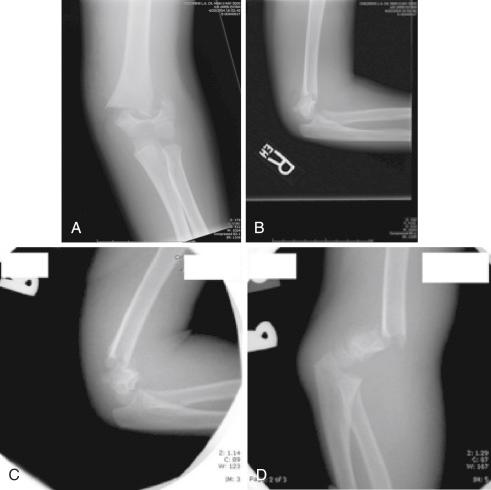
Supracondylar humeral fractures may be classified as extension or flexion-type injuries. Extension injuries are much more common (>95%) and are further defined as to the extent of displacement by the Gartland classification ( Fig. 17.11 ). Type I fractures are nondisplaced. The fracture line may be easily visible or indistinct. Good lateral views and observation of fat pad elevation help identify this fracture radiographically. Type II fractures are angulated but have an intact posterior periosteal hinge. Radiographically, the anterior angulation of the capitellum (normally 30 degrees) is diminished, and the anterior humeral line is positioned anterior to the middle of the capitellum (see Fig. 17.7 ). Type III fractures are completely displaced, with loss of all continuity between the two fragments. Displacement is most often posteromedial and less frequently posterolateral. Wilkins modified the Gartland classification by dividing type II fractures into A and B subtypes. Type IIA fractures are extended but not rotated or translated; type IIB fractures have some component of rotational displacement or translation. Clinical implications are that IIA fractures are stable after manipulative reduction and may be treated closed, whereas IIB injuries are usually unstable and more likely to require reduction and fixation ( Fig. 17.12 ).
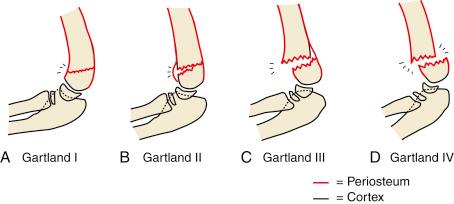
Leitch and colleagues proposed the addition of a type IV fracture to the original Gartland classification. Type IV fractures are multidirectionally unstable in both flexion and extension because of complete loss of both anterior and posterior periosteal hinges and are thought to be caused by either excessive trauma or overzealous anteriorly directed force during attempted reduction of a type III fracture ( Fig. 17.13 ).
Children who are old enough to provide an adequate history usually describe a fall while running or from a height (typically playground equipment) and landing on an outstretched hand with the elbow extended. Children with supracondylar fractures have pain and swelling about the elbow, decreased range of motion, and variable amounts of deformity. Skin in the antecubital fossa is usually ecchymotic, and if the fracture is significantly displaced, it may cause tenting of the skin or puckering if the metaphyseal fragment is buttonholed through the brachialis muscle and fascia. A thorough assessment of skin integrity must be performed to be sure the fracture is not open, and an assessment of the rest of the upper extremity must be performed to be sure no other skeletal injury is present.
A complete neurologic evaluation of the arm is essential because of the frequency of nerve injuries associated with this fracture. The neurologic evaluation also establishes a baseline to compare to after treatment has been completed. AIN injuries are most common, followed by injuries to the median, radial, and ulnar nerves. Ulnar nerve injury is usually associated with flexion-type fractures. The neurologic examination should include motor and sensory assessment of the median, ulnar, and radial nerves. However, formal testing of nerve function is not always possible because of pain, anxiety, or lack of cooperation, and baseline assessment of nerve function often has to be made based on observation of spontaneous movement over several encounters with the child, particularly in the very young. In general, nerve deficits that are present immediately after the injury represent neurapraxia and will resolve spontaneously. In contrast, a change in neurologic status after treatment is more likely to be indicative of injury during manipulation, with pinning or by entrapment in the fracture site. This type of injury usually requires exploration and intervention.
Assessment of vascular status is also paramount. Assessment of perfusion of the hand is the most important indicator of vascular status of the extremity. The distal radial pulse should be palpated and, if not present, checked by Doppler ultrasonography. It is not uncommon for the radial pulse to be absent at the time of initial evaluation because of compression or tethering of the brachial artery over the anterior aspect of the proximal fragment of the fracture or arterial spasm. If the pulse is absent, the elbow should be flexed to relieve any pressure on the artery. Although absence of the radial pulse causes concern, the artery is rarely torn or damaged permanently. The extensive collateral circulation around the elbow allows for sufficient perfusion to the forearm and hand in most instances to maintain viability, even if the artery is damaged. Regardless of whether the radial pulse is present, indicators of adequate distal perfusion include color (“pink”), temperature (“warm”), and normal capillary refill. The vascular status of the extremity can be described as normal, perfused (pink hand) but pulseless, or dysvascular (pulseless and white). Ischemia leading to compartment syndrome has been associated with this fracture and therefore should be carefully evaluated. Children with impending compartment syndrome may appear agitated and anxious and have increasing analgesic requirements long before the classic signs of ischemia—pain, paresthesia, pallor, paralysis (loss of motor function), and absent pulse—are evident. In patients in whom compartment syndrome is a concern, forearm compartment pressures should be measured. The risk of compartment syndrome is higher in patients with floating elbow injuries.
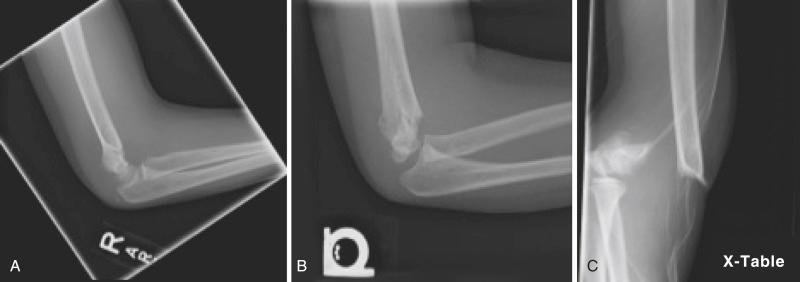
Accurate radiographic diagnosis of a type III fracture of the supracondylar region of the distal end of the humerus is not usually difficult. Correct diagnosis of type I and occasionally type II fractures may be more difficult. As previously mentioned, use of the fat pad signs on lateral radiographs is helpful in localizing the trauma to the region of the elbow joint when no fracture line is clearly identified. In addition, on a lateral radiograph, one should look for any alteration in the intersection of the capitellum with the anterior humeral line; if this line crosses anterior to the middle of the capitellum, a type I or II supracondylar fracture is likely to be present.
A patient with a supracondylar humeral fracture and a pale, pulseless hand requires emergent surgical attention. Closed supracondylar humeral fractures in children without associated neurovascular injury can be successfully managed by closed methods or surgery on an urgent basis. Surgical options include closed or open reduction and stabilization with Kirschner wires (K-wires). Specific treatment of extension-type supracondylar humeral fractures is determined by Gartland type.
Type I fractures are typically treated closed with 3 to 4 weeks of immobilization in a long arm cast with the elbow flexed to 90 degrees. If there is a large degree of swelling, a posterior splint may also be used. A small degree of posterior angulation of the distal fragment in a younger child may be accepted in anticipation of remodeling, although there may be hyperextension of the elbow and decreased elbow flexion until it does. Normally, the capitellum is angulated anteriorly about 30 degrees, and reduction is not required if the posterior angulation is 20 degrees or less, or if the anterior humeral line intersects any part of the capitellum.
One pitfall in treating a type I fracture is not recognizing impaction of the medial column that, if left uncorrected, can result in a varus deformity, which will not correct with growth ( Fig. 17.14 ). De Boeck and associates identified 13 patients with medial compression of the distal end of the humerus in otherwise innocent-looking type I fractures that developed varus deformities. A type I fracture with medial compression must be reduced so to avoid development of cubitus varus. With the patient under general anesthesia, the fracture is reduced by application of longitudinal traction with the elbow in full extension. An assistant applies countertraction to the upper part of the arm. Valgus correction is obtained with the use of the forearm as a lever. Once reduced, the fracture may be treated in a long arm cast in extension or preferably, because the medial column may be inherently unstable, with crossed K-wires to prevent drift back into varus. Once the fracture is stabilized, the elbow may be flexed to 80 degrees to 90 degrees and immobilized for 3 weeks.
Management of type II fractures is controversial. Type IIA fractures, which are extended but have no rotation or translation, may be successfully treated with closed reduction and casting. Alignment of these fractures does need to be closely monitored for loss of reduction, primarily in the coronal plane because varus or valgus angulation will not remodel. Residual posterior angulation can be expected to remodel with growth because the deformity is close to and primarily in the plane of motion of the joint. Two pitfalls in managing type IIA fractures closed are accepting reduction when the distal fragment is angulated too far posteriorly such that the capitellum is posterior to the longitudinal axis of the anterior humerus, and mistaking posterior translation of the distal fragment for angulation. In the former scenario, the patient may be left with a significant hyperextension deformity of the elbow that is more of a cosmetic problem than a functional one, and in the latter, the patient may have a significant flexion lag that can be a functional problem, particularly if it involves the dominant extremity. Type IIB fractures ( Fig. 17.15 ) should be treated operatively with closed reduction and percutaneous pinning.
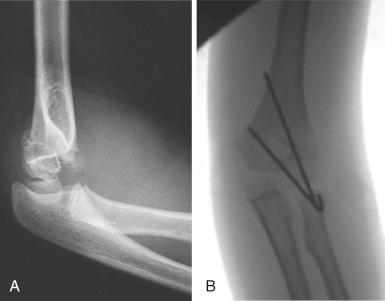
In all type II fractures, coronal angulation of the elbow must be carefully assessed. Clinical examination of the fully extended elbow under general anesthesia is the best means of accurately assessing the carrying angle. Type II fractures with posterior medial compression may be reduced and casted with the use of the technique described previously for type I fractures with impaction of the medial column. Reduction may be achieved by first extending the elbow and correcting the coronal plane deformity and then flexing the elbow while pronating the forearm to address sagittal plane angulation. Millis has shown that hyperflexion of the elbow to more than 120 degrees may be necessary to maintain reduction. Despite techniques like the figure-of-eight cast described originally by Rang to avoid circumferential wrapping of the elbow to achieve this position, maintenance of hyperflexion carries a high risk of skin problems, neurovascular compromise, and compartment syndrome. Therefore, if there is significant soft tissue swelling, any question of vascular compromise, or fracture instability in any type II fracture, the fracture should be pinned percutaneously.
Type II fractures with translational or rotational deformity are unstable and are best treated in the same way as type III fractures (see next section). With the patient under general anesthesia and fluoroscopy available, the fracture may be reduced by application of a valgus force on the extended elbow to correct any medial compression/angulation. Next, the elbow is maximally flexed, and the olecranon is pushed anteriorly to reduce posterior angulation/translation. Pin fixation with K-wires is performed to maintain the reduction. Fixation with two lateral pins is generally sufficient for type II fractures, which are inherently more stable than type III fractures. The arm is immobilized in a posterior splint or cast with the elbow at 60 to 80 degrees of flexion.
Type III fractures are best managed by closed reduction and percutaneous pin fixation. Type III fractures are more severe injuries that are associated with more significant swelling and soft tissue injury, are more difficult to reduce, and are more likely to have neurovascular injuries or complications. These fractures are completely displaced. In most instances, the proximal and distal bone fragments are not in contact, and only a small bridge of periosteum may be preserved posteriorly. These fractures are prone to developing residual deformity, particularly cubitus varus, when treated by closed reduction and casting. In separate retrospective studies by Kurer and Regan and Pirone and associates of completely displaced supracondylar fractures, closed reduction and splinting or casting resulted in significantly fewer good results and more complications than traction or percutaneous pinning, which provided the best results. Based on the best current evidence and a systematic review of published studies as reported by Howard and associates, closed reduction with percutaneous pinning is widely accepted as the best method of treatment for all type III fractures, as well as for any type II fractures that are not reducible or that are associated with neurovascular status changes during fracture reduction.
Type IV fractures are unstable in both flexion and extension, have complete disruption of the periosteum both anteriorly and posteriorly, and need to be managed operatively (see Fig. 17.13 ). This fracture is inherently unstable and requires internal fixation. Leitch and colleagues described a method of closed reduction and percutaneous fixation that involves placement of K-wires before reduction of the fracture and rotation of the fluoroscopy unit, rather than the extremity, to obtain orthogonal views. However, these fractures may need to be opened to obtain adequate reduction.
Accurate closed reduction of supracondylar humeral fractures is critical for preventing cubitus varus deformity, regardless of whether the fracture is treated closed or pinned. Reduction of the fracture is accomplished with the patient under general anesthesia and with fluoroscopic guidance. With the elbow extended, gentle longitudinal traction is applied to the supinated forearm while countertraction is applied to the upper part of the arm by an assistant. The distal fragment is then translated medially or laterally, depending on the position of displacement, by application of digital pressure to the appropriate condyle. Most of these fractures are displaced posteromedially, and reduction of this component of the deformity is achieved by having the surgeon flex the patient’s elbow to 120 degrees while simultaneously pronating the forearm and applying digital pressure with the thumb of the opposite hand to the olecranon to reduce the posterior displacement of the distal fragment. Pronating the forearm is thought to engage the intact posteromedial periosteum and allow the fracture to be reduced. Pronation also causes the wrist extensors and brachioradialis to tighten, which helps close down and stabilize the fracture laterally. If the fracture is displaced posterolaterally, the forearm is supinated so that the intact posterolateral periosteal bridge can be used in a reciprocal manner.
The elbow must be flexed maximally so that reduction of the fracture is maintained while imaging is performed to assess alignment. A lateral view is relatively easily obtained by external rotation of the shoulder. Alternatively, if the reduction is tenuous, this view can be obtained by rotation of the fluoroscopy tube to a cross-table position so that the arm does not have to be moved. Adequate sagittal alignment can be assessed by the relationship of the capitellum to the anterior humeral line and the presence or absence of overlap of the ossification center of the capitellum on the olecranon. Coronal alignment of the fracture is assessed with the so-called Jones view , which is, in essence, an AP image of the distal humerus taken through the flexed, overlying forearm ( Fig. 17.16 ). Although detail of the distal humerus is slightly obscured, the image is usually adequate for assessment of the alignment of the medial and lateral columns of the distal humerus and restoration of the olecranon fossa by slight internal and external rotation of the arm. The Baumann angle and the medial epicondylar epiphyseal angle can be measured on this view. Otsuka and Kasser believed that the Baumann angle, the relationship of the capitellum to the anterior humeral line, and restoration of the normal anatomy of the olecranon fossa represented the best indicators of a satisfactory reduction.
Once the fracture has been reduced, immediate flexion of the elbow with either pronation (for posteromedial displacement) or supination (for posterolateral displacement) of the forearm is required to maintain reduction in a cast. The problem with this position is that immediate flexion of the elbow increases the tension in an already swollen extremity, thus increasing the risk of vascular compromise by reduction of arterial flow into and venous flow out of the forearm. In a clinical study, Mapes and Hennrikus showed that the Doppler pulse became weaker and even disappeared in patients with supracondylar humeral fractures whose elbows were flexed. The more the elbow was flexed, the weaker the pulse became. Because of the dilemma of potential vascular compromise with cast immobilization in the position necessary to prevent loss of reduction, percutaneous pinning has emerged as the treatment of choice for maintaining alignment after closed (or open) reduction of displaced supracondylar humeral fractures.
The modern era of treatment of this fracture began in 1948 with a description by Swenson of percutaneous pinning of distal humeral fractures in adults. In 1961, Casiano reported the use of this technique in children. Since this description, multiple reports of the use of percutaneous pinning for the maintenance of reduction of a displaced supracondylar fracture have appeared. Traditionally, a crossed pin configuration has been used to stabilize these fractures, but increasingly, the recent trend has been to stabilize only with lateral entry pins because of concerns of ulnar nerve injuries that have been reported in up to 10% of patients treated with a medial entry pin.
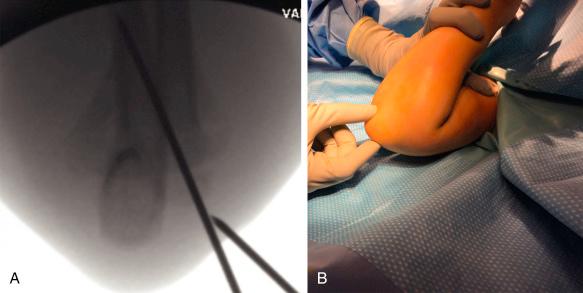
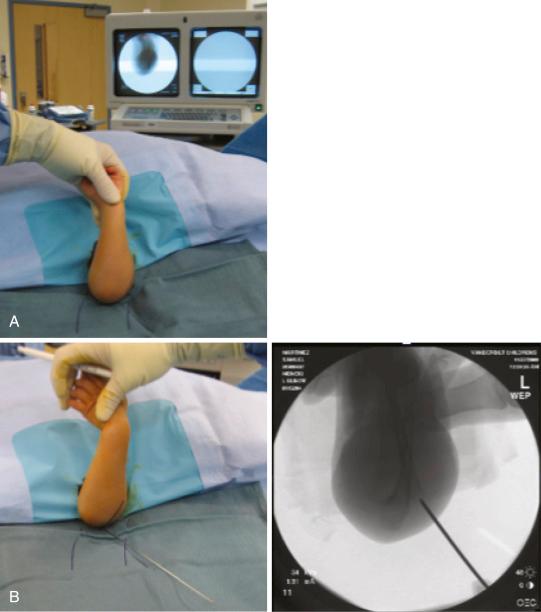
Closed reduction and pinning are typically done with the child positioned supine and the injured limb suspended over the side of the table. This position allows free access to the C-arm, which is placed directly under the arm parallel to the operating table ( Fig. 17.17 ). The procedure is usually done with sterile preparation and draping, with incorporation of the C-arm unit as the operating table. Alternatively, the procedure can be done in a semisterile fashion, as described by Lobst and associates. Their technique does not require drapes or gowns, hence reducing operating room time and costs. In a series of more than 300 cases, there were no superficial or deep pin infections. Reduction of the fracture is carried out as described in the previous section.
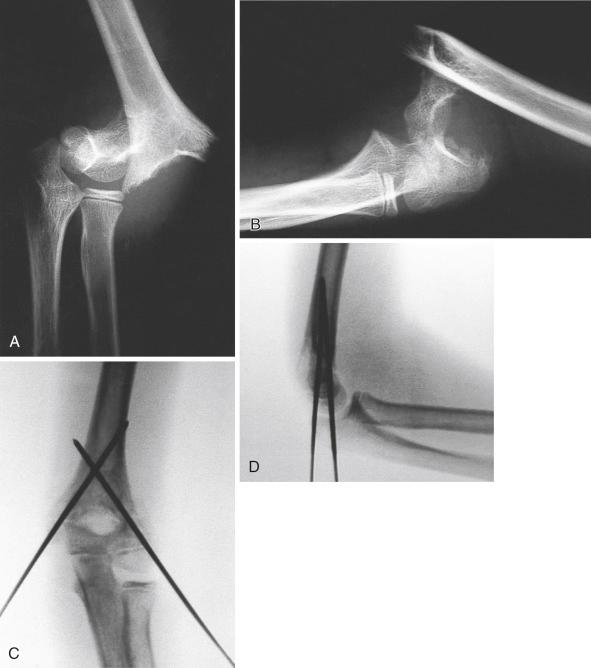
Most fractures can be reduced with this technique; however, if the posterior periosteum is torn, the fracture will be completely unstable, as the hinge that the intact posterior periosteum provides for reduction is lost. Fracture reduction can also be hindered by soft tissue interposition in the fracture. If a spike of the proximal fragment penetrates the brachialis muscle, the muscle must be removed before the fracture is reduced. Peters and colleagues described a technique for dislodging the entrapped brachialis muscle by grasping the arm close to the axilla and gradually “milking” the anterior musculature in a proximal-to-distal direction toward the spike of the proximal fragment while an assistant applies countertraction through the axilla. Pressure is placed primarily on the lateral side to avoid injury to the medial neurovascular structures. Freeing of the entrapped muscle may be accompanied by a palpable sensation of the bone disengaging the soft tissues. Once the brachialis muscle has been cleared, the reduction can usually be completed ( Fig. 17.18 ). One must be cautious manipulating a supracondylar humeral fracture that is displaced in a posterolateral direction with buttonholing of the brachialis muscle, particularly when a neurologic deficit exists, because of the risk of further neurovascular injury. In 27 children with vascular deficits (22 of whom also had median nerve deficits) and posterolaterally displaced fractures, Rasool and Naidoo found that the neurovascular bundle was trapped just anterior to the metaphyseal spike in 18 patients, trapped behind the fracture in five, and separated by the fracture spike in four.
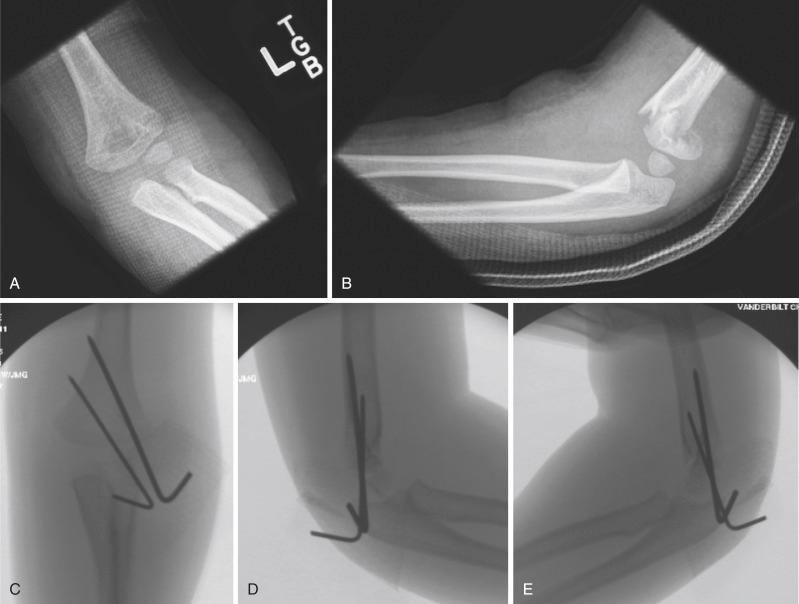
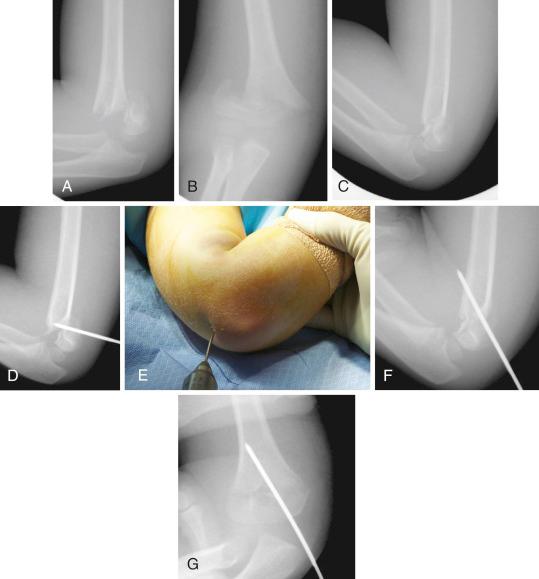
Once obtained, fracture reduction is confirmed fluoroscopically. Sagittal alignment may be checked by either external rotation of the shoulder on the C-arm or, if the reduction is tenuous, by rotation of the C-arm to prevent loss of reduction. The Jones (transcondylar) view is used to assess coronal alignment with the elbow flexed, and alignment of the medial and lateral columns of the distal humerus can be further assessed by slight internal and external rotation of the arm. If reduction is satisfactory, percutaneous pinning is performed. It is debatable whether a medial entry pin should be used or whether two lateral pins are sufficient. It has been shown that crossed pins are the most biomechanically stable ; however, in comparative clinical studies, the stability afforded by two lateral pins has been shown to be adequate for maintenance of reduction in type II and most type III fractures. For type II fractures, the author uses two lateral pins, which afford ample stability ( Fig. 17.19 ), and for type III fractures, the author adds a third pin, either lateral or medial entry, depending on fracture characteristics and surgeon preference, as recommended by Bloom and colleagues ( Fig. 17.20 ). In children younger than 4 years, 0.062-inch K-wires are adequate. In older children, 2-mm wires are more suitable.
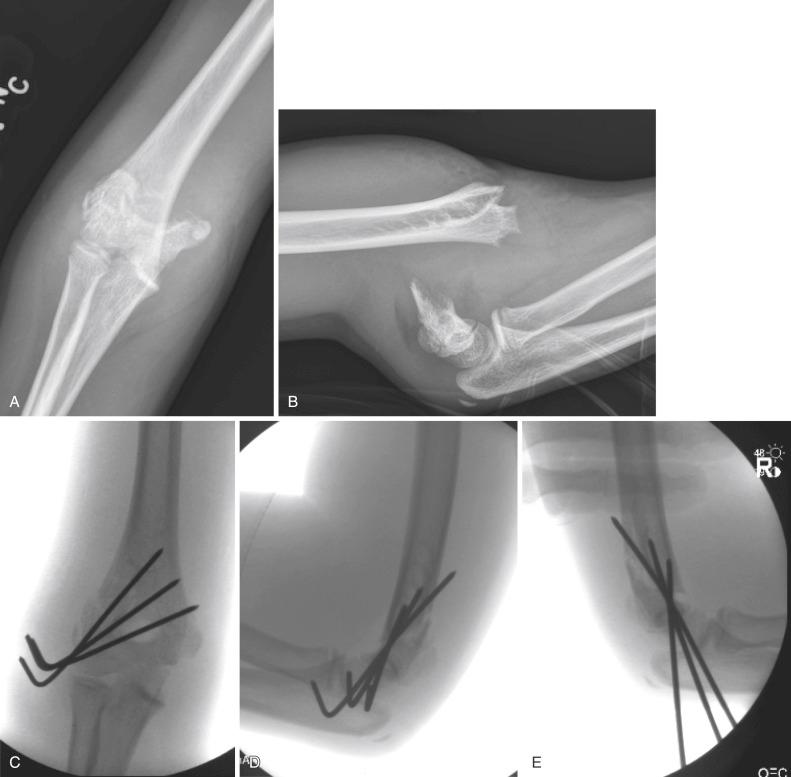
Pinning is performed with the elbow maximally flexed and resting on the C-arm tube. With the arm rotated to the neutral position and a small towel bump placed under the elbow to facilitate clearance from the C-arm, the first K-wire is inserted through the lateral condyle and across the distal humeral physis, ideally aiming up the lateral column of the metaphysis. The path of the pin in the coronal plane can be checked fluoroscopically with the use of the Jones (transcondylar) view. The position and direction of the pin in the sagittal plane can be checked by rotating the shoulder externally. The arm is then rotated back to neutral and the pin advanced to engage the opposite cortex. A second and, if necessary, a third lateral entry pin can be placed in similar fashion with the use of a parallel to slightly divergent path. The surgeon should attempt to achieve as much spread of the pins across the fracture site as possible, avoiding convergence or crossing at the fracture and engaging the cortices of both the lateral and medial columns. A biomechanical study of pin configurations by Lee and colleagues comparing crossed pins, parallel lateral pins, and divergent lateral pins found that the stability provided by divergent lateral pins exceeded that of parallel lateral pins and was similar to crossed pins in all but torsional loading; the study prompted the recommendation for divergent placement for lateral entry pins so that stability could be maximized. If the fracture is comminuted or deemed to be very unstable, a medial entry pin may need to be placed. Zenios and colleagues performed prospective intraoperative evaluation of stability in 21 consecutive patients with type III fractures by taking lateral images of the elbow in external and internal rotation after pinning with two lateral pins; the authors found only six (28%) to be stable, necessitating placement of a third lateral or medial column pin in the remaining 15 fractures. Similarly, Bauer and colleagues found internal rotation stress testing to be helpful in assessing alignment and need for additional fixation in type III supracondylar humerus fractures.
There is no doubt that a crossed pin configuration is biomechanically the most stable construct and historically has been the technique of choice. Zionts and colleagues studied the torsional strength of different pin configurations in displaced supracondylar humeral fractures and found that 37% less torque was required to produce 10 degrees of rotation with the use of two lateral pins than with the use of medial and lateral pins, and it was 80% less if the lateral pins were crossed at the fracture site.
Despite the superior biomechanically stability of crossed pins, the use of this technique has fallen out of favor because of reports of ulnar nerve injury in up to 10% of patients treated with this pin configuration. Injury to the ulnar nerve may occur by direct penetration, particularly when the nerve is unstable and subluxates in flexion. Nerve injury can also occur by tethering if adjacent soft tissues are wrapped up as the pin is inserted, or by stretching around the pin when the elbow is flexed. These potential problems can be mitigated when the surgeon performs medial entry pin placement by extending the elbow to less than 80 degrees of flexion (permissible once fixation of the lateral column has been performed), allowing the ulnar nerve to move posteriorly away from the medial epicondyle. Additionally, a very small incision can be made over the medial epicondyle to allow direct placement of the K-wire on the bone, which ensures that the nerve is avoided during pin placement ( Fig. 17.21 ). The position of the medial pin is then checked with fluoroscopy and advanced through the lateral cortex of the humerus. The pins should cross proximal to the fracture for maximal stability. The elbow can then be extended so that the carrying angle and coronal alignment of the fracture can be assessed on a true AP radiograph. The pins are padded and bent outside the skin, and the arm is splinted or placed in a bivalved cast with the elbow in 70 to 80 degrees of flexion. Using this technique, Green and associates reported no ulnar nerve motor injuries and just one transient ulnar sensory neurapraxia in their single-cohort retrospective study of 71 consecutive children treated operatively for Gartland type II or III supracondylar humeral fractures.
Children should be hospitalized until they are comfortable, their neurologic status can be confirmed, and the risk of circulatory compromise is past. Perioperative ketorolac has been shown to decrease pain, opioid usage, and length of stay. Alignment is checked with radiographs 1 week postoperatively, and the splint is converted to a long arm cast, or the bivalved cast is overwrapped. Pins may be removed and immobilization discontinued 3 to 4 weeks later if radiographs demonstrate adequate healing, although the utility of routine radiographs at this time, in the absence of clinical concerns, has been questioned. Unprotected motion, with activity restrictions, is allowed. Formal physical therapy is not typically necessary because most children regain normal motion on their own.
The relative safety of medial entry pins has been addressed in several recent studies of type III supracondylar humeral fractures. A systematic review by Brauer and associates of 2054 children from 35 studies found the probability of iatrogenic nerve injury to be 1.84 times higher with medial pins. Slobogean and associates performed a metaanalysis of 32 studies with 2639 patients and found a higher rate of ulnar nerve injury with crossed pinning and calculated the number needed to harm to be 28 (i.e., one ulnar nerve injury for every 28 patients treated with cross pinning). In their analysis of pooled data from 5154 fractures, Babal and associates found that medial entry pinning was associated with a 4% risk of injury to the ulnar nerve and that third lateral entry pins were associated with a 3% risk to the median nerve. A prospective randomized clinical study by Kocher and colleagues in 2007 comparing lateral entry pins to medial and lateral entry pins found no statistically significant difference in the rate of ulnar nerve injury. More recently, Garg and colleagues performed a retrospective comparative study of 872 patients with type III supracondylar humeral fractures, representing the largest single-center study of severe supracondylar humeral fractures (all type III), and found a slightly higher but statistically insignificant ( P > .5) increase in the rate of ulnar nerve palsy with medial entry pins. Specifically, 5 of 335 patients with medial pins and 4 of 537 patients without medial pins developed an ulnar nerve palsy.
Sawaizumi and colleagues described a technique referred to as “leverage pinning” to facilitate percutaneous reduction of displaced supracondylar humeral fractures that avoids the need for hyperflexion of the elbow. In their original description, the authors positioned the patient laterally and placed the elbow in a reduction bracket attached to the operating room table with the forearm allowed to hang free. Under C-arm guidance, the medial and lateral alignment was corrected and confirmed on an AP view. Next, a 2-mm K-wire was introduced percutaneously through the triceps, in a proximal-to-distal direction, into the fracture site, engaging the proximal fragment just past the posterior cortex. The K-wire was then used as a “joystick” to lever the distal fragment in an anterior direction by movement of the pin distally. Once reduction was obtained, the K-wire was advanced through the anterior cortex of the humerus, thus stabilizing the fracture. An additional lateral pin was added to complete fixation. Another option is to place two lateral or medial and lateral pins and remove the reduction pin.
Yu and colleagues reported their experience with a similar technique, done with the patient supine, using a temporary 3-mm K-wire to facilitate reduction of type III supracondylar humeral fractures that could not be realigned with traditional closed maneuvers ( Fig. 17.22 ). They used this technique in 42 of 118 patients and did not have to resort to open reduction of the fractures. Their results with this technique were similar to conventional closed reduction and cross pinning.
The indications for open reduction of supracondylar fractures include fractures that cannot be reduced by closed methods, those that are not amenable to closed treatment because of intraarticular comminution, open fractures that require irrigation and débridement, and fractures with vascular compromise or neurologic loss after reduction that require exploration and possible repair of neurovascular structures. It is unusual for fractures to be irreducible by closed means; however, when it occurs, the most common cause is interposition of soft tissue or neurovascular structures. The brachialis is a common soft tissue impediment to reduction. Entrapment of the brachial artery within the fracture may be heralded by vascular compromise after closed reduction. Fleuriau-Chateau and associates reported on 41 open reductions performed for irreducible supracondylar fractures. The most common finding intraoperatively was buttonholing of the brachialis muscle by the distal end of the proximal fragment. They also found tethering of the median or radial nerve (or both), with or without the brachial artery, that was not expected on the basis of preoperative evaluation.
Failure to achieve adequate reduction is the most common cause of a poor outcome after supracondylar humeral fractures, and open reduction is preferable to repeated attempts at closed reduction or accepting suboptimal alignment. Results after open reduction compare favorably to closed reduction and pinning. Cramer and colleagues found comparable results for open reduction with pin fixation and closed reduction with percutaneous pin fixation, despite the fact that the fractures in the open reduction group were more severe and were unable to be reduced by closed means. Ozkoc and colleagues reported on 99 patients with displaced extension-type supracondylar fractures of the humerus. The first 44 were treated with open reduction and internal fixation because of a lack of an image intensifier. The next 55 patients were treated with closed reduction and percutaneous pin fixation. These authors found that the open surgical group had slightly worse functional outcomes. They lost an average of 6 degrees of extension and 8 degrees of flexion compared with 0.6 and 8 degrees, respectively, in the closed group. There were no cosmetic differences. This report confirms that closed reduction and percutaneous pinning is the preferred treatment but that open reduction also leads to very good results, when indicated.
Anterior, medial, lateral, and posterior surgical approaches to the distal humerus have all been described. A guiding principle in choosing an approach for supracondylar fractures is that it should be performed through the area of disrupted periosteum. The presence of a neurovascular deficit should also be considered when an approach is chosen. An anterior approach through a transverse incision in the antecubital fossa provides access to the common soft tissue impediments to reduction and the best exposure of the neurovascular structures ( Fig. 17.23 ). The scar runs parallel with the normal skin folds and, as such, is very cosmetic and avoids the contracture that can be associated with a longitudinal incision made across the flexor surface of the elbow. In addition, it can be converted to an extensile exposure by extending the incision proximally in a longitudinal fashion along the medial side of the brachium and/or distally along the radial side of the forearm as needed. The medial approach is preferred for flexion-type injuries in which the ulnar nerve is likely to be trapped in the fracture.
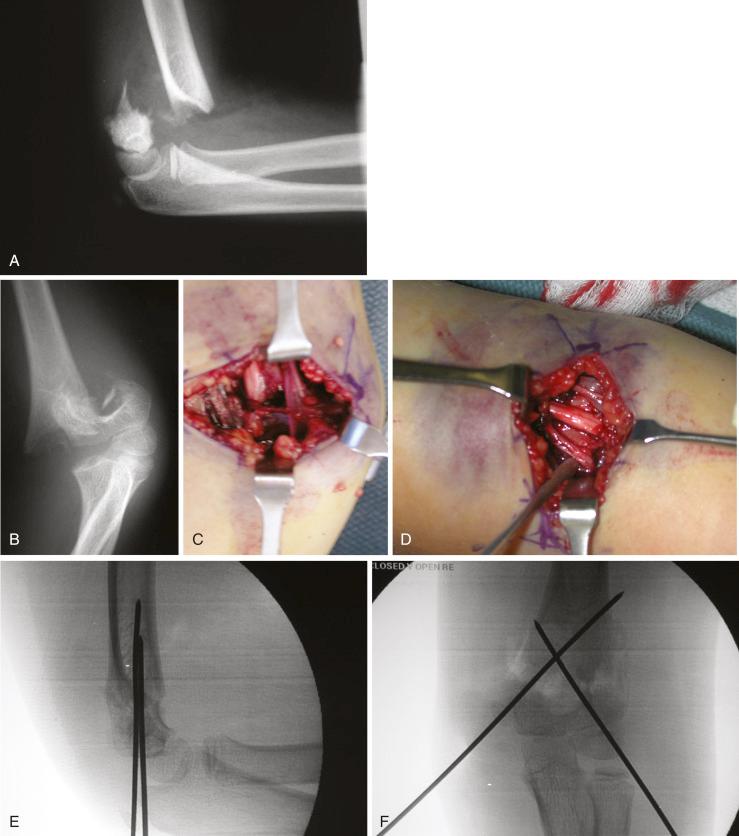
Koudstaal and colleagues reported their outcomes in 26 patients in whom the anterior approach was used and compared them with a historical group of their own patients whose fractures were treated through a lateral or combined medial and lateral approach. They used a transverse incision in the antecubital fossa. Results were evaluated with the use of Flynn’s criteria, and no statistically significant difference was noted between the three approaches. Advantages of the anterior approach were as follows: (1) a more thorough hematoma evacuation from the antecubital fossa, (2) excellent fracture visualization with concurrent visualization of entrapped muscle and vascular and neural structures, and (3) the ability to directly palpate the medial and lateral epicondyles, which allows the surgeon to correct any residual displacement. Ay and colleagues also performed a retrospective review of 61 children with displaced supracondylar humeral fractures who had open reduction and K-wire fixation via an anterior approach and found that all patients had either an excellent (73%) or good (27%) outcome by Flynn’s criteria.
The medial approach is performed with the shoulder externally rotated. A 3- to 4-cm longitudinal incision is made on the medial side of the distal end of the humerus and elbow. Once the skin and subcutaneous fat have been incised, the fracture hematoma and the fracture are encountered. The ulnar nerve is protected but does not have to be visualized. The periosteum over the anterior aspect of the proximal fragment is usually stripped by the injury. The fracture hematoma can be evacuated and the fracture explored by visual and digital inspection; this ensures that no neurovascular structures are trapped. Adequate visualization of the fracture is necessary to ensure anatomic reduction, but care must be taken not to disrupt the intact posteromedial periosteal hinge. The fracture is reduced and cross pinned. If anatomic reduction is not possible, a lateral approach is made to ensure a perfect reduction. The lateral incision, which is also longitudinal, is made over the lateral condyle of the distal end of the humerus; such an incision allows exposure of the lateral side of the fracture. As with closed reduction, the pins are left protruding from the skin and are bent over felt.
The posterior approach to the elbow is preferred when there is intraarticular extension or comminution of the distal humeral condyles in older patients. It is not generally indicated in young children with type III supracondylar fractures because it may compromise the only intact periosteum and blood supply to the distal humerus.
Postoperative discomfort is usually minimal, most likely attributable to decompression of the hematoma and neurovascular structures and stabilization of the fracture. Patients may typically be discharged from the hospital when they are comfortable, their neurologic status can be confirmed, and there is no concern for circulatory compromise. Follow-up care and course are the same as for closed pinning, with pin alignment check at 7 to 10 days and pin removal at 3 to 4 weeks after surgery.
Historically, closed reduction and pin fixation of type III supracondylar humeral fractures has been done on an emergent basis because of concerns about increased swelling, neurovascular compromise, compartment syndrome, and difficulty with closed reduction. It had been thought that early treatment enhances the likelihood of obtaining an anatomic reduction and reduces the risk of complications such as vascular compromise. The standard of care has evolved based on results of several studies indicating that these fractures can be treated on an urgent rather than emergent basis unless there is vascular compromise. Both Green and Mehlman and colleagues compared the effect of surgical timing on perioperative complications. Mehlman’s group found no significant difference in fractures that were managed 8 hours or earlier after injury ( n = 52) compared with those treated more than 8 hours after the injury ( n = 146). Treatment of the fracture was delayed so that it could be undertaken during normal working hours. No increase in the incidence of cubitus varus, pin tract infection, or vascular complications was detected. There was no increased need for open reduction in the delayed-treatment group. Iyengar and colleagues came to similar conclusions when comparing results of early versus delayed pinning of completely displaced supracondylar humeral fractures. Another report by Leet and colleagues provides further support that delaying reduction in the operating room does not lead to further adverse events. Increased operative time, need to open the fracture site, hospital length of stay, and complications were not correlated with an increase in the time to surgical intervention. Likewise, Gupta and colleagues compared 50 children with type III supracondylar humeral fractures treated in less than 12 hours with 100 children treated more than 12 hours after injury. They found no significant difference between groups in rates of open reduction, pin tract infections, compartment syndromes, vascular compromise, or nerve injuries.
More recently, a prospective study of 145 fractures showed no increase in the need for open reduction or the number of perioperative complications with a delay in surgical treatment up to 21 hours after injury. In the study by Garg and associates, the time from injury to surgical treatment for 872 severe (type III) supracondylar fractures averaged 16.3 hours. Patients were grouped into four cohorts based on time to surgery (<6 hours, 6–12 hours, 12–24 hours, >24 hours). The study found no increased rate of morbidity or complications with increased lengths of time from presentation to surgery. The authors of both of these studies emphasize the need for a thorough and accurate baseline assessment of neurovascular status, immobilization of the limb without fracture reduction, frequent neurovascular checks while awaiting surgery, and the availability of an operating room in an acceptable time frame.
There are numerous complications of supracondylar fractures and their treatment. Early complications are specifically related to the initial injury and treatment. They include vascular compromise, compartment syndrome, neurologic deficit, loss of reduction, and pin track infections; delayed problems include cubitus varus, elbow stiffness, myositis ossificans, nonunion, osteonecrosis of the epiphysis, and hyperextension deformity.
Vascular problems can be grouped into two types: acute, from occlusion of the brachial artery, and subacute, or Volkmann ischemia. Fortunately, acute vascular insufficiency is relatively uncommon, occurring in 5% to 12% of children with supracondylar humerus fractures. The vascular status of the extremity can be assessed by color of the hand (pink or white), temperature of the extremity (warm or cold), neurologic status, amount of pain, and status of the radial pulse (presence or absent). The elbow has excellent collateral circulation that usually provides sufficient blood flow to the distal extremity, even if the brachial artery is damaged, hence the term pink, pulseless hand.
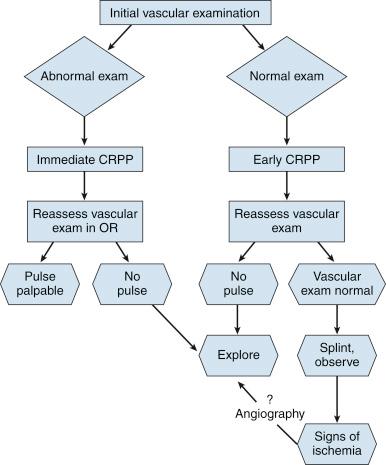
The absence of the radial pulse on palpation is never normal, but it is not always an indication of a true arterial injury. In fact, the radial pulse may be absent because of spasm and may return after reduction of the fracture. Loss of the pulse during reduction may indicate obstruction from too much elbow flexion or entrapment of the artery in the fracture with reduction. Although arterial exploration is always indicated in the event of a truly dysvascular extremity, the simple absence of the radial pulse with good peripheral circulation may not always be an indication for arterial exploration. When the radial pulse is absent (regardless of the perfusion status of the hand), fracture reduction with pin fixation and reassessment of the vascular status are the recommended treatment steps. If the radial pulse is absent but concomitant median or AIN palsy is present, immediate exploration may be warranted. If the hand is perfused but remains pulseless after reduction, opinion is divided about the need for immediate arterial exploration in all children.
The persistent absence of a radial pulse after fracture reduction is always worrisome. Campbell and colleagues studied 59 children with supracondylar humeral fractures in whom 11 (19%) had absent pulses; pulses returned after reduction in five patients and required no further treatment. The other six patients underwent exploration of the brachial artery. The artery was interposed in the fracture in one patient and lacerated in one, and one had an intimal tear. The other three patients were found to have spasm that resolved without other treatment. They recommend vascular exploration if the radial pulse is absent after reduction of the fracture.
Copley and colleagues reviewed 128 children with grade III supracondylar fractures of the humerus. Seventeen of the children had absent or diminished (detected on Doppler but not on palpation) radial pulses on initial examination. A total of 14 of the 17 recovered pulses after fracture reduction, but the remaining three had persistent absence of the radial pulse. These patients underwent exploration of the brachial artery immediately, and a significant vascular injury requiring repair was found in each. In two of the 14 patients whose pulses returned after fracture reduction, progressive postoperative deterioration in their circulation developed during the first 24 to 36 hours after reduction, with loss of the radial pulse. In both, arteriography identified arterial injuries, and both underwent exploration and vascular repair. These investigators concluded that absence of the radial pulse after reduction of a supracondylar humeral fracture indicated the existence of a significant arterial injury requiring surgical exploration and vascular repair ( Fig. 17.24 ).
Schoenecker and colleagues agreed with these authors in recommending arterial exploration in the absence of a Doppler-detectable radial pulse. They performed surgical exploration in seven limbs without a radial pulse after a supracondylar fracture and found the brachial artery to be either kinked or entrapped in the fracture in four. They repaired three of the arteries.
On the other hand, Garbuz and associates reviewed 326 patients with supracondylar fractures of the distal end of the humerus and found 22 whose radial pulses were absent on examination. Radial pulses returned in 15 patients after fracture reduction, and these extremities were monitored without exploration of the brachial artery. In seven patients, absence of the radial pulse persisted after reduction, and the hand was dysvascular. All of these patients underwent arterial exploration with arterial repair. Based on these results, the authors recommended that patients with an absent radial pulse but good circulation to the hand be observed after fracture reduction.
Sabharwal and colleagues reviewed patients with supracondylar fractures of the humerus and found that 13 of 410 fractures did not have radial pulses. All patients without a radial pulse had adequate collateral circulation, as demonstrated by magnetic resonance angiography, duplex scanning, or both. They performed arterial exploration in all these patients and found arterial injuries in all of them. Arterial repair was performed in all patients; however, asymptomatic reocclusion and residual stenosis were observed in these patients at follow-up. They recommended observation of patients with supracondylar humeral fractures and an associated absence of a radial pulse. They reasoned that the collateral circulation is adequate for the normal survival and use of the upper extremity, and they further stated that if the brachial artery is repaired, it is likely to become occluded as a result of insufficient flow.
Choi and colleagues determined that 2.6% of supracondylar fractures (33/1255) evaluated did not have a pulse. Of the 33 patients without radial pulses in this study, 24 were considered to be pulseless but well perfused at presentation, and all of these remained so immediately after reduction and internal fixation. Fourteen (58%) were also noted to have a palpable pulse postoperatively. Four more patients subsequently had return of a palpable pulse at an average of 8 weeks after surgery. In all, 75% of the perfused and pulseless limbs (18/24 patients) normalized after reduction and internal fixation. The other six patients in this group never had a documented return of pulse but sustained no known complications. Of the nine pulseless and poorly perfused limbs, only two had return of both pulse and perfusion, and another two regained perfusion but remained pulseless. The other five patients with pulseless, dysvascular limbs underwent exploration and repair at an average of 7.3 hours after injury and had good results. Garg and colleagues observed restoration of a palpable pulse by the first postoperative visit in all of the children in their study of type III supracondylar fractures treated by closed reduction and pinning; these patients had initially been seen with absent palpable or Doppler-detected pulses but clinically perfused limbs.
More recent studies suggest that a “watchful, waiting” strategy for the pulseless, perfused limb after a supracondylar humeral fracture may underestimate the significance of the vascular injury. White and colleagues performed a metaanalysis in which they studied pooled data for 313 pulseless supracondylar fractures. They found a 77% rate of true vascular injury in the setting of a pulseless, perfused supracondylar fracture and a 91% patent artery rate after brachial artery repair at 2 years’ follow-up. Mangat and colleagues found that 80% of patients with a pulseless, perfused hand had tethering or entrapment of the vessel when a concomitant nerve palsy (median or AIN) was present and recommended immediate arterial exploration in these circumstances. They also documented that all vessels repaired remained patent at follow-up. Reigstad and colleagues performed exploration on five patients with type III supracondylar humeral fractures whose extremities remained pulseless with slow or absent capillary refilling after closed reduction and percutaneous pinning. All were found to have an entrapped brachial artery, of whom four required microvascular repairs, and two had entrapment of the median nerve. Normal function was documented at more than 1 year after exploration.
Blakey and colleagues found that 23 of 26 patients with “pink, pulseless hands” who did not immediately undergo surgical exploration had some degree of ischemic contracture when they were examined at an average follow-up of 25 years (range, 4–26 years). These authors concluded that a pink, pulseless hand after fracture reduction is ischemic and that persistent and increasing pain with a deepening nerve lesion was indicative of critical ischemia. They recommended urgent surgical exploration when the pulse is absent to prevent the long-term sequelae observed in their study.
Consensus current practice for the management of displaced, type III supracondylar humeral fractures when the pulse is absent, regardless of the status of the vascularity of the hand, is to perform closed reduction and percutaneous pinning on an emergent basis. If the pulse returns, the extremity can be splinted, neurovascular status can be monitored to ensure that there is no deterioration, and the patient can be discharged when comfortable. Regardless of the status of the pulse, if the hand is well perfused, which indicates adequate distal circulation, observation is appropriate. Current evidence does not support exploring all limbs without a palpable radial pulse. Findings by White and colleagues and a poll of members of the Pediatric Orthopaedic Society of North America suggest that the common practice of watchful waiting for pulseless and perfused hands after supracondylar fractures should be questioned. There is, however, consensus that exploration of the brachial artery is indicated if the pulse returns but the vascular examination is equivocal, if the extremity is dysvascular, if there are signs of forearm ischemia, or if there is a concomitant median or AIN palsy. However a recent study by Harris et al of 71 patients from four trauma centers found that the presence of a pulseless extremities and concomitant anterior interosseous or median nerve palsy was not an absolute indication for open reduction. Arteriography is rarely indicated preoperatively because the location of the lesion is at the level of the fracture, and an arteriogram is unlikely to reveal anything that is not already known and will only delay treatment that is otherwise necessary.
Compartment syndrome is a rare complication of supracondylar fractures of the humerus in children. Historically, Volkmann ischemia was more common after closed reduction and immobilization of the elbow in a hyperflexed position, which is a practice that has been largely abandoned in favor of current treatment with pin fixation that allows the elbow to be splinted in much less flexion. The rate of compartment syndrome in supracondylar humerus fractures is estimated to be 0.1% to 0.3%. The risk is highest in fractures with an absent radial pulse and dysvascular hand, even after successful vascular repair. Posterolaterally displaced supracondylar fractures have a higher risk of vascular injury and compartment syndrome, whereas ipsilateral supracondylar and forearm fractures (“floating elbows”) are a marker of significant trauma and have the highest risk of neurovascular injury and compartment syndrome.
The traditional signs and symptoms of forearm ischemia in adults—pain, paresthesia, paralysis, pallor, and pulselessness—are less reliable in predicting the presence of impending compartment syndrome in children. Pain and paresthesia are early signs of ischemia and nerve compression. Paralysis, pallor, and an absent pulse are later findings of more prolonged ischemia and may reflect irreversible change. In children, the combination of increasing analgesic requirement plus anxiety and agitation, referred to as the three As, may be more predictive of impending compartment syndrome. An increasing analgesic requirement is the most sensitive indicator of impending compartment syndrome in children, preceding changes in vascular status by more than 7 hours. Progressively deteriorating neurologic status in the absence of pain or other “typical” clinical findings may also be a sign of an evolving compartment syndrome. This so-called “silent compartment syndrome” should be suspected particularly if a median nerve injury is present. In a young child, if there is suspicion of compartment syndrome, tissue pressure measurements should be obtained. This is best done emergently in the operating room. If compartment pressures are found to be elevated, a fasciotomy should be performed through an extensile volar approach extending from the elbow through the carpal tunnel. A fasciotomy performed within a mean of 30 hours from diagnosis has been shown to be effective in reducing the risk of permanent damage in 90% of children.
It has been shown that the risk of nerve injury after supracondylar humeral fractures increases with increasing fracture displacement. Based on the findings of two recent studies—a metaanalysis of 5154 fractures by Babal and colleagues and a single-institution, consecutive cohort study of 872 type III fractures by Garg and colleagues —the incidence of neurologic injury after extension-type supracondylar humeral fractures is 12%. The most common nerve injured is the AIN, which is observed in one-third of cases, followed by injury to the radial nerve. Ulnar nerve injury was the least frequent. Garg and colleagues found a 28% rate of iatrogenic nerve injury but observed no difference with pin configuration.
Most nerve injuries that occur at the time of fracture are neurapraxias, regardless of the nerve injured. Motor recovery typically takes about 2 to 3 months, whereas sensory function may take up to 6 months Routine (early) nerve exploration is not recommended unless no clinical or electromyographic evidence of recovery has been noted by 5 months. Amillo and Mora recommended that if nerve recovery is not seen within this time frame, nerve exploration should be undertaken as soon thereafter as possible because recovery is less predictable with further delay. Results were poor when exploration was performed beyond 1 year after injury. The outcomes of neurolysis are predictably good for chronic nerve palsies in which the nerve is in continuity. Early nerve exploration is indicated if nerve function deteriorates after closed reduction and pinning of the fracture because of the likelihood of nerve entrapment in the fracture site or iatrogenic injury. Extraction of the nerve from the fracture or from constricting soft tissue structures and removal of any compromising hardware should be performed as soon as the deficit is identified. Provided the nerve is not lacerated, observation is recommended for these iatrogenic injuries.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here