Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Flap is tissue(s) with its own vascular supply, allowing transfer from one site to another.
Flaps come in various forms, shapes, and function. They may be composed of a simple skin tissue or multiple composite tissues.
The purpose of classification is to understand the anatomy and the features that each flap provides. It also allows communication not only with peers but with the patients as well to achieve the common goal of reconstruction.
Various methods can be used to classify flaps, but commonly used classifications are based on the locality of the flap, vascular supply of the flap, and tissue components of the flap.
By applying a precise knowledge of the anatomy of skin, muscle, bone, fascia, and other tissue in planning reconstructive procedures, the surgeon has the ability to restore function in congenital and acquired defects. Flap physiology and pharmacology are also important to understand.
This chapter reviews flap classification and gives examples of its applications. This chapter also reviews flap physiology and pharmacology.
![]() Access video lecture content for this chapter online at Elsevier eBooks+
Access video lecture content for this chapter online at Elsevier eBooks+
The use of flaps for reconstructive plastic surgery dates back to 600 BC, when the earliest recorded application of pedicled flaps for nasal reconstruction is attributed to Sushruta Samhita (translated by Bhishagratna in 1916). The earliest flaps centered on the head and neck as well as the lower extremities because wounds in these regions failed to heal by secondary intention. The initial flaps used would now be considered random-pattern flaps, as they were not based on a specific blood supply and were used without an understanding of how and why they survived. All possible flaps were based on the simple idea of random flaps. Tagliacozzi used a distally based arm flap in a two-staged procedure. His work was published in Venice in 1597. Much of this knowledge was forgotten until the nineteenth century, when the English surgeon Carpue successfully used forehead flaps to reconstruct the noses of two officers. The publication of Rhinoplastik by von Graefe in 1818 further advanced the use of these techniques. Attention in the early twentieth century remained focused on tubed random flaps. It was found that the only way to increase survival of these flaps was to perform a surgical delay. A German anatomist, Carl Manchot, demonstrated the concept of anatomic skin territories supplied by consistent vessels in Die Hautarterien des menschlichen Körpers , published in 1889. Further development into the shape of modern flaps came with Tansini when he described the skin island of the latissimus dorsi musculocutaneous flap in 1906. Davis, crediting Manchot, demonstrated axial and pedicled muscle and fascial flaps, as well as composite flaps in 1919. McGregor introduced the temporalis flap, which allowed midface and lower face coverage without the donor site deformity associated with the previously popular forehead flap. Coverage of the lower third of the face as well as of the oral and esophageal defects was accomplished by Bakamjian with the use of the deltopectoral flap. The availability of the forehead, temporalis, and deltopectoral flaps changed the approach to head and neck cancer extirpative surgery with an emphasis on immediate reconstruction. A slow evolution of flaps was based on experience and gradual understanding of anatomy.
The muscle flap for lower extremity reconstruction was initially described by Stark for coverage of debridement sites for osteomyelitis. Unfortunately, this went unnoticed until Ger recognized that the leg muscles are a source of well-vascularized tissue for leg coverage. Although Owens, in 1955, used a compound flap consisting of the sternocleidomastoid muscle with overlying skin for head and neck reconstruction, the concept of musculocutaneous perforating vessels providing a cutaneous territory for superficial muscles was first reported by Orticochea in 1972. Shortly thereafter, surgeons made significant contributions toward definition of flaps, expansion, and use of muscle and musculocutaneous flaps in reconstructive surgery. The contributions included the concept of cutaneous territory of superficial muscles ; anatomy of the muscles including specific arcs of rotation ; applications of muscle and musculocutaneous flaps for breast, chest, extremity, and head and neck reconstruction; and microsurgical transplantation. In 1981, Pontén recognized the input of septocutaneous perforating vessels to the overlying skin circulation. On the basis of this observation, the concept of fasciocutaneous flaps was introduced. Like the muscle flap, the septocutaneous flap was initially described in the lower extremity, but the principle of the fasciocutaneous flap, based on muscle, fascia, and associated cutaneous territories, was rapidly applied to all body regions ( Table 24.1 ).
| 600 bc | Sushruta Samhita | Pedicle flaps in the face and forehead for nasal reconstruction |
|---|---|---|
| 1597 | Tagliacozzi | Nasal reconstruction by tubed pedicle flap from arm; described “delay” of pedicle flap |
| 1896 | Tansini | Latissimus dorsi musculocutaneous flap for breast reconstruction (post-mastectomy) |
| 1920 | Gillies | Tubed pedicle flap |
| 1946 | Stark | Muscle flaps for osteomyelitis |
| 1955 | Owens | Compound neck flap |
| 1963 | McGregor | Temporalis flap |
| 1965 | Bakamjian | Deltopectoral flap |
| 1971 | Ger | Lower extremity musculocutaneous flap |
| 1972 | McGregor and Jackson | Groin flap |
| 1972 | Orticochea | Musculocutaneous flaps |
| 1977 | McCraw et al . | Musculocutaneous territories |
| 1981 | Mathes and Nahai | Classification of muscle flaps based on vascular anatomy |
| 1981 | Pontén | Fasciocutaneous flaps |
Starting in the late 1970s, a proliferation of innovative techniques reported in the surgical literature throughout the world defined new anatomic data and applications of the muscle, musculocutaneous, fascial, and fasciocutaneous flaps. These advances in flap definition and application have changed the entire approach to plastic surgery. With the isolation of the vascular pedicles to muscle- and fascia-based flaps, microsurgical transplantation is selected when the ideal flap is outside of a traditional and safe arc of rotation to the recipient site. With the definition of musculocutaneous and fasciocutaneous territories, an expander may be used to enlarge flap dimensions and still ensure direct closure of the donor site. The reconstructive ladder, introduced in 1982, was appropriate for use in choosing reconstructive methods that ensure safety in soft-tissue reconstruction. With increased understanding of the flap anatomy, physiology and even in regards to donor morbidity, ideal form and function can be achieved by complex procedures without compromising safety. This new approach can be considered as an elevator approach to defects entailing most of the issues to achieve a minimal staged and ideally functional reconstruction. Furthermore, donor site deformity is frequently avoided because the flap can be precisely tailored to fit the defect. In 1997, the reconstructive triangle was introduced to guide the thinking of complex reconstruction. The surgeon may choose the transposition flap, microsurgical transplantation, or tissue expansion with the goals of achieving form and function at the recipient site, avoiding donor site deformity, and providing safety throughout the reconstructive endeavor. The more recent introduction of perforator flaps has expanded our options even more and brought us to the era of free-style flaps. With all the explosive introduction of new flaps, new ideas and new findings, a classification may not describe all the innovations involved in the field of reconstruction. Furthermore, the introduction of the corresponding ideas and nomenclature increases the confusion.
This chapter describes the flap classification based on the important characteristics of the flap ( & ![]() ). The atomic classification of Tolhurst naturally involved the early flap characteristics and further refinements from Lamberty and Healy describe the most important factors for flap selection; circulation, constituents, conformation, contiguity, construction, and condition. Further modifications have been made to accommodate the recent introduction of flaps. In addition, application of these flaps will be introduced in this chapter.
). The atomic classification of Tolhurst naturally involved the early flap characteristics and further refinements from Lamberty and Healy describe the most important factors for flap selection; circulation, constituents, conformation, contiguity, construction, and condition. Further modifications have been made to accommodate the recent introduction of flaps. In addition, application of these flaps will be introduced in this chapter.
A flap consists of tissue that is mobilized on the basis of its vascular anatomy. Flaps can be composed of skin (including subcutaneous fat), skin and fascia, skin and muscle, or skin, muscle and bone, or various compositions of tissues. Because the circulation to the tissue to be mobilized is crucial for flap survival, the development of flap techniques has depended on defining the vascular anatomy of the skin and underlying soft tissue.
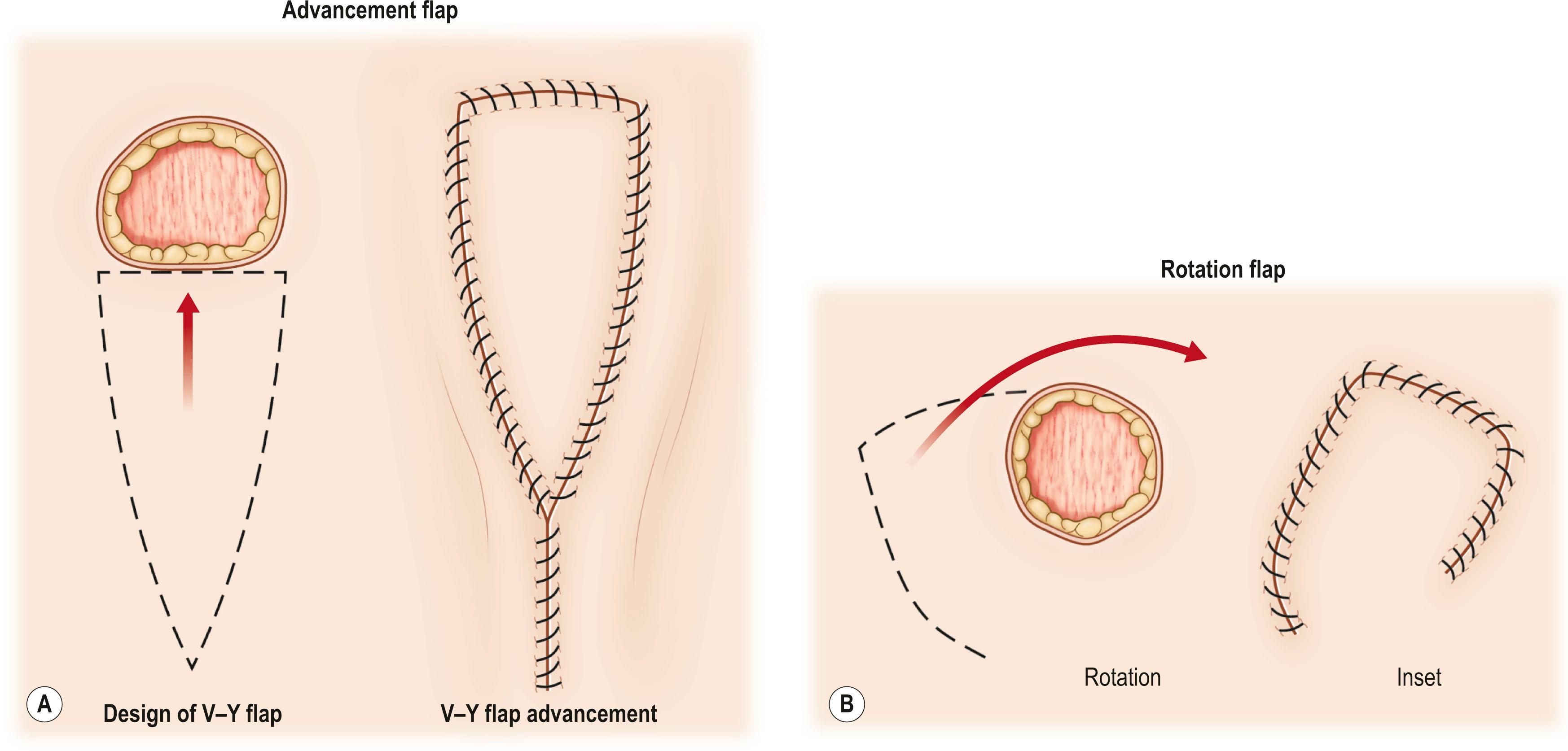
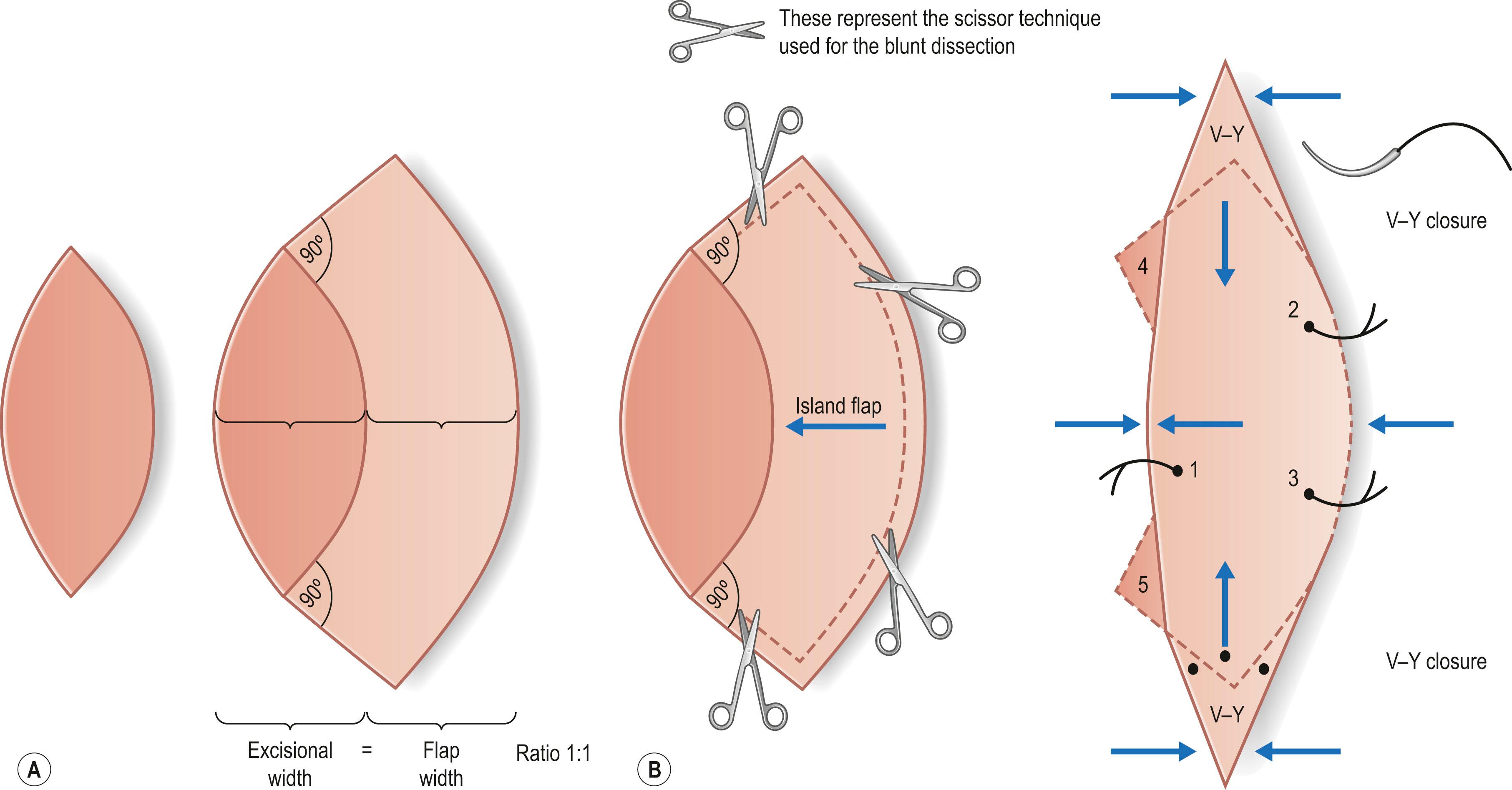
The first classification can be made for a random pattern flap with a non-identified pedicle versus flaps with an identified pedicle. An early concept of vascular anatomy as it pertained to flap surgery was the belief that skin circulation was based on the longitudinal subdermal plexus. A random pattern flap based on this subdermal plexus was designed to allow elevation of skin and subcutaneous tissue with a certain length-to-width ratio in the range of 2–1.5 : 1. Based on the simplicity of circulation, the shape and movement of the flap were important distinguishing factors ( Fig. 24.1 ). Milton subsequently disproved the concept of length-to-width ratios as viability depended on the extent of circulation. He further proved his concept by showing that a delay procedure on the flap can further increase the extent of the flap in a pig model. Historically, attempts to use a random pattern flap based on subdermal circulation distant from the wound location eventually resulted in the introduction of the tubed pedicle flap. Through a series of delays using the initial bipedicle flap design, the arc of rotation of the skin flap was increased. Alternatively, the flap was attached to an arm carrier, which later required transferring the arm carrier of the random pattern flap from one body region (donor site) to another (recipient site). This use of the random pattern flap with multiple delays or the arm carrier allowed reconstruction of distant complex defects, particularly in the head and neck region, and for coverage of composite wounds when local tissue was unavailable or severely damaged. Despite this, the random pattern flap provided no new source of circulation when transferred to a distant site. Thus, the success of these flaps ultimately depended on the local wound environment for nourishment. Although limited in its reach, the random pattern flap can be elevated and rotated to provide viable skin and subcutaneous tissue to cover an adjacent wound. Common flaps based on the subdermal plexus or the underlying vascular source without identification include the bipedicle flap, advancement flaps (i.e., V–Y), and rotation or transposition flaps. Today these techniques are still widely used for small or medium-sized defects that can be reconstructed with regional skin. A similar concept used for larger wounds on the trunk and extremities is the keystone flap. Described by Behan, the keystone flap is a curvilinear-shaped trapezoidal-design flap, essentially being two V–Y advancement flaps along the long axis of the flap ( Fig. 24.2 ). The longitudinal tension of the flap is released, thus creating laxity and redundancy in the midportion allowing movement horizontally toward the defect with minimal tension. Because of the large size of the flap, identification of the vascular source is not necessary and it is presumed to have various blood supplies from the perforators entering from the deep fascia. But when perforators are identified using a Doppler, one can also call this a keystone-design perforator island flap.
Previously with random pattern flaps, type of movement, shape of the flap or anatomical region was the focus for differentiating the types. But as the anatomy of the vessel became more evident and the importance recognized, flap description began to depict the circulation. The evolution from random pattern flaps to fasciocutaneous flaps, myocutaneous flaps and then recently to perforator flaps was based on the development and findings of vascular anatomy throughout the body. Pioneering work from Manchot, Salmon, Cormack, Lamberty, Taylor, Morris, Tang, Nakajima and others clarified the vascular anatomy for skin territory originating from a source vessel. The flap elevation practice changed with the work of Taylor and Palmer presenting the angiosome concept, which increased our knowledge of vascular territory of the skin flap. Now the concept of vascular territory has evolved from thinking of the angiosome as the basic unit to applying the perforator as the basic unit, termed “perforasome”.
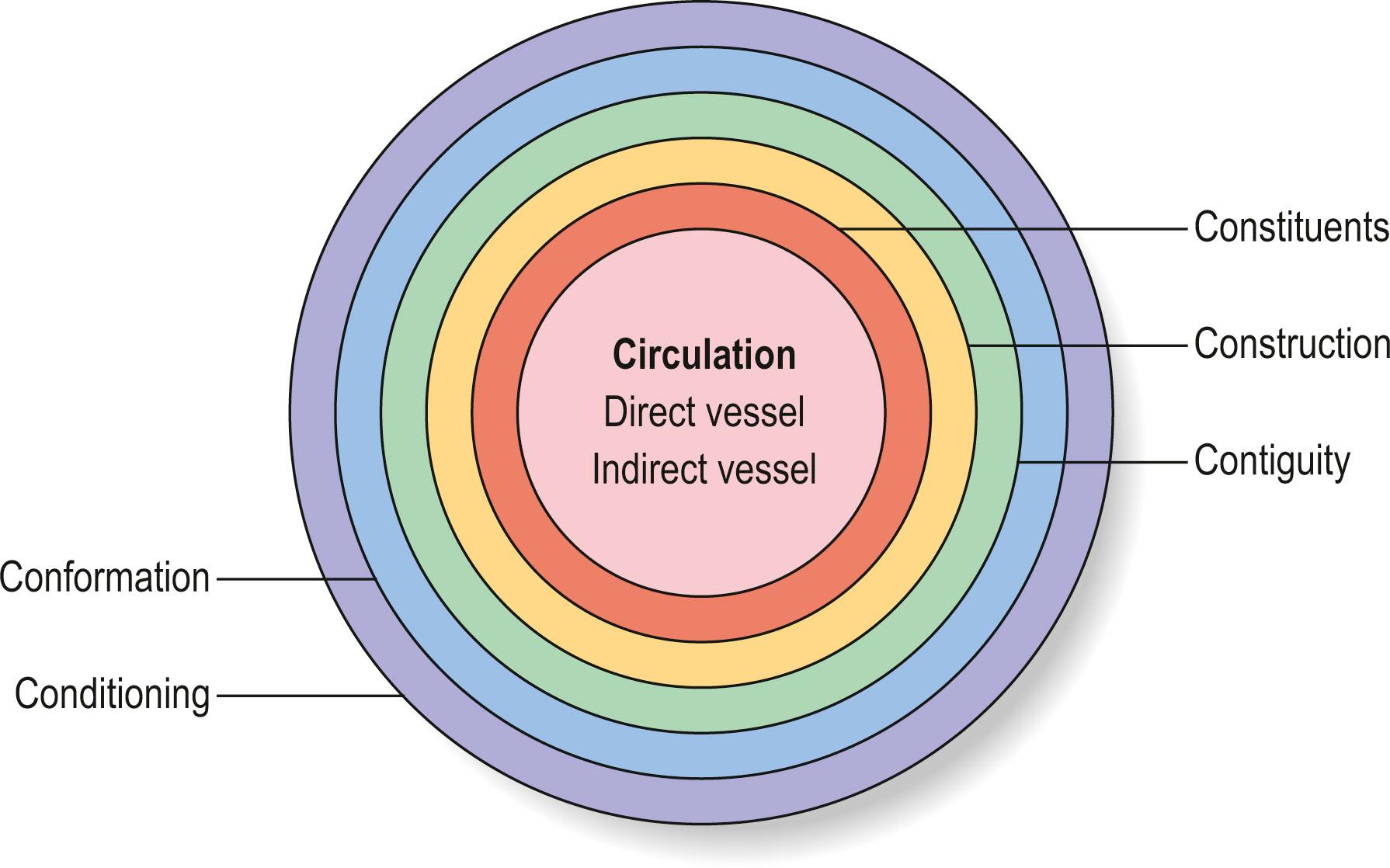
Circulation being clearly the most important characteristic of flaps with identified pedicle, Cormack and Lamberty were the first to advocate the source of the circulation as the most important characteristic of flap selection. The six Cs included, in addition to circulation, constituents (composition), conformation (form/shape), contiguity (destination), construction (type of pedicle), and conditioning (preparation) ( Fig. 24.3 ). Hallock further outlined this classification in accordance with the six Cs and named it “complete classification of flaps”. With the addition of recent flaps and advances, a modified outline for the basis of flap classification is shown in Box 24.1 .
Direct vessels
axial
septocutaneous
endosteal
Indirect vessels
myocutaneous
periosteal
Skin (with subcutaneous fat)
Fasciocutaneous/fascia
Muscle/musculocutaneous
Visceral
Nerve
Bone
Cartilage
Lymph node (with subcutaneous fat)
Other
Local
Regional
Distant (free)
⁎ *D *Denotes default or standard for flaps and can be omitted in the use of complete classification.
Bipedicle
⁎ *D enotes default or standard for flaps and can be omitted in the use of complete classification.
Retrograde (reverse)
Turbocharged
Supercharged
Arterialized venous
Special shapes
Tubed
Combined flaps
None ⁎ **
⁎ *D enotes default or standard for flaps and can be omitted in the use of complete classification.
This approach to muscle flaps can be demonstrated with the gracilis muscle flap shown in Fig. 24.4 :
Circulation: medial circumflex femoral artery
Constituents: gracilis muscle
Contiguity: free flap
Construction: antegrade flow
Conditioning: none
Conformation: muscle only
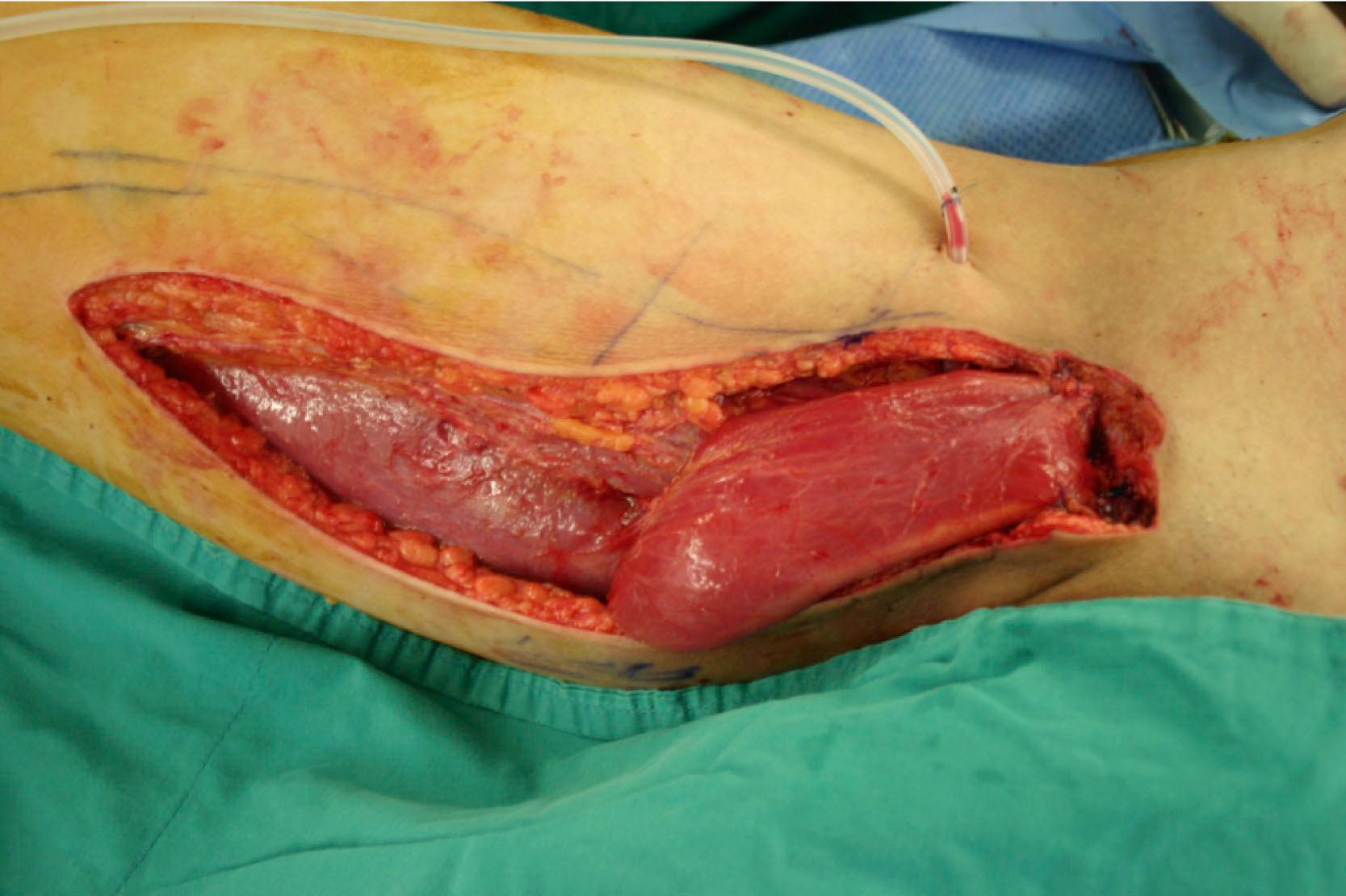
Thus the gracilis muscle flap from Fig. 24.4 can be named medial circumflex femoral artery-based (circulation) gracilis muscle (constituents) free (contiguity) flap with antegrade flow (construction) with no conditioning (conditioning) and muscle only (conformation) according to the complete classification. But, as default, “antegrade flow with no conditioning” can be omitted from the nomenclature. Also, since there is one conformation, “muscle” does not have to be repeated and, finally, since the circulation from the “medial circumflex femoral artery” is an established one for any muscle flaps, this can be considered to be understood and does not have to be reiterated. Thus the final classification using the “complete classification” can be described as “gracilis muscle free flap”.
For muscle flaps, the circulation can be from multiple variations and these variations play an important part in survival of each muscle flap. Therefore further classification of muscle flaps should be addressed.
The next example would be a musculo-fascio-cutaneous flap from the same region as shown in Fig. 24.5 :
Circulation: medial circumflex femoral artery
Constituents: gracilis muscle and upper medial thigh fasciocutaneous
Contiguity: free flap
Construction: antegrade flow
Conditioning: none
Conformation: combined muscle and fasciocutaneous flap
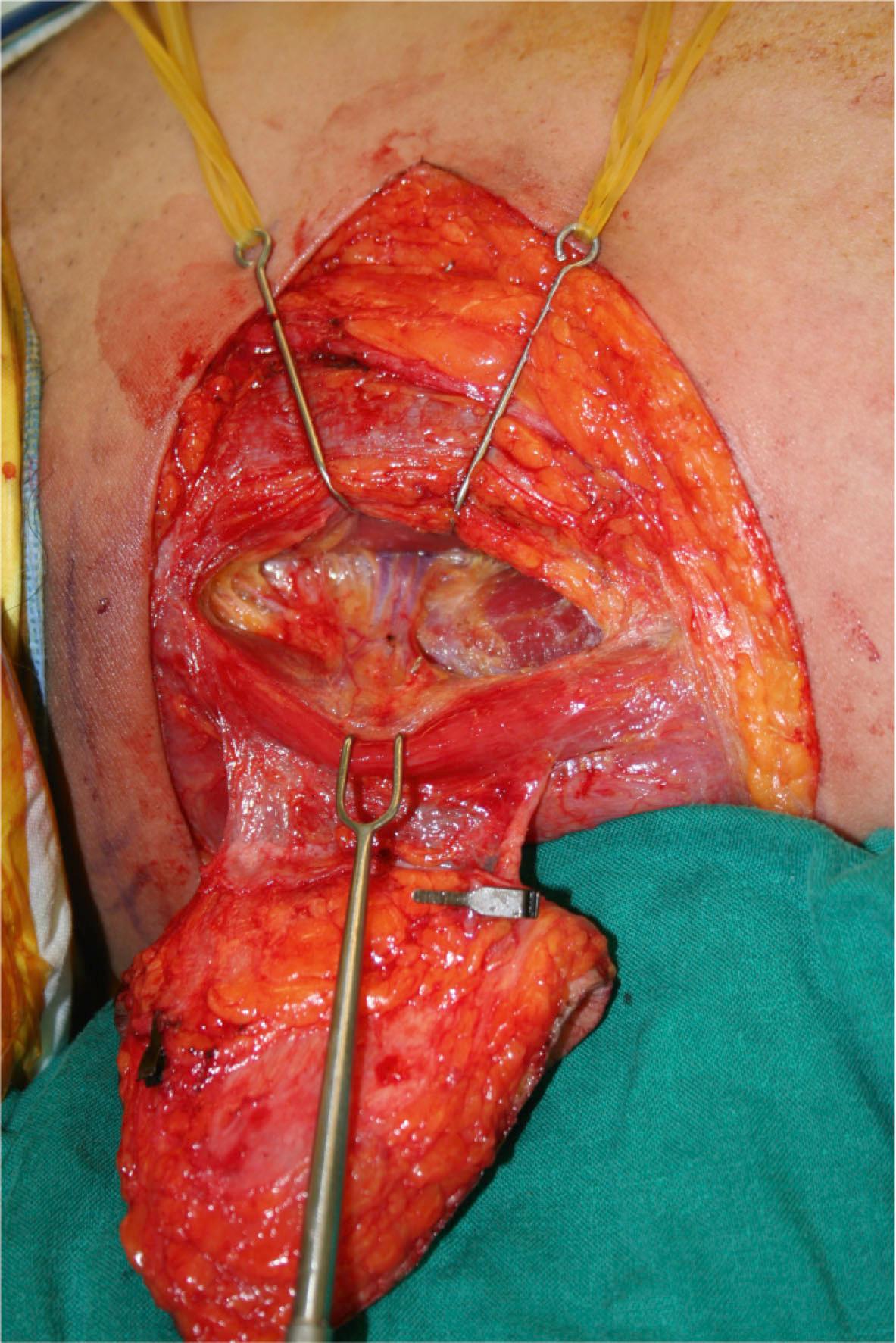
The flap in Fig. 24.5 can be named as medial circumflex femoral artery-based (circulation) gracilis muscle and upper medial thigh fasciocutaneous (constituents) free (contiguity) flap with antegrade flow (construction) with no conditioning (conditioning) and combined with muscle and fasciocutaneous component (conformation) according to the complete classification. As defaults are omitted from the nomenclature, the “complete classification” should be: “combined gracilis musculo(-fascio-)cutaneous free flap”.
The actual source of circulation feeding the subdermal plexus of the skin was described in a tripartite system from Cormack and Lamberty. The system consisted of axial (direct cutaneous), musculocutaneous, and fasciocutaneous types of free flaps. They further described the fasciocutaneous flap into subtypes leading to the fasciocutaneous flap classification. These classifications should be recognized as they rely on the anatomical vascular system of the flap and improve the design and survival of the flaps.
When the skin portion of this flap is isolated on a single perforator, the constituents become a combination of medial circumflex artery perforator flap and gracilis muscle flap. Combined flaps with identified circulation can also undergo a classification for combined/compound flaps. Thus, this variation of flap should be named “combined (conjoint) medial circumflex femoral perforator and gracilis muscle free flap”.
The final example is shown in Fig. 24.6 . Note that this flap from the upper medial thigh is supplied only with a perforator.
Circulation: medial circumflex femoral artery (perforator)
Constituents: upper medial thigh skin (with fat)
Contiguity: free flap
Construction: antegrade flow
Conditioning: none
Conformation: skin (with fat) only
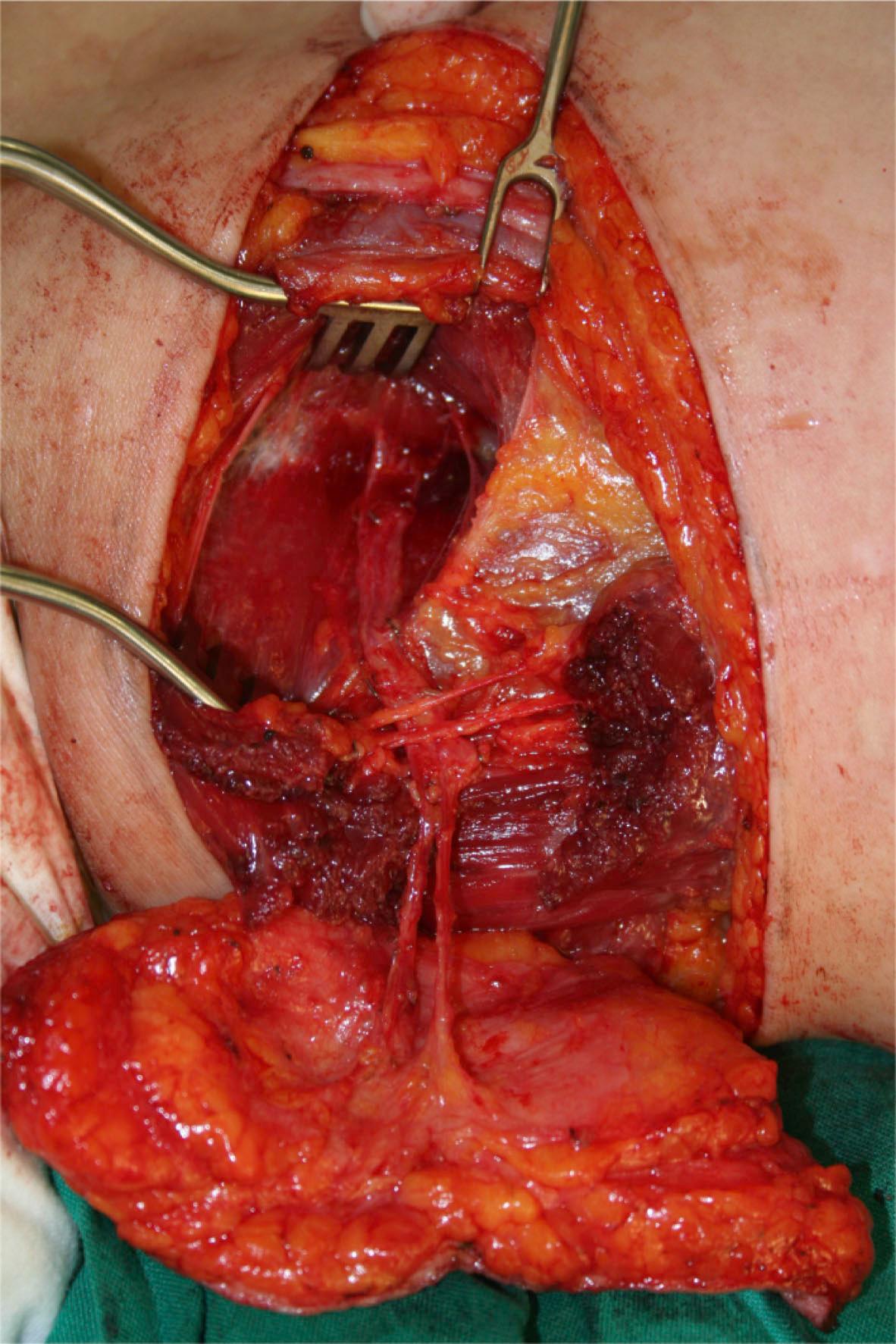
The circulation to this flap is the same source vessel as the gracilis muscle or musculocutaneous flap. But this is a flap supplied with an identified perforator, so recognition should be made of the perforator as the main circulation. Thus, this flap should be named as “medial circumflex femoral artery perforator-based (circulation) upper medial thigh skin (with fat) (constituents) free (contiguity) flap with antegrade flow (construction) with no conditioning (conditioning) and skin only component (conformation) according to the complete classification. As defaults are omitted from the nomenclature, the “complete classification” should be “medial circumflex femoral artery perforator skin flap”.
This type of perforator-based flap has been subject to controversy as there have been many classifications proposed. The Gent consensus for classification of perforator flaps will be reviewed as an example of perforator flap classification.
In 1981, Mathes and Nahai described a classification system for muscles based on the following anatomic relationships between the muscle and its vascular pedicles:
The regional source of the pedicle entering the muscle
The number and size of the pedicle
The location of the pedicle with respect to the muscle's origin and insertion
The angiographic patterns of the intramuscular vessels.
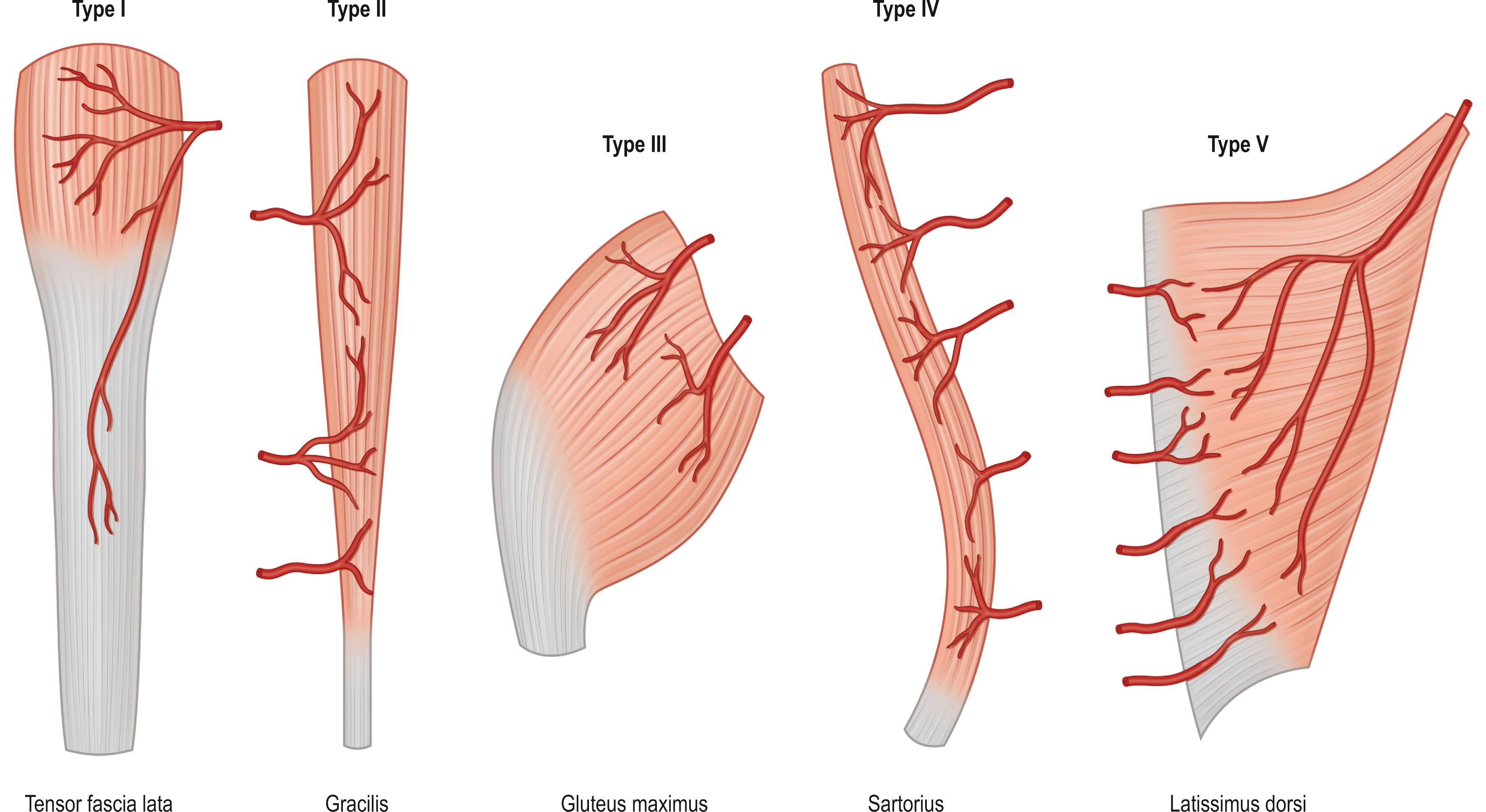
This classification system enables the surgeon to categorize the various muscle and musculocutaneous flaps into distinctly different, clinically applicable groups based on the vascular anatomy. There are five different vascular patterns by which the various muscles are categorized ( Fig. 24.7 ).
Type I: one vascular pedicle – type I muscles are supplied by a single vascular pedicle ( Box 24.2 ).
Abductor digiti minimi (hand)
Abductor pollicis brevis
Anconeus
Colon
Deep circumflex iliac artery
First dorsal interosseous
Gastrocnemius, medial and lateral
Genioglossus
Hyoglossus
Jejunum
Longitudinalis linguae
Styloglossus
Tensor fascia lata
Transversus and verticalis linguae
Vastus lateralis
Type II: dominant vascular pedicle and minor pedicle – type II muscles are supplied by both a dominant and minor vascular pedicle. The larger dominant vascular pedicle will usually sustain circulation to these muscles after the elevation of the flap when the minor pedicles are divided. This is the most common pattern of circulation observed in human muscle ( Box 24.3 ).
Abductor digiti minimi (foot)
Abductor hallucis
Brachioradialis
Coracobrachialis
Flexor carpi ulnaris
Flexor digitorum brevis
Gracilis
Hamstring (biceps femoris)
Peroneus brevis
Peroneus longus
Platysma
Rectus femoris
Soleus
Sternocleidomastoid
Trapezius
Triceps
Vastus medialis
Type III: two dominant pedicles – type III muscles possess two large vascular pedicles from separate vascular sources. These pedicles have either a separate regional source of circulation or are located on opposite sides of the muscle. Division of one pedicle during flap elevation rarely results in loss of muscle within its vascular distribution. The muscle will usually survive on one of its two dominant vascular pedicles. This vascular pattern allows the muscle to be split, allowing the use of only part of the muscle as a muscle or musculocutaneous flap ( Box 24.4 ).
Gluteus maximus
Intercostal
Omentum
Orbicularis oris
Pectoralis minor
Rectus abdominis
Serratus anterior
Temporalis
Type IV: segmental vascular pedicles – type IV muscles are supplied by segmental vascular pedicles entering along the course of the muscle belly. Each pedicle provides circulation to a segment of the muscle. Division of more than two or three of the pedicles during elevation as a flap may result in distal muscle necrosis ( Box 24.5 ).
Extensor digitorum longus
Extensor hallucis longus
External oblique
Flexor digitorum longus
Flexor hallucis longus
Sartorius
Tibialis anterior
Type V: one dominant vascular pedicle and secondary segmental vascular pedicles – type V muscles are supplied by a single dominant pedicle and secondary segmental vascular pedicles. These muscles have one large dominant vascular pedicle near the insertion of the muscle with several segmental pedicles near the origin. The internal vasculature can be supplied by either the dominant or the segmental pedicles, and therefore the muscle may be elevated as a flap on either vascular system ( Box 24.6 ).
Fibula
Internal oblique
Latissimus dorsi
Pectoralis major
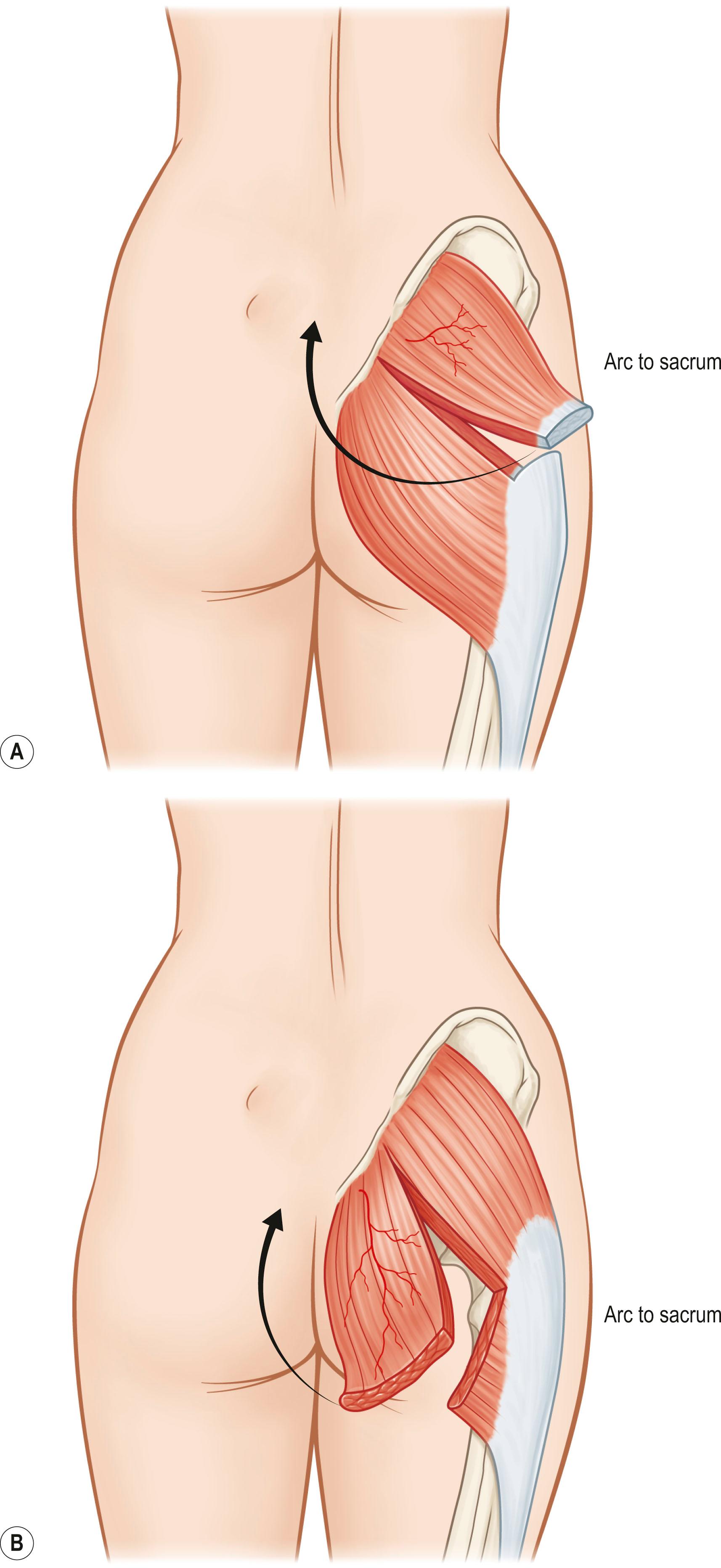
When a flap is used based on the pedicle as a local or an island flap, the dominant vascular pedicle to the flap is preserved. A factor that may prevent successful flap transposition is the flap's arc of rotation. The arc of rotation of a muscle is determined by the extent of elevation of the muscle from its anatomic bed and the ability of the muscle to reach adjacent areas without devascularization. The mobility of a muscle depends on the number of vascular pedicles and the location of the dominant vascular pedicle relative to the muscle's origin and insertion ( Fig. 24.8 ). The area covered by the arc of rotation varies among individuals. On the basis of the flap length distal to the point of rotation and the length of the vascular pedicle, a safe standard arc of rotation is measured for each flap. A modified arc of rotation is also available based on refinements in design and specific modifications of the flap. Precise knowledge of the safe standard and modified arc of rotation is necessary to avoid loss of the flap from excessive tension or damage to the pedicle from overzealous dissection.
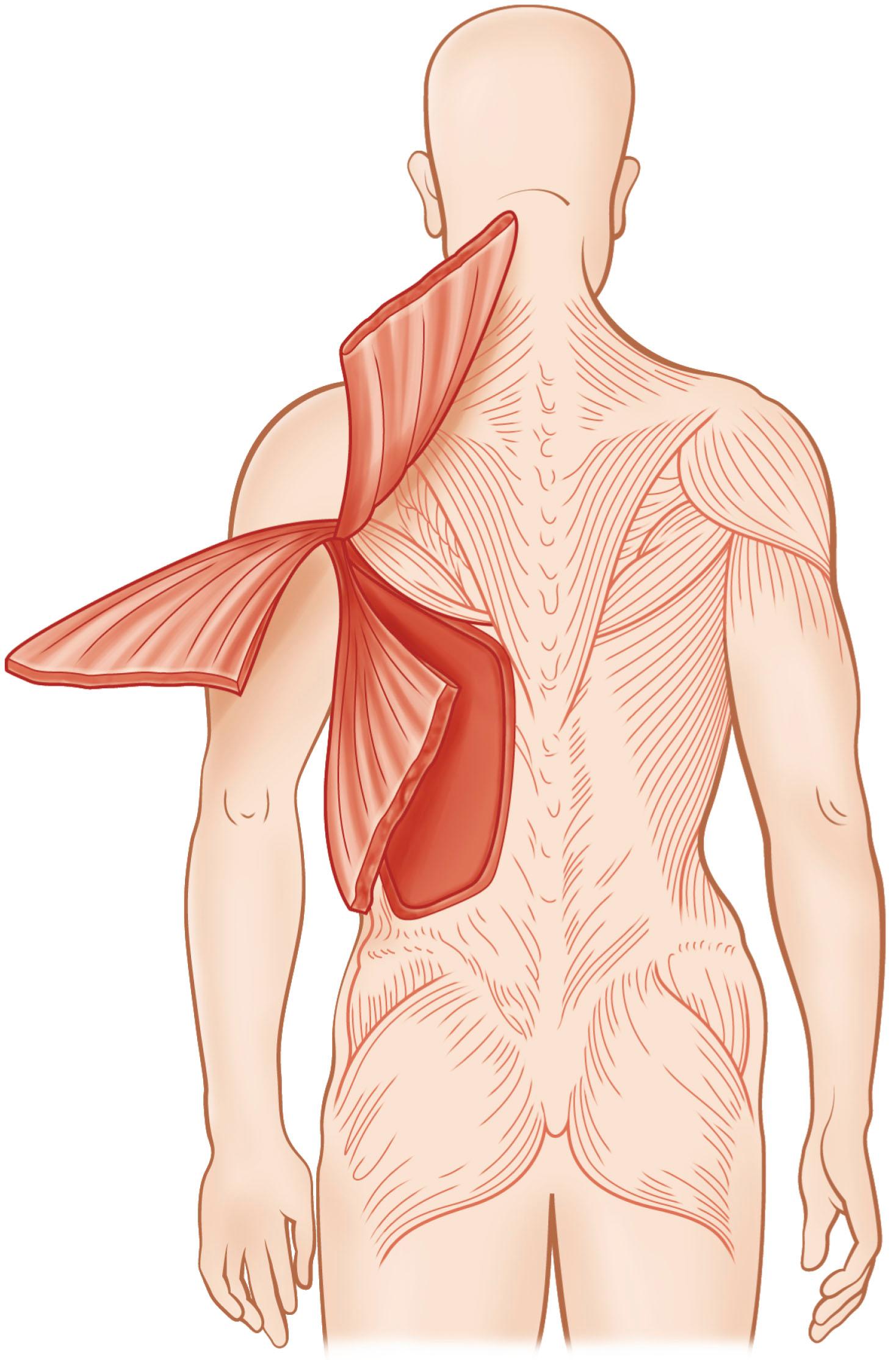
In general, the arc of rotation is inversely proportional to the number of vascular pedicles. If a muscle has a large number of pedicles, it usually has a limited arc of rotation. Type IV muscles, such as the sartorius and tibialis anterior, are examples of muscles with multiple segmental vascular pedicles and limited arcs of rotation. Similarly, the location of the dominant vascular pedicle relative to the muscle's origin and insertion greatly determines the arc of rotation. The closer the dominant vascular pedicle is to either the origin or the insertion of the muscle, the greater the arc of rotation. The point of rotation for types I, II, III, and V muscles generally are located at one end or the proximal third of the muscle. For example, type V muscles, such as the pectoralis major and latissimus dorsi, have their major vascular pedicle near their insertion, and correspondingly have a wide arc of rotation. Certain muscles, such as type V muscles, have two arcs of rotation. The first arc of rotation is based on the dominant blood supply, while the second arc is based on the secondary segmental vascular pedicles. Reverse arc of rotation refers to the degree of transposition of a flap based on its secondary segmental vascular pedicles.
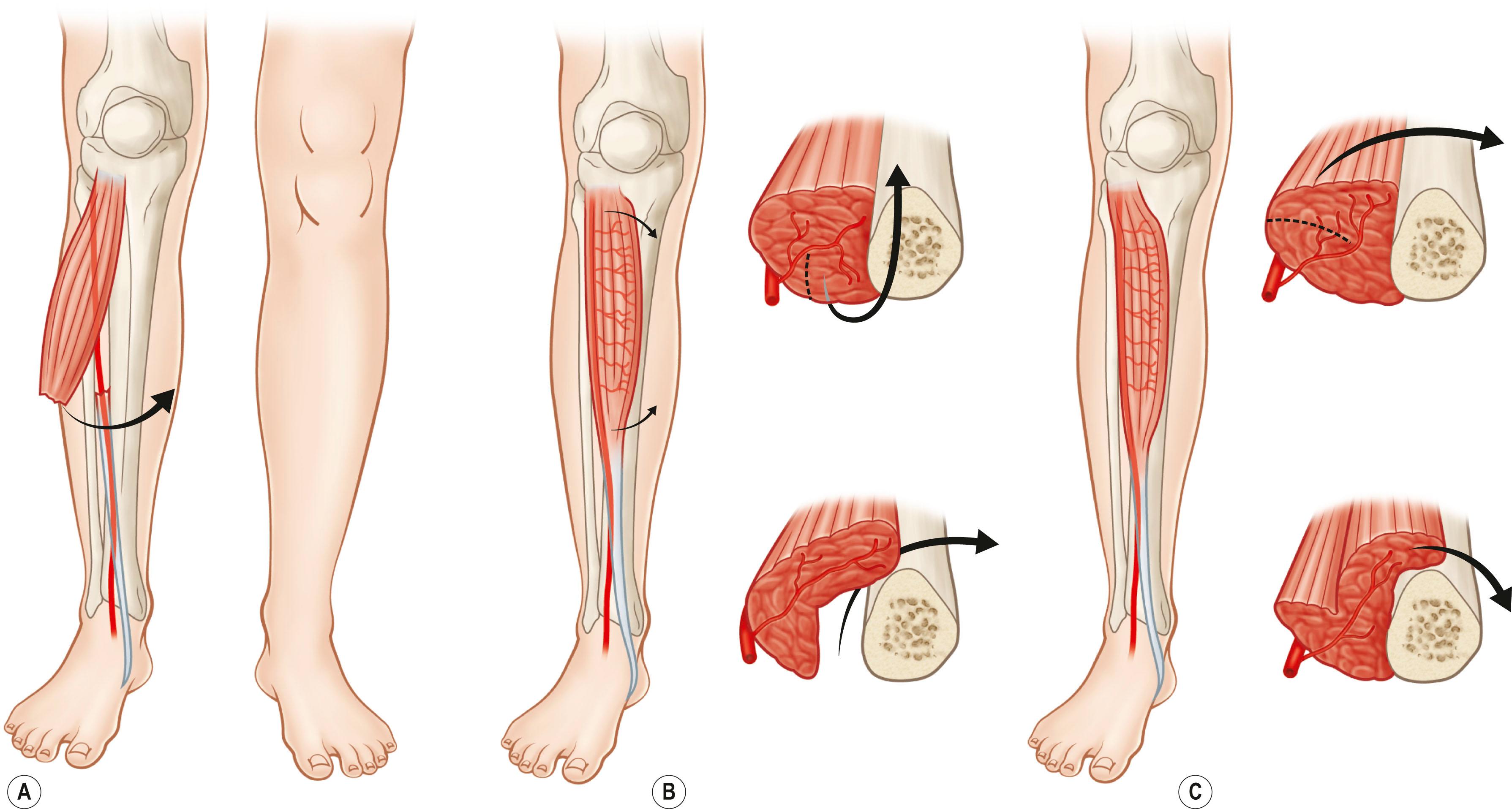
A muscle can be split and a portion in continuity with the dominant vascular pedicle can be used as a transposition flap using just a segment of the muscle. Techniques of muscle splitting to preserve tissue and function have been described. The remaining muscle with its origin and insertion is maintained to preserve function. Alternatively, the entire muscle may be split and used to cover two defects simultaneously. Frequently, only a part of the muscle in proximity to the dominant vascular pedicle is elevated for microvascular transplantation. The skin territory may also be modified and split into two separate skin islands or elevated with only a segment of the muscle flap. However, the skin territory must include vascular connections via musculocutaneous perforating vessels from the segmental flap ( Figs. 24.9 & 24.10 ).
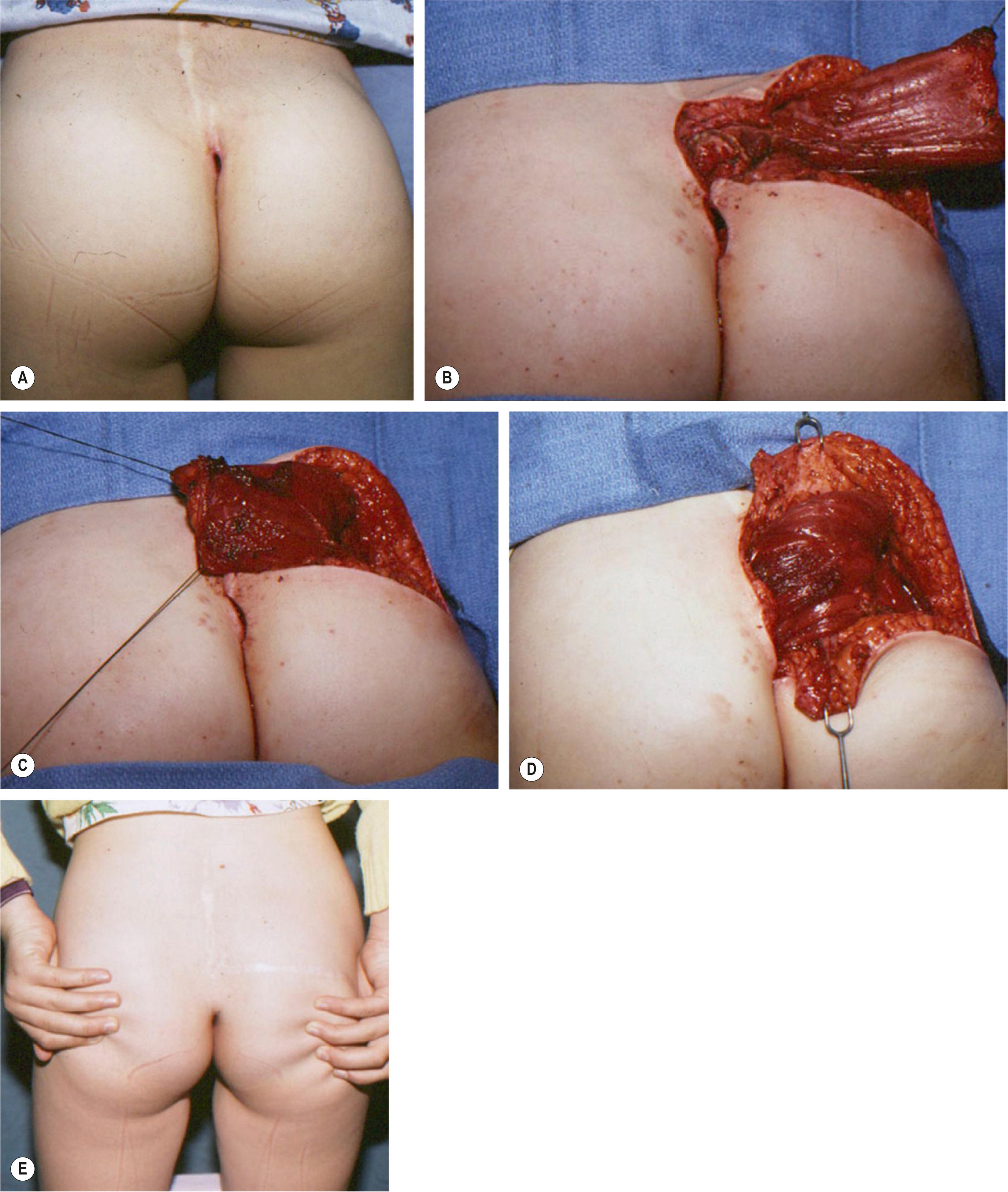
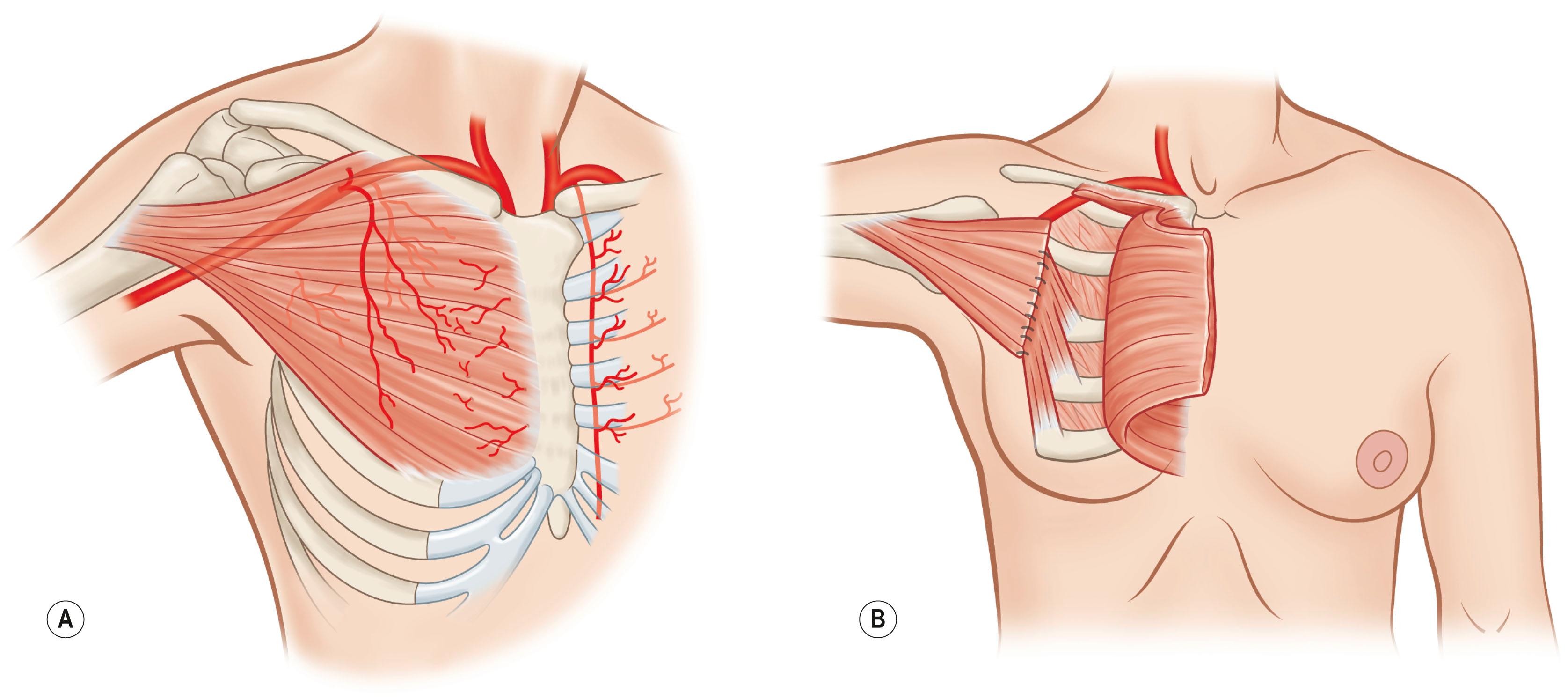
The basis for splitting the pectoralis major muscle was demonstrated by Tobin in 1985. The pectoralis has three segmental neurovascular subunits: the clavicular, the sternocostal, and the external subunit. These can be surgically split and independently transferred on vascular pedicles from the thoracoacromial, internal mammary, and lateral thoracic vessels. Splitting of the pectoralis major muscle into segments has been performed when the segmental transfer of a single intercostal portion of the pectoralis muscle, based on a single medial perforating branch of the internal thoracic artery, is required for chest wall and neck reconstruction ( Figs. 24.11 & 24.12 ). The concept of segmental transposition of muscle allows for the transplantation of independent neuromuscular units (segments of muscle innervated by a single nerve fascicle).
Selection of the most appropriate reconstructive method can be difficult. Careful consideration must be given to all the possible methods of repair, and the advantages and disadvantages of each technique must be weighed accordingly.
The advantages of muscle or musculocutaneous flaps include the following:
The vascular pedicles are specific and reliable.
The vascular pedicle is often located outside the surgical defect, which can be particularly important for wounds with an extensive zone of injury beyond the actual wound (e.g., after irradiation, trauma).
The muscle provides bulk for deep, extensive defects and protective padding for exposed vital structures (e.g., tendons, nerves, vessels, bones, and prostheses).
Muscle is malleable and can be manipulated (e.g., folded on itself) to produce a desired shape or volume.
Well-vascularized muscle is resistant to bacterial inoculation and infection.
Reconstruction by use of muscle or musculocutaneous flaps is often a one-stage procedure.
Restoration of function, whether motor or sensory, is possible with certain flaps.
The reliability and availability of muscle and musculocutaneous flaps make them an excellent alternative means of reconstruction when the closure method of choice for a particular defect is unavailable or inadequate.
The disadvantages of muscle and musculocutaneous flaps include the following:
The donor defect may lose some degree of function.
The donor defect may be aesthetically undesirable.
Reconstruction with muscle or musculocutaneous flaps may provide excessive bulk, leaving an aesthetically unacceptable result.
Muscle or musculocutaneous flaps may atrophy over time and thus fail to provide adequate coverage.
Removal of the muscle or musculocutaneous flap may result in contour deformities at the donor site.
The preservation of function can be extremely important when nonexpendable muscles are used as flaps. The techniques of function preservation generally involve transposing part of the muscle without completely interrupting the origin or insertion of the donor muscle. For example, the transposition of the superior half of the gluteus maximus muscle for sacral coverage in the ambulatory patient can be performed without loss of thigh extension or hip stability because the remainder of the gluteus maximus is functionally intact.
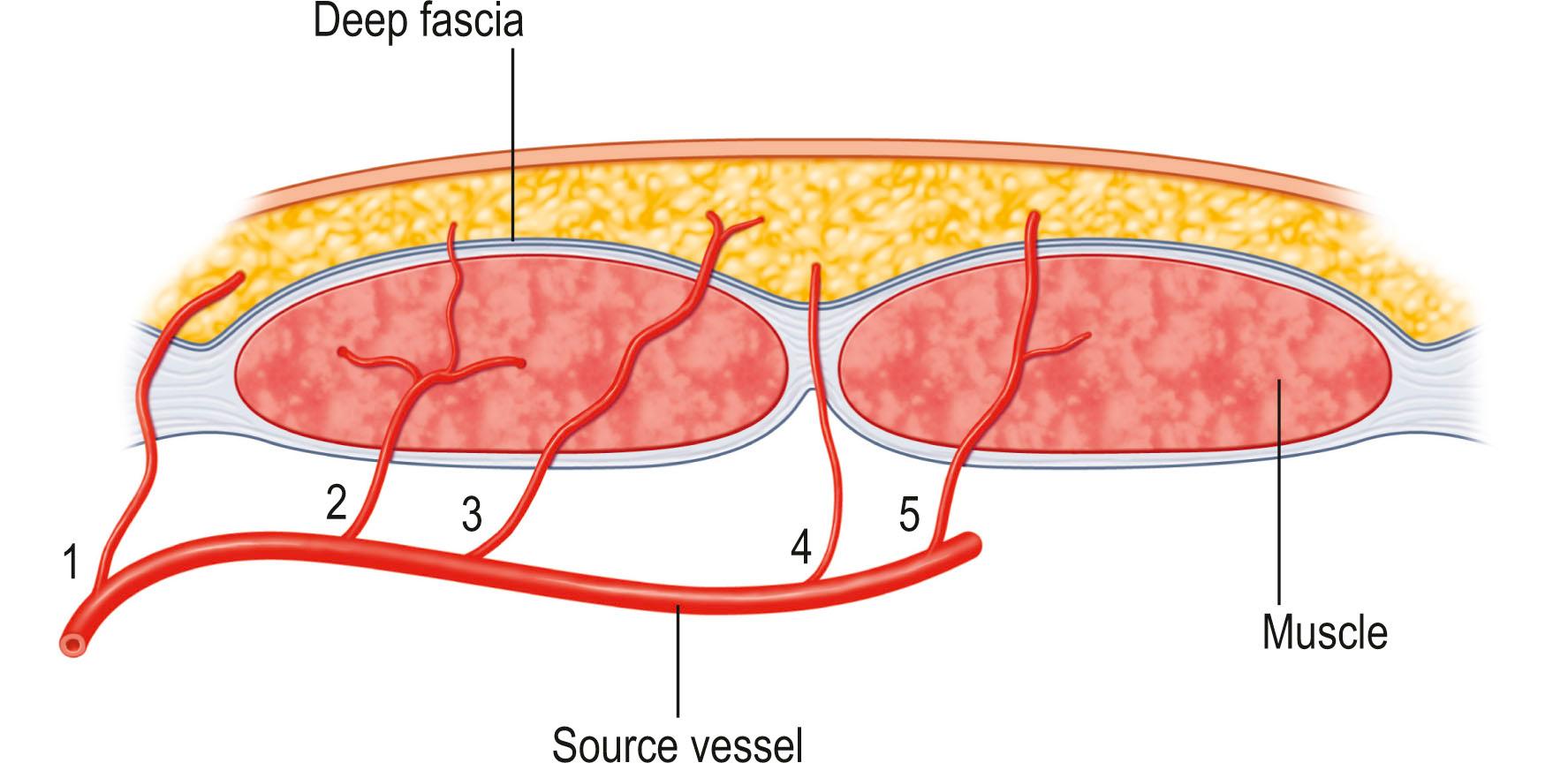
A growing knowledge of the source of skin circulation after the recognition of the muscle and musculocutaneous system led to the identification of vascular pedicles emerging between muscles (septocutaneous pedicles) and entering the deep fascia. Elevation of the skin with its deep fascia represented a new vascular basis for flap design.
A fascial flap consists of fascia detached from its normal origin or insertion and transposed to another location. Without the overlying skin and fat, this represents a delicate flap. A fasciocutaneous flap, originally called an axial flap, includes the skin, subcutaneous tissue, and underlying fascia, which may be distinct from the fascia covering the underlying muscle. The vascular supply is derived at the base of the flap from musculocutaneous perforators or direct septocutaneous branches of major arteries.
The first fascia and fasciocutaneous flaps were described by Pontén in 1981 for lower extremity reconstruction and Tolhurst in 1983 for trunk and axillary reconstruction. Investigations have shown that the fasciocutaneous system consists of perforating vessels that arise from regional arteries and pass along the fibrous septa between muscle bellies or muscle compartments. The vessels then spread out at the level of the deep fascia, both above and below, to form plexuses, which in turn give off branches to the skin. In 1975, Schafer found three major vascular systems of the deep fascia:
Perforating arteries from underlying muscle giving off several radiating branches, which perforate the fascia before continuing to the subdermal plexus
Subcutaneous arteries running in the fat and anastomosing frequently with the superficial plexus of the deep fascia and with each other
Subfascial arteries arising from the intermuscular septa and running in the loose areolar tissue beneath the deep fascia and adjoining the deep and superficial plexus.
These pedicles consist of an artery (generally a branch of the artery to the specific anatomic region of the fascia and regional musculature) and paired venae comitantes that drain into corresponding major regional veins. Direct cutaneous and septocutaneous pedicles are fairly constant in location. There is a greater variability in location of the musculocutaneous perforators. These pedicles provide a vascular basis for specific fascial or fasciocutaneous flaps. On this basis, Mathes and Nahai have classified fascia and fasciocutaneous flaps as types A, B, and C ( Fig. 24.13 ).
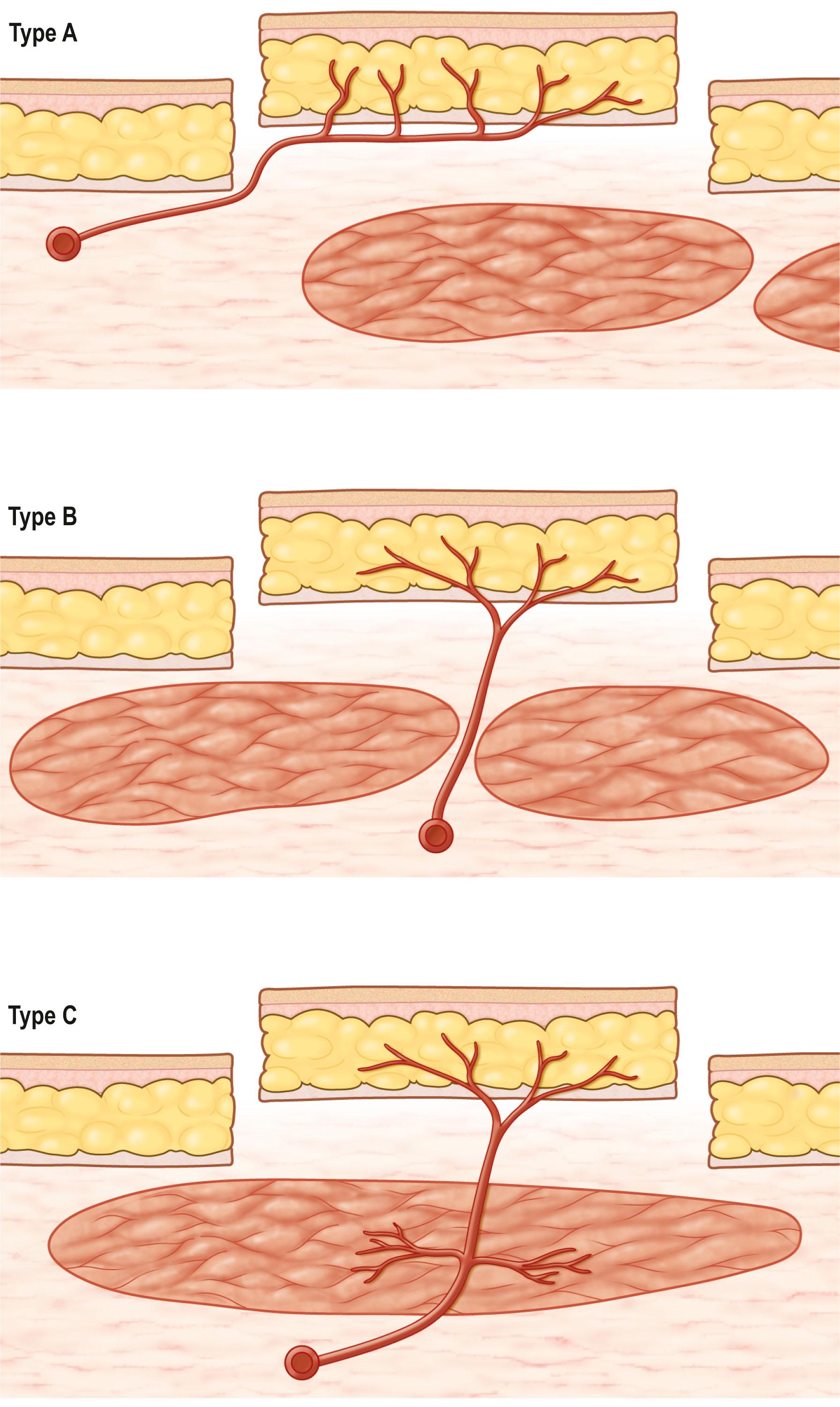
Anatomic studies demonstrate that type A fasciocutaneous flaps have a vascular pedicle to the deep fascia that emerges from a regional source coursing initially beneath the deep fascia and eventually continuing its course superficial to the deep fascia. This pedicle provides numerous fasciocutaneous perforators to the skin. Because the pedicle tends to course in a radial fashion from its regional source into its distal cutaneous distribution, the flap is often referred to as an axial flap. The long, relatively superficial course of the dominant pedicle permits evaluation by palpation or Doppler probe ( Box 24.7 ).
Deep external pudendal artery
Digital artery
Dorsal metacarpal artery
Gluteal thigh
Great toe (hallux)
Groin
Lateral thoracic (axillary)
Pudendal – thigh
Saphenous
Scalp
Second toe
Standard forehead
Superficial external pudendal artery
Superficial interior epigastric artery
Sural artery
Temporoparietal fascia
Type B fasciocutaneous flaps have a septocutaneous pedicle, which courses between major muscle groups in an intermuscular septum or between adjacent muscles. This pedicle is located within the intermuscular septum or the potential space between adjacent muscles and supplies a regional fascial vascular system. The largest septocutaneous pedicles are dominant pedicles to specific fasciocutaneous flaps and are fairly constant in location ( Box 24.8 ).
Anterolateral thigh
Anterior tibial artery
Deltoid
Dorsalis pedis
Inferior cubital artery (antecubital)
Lateral arm
Lateral plantar artery
Lateral thigh
Medial arm
Medial plantar artery
Medial thigh
Peroneal artery
Posterior interosseous
Posterior tibial artery
Radial forearm
Radial recurrent
Scapular
Ulnar recurrent
In certain regions, larger musculocutaneous perforators enter the deep fascia and contribute to both the deep fascia and cutaneous circulation. The design of a fasciocutaneous flap can be based on these dominant perforating vessels without incorporation of the underlying muscle; this vascular pattern represents the type C fasciocutaneous flap. However, increasing pedicle length will necessitate proximal dissection of the pedicle through muscle to its regional source or incorporation of all or part of the muscle in the flap design. Type C flaps are generally the anatomic model used for the perforator flap in microsurgical transplantation ( Box 24.9 ).
Anterolateral thigh
Deltopectoral
Nasolabial
Median forehead
Thoracoepigastric (transverse abdominal)
Transverse back
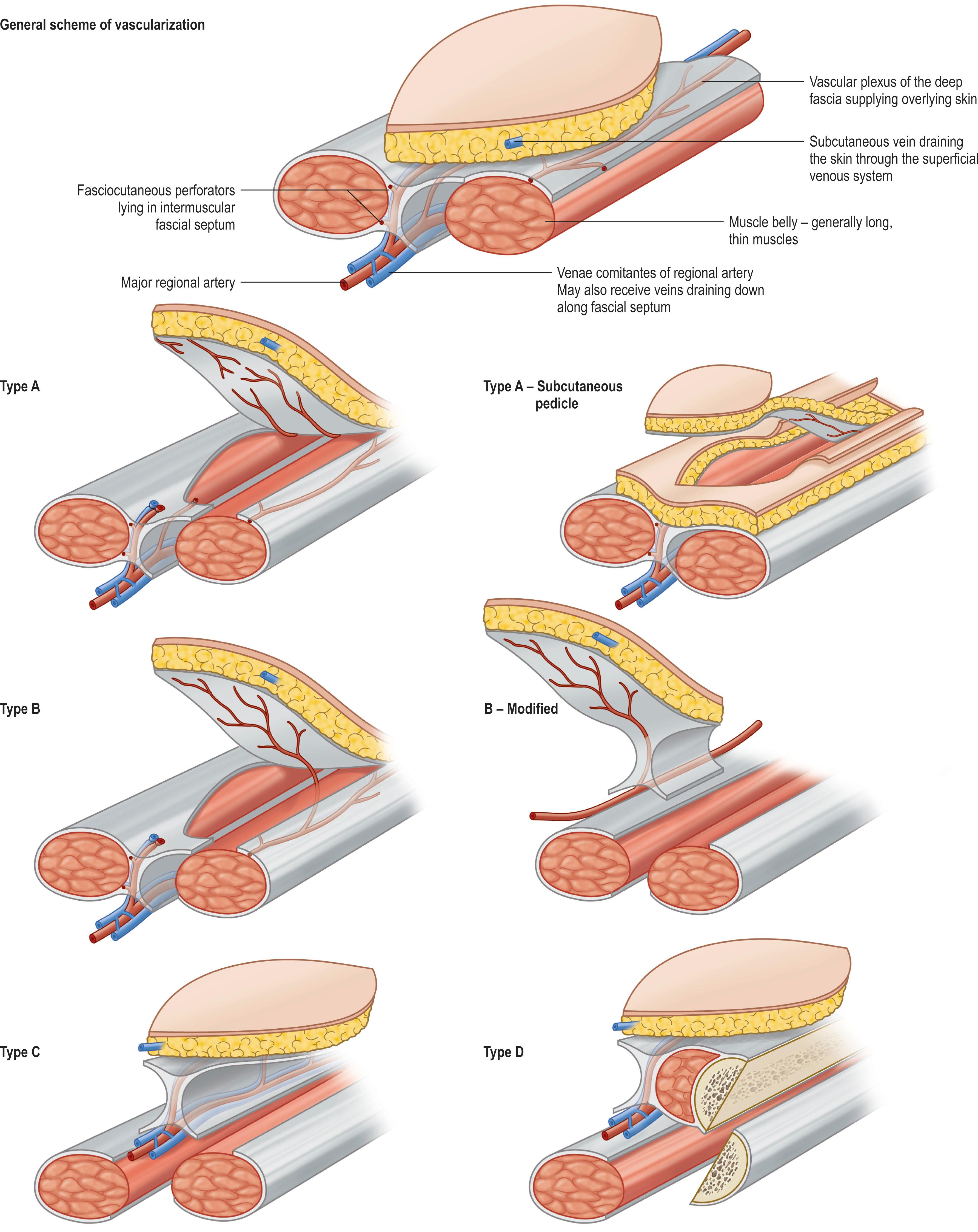
Cormack and Lamberty also classified fasciocutaneous flaps based on vascular anatomy. The type A flap is supplied by multiple fasciocutaneous perforators that enter at the base of the flap and extend throughout the longitudinal length. The flap can be based proximally, distally, or as an island. The type B flap has a single fasciocutaneous perforator, which is of moderate size and is fairly consistent. It is intended for use as a free flap. The type C flap is based on multiple small perforators that run along a fascial septum. The supplying artery is included within the flap. It may be based proximally, distally, or as a free flap. The type D flap is an osteomusculofasciocutaneous flap, and is based on multiple small perforators similar to the type C flap, but also includes a portion of adjacent muscle and bone. It may be based proximally or distally on a pedicle or used for microvascular tissue transplantation ( Fig. 24.14 ).
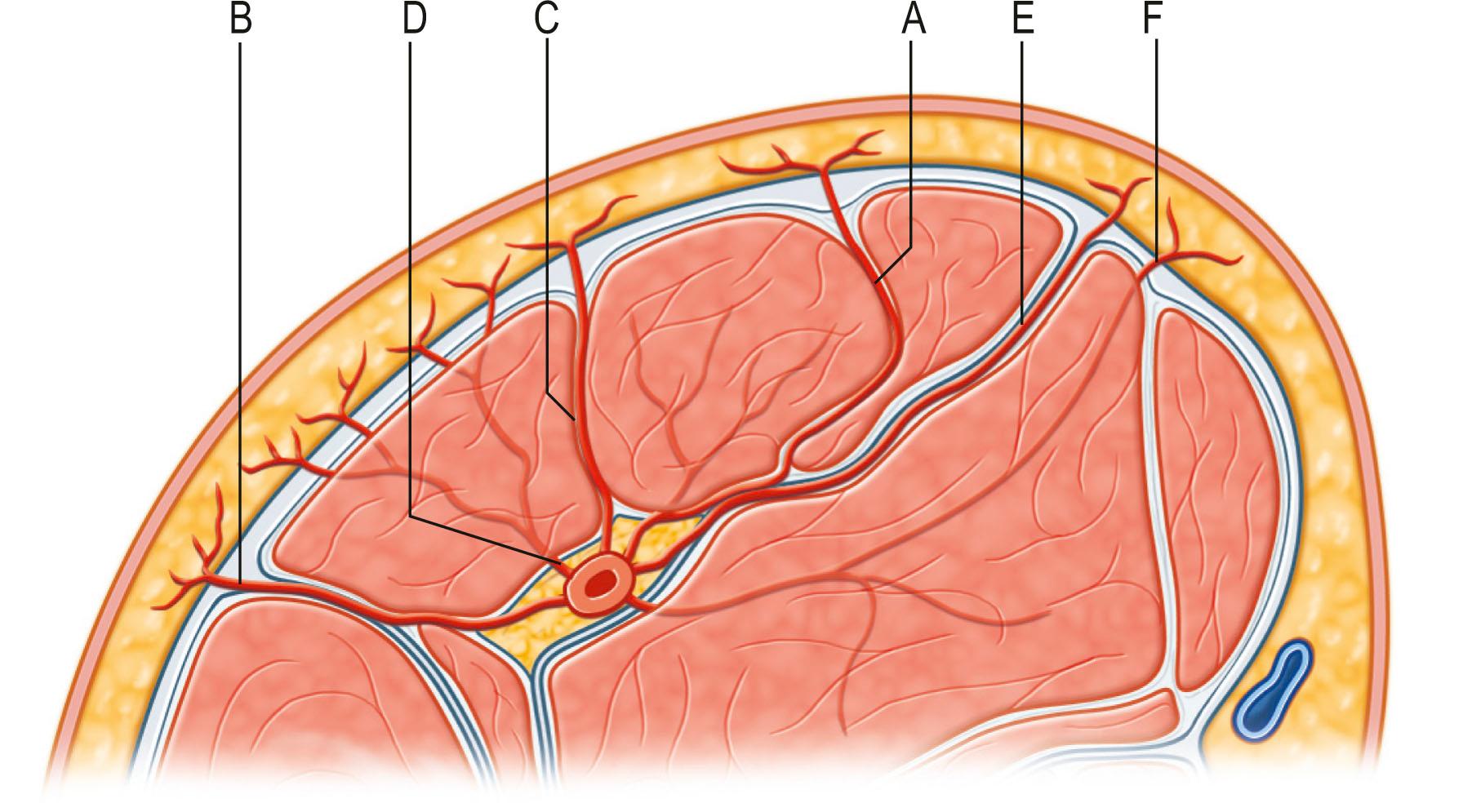
Nakajima et al . expanded the types of fasciocutaneous flaps and described them originating from six different types of perforators piercing the deep fascia feeding to the plexus of the skin ( Fig. 24.15 ). Type A, direct cutaneous branch of muscular vessel was described in the same manner as axial pattern flaps of McGregor and Morgan. Type B, septocutaneous perforator was the same as type B from the classification of Cormack and Lamberty. Type C, direct cutaneous branch, and type D, musculocutaneous perforator supplying the fasciocutaneous flap, were not quite described in previous classifications. These types, especially type D from the Nakajima et al . classification, further play a pivotal role for setting up the concept of true perforator flaps. Type E, direct septocutaneous, describes type C from the classification of Cormack and Lamberty and type F, perforating cutaneous branch of muscular vessel resembles traditional musculocutaneous flaps.
The fasciocutaneous flap is used as a local flap, the standard arc of rotation is determined by the extent of elevation of the deep fascia from its normal anatomic position to reach adjacent defects. The point of rotation is based on the site of entrance of the dominant vascular pedicle into the fascia. The fascia or fasciocutaneous flap is elevated to the point of entrance of the flap pedicle, and the fascia and overlying skin distal to this point are rotated into the defect.
The advantages and disadvantages of these flaps are somewhat similar to those of muscle flaps, although there are a few exceptions. The advantages include the following:
They are thin and pliable.
The blood supply is reliable and robust.
The donor site morbidity is minimal in regard to function.
They are muscle sparing.
They have the ability to restore sensation.
There are many potential donor sites.
The disadvantages of fascia, fasciocutaneous, and perforator flaps include the following:
There is a lack of bulk for deep defects.
They are technically more challenging (pedicle dissection; many require microvascular anastomosis or at least microvascular techniques).
There are size limitations.
The arc of rotation is sometimes limited though often better than the similar muscle flap because of the longer pedicle.
Donor site may require skin graft closure, resulting in donor site deformity.
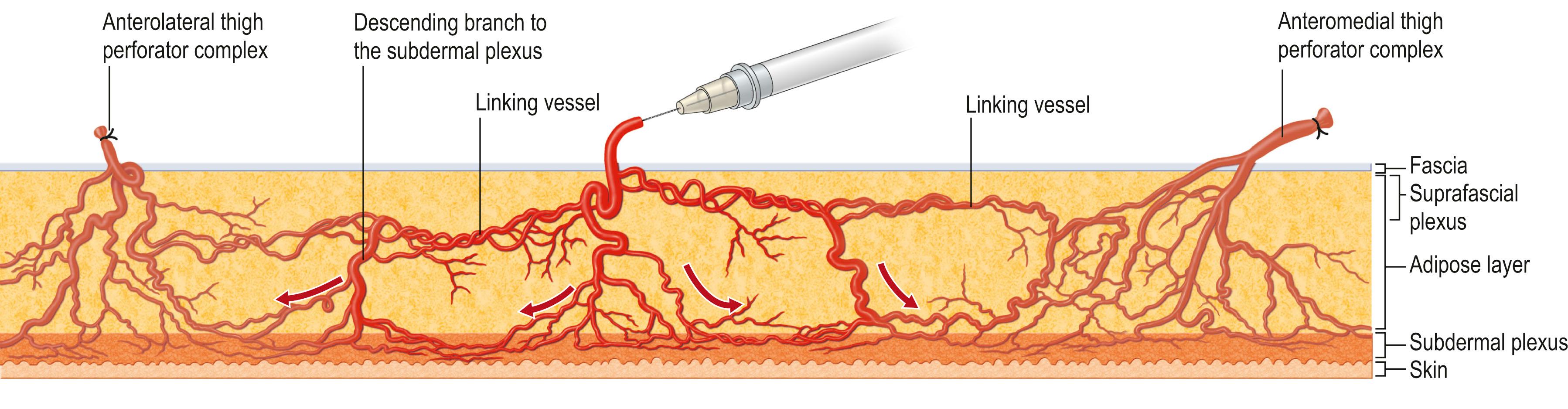
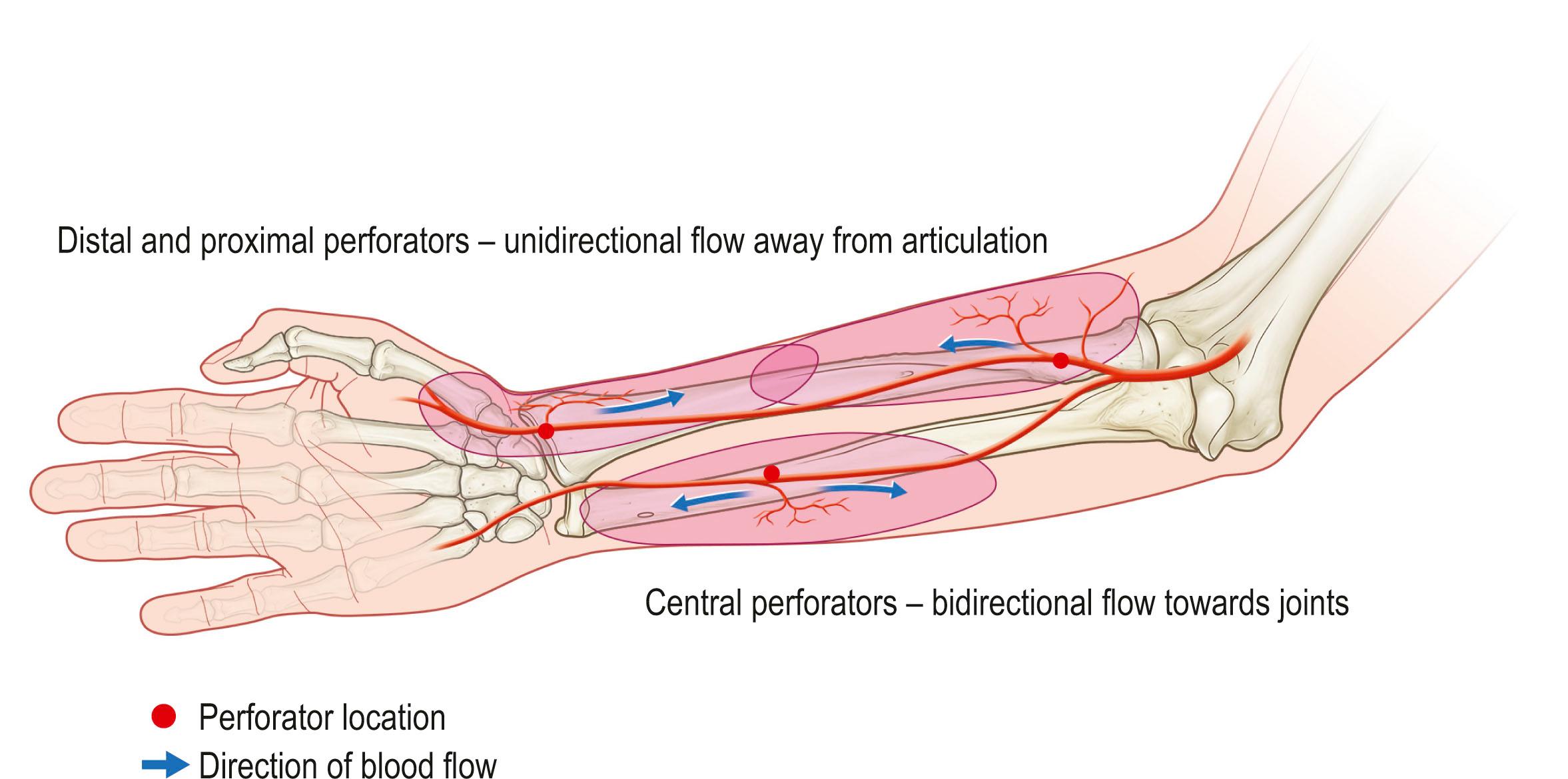
Further refinements in flap application have led to the development of perforator flaps. Perforator flaps have evolved from musculocutaneous and fasciocutaneous flaps without the muscle or fascial carrier. It was a natural evolution as reconstruction needed fine-tuning while aiming to minimize donor morbidities. A good example of this evolution would be the change from transverse rectus abdominis muscle (TRAM) flaps to muscle-sparing TRAM to deep inferior epigastic perforator (DIEP) flaps ( Fig. 24.16 ). Transformation of these flaps has shown that neither a passive muscle carrier nor the underlying fascial plexus of vessels are necessary for flap survival. Thus, a perforator flap is a skin flap (with or without fascia) based on a single perforator. Like the angiosome concept showing the vascular territory of a source vessel, one must understand the anatomy and physiology of a single perforator territory to obtain the ideal design of the perforator flap. The perforasome theory by Saint-Cyr reported four major characteristics of a perforator flap: (1) each perforasome is linked with adjacent perforasomes by means of direct and indirect linking vessels ( Fig. 24.17 ); (2) flap design and skin paddle orientation should be based on the direction of the linking vessels, which is axial in the extremities and perpendicular to the midline in the trunk ( Fig. 24.18 ); (3) filling of the perforasomes occurs within perforasomes of the same source artery first followed by perforators of the other adjacent source arteries; (4) mass vascularity of a perforator found adjacent to an articulation is directed away from that same articulation ( Fig. 24.19 ). This theory provides insights into perforator flap vascularity and can clinically guide to the harvest of a safer free or pedicle perforator flap.
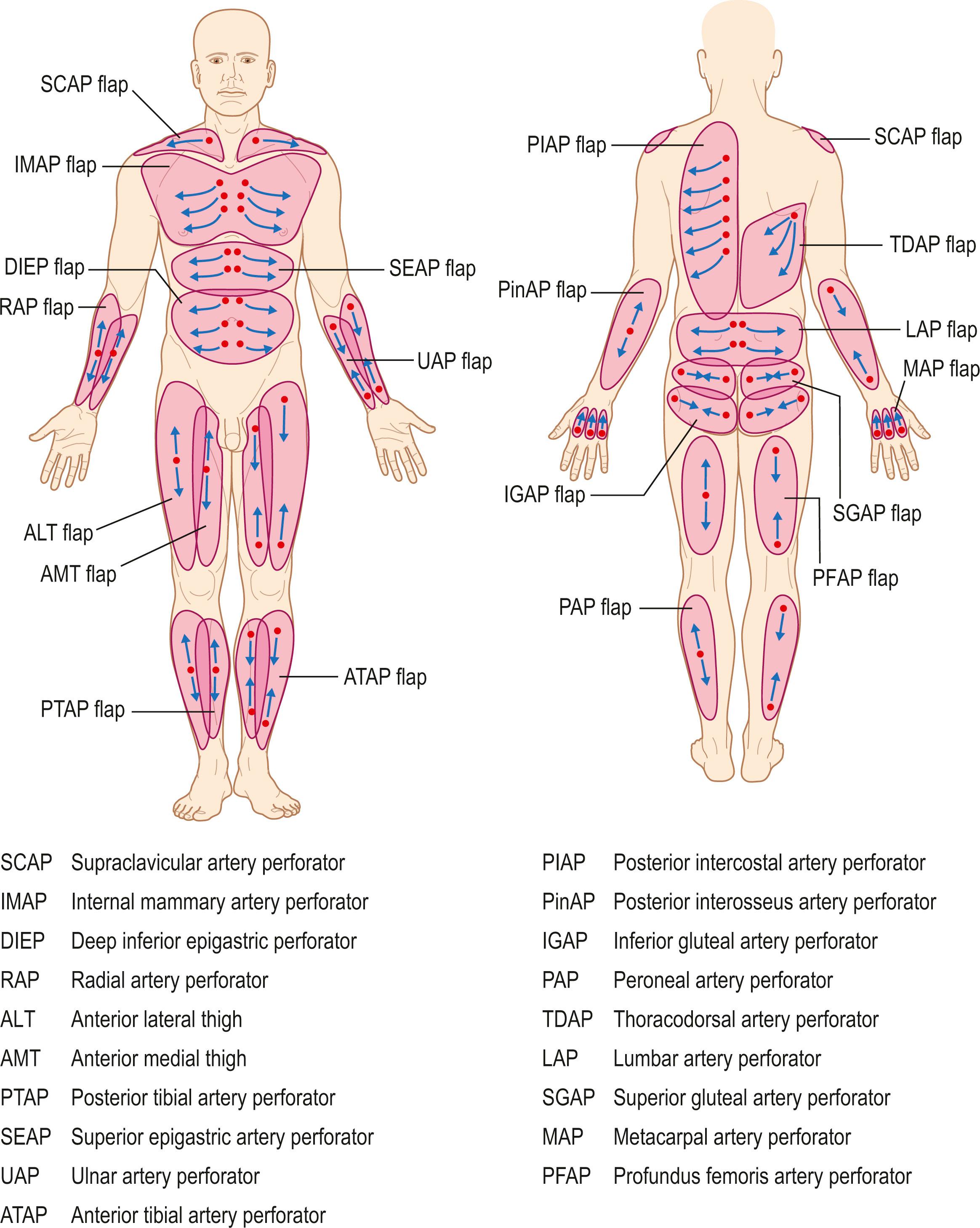
In 1989 Koshima and Soeda used the term “perforator flaps” in their harvest for paraumbilical skin and fat island flaps based on a muscular perforator. Since then, perforator-based flaps using lower abdomen skin flaps have been applied for breast reconstruction. Slowly other perforator-based flaps have been introduced from various parts of the skin.
The nomenclature of perforator flaps is confusing and oftentimes misstated. Perforator flaps have been designated by their location (e.g., anterolateral thigh flap), arterial supply (e.g., deep inferior epigastric artery perforator flap), or the muscle of origin (e.g., gastrocnemius perforator flap). Thus, a clear definition and classification for perforator flaps was needed. Hallock defines a perforator as any vessel that pierces through the fenestration in the deep fascia regardless of origin. In a consensus document for perforator flap terminology, perforators that pierce through the deep fascia without traversing any other structural tissues were called direct perforators while indirect perforators run through deeper structures such as muscles, septum and perimysium. Evolved from the six patterns of fascial perforators of Nakajima et al ., the consensus paper simplified it into five types of perforator based on the surgical approach for dissection of the perforators ( Fig. 24.20 ). Type 1: direct perforators perforate the deep fascia only; type 2: indirect muscle perforators predominantly supply the subcutaneous tissues; type 3: indirect muscle perforators predominantly supply the muscle but have secondary branches to the subcutaneous tissues; type 4: indirect perimysial perforators travel within the perimysium between muscle fiber before piercing the deep fascia; type 5: indirect septal perforators travel through the intermuscular septum before piercing the deep fascia. Based on this distinction of different perforators, the consensus paper gives definitions and classification for perforator flaps as follows:
Definition 1 : A perforator flap is a flap consisting of skin and/or subcutaneous fat. The vessels that supply the blood to the flap are isolated perforator(s). These perforators may pass either through or in between deep tissues (mostly muscle).
Definition 2 : A muscle perforator is a blood vessel that traverses through septum to supply the overlying skin.
Definition 3 : A septal perforator is a blood vessel that traverses only through septum to supply the overlying skin.
Definition 4 : A flap that is vascularized by a muscle perforator is called a muscle perforator flap.
Definition 5 : A flap vascularized by a septal perforator is called a septal perforator flap.
Definition 6 : A perforator flap should be named after the nutrient artery or vessels and not after the underlying muscle. If there is a potential to harvest multiple perforator flaps from one vessel, the name of each flap should be based on its anatomical region or muscle.
This terminology and the classification into indirect and direct perforator flaps and further into septal and muscle perforator flaps were set up to clearly identify the course of these small terminal branches before they pierce the deep fascia and the technical implications during the surgical procedure. Table 24.2 shows some of the popular perforator flaps based on this terminology. Recent innovations in this field have made some of the terminology and classification somewhat misleading. As with all evolution, new classification and terminology will be constantly updated.
| Flap – abbreviation | Flap – full name | Nutrient artery |
|---|---|---|
| Muscle perforator flaps | ||
| DIEP | Deep inferior epigastric perforator | Deep inferior epigastric vessels |
| TAP | Thoracodorsal artery perforator | Thoracodorsal vessels |
| SGAP | Superior gluteal artery perforator | Superior gluteal vessels |
| IGAP | Inferior gluteal artery perforator | Inferior gluteal vessels |
| IMAP | Internal mammary artery perforator | Internal mammary vessels |
| ICAP | Intercostal perforator | Intercostal vessels |
| PLP | Paralumbar perforator | Paralumbar perforating vessels |
| GP | Gracilis perforator | Medial circumflex femoris vessels |
| TFLP | Tensor fasciae latae perforator | Transverse branch of the lateral circumflex femoris vessels |
| ALTP | Anterolateral thigh perforator | Descending branch of the lateral circumflex femoris vessels |
| AMTP | Anteromedial thigh perforator | Innominate branch of the descending branch of the lateral circumflex femoris vessels |
| SAP | Sural artery perforator | Sural vessels |
| PTAP | Posterior tibial artery perforator | Posterior tibial vessels |
| ATAP | Anterior tibial artery perforator | Anterior tibial vessels |
| Septal perforator flaps | ||
| RAP | Radial artery perforator | Radial vessels |
| AP | Adductor perforator | Medial circumflex femoris vessels |
| AMTP | Anteromedial thigh perforator | Innominate branch of the descending branch of the lateral circumflex femoris vessels (if perforator runs only in septum) |
| ALTP | Anterolateral thigh perforator | Descending branch of the circumflex femoris lateralis vessels (if perforator runs only in the septum) |
One of the recent evolutions in perforator flaps has been in producing thinner flaps. Although post-reconstruction debulking achieves pleasing aesthetic results and is widely practiced, customized approach during elevation to achieve the ideal thickness will increase efficiency while achieving the best possible aesthetic outcome in accordance with the elevator reconstruction concept. Multiple planes for elevation have been reported along with different techniques. To date, the elevation layers can be categorized into five planes; subfascial (immediately under and including the deep fascia same as fasciocutaneous structure), suprafascial (immediately above and excluding the deep fascia), superficial fascia (also termed superthin, on the fascia-like film layer between the deep and the superficial fat), fascia-like plane within the superficial fat (also termed ultrathin) and the subdermal plane (also known as the pure skin perforator flap) ( Fig. 24.21 ). These planes all have their unique properties and challenges and the surgeon should understand the anatomy, technical aspect, and possible pitfalls to safely elevate the perforator flaps on these different planes.
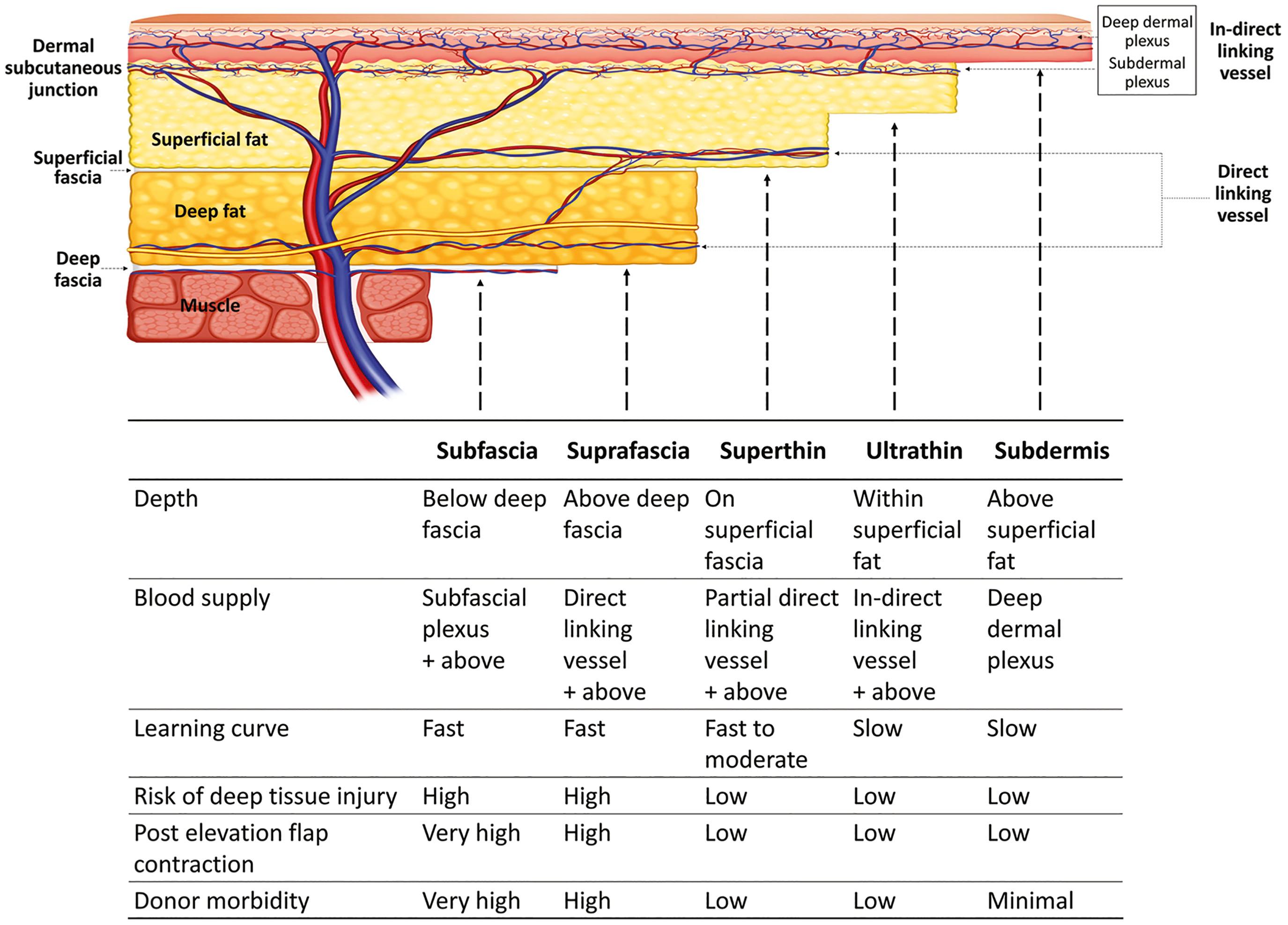
When selecting the perforator flap as a free flap, multiple factors can be considered. A recent algorithm for perforator flap selection recommends to consider the patient position on the surgical table, flap size needed for coverage, thickness of the perforator flap, composition of the flap and the pedicle length required to sufficiently reach the recipient vessel. This approach can help to optimize form and function, decrease operative time, while minimizing donor site morbidity and secondary procedures.
The perforator flap concept simplified and overcame the applications and limits of classic local flaps. By identifying the perforator as the pedicle and further dissecting toward the source vessel, it allowed improved movement and better survival of flap. One form of perforator-based local flap, the propeller flap, is an island flap that reaches the recipient site through an axial rotation. When a perforator propeller flap is being elevated, the perforator is dissected free from the fascial and fat adhesions to minimize the chance of kinking. Although less rotation reduces the chance for kinking, the skin island may be safely rotated up to 180° ( Fig. 24.22 ).
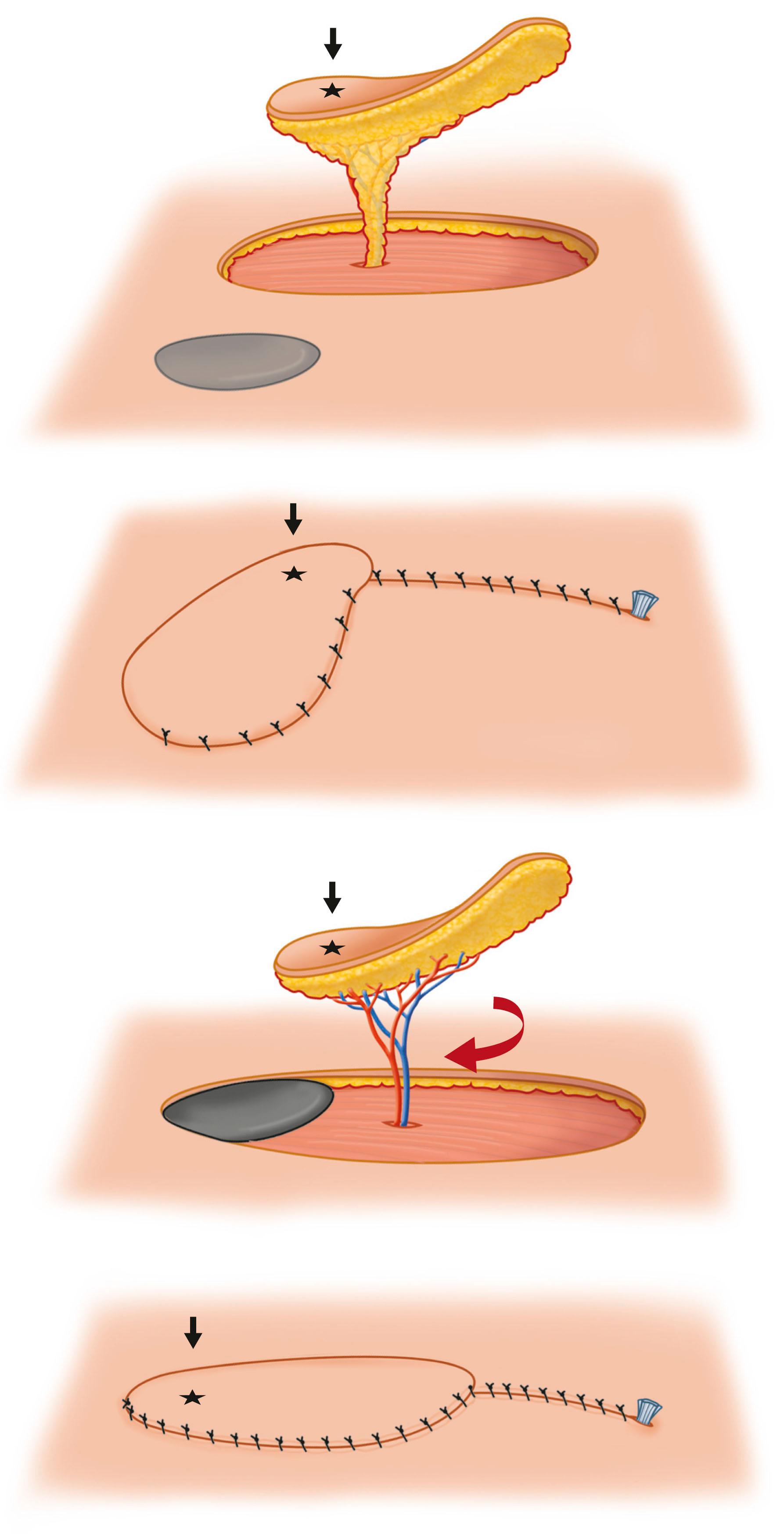
Advantages of perforator flaps include:
Reduced donor site morbidity
Versatility in flap design
Muscle sparing (less functional deficit)
Improved postoperative recovery of the patient.
Disadvantages of perforator flaps may include:
Meticulous dissection needed to isolate the perforator vessels
Increased operative time, especially in muscle perforators
The variability in the position and size of the perforator vessels
Steep learning curve.
However, modifications such as the free-style approach and supermicrosurgery have further simplified the approach and increased the use of perforator flaps. The free-style approach identifies the perforator feeding the skin flap first and then dissects proximally toward the source vessel contrary to the classic approach where identification of the source vessel was made first and then dissection toward the perforator. This allows the freedom to design flaps based on any perforator as well as alleviate the risk for pedicle variation. The supermicrosurgery approach, perforator to perforator anastomosis, allows harvesting the flap as a short pedicled flap, reducing the dissection time and minimizing the risk for traumatizing the pedicle during dissection.
The abdominal viscera are not easily classified; however, for the purposes of flap transposition or microvascular tissue transplantation, the colon, jejunum, and omentum fall conveniently into the muscle classification system ( Table 24.3 ). For microvascular transplantation, the segment of bowel (jejunum or colon) is elevated on one vascular arcade with a single dominant vessel, a type I pattern of circulation. In unusual circumstances where a longer segment of bowel extends beyond the vascular territory of one arcade, two vascular arcades must be included to ensure viability of this longer segment of bowel. In this instance, the pattern of circulation will be type III (two dominant arcades or pedicles). It is possible to reconstruct the esophagus from the base of the tongue to the stomach with a long segment of jejunum where one pedicle is revascularized in the upper chest or neck and the second pedicle is left intact. Additional uses of the colon or jejunum as flaps have been for vaginal reconstruction. The appendix, as a type I pattern based on the appendiceal artery and vein, has been used for reconstructing the urethra and for voice reconstruction.
| Flap | Type | Circulation pattern | Size |
|---|---|---|---|
| Colon | Bowel | Type I | 20–25 cm in lengthLumen diameter of 8 cm |
| Jejunum | Bowel | Type I | 7–25 cm may be transferred on one pedicleLumen diameter of 3–5 cm |
| Omentum | Omentum | Type III | Variable; up to 40×60 cm |
The omentum may be based as a transposition flap on either the right or left gastroepiploic vessels and is thus classified as having a type III pattern of circulation. The omentum is also commonly transferred microsurgically. The omentum can be used to reconstruct a wide range of extraperitoneal defects and has been shown to have immunologic and angiogenic properties. Although useful for reconstruction, donor site complications can be significant, including abdominal wall infection and hernia. With the advances in minimally invasive surgery, the abdominal viscera can successfully be harvested laparoscopically obviating the need for a large midline incision providing a better cosmetic result and less donor site morbidity.
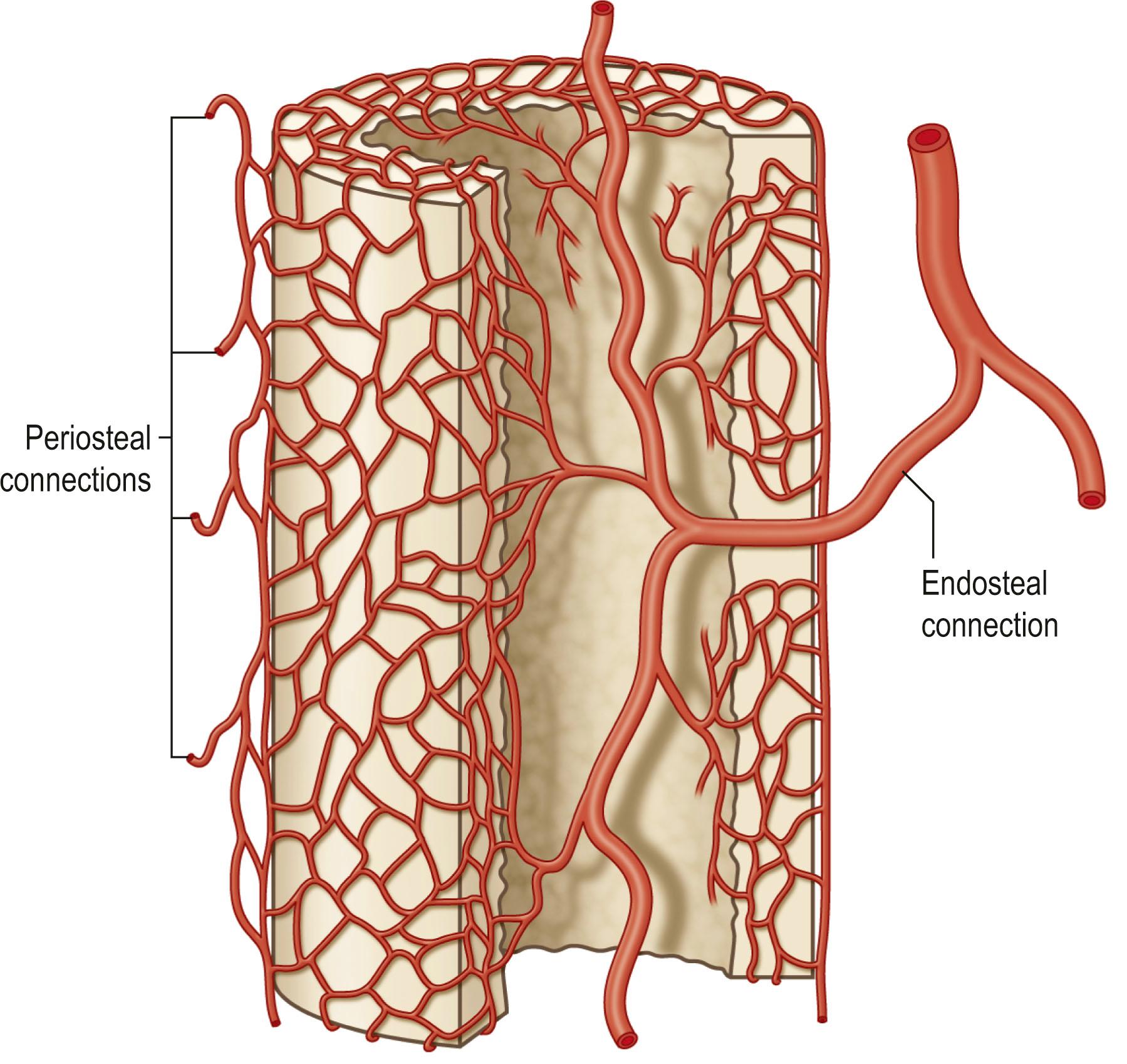
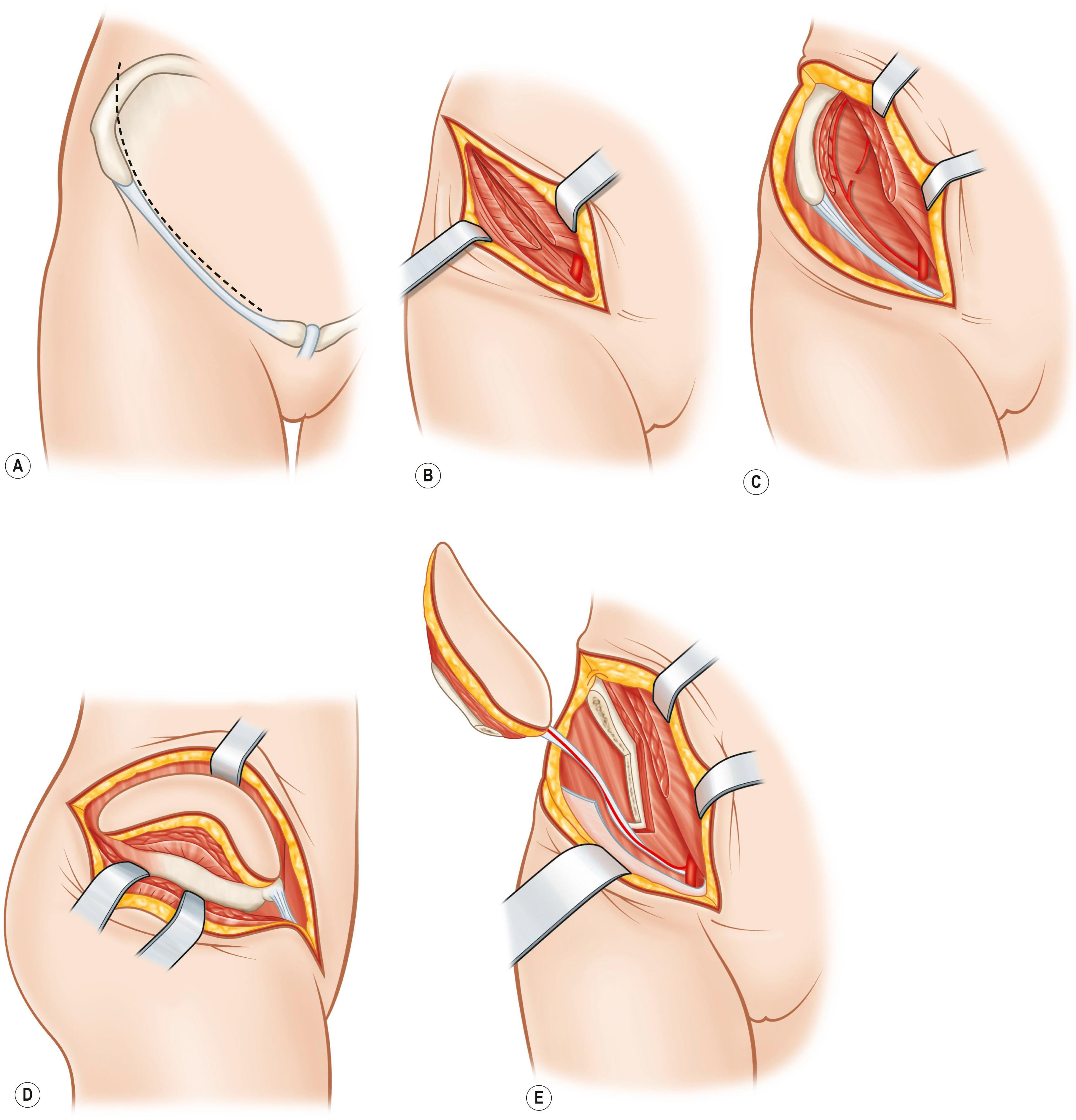
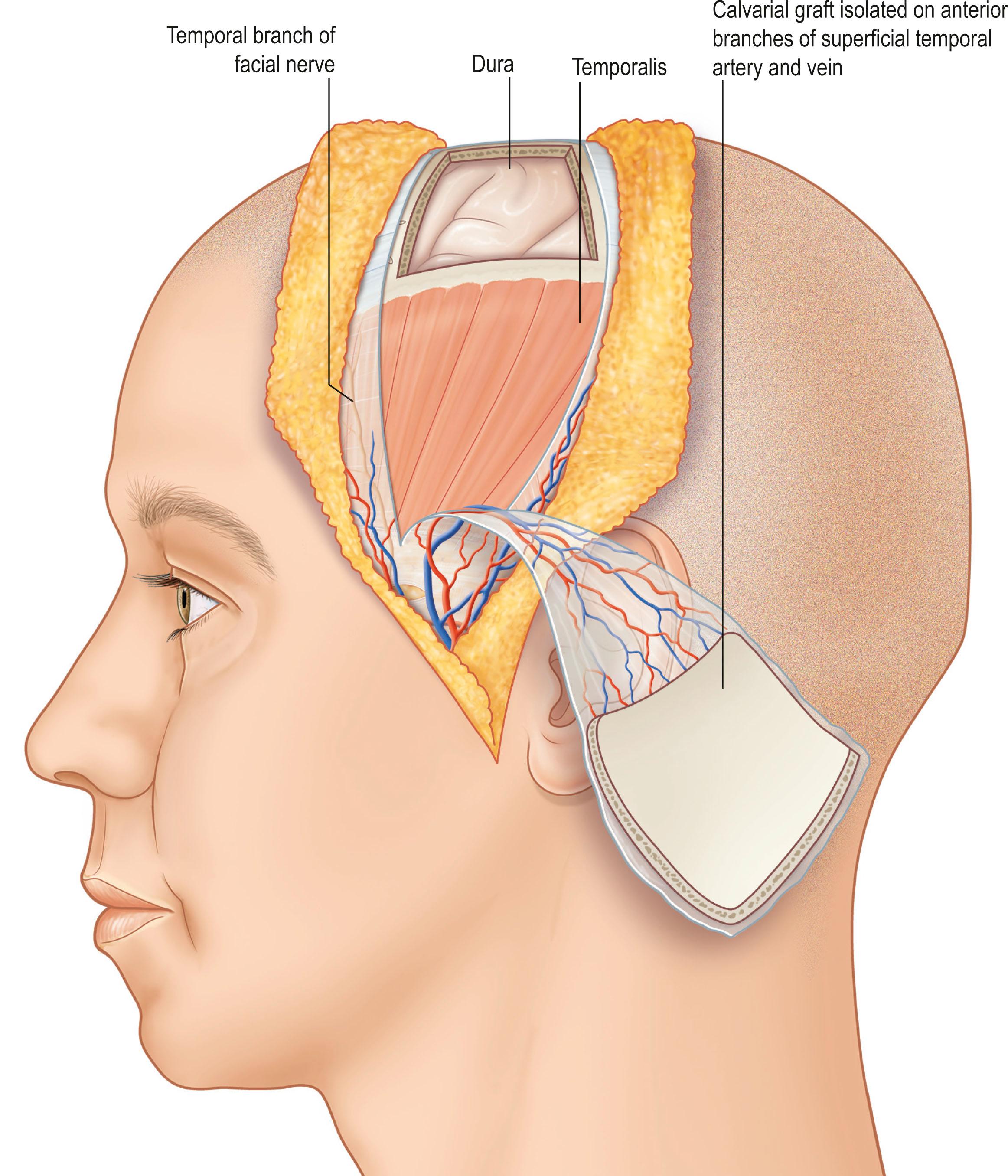
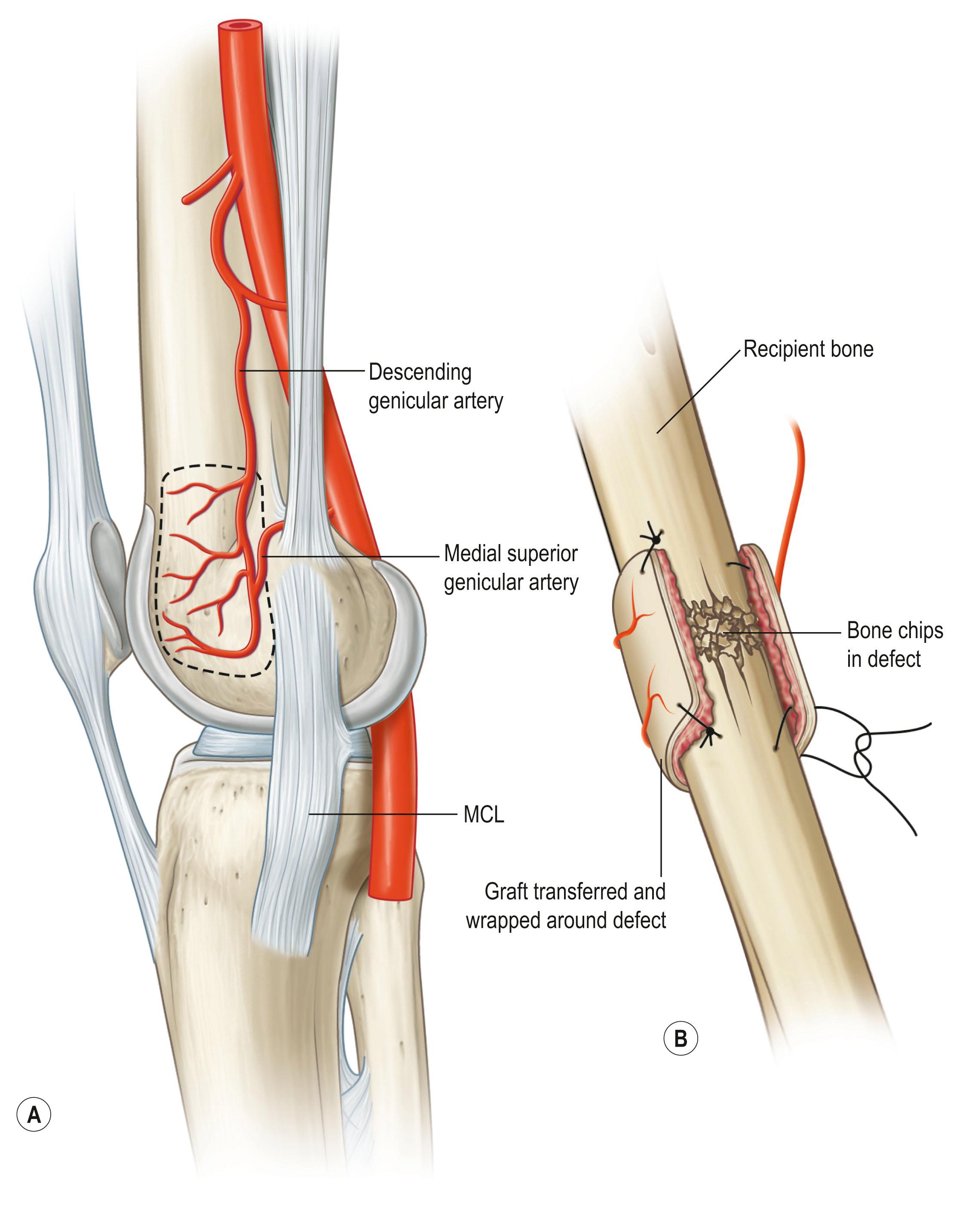
Bone is vascularized through endosteal and periosteal sources ( Fig. 24.23 ). The complex blood supply of bone is based on nutrient vessels entering the bone directly and through vascular connections between muscles and bone, typically where the muscle has a large bony origin or insertion. Vascularized bone is useful in muscles suitable for microvascular transplantation or in those muscles designed for transposition when the vascular attachments to bone are distal to the point of rotation. The commonly transferred bones include the fibula based on the peroneal artery, iliac crest based on the deep circumflex iliac artery ( Fig. 24.24 ), the scapula based on the circumflex scapula or thoracodorsal arteries ( Fig. 24.25 ), and the radius based on the radial artery ( Fig. 24.26 ). The calvarial osseous flap based on the superficial temporal artery or occipital artery with partial- or full-thickness bone is also useful for reconstructing facial anomalies and deformities ( Fig. 24.27 ). The use of periosteum and part of the cortical bone as an osseous–periosteal flap is being widely used for non-union of the bone and small bone defects. The genicular osseous–periosteal flap, also known as the medial femoral condyle flap, based on the articular branch of the descending genicular artery and vein with periosteum and a thin (0.5–1.0 mm) layer of outer cortical bone, was first reported by Sakai et al . to treat fracture non-union ( Fig. 24.28 ). Further applications of this flap with or without skin and cartilage expanded to reconstructing small bone defects, avascular necrosis of the bone and other complex defects of the hand.
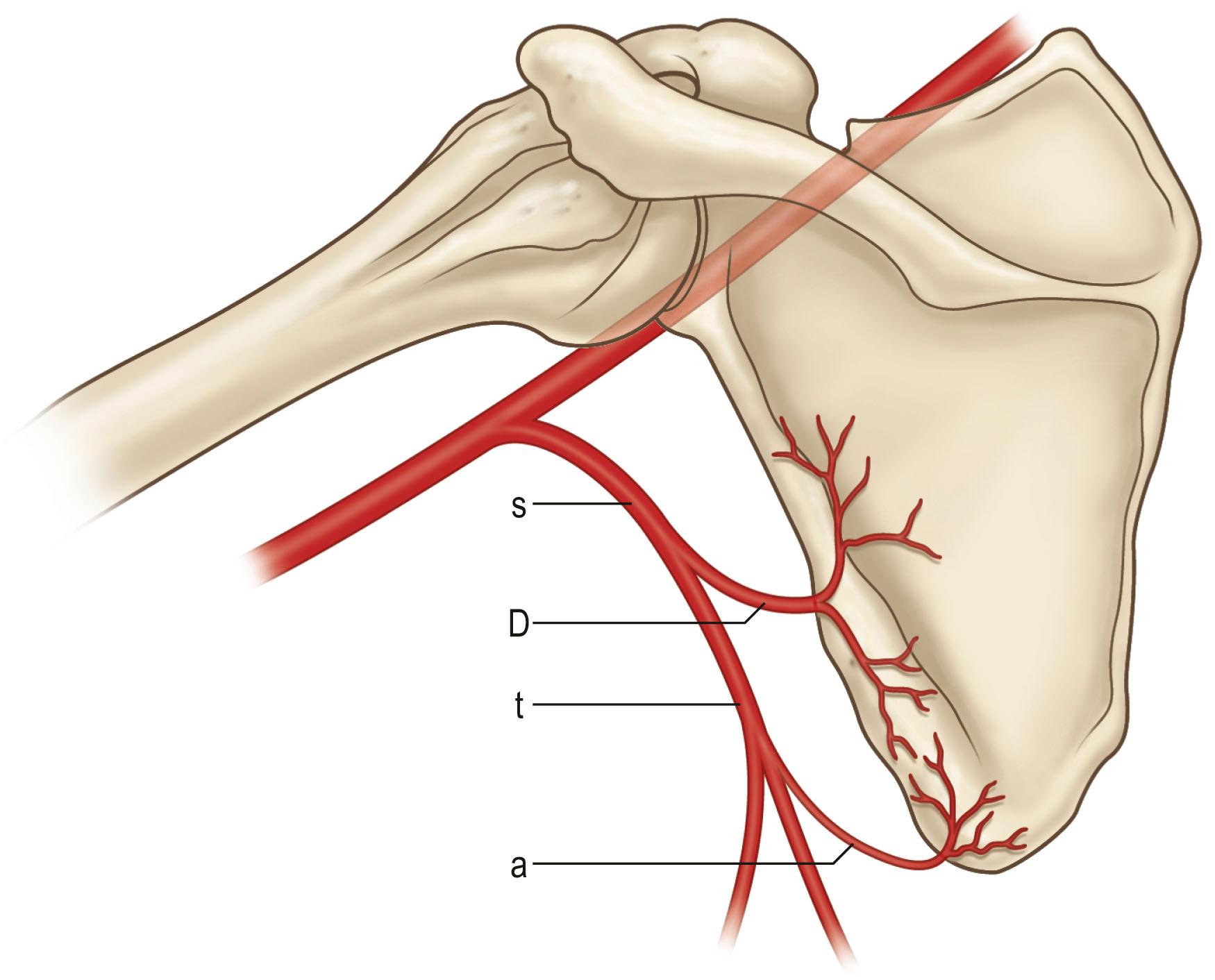
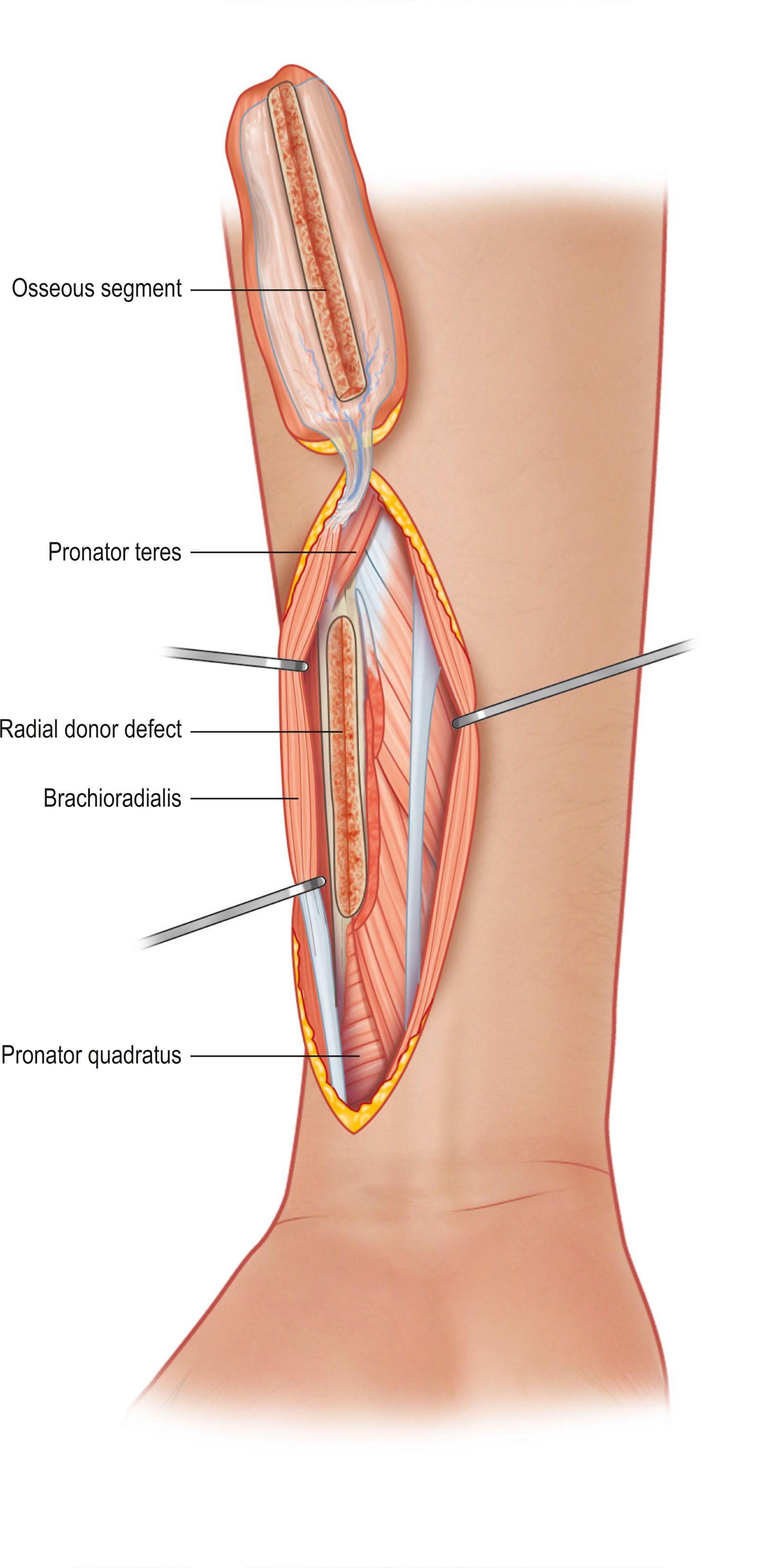
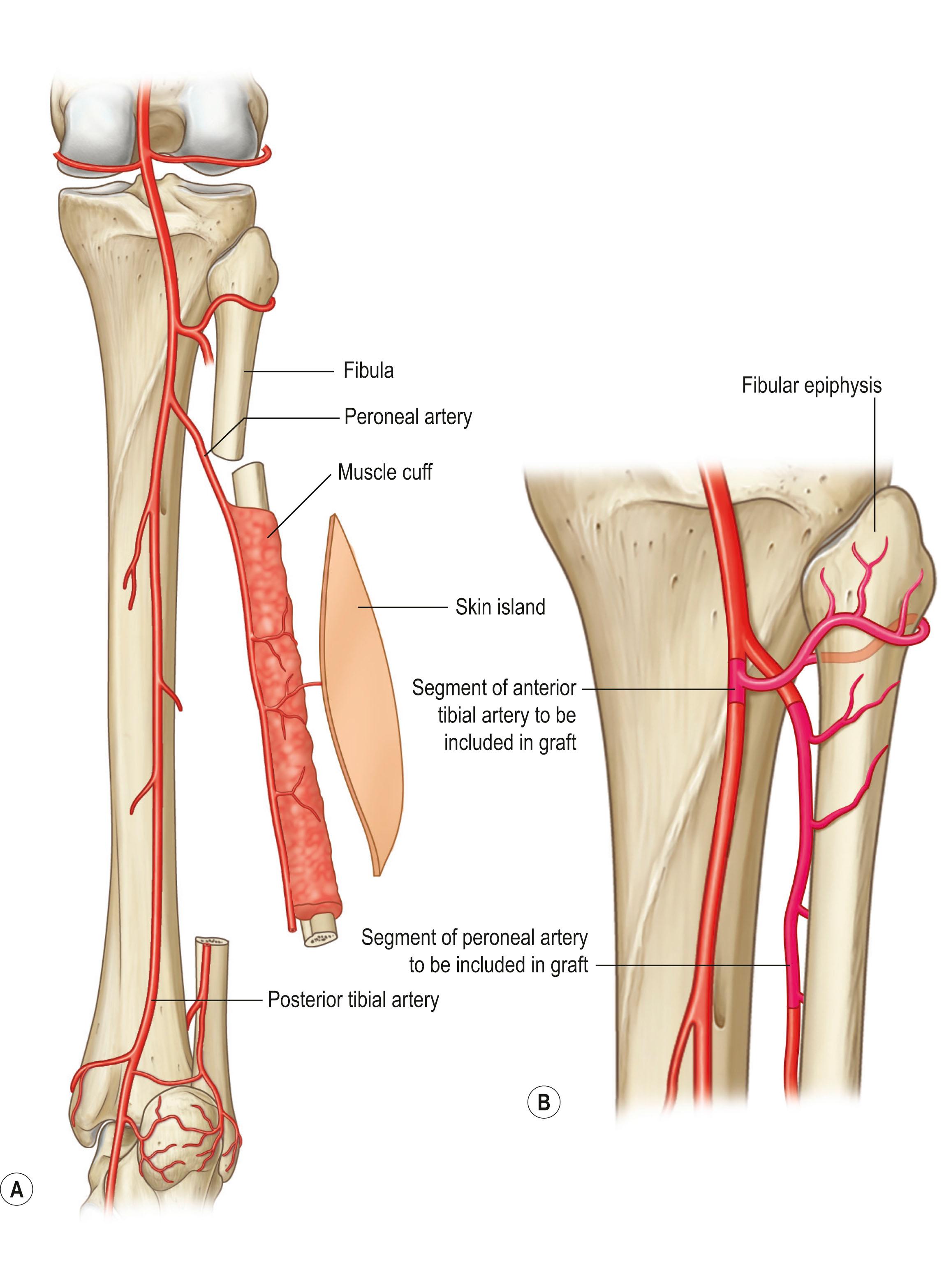
There is no widely accepted classification of bone flaps alone. Perhaps it is due to the complexity of the vasculature to each bone. One of the most widely used, the fibula, has vascular supply from the branches of the anterior tibial artery supplying the head, neck, and epiphysis while the peroneal artery gives rise to multiple arcuate vessels along the fibula and a nutrient vessel in the mid third of the fibula bone ( Fig. 24.29 ). Thus, depending on the portion of the fibula bone being harvested, the pedicle of the bone flap can differ not only in anatomical region but also in type of vascular supply to the bone. The fibula bone flap with or without the skin (fasciocutaneous) flap is frequently used for segmental bone defects especially after mandibular resection, long bone resection, and for pelvis reconstruction (see Fig. 24.24 ). The fibular bone including the head and epiphyseal growth plate is used in patients who need further growth of the long bone such as reconstruction after sarcoma resection of the proximal humerus.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here