Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Whether present in the vasculature or in the soft tissues, foreign bodies left unchecked can erode into adjacent vital organs or migrate into remote locations. There are extreme reports of a lumboperitoneal shunts, ingested toothpicks, septal occluders, vertebroplasty cement, Onyx subdermal contraceptive implants, and embedded knitting needles traveling to locations far distant from their original location and causing tissue injury.
Although many foreign bodies require surgical removal, many can be easily extracted by an interventional radiologist with the use of fluoroscopy and ultrasound.
Foreign bodies left unattended in the vascular system have been associated with a 71% major complication rate and 24% to 60% mortality rate. Those located within the cardiopulmonary system pose the greatest risk. Foreign objects find their way into the vascular system through trauma, instrumentation, or transmural penetration from adjacent tissues (e.g., ingested foreign bodies). Upon arriving in the heart, wires, stents, inferior vena cava (IVC) filters, and filter fragments can cause cardiac arrhythmias, septal or ventricular wall perforation, myocardial infarction, myocarditis, sepsis, pericardial effusion, and pericardial tamponade. Continued migration into the pulmonary arteries can result in thromboembolism and sepsis.
Percutaneous techniques have dramatically altered the management of foreign bodies over the last several decades. Earliest reports of retrieving misplaced objects were limited to removal of catheter fragments and wires with a Dormia basket, forceps, or a self-made wire loop. Unable to be directed over a guidewire, baskets were difficult to manipulate into position. Stiff forceps were prone to traumatizing the vascular media, resulting in spasm, dissection, and perforation. After reports of the less traumatic self-made wire loop, several improved devices became commercially available. This niche market of retrieval devices has since been driven by the increasing use of stents, filters, coils, and other intravascular devices. Medical literature relating to foreign body retrieval is limited to case reports and short series. This chapter describes the rationale for removing foreign bodies and discusses the various techniques that can be used.
The most commonly used retrieval devices today are snares. The Amplatz Goose Neck snare (ev3/Covidien, Plymouth, MN) is constructed of a Nitinol cable and a gold-plated tungsten loop. The snare’s superelastic construction prevents it from deforming during use. The platinum-iridium radiopaque marker band at the tip of the guiding catheter is highly visible under fluoroscopy. When fully deployed, the snare is oriented at a 90-degree angle with respect to the guiding catheter tip. It is cinched around the foreign body by holding it stationary and advancing the catheter forward. Amplatz Goose Neck snares are available in 5- to 35-mm diameters.
Snares are successful in removing foreign bodies 95% of the time. Objects that are too large or noncompressible for removal through a vascular sheath can be relocated to a less noxious location or moved to a position that is easily accessible through a surgical cutdown.
A filter may be tipped or have its hook embedded into the wall, making it impossible to engage with a gooseneck snare. It may be possible to deflect the tip away from the wall, using a shepherd’s crook catheter or an angioplasty balloon. More recently, endobronchial forceps have been used to grasp the hook and remove the filter.
If a filter has been left in place for a long period of time, the legs can become incorporated into the IVC wall, preventing filter removal. A 12F laser-tipped sheath used to extract pacemaker leads can assist in the removal of embedded filters. Photochemolysis and photothermal ablation causes tissue adjacent to the sheath tip to disintegrate into particles up to 5 microns in diameter. The laser affects only tissue 100 microns deep to the sheath tip.
A guidewire is usually lost in the vascular system by an inexperienced operator who loses sight of the wire during dilator or catheter exchange while placing a central venous catheter. Catheter fragmentation usually occurs during catheter removal. Catheter shearing may also occur when the catheter is “pinched off” between the clavicle and the anterior portion of the first rib. Peripherally inserted central catheter (PICC) lines usually become torn when the peripheral arm vein goes into spasm during catheter removal.
The interventional radiologist may be called to remove a PICC line while the catheter is still intact but cannot be removed. In this case, a 0.025-inch guidewire is advanced through the PICC line under fluoroscopy until the wire tip exits the catheter. The guidewire and PICC line are then removed by applying traction to both as a unit.
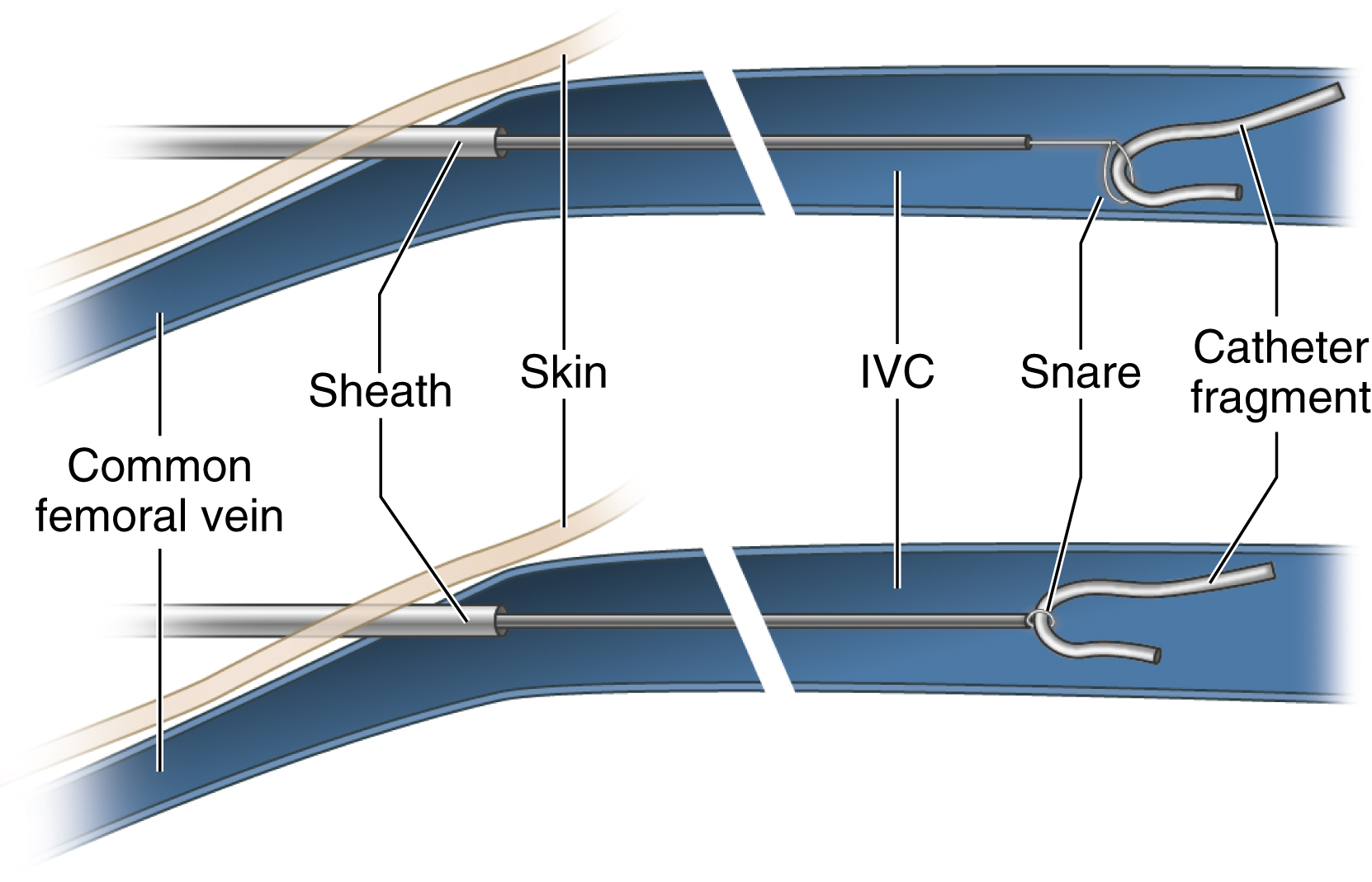
When a catheter of any type has become torn or is found incidentally on imaging or a wire has been lost, use of an endovascular snare becomes necessary. A vascular sheath is placed in either the internal jugular or common femoral vein. A long sheath provides greater support and stability for the snare. The snare is passed through the sheath and advanced or retracted over the fragment tip. The snare is then cinched and drawn out with the fragment through the sheath ( Fig. 74.1 ).
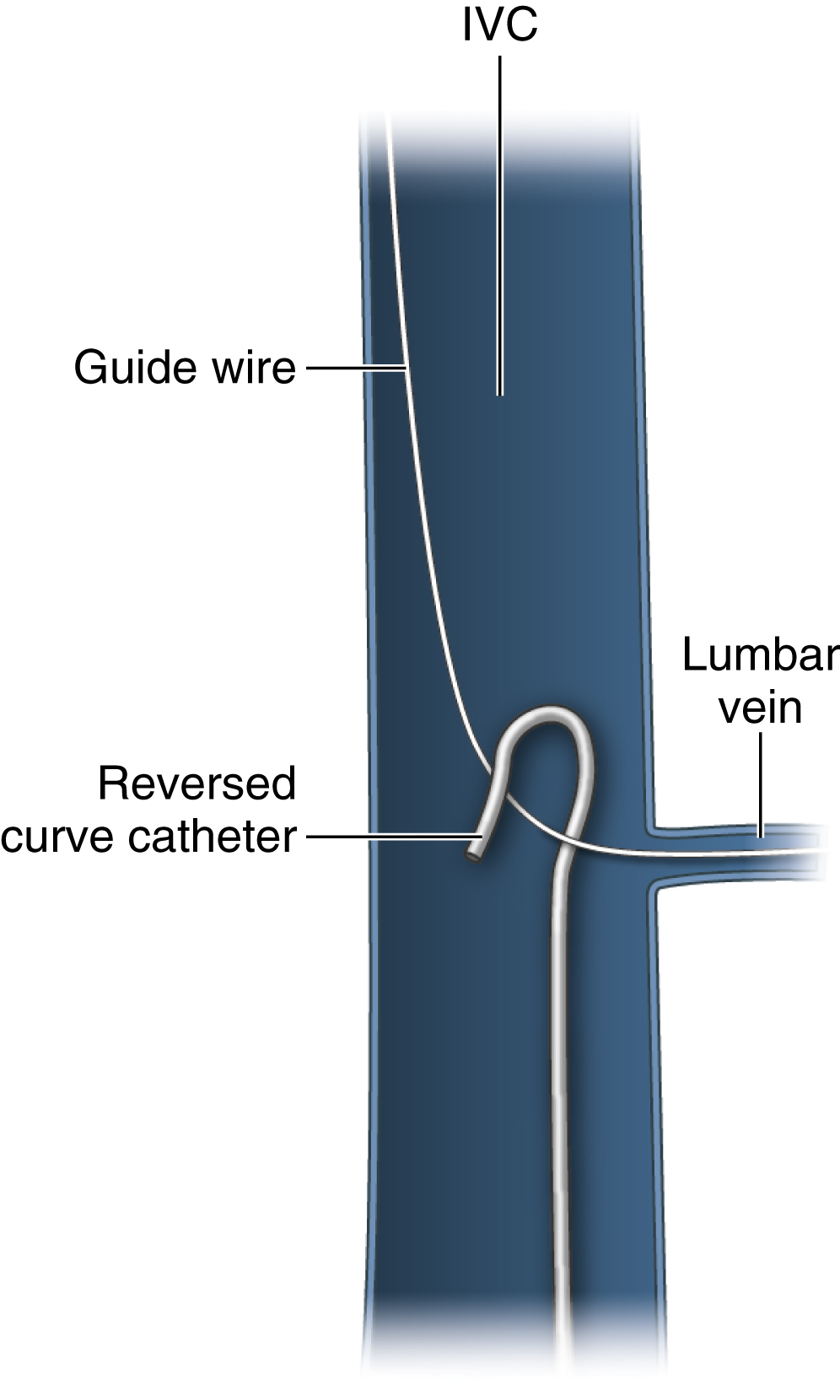
A fragment tip that is caught in a small branch vessel or chronically embedded in the vessel wall must first be freed from the vessel wall to be snared. A reverse curve catheter passed through the same sheath or a separate sheath can be used to pull down on the shaft of the foreign body, freeing the tip ( Fig. 74.2 ). A braiding technique can also be used for this purpose. With this method, a pigtail catheter tip is wrapped around the fragment and rotated, braiding itself with the fragment and freeing the tip to allow successful snaring.
The end of a fragment may sometimes extend into the subcutaneous tissues while remaining invisible at the skin surface. This occurs when the catheter is torn during attempted removal. With the help of fluoroscopy or ultrasound, one can incise the skin and dissect down onto the fragment. The fragment is then secured and extracted along with its intravascular component. An 18- to 20-gauge needle can be formed into a hook on the table for this purpose. If the tip of the foreign body is too deep for a safe or successful cutdown, the intravascular end of the fragment can be snared and removed through a vascular sheath, bringing the soft tissue component along with it.
Rarely, a catheter will become sutured to the wall of a vein during an operation. Snaring the catheter would risk tearing the vessel wall. In this setting, two vascular sheaths are placed via femoral and internal jugular access. One snare is passed through each sheath. The catheter is snared on each side of the suture, immediately adjacent to the suture. Steady, equal traction is applied to the catheter fragment from each direction until the fragment is torn into two pieces. Each fragment is subsequently removed through the sheaths. Because of the risks associated with this procedure, anesthesiology and surgery services should first be consulted.
A self-expanding stent can migrate during deployment when the constrained stent is not centered on a stenosis and it “trumpets” or “melon seeds” in either direction during release. A stent can also migrate after a technically successful deployment in a vein as the vessel diameter increases with respirations or Valsalva maneuvers. Using a T-fastener to anchor an IVC stent to the wall of the intrahepatic IVC has been described in this setting. A stent may become irretrievable if left to migrate into the heart chambers, requiring open-heart surgery for its removal.
Maintaining a guidewire through the lumen of a lost stent is key to its successful capture and removal. Keeping the wire in both the superior and inferior venae cavae will prevent the stent from passing into the heart chambers. The vascular sheath is exchanged for one that is wide enough to pull the captured stent through. The snare is cinched around the wire outside of the sheath. The snare and its guiding catheter are passed through the sheath along the guidewire. The snare is opened around the proximal-most end of the lost stent. The snare is then cinched down on the proximal end of the stent, drawing the constrained end of the stent out through the sheath.
A lost stent through which a wire does not pass presents a greater challenge. The snare will tend to get caught on the struts of the stent, constraining only a part of the end of the stent. It will not be possible to pull this stent into the vascular sheath for removal. Passing a wire through the stent before snaring will assist in stent capture. Using a J-tipped wire for this purpose will help avoid passing the wire tip through the side wall of an uncovered stent.
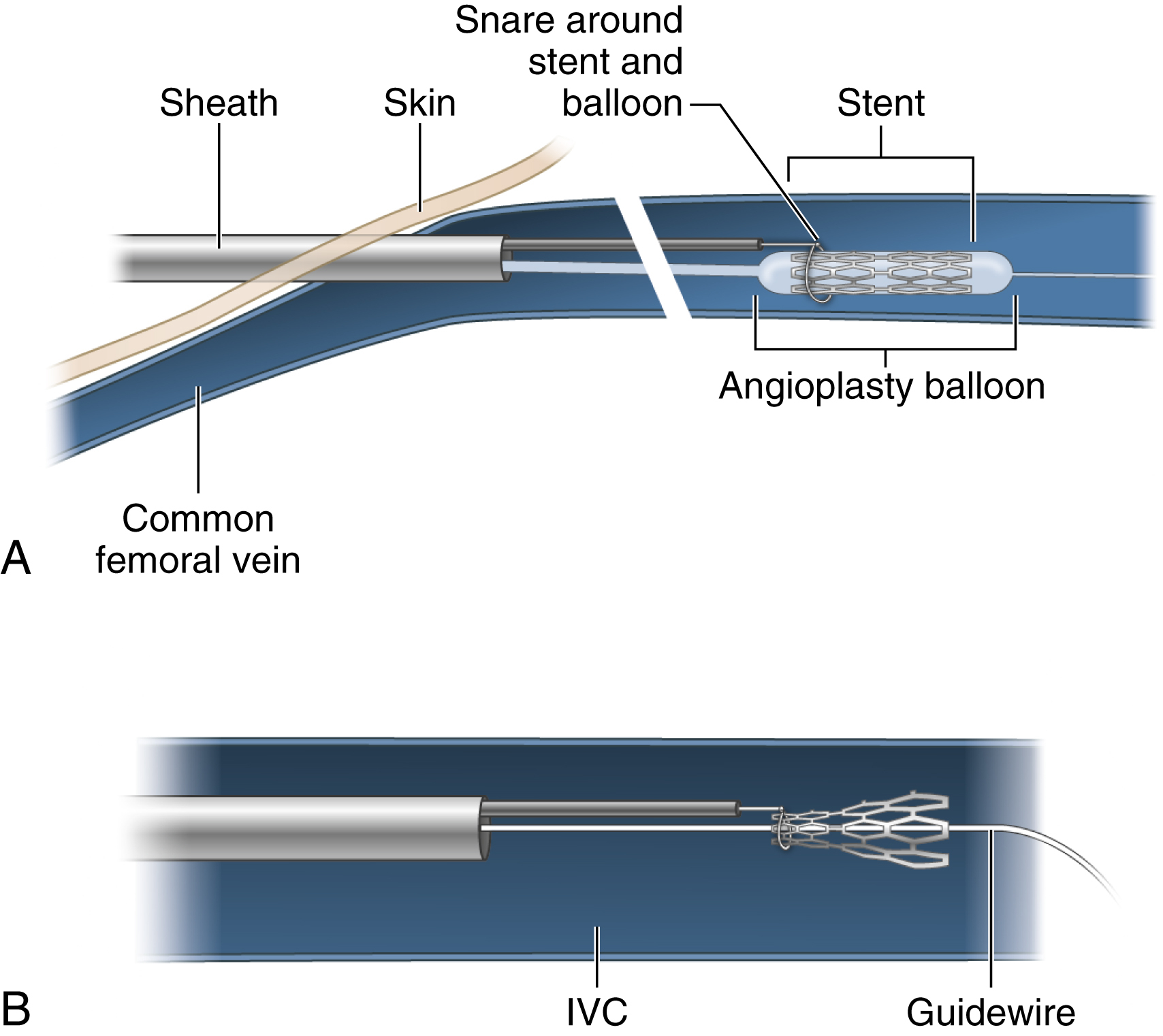
Having successfully passed a wire through the stent, the operator may still find it difficult to avoid hang-up of the snare on the struts at the end of the stent. Use of an angioplasty balloon will provide a transition zone for the snare to approach and slip over the end of the stent. The vascular sheath is first exchanged for an appropriately sized one. An angioplasty balloon 1 to 2 mm greater in diameter than the stent and longer than the stent is selected. A snare is cinched around the balloon catheter shaft outside of the sheath. The balloon/snare combination is passed over the wire just proximal to the stent. The snare is opened. The balloon is advanced into the stent and inflated. The open snare is advanced over the tapered end of the balloon and onto the proximal end of the stent ( Fig. 74.3A ). The balloon is deflated and pulled out of the stent as the end of the stent is constrained by the snare ( Fig. 74.3B ). The deflated balloon and captured stent are then drawn out sequentially through the sheath.
Once a stent lies in the right ventricle, the struts may lodge in the chordae tendineae, or access to the stent may be blocked by the tricuspid valve. Capture is made even more difficult by the moving target created by cardiac motion during fluoroscopy. Changing the angle of approach from femoral vein access to internal jugular vein access (or vice versa) may aid in stent capture. Foreign bodies trapped within the heart pose a greater risk for capture. Attempts at their removal may cause dysrhythmia or damage to the myocardium, chordae tendineae, or tricuspid valve. A defibrillator, code cart, and transvenous pacer should be available for use, and a cardiothoracic surgeon on standby. If percutaneous retrieval is felt to be associated with high risk or if attempts fail, the interventional radiologist should consult a cardiothoracic surgeon to determine whether he or she can remove the intracardiac foreign body more safely and expeditiously.
Foreign bodies that have traveled through the heart and into the pulmonary arteries can usually be extracted by the radiologist with a snare.
Once a stent has traveled through the heart and into the pulmonary arteries, apposition of the stent against the vessel wall may make it impossible to pass a snare around it. If this occurs, an appropriately sized angioplasty balloon can be inflated inside the stent and used to pull the stent from the artery, through the cardiac chambers, and into the venae cavae or iliac veins for snaring through a second vascular sheath.
Once lost stents are snared and constrained, it may not be possible to pull the stent into the sheath. Stent struts or a severely deformed stent may keep the stent from entering the sheath. Three options are available at this point:
The stent can be deployed in the iliac vein if it is appropriately sized. If the stent is too small in diameter, a larger and longer self-expanding stent can be deployed within it to trap it in place.
A second snare can be used to capture and constrain the other end of the stent from a different approach.
The stent can be drawn into the common femoral vein and removed by surgical cutdown.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here