Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
autosomal dominant polycystic kidney disease
adaptor protein complex 1
autosomal recessive polycystic kidney disease
cyclic adenosine monophosphate
cell division cycle 25A
congenital hepatic fibrosis
chemokine (C-X-C motif) receptor 2
ductal plate malformation
epithelial-derived neutrophil-activating protein 78
endoplasmic reticulum
endoscopic retrograde cholangiopancreatography
extracellular signal–regulated kinase
histone deacetylase 6
insulin-like growth factor 1
interleukin
Janus kinase
LDL receptor–related protein 5
matrix metalloprotease
magnetic resonance cholangiopancreatography
mammalian target of rapamycin
polycystin 1
polycystin 2
polycystic liver disease
protein kinase C
polycystic kidney disease gene 1
polycystic kidney disease gene 2
polycystic kidney and hepatic disease gene 1
protein kinase C substrate 80K-H gene
percutaneous transhepatic cholangiography
homolog, protein translocation regulator gene
signal transducer and activator of transcription
vascular endothelial growth factor
VEGF receptor 2
wingless-type
Fibrocystic liver diseases constitute a group of congenitally acquired conditions that target bile ducts and surrounding portal tracts within the liver and biliary tree. These diseases include autosomal dominant forms in isolation (polycystic liver disease, PCLD) or in association with autosomal dominant polycystic kidney disease (ADPKD), congenital hepatic fibrosis (CHF), autosomal recessive polycystic kidney disease (ARPKD), Caroli disease, choledochal cysts, and solitary hepatic cysts ( Table 64-1 ). Although these diseases are distinct from one another, they share common features, including proliferation of biliary ductular epithelium, biliary ectasia, cyst formation, and periductular fibrosis. The last decade has witnessed enormous advances in our understanding of the genetic, molecular, and cellular events that underlie fibrocystic liver diseases. These advances are highlighted in the first section of this chapter, “ Biology of Fibrocystic Liver Diseases .” In the second section, “ Histopathology of Fibrocystic Liver Diseases ,” the gross anatomic and histopathologic features of these disorders are presented. In the third section, “ Clinical Manifestations and Treatments of Fibrocystic Liver Diseases ,” the clinical features and therapies for each form of fibrocystic liver disease are described. Since the previous edition of this chapter, mechanism-based therapies have been initiated and are showing clinical potential. It is hoped that continued advances in the laboratory will translate into improved medical therapies for patients with fibrocystic liver diseases.
| ADPKD | PCLD | Congenital Hepatic Fibrosis | Choledochal Cysts | Caroli Disease | Solitary Hepatic Cyst | |||
|---|---|---|---|---|---|---|---|---|
| Gene affected | PKD1 (85-90%). Chromosome locus 16p13.3-13.12; 230 distinct mutations in PKD1 have been described | PKD2 (10-15%). Chromosome locus 4q21-23; 60 mutations have been described | PRKCSH . Chromosome locus 19p13 | SEC63 , LRP5 | Given its association with ARPKD, thought to be related to mutations in PKHD1 (chromosome locus 6p) | Unknown | Associated with mutations in PKHD1 and possibly PKD1 , or some other site | Unknown |
| Protein affected | Polycystin 1 (~460 kDa) | Polycystin 2 (110 kDa) | Hepatocystin (59 kDa) | SEC63, Lrp5 | Possibly fibrocystin (447 kDa) | Unknown | Possibly fibrocystin | Unknown |
| Anatomic features | Development of multiple large cysts in kidneys and liver | Development of multiple large cysts in liver only | Extensive fibrosis and malformations of the interlobular bile ducts | Cystic dilatations or diverticula of the extrahepatic bile ducts | Cystic dilatation of the intrahepatic bile ducts that communicate with the extrahepatic biliary tree | Intrahepatic cyst formation that does not communicate with the biliary tree | ||
| Hepatic histopathologic features | Macrocystic disease; cysts lined by simple flattened to columnar epithelium; immunohistochemical profile similar to that of biliary epithelium | Macrocystic disease; cysts lined by simple flattened to columnar epithelium; immunohistochemical profile similar to that of biliary epithelium | Ductal plate malformations of the interlobular bile ducts, associated with extensive fibrosis | Biliary or intestinalized epithelium; often with extensive denudation; inflammation and reactive epithelial changes | Dilatation of large bile ducts with marked periductal inflammation; bridges, and soft-tissue protrusions into dilated ducts | Simple epithelium; rounded contour | ||
| Clinical presentation of liver cysts | Hepatomegaly and abdominal pain; prevalence is higher in females; number and size of cysts increase with age; disease related to PKD2 mutations typically has later onset and is associated with greater life expectancy | Similarly to the hepatic presentation of ADPKD, PCLD typically presents with hepatomegaly and abdominal pain | Portal hypertension, recurrent cholangitis; typically diagnosed early in childhood; incidence of 1 : 20,000-1 : 40,000 | Chronic intermittent abdominal pain, jaundice, and recurrent cholangitis. Can be congenital or acquired | Typically presents with recurrent cholangitis or complications of portal hypertension | Typically asymptomatic and discovered incidentally, although right upper quadrant pain can occur when cysts exceed 5 cm in diameter | ||
| Radiographic findings | CT, US, and MRI scanning shows multiple, large noncommunicating cysts within the hepatic and renal parenchyma | CT, US, and MRI scanning shows multiple, large noncommunicating cysts within the hepatic parenchyma only | Large multilobulated liver with rare cysts | Cholangiography shows cystic dilatation of the bile duct without overt obstruction. Dilatations can also be seen on CT, MRCP, or EUS | Cholangiography shows nonobstructing cystic dilatations that communicate with the biliary tree | Ultrasound can distinguish simple hepatic cysts from other cystic lesions | ||
| Treatment options | Radiographic cyst aspiration and sclerosis; surgical fenestration; liver resection; liver transplant | Radiographic cyst aspiration and sclerosis; surgical fenestration; liver resection; liver transplant | Management focused on treatment of complications of cholangitis and portal hypertension | Given increased risk of cholangiocarcinoma, surgical resection is indicated | Adequate biliary drainage is the mainstay; often requires lobectomy or liver transplant | Conservative management usually appropriate, if symptomatic radiographic aspiration and sclerosis | ||
In humans, formation of the biliary tree begins during the first trimester of fetal life, when precursor cells in contact with the mesenchymal tissue of the portal tracts differentiate to a glandular morphology and give rise to the ductal plate. This intermediary biliary structure consists of a double-layered tube of biliary epithelial cells surrounding the periphery of the future portal tracts, and its formation proceeds from the central portion of the liver toward progressively smaller and more peripheral branches of the biliary tree ( Fig. 64-1 ). During the beginning of the second trimester of fetal life, remodeling of the ductal plate normally begins with one portion, which is destined to become the functional bile duct, becoming embedded within the connective tissue of the portal tract, whereas other sections of the circumferential ductal plate gradually degenerate and disappear. This process is completed after birth, and a discontinuous vestige of the ductal plate can be identified by cytokeratin staining in newborns.
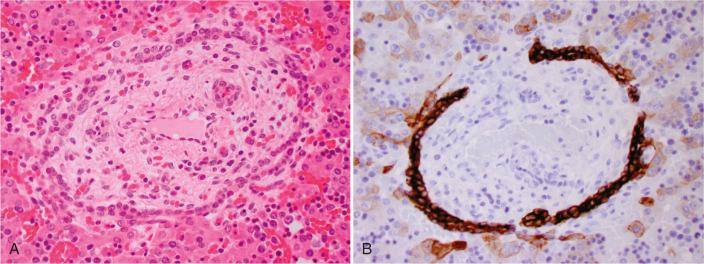
Histopathologic examination of livers from patients with fibrocystic liver diseases commonly shows abnormal biliary structures that are reminiscent of the ductal plate stage of fetal development. This similarity was first noted by Jorgensen, who termed the lesion ductal plate malformation (DPM) . DPM may be caused by (1) inability of biliary precursors cells to differentiate, (2) defects in primitive bile ducts maturation, and/or (3) abnormal bile duct enlargement. Isolated lesions are often referred to as biliary microhamartoma or von Meyenburg complexes , and may be observed in normal livers. Desmet expanded on the observations of Jorgensen to hypothesize that many cystic liver diseases represent malformations of biliary development. Although studies have not yet completely validated this hypothesis, the concept is useful to explain the similarities in the histologic findings in patients with various fibrocystic diseases. Immunohistochemical characterization of the epithelia within DPMs in a variety of fibrocystic diseases demonstrates similarity to the phenotype of normal embryonic ductal plates after 20 weeks of gestation. Anomalous ductal plate morphology is also observed in the hepatic conditions associated with renal polycystic disease and a number of other genetic syndromes, including Meckel syndrome.
Although the pattern of expression of ADPKD and PCLD within families is consistent with these diseases being autosomal dominant in nature, ADPKD and PCLD are likely molecular recessive diseases. In this case affected individuals have a germline mutation in one copy of the gene responsible (i.e., first hit in PKD1 , PKD2 , PRKCSH , SEC63 , and LRP5 ) and acquire a mutation in the second copy of the gene responsible within individual bile duct epithelial cells during the individual's lifetime (i.e., second hit). The developmental pattern of liver cysts in ADPKD and PCLD is consistent with this two-hit hypothesis. Unlike the early and pervasive involvement of bile ducts in ARPKD/Caroli disease, liver cysts in ADPKD and PCLD develop focally along the bile duct during the lifetime of the individual. Genetic screening of the cyst-lining epithelial cells showed that these cells have lost the remaining copy of the gene through somatic mutation. The fact that each cyst has acquired a different somatic mutation, ranging from a small point mutation to a large region with loss of heterozygosity, indicates that cysts develop independently and one somatic mutation is sufficient to trigger cyst formation. Genetic mouse models of ADPKD also support the two-hit hypothesis. Homozygous knockout models of ADPKD generally die in the late embryonic stages. In contrast, pkd2 WS25 /− mice develop kidney and liver cysts in a postpuberty pattern that mirrors the human condition. Importantly, these mice have one true knockout in the pkd2 gene (homolog of one of the two genes linked to ADPKD in humans; first hit) and a recombinant sensitive allele (i.e., WS25 ) in the second copy of the pkd2 gene. Appropriate recombination of the WS25 allele results in a loss of its function (second hit) and initiation of cyst development. In another mouse model it was shown that a reduction in pkd1 gene expression levels was sufficient to trigger cyst formation.
Epithelial cells, including bile duct epithelial cells, have a single primary cilium that extends from their luminal surface ( Fig. 64-2 ). Primary cilia are a nonmotile, microtubule-based organelle that arise from the basal body and can extend several microns in length into the lumen of the duct. The primary cilia serve to sense and transduce information about the luminal fluid osmolarity, composition, and flow rate. As the proteins that contribute to the structure and function of the primary cilium have been discovered, it has become apparent that a variety of genetic syndromes and diseases are associated with the dysfunction of primary cilia. Shortened, unusually long or entirely absent cilia are present in cystic cholangiocytes of animal models and patients with ADPKD and/or ARPKD. These diseases, coined ciliopathies , have a number of shared features and often include the development of cysts. These abnormalities are also accompanied by atypical centrosome positioning, supernumerary centrosomes, and multipolar spindles that participate in cystogenesis.
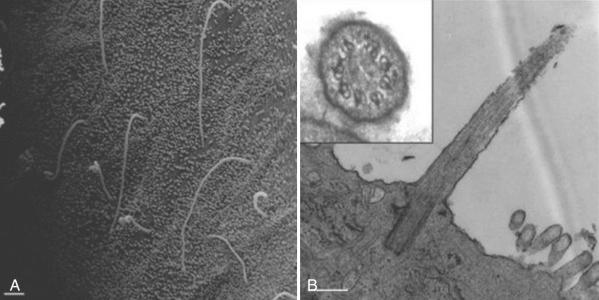
In the liver the proteins linked to ADPKD (polycystin 1 [PC-1] and polycystin 2 [PC-2]) and ARPKD/CHF/Caroli disease (fibrocystin, also known as polyductin ) localize to the primary cilium of biliary epithelial cells. Parenthetically, the proteins linked to PCLD (hepatocystin, Sec63, and LDL receptor–related protein 5, Lrp5) are among the few cystogenic proteins that have not yet been found to localize to the primary cilium or basal body.
The last decade has witnessed the discovery and characterization of the genes and proteins responsible for different forms of cystic diseases. The following sections describe the genes, proteins, and functions linked to human ADPKD, PCLD, and ARPKD/CHF/Caroli disease. For more comprehensive descriptions of the genes and proteins responsible, there are a number of excellent reviews.
The most common form of PCLD occurs in conjunction with ADPKD, has a prevalence of approximately 1 in 500 to 1 in 1000, and is linked to mutations in either PKD1 or PKD2 . Eighty-five percent of cases are due to mutations in PKD1 and 15% are due to mutations in PKD2 . The phenotypic characteristics stemming from mutations in PKD1 and PKD2 are quite similar but patients with mutations in PKD2 have a later onset of disease and approximately 16 years of increased life expectancy compared with patients with mutations in PKD1 .
In 1957 Dalgaard demonstrated autosomal dominant inheritance in more than 90% of cases of polycystic renal disease. In 1985, linkage techniques localized the first gene for ADPKD, coined PKD1 , to chromosome locus 16p13.3-13.12. PKD1 encodes a 14.1-kb message that translates into a 4304–amino acid protein, PC-1. Additional copies of exons 1 to 34 lie adjacent to the active PKD1 locus. These duplicated copies are probably nonfunctional and fail to express protein but their presence has hampered the development of molecular genetic testing.
At least 270 distinct mutations have been identified within the PKD1 gene. Evenly dispersed without evidence for clustering, most of these mutations are missense or nonsense mutations, but splicing mutations and gene rearrangements have also been reported. Approximately 60% of all mutations introduce premature stop codons that result in truncated proteins. Specific mutations and their locations have been associated with intracranial aneurysms and severer polycystic disease within individual ADPKD families. For example, mutations in the 5′ end of PKD1 are predictive of a more rapid development and greater severity of end-stage renal disease. An effect of mutational type or location on hepatic cystic disease has not been specifically analyzed.
PKD1 encodes PC-1, a 460-kDa integral membrane protein with a large extracellular NH 2 -terminal domain, 11 transmembrane domains, and a comparatively small intracellular COOH-terminal domain. Although its function remains to be clarified, PC-1 is predicted to help transduce extracellular signals from the cell surface to the cell interior. Constituting two thirds of the protein, the NH 2 terminus of the protein contains a number of domains that are consistent with initiating signaling through protein-protein and protein-carbohydrate interactions. The extracellular NH 2 terminus contains a region of leucine-rich repeats, a segment with C-type lectin characteristics, a segment with LDL-like features, 12 immunoglobulin-like PKD repeats, an REJ domain and a G protein–coupled receptor site. The PKD repeat domains permit direct PC-1-PC-1 interaction. In other proteins, REJ domain moderate fluxes in ion channel complexes. Its presence supports the hypothesis that PC-1 serves, in part, to regulate Ca 2+ signaling. Specific protein-protein interactions of the intracellular COOH-terminal domain with other proteins, including heterotrimeric G proteins, Janus kinase 2 (JAK2), and PC-2, further the notion that PC-1 serves to transduce extracellular cues into intracellular signals. PC-1 also has cleavage-sensitive sites, one within its extracellular G protein–coupled receptor site and two within its intracellular tail. All three sites are anticipated to have physiologic significance and contribute to cyst formation in pathologic states. Regarding its localization and site of activity, PC-1 was originally described to reside along the basolateral membrane of epithelial cells, where it could participate in cell-cell and cell-matrix interactions. The localization of PC-1, along with PC-2, within the membrane of primary cilia has focused investigative efforts into understanding the function and relationship of these two proteins within this organelle.
In 199, a second genetic locus linked to ADPKD was discovered at chromosome locus 4q21-23, and 3 years later the PKD2 gene was identified, sequenced, and cloned. PKD2 produces a 5.3-kb message that codes for the 968–amino acid PC-2. At least 73 mutations have been identified in PKD2 and, like PKD1 , mutations of PKD2 are evenly dispersed throughout the gene, without clustering at any particular position. Although the type of mutation within the PKD2 gene influences the clinical outcome of renal cystic disease, its relationship with the liver cystic phenotype remains unknown.
PC-2 (also known as transient receptor potential polycystic 2 ) is a 110-kDa integral membrane protein with six transmembrane domains and intracellular NH 2 and COOH tails. Sequence homology and functional characteristics place PC-2 within the transient receptor potential superfamily of ion channels. PC-2 can form homotetramers and function as a cation channel. The PC-2-dependent Ca 2+ transients may be further amplified by heteromultimerizing with other transient receptor potential channels. Initially found along the lateral membranes and endoplasmic reticulum (ER) of epithelial cells, PC-2 was subsequently discovered within the primary cilium as well. A wide variety of distinct proteins have been shown to bind with PC-2, including PC-1, supporting the prediction that PC-2 participates in organizing an extracellular signal transduction complex.
ARPKD is a rare genetic condition occurring in approximately 1 in 20,000 live births with a high risk of morbidity and death. It is a severe, typically early-onset form of cystic disease that primarily involves the kidneys and biliary tract. Phenotypic expression and age at presentation are highly variable.
The gene affected in ARPKD is PKHD1 , a huge gene on chromosome band 6p12 that extends over a genomic segment of almost 500 kb. The longest open reading frame comprises 66 exons encoding fibrocystin, a type I single-pass transmembrane protein of 4074 amino acids. Because of allelic heterogeneity and a high level of missense mutations, mutation analysis for PKHD1 is laborious but well established. Mutation detection rates of approximately 80% for the entire clinical spectrum of ARPKD patients have been shown; at least one PKHD1 mutation can be identified in more than 95% of families. There are at least 300 PKHD1 gene mutations, and clear genotype-phenotype correlations are absent. Patients with two truncating mutations generally display a severe phenotype with perinatal or neonatal death, whereas patients surviving the neonatal period usually carry at least one missense mutation.
Although PKHD1 is the main gene affected in ARPKD, there is compelling evidence for locus heterogeneity. Fibrocystin probably has receptor-like properties and localizes to primary cilia. The COOH-terminal domain contains a nuclear localization signal, and fibrocystin may participate in nuclear signaling and transcriptional regulation.
PCLD gives rise to polycystic liver with few to no discernible renal manifestations (~1 : 100,000). This phenotypic distinction is paralleled by disparate genetic linkages. PCLD has been definitively linked to three genes: PRKCSH (15%), SEC63 (5.7%), and LRP5 (2.7%). Early analysis suggests these three genes account for a minority of PCLD cases, indicating there is at least one more genetic locus to be linked to PCLD. The genetic mutations responsible for the phenotype of polycystic liver alter the expression, structure, or processing of proteins of the primary cilium.
The first demonstration that PCLD was genetically distinct from ADPKD was from phenotypic and genetic studies of a family affected by PCLD without renal cystic disease in three generations. PCLD was initially linked to mutations in the protein kinase C (PKC) substrate 80K-H gene ( PRKCSH ) at chromosome locus 19p13.2-13.1. PRKCSH encodes a 527–amino acid protein of 59 kDa, termed hepatocystin , that is expressed in a number of different tissues. The protein contains a membrane translocation signal sequence for the ER at its NH 2 end, an LDLα domain, two EF-hand domains, a glutamic acid–rich region, and an ER retrieval sequence at its COOH end. Several functions for hepatocystin have been reported but the weight of evidence indicates a primary role as the noncatalytic subunit of the glucosidase II protein complex. Glucosidase II is localized in the ER, where it modifies protein glycosylation and contributes to posttranslational processing of newly synthesized glycoproteins. Mutations that truncate or alter messenger RNA splicing of hepatocystin have been reported in PCLD families.
The association of PCLD with the ER and protein processing was bolstered by the linkage of a second gene, SEC63 , to PCLD. In humans the SEC63 gene is on chromosome band 6q21, and mutations are distributed throughout the gene. The protein product of the SEC63 gene, Sec63, is an integral membrane protein within the ER that functions as a component of the protein translocation complex. This protein processing step is upstream of the glycosylation modification step that is performed by glycosidase II. Despite the apparent liver specificity of PCLD that is initiated by SEC63 mutations, SEC63 is broadly expressed in a number of tissues, including the kidney.
The LRP5 gene (NM_002335.2) is associated with hepatic cystogenesis as evidenced by whole-exome sequencing in an extended PCLD family. The protein product Lrp5 consists of a large extracellular region, a single-span transmembrane region, and a relatively short intracellular region. LRP5 variants are identified in both intracellular and extracellular protein domains. Accordingly, Lrp5 has a wide tissue expression and is abundantly present in cholangiocytes. Previous studies presented variants of the LRP5 gene, located at chromosome locus 11q13.2, linked to bone, retinal disorders, and metabolic disease. Lrp5 functions as a co-receptor in the canonical wingless-type (Wnt) signaling responsible for fundamental physiologic mechanisms and developmental processes. LRP5-associated diseases are marked by an imbalanced canonical and noncanonical Wnt signal transduction. Likewise, LRP5 mutations in PCLD show a reduced canonical Wnt signaling activity which renders the protein less functional.
The developmental characteristics of liver cysts in ADPKD and PCLD are markedly similar. In both diseases liver cysts develop after puberty, clinical manifestations generally appear around the fourth decade of life, and disease expression is sexually dimorphic, with women having a greater degree of cyst progression. The striking difference between these two diseases is that ADPKD affects the kidneys, vasculature, and liver, whereas manifestation of PCLD is largely limited to the liver. As described already, the proteins linked to ADPKD and ARPKD form a mechanosensory signal transduction complex within the primary cilia. In contrast, proteins linked to PCLD participate in the translocation, processing, and quality control of ER proteins.
The biologic significance of the Wnt signaling in both ADPKD and PCLD has been proven by several animal and functional studies. ADPKD rodents showed PC-1 interactions and reduced β-catenin activity leading to inhibited canonical Wnt signaling. Fundamental studies identified the Wnt signaling–associated cytosolic protein nucleoredoxin as an interaction partner of human Sec63. Therefore the SEC63 gene is linked to the Wnt pathway. Finally, the discovery of the LRP5 gene associated with PCLD in humans has proven that the Wnt signal transduction pathway is also of clinical significance. Functional studies revealed a reduced activated canonical Wnt signaling.
Understanding how the loss of function of the disparate proteins associated with ADPKD versus PCLD results in the phenotypic emergence of liver cysts may reveal the pivotal steps that underlie liver cystogenesis. In this regard, hepatocystin and Sec63 are necessary for the adequate expression of PC-1, PC-2, and fibrocystin, PC-1 being considered the rate-limiting component that determines both cyst formation and the severity of all forms of PCLD. Thus this regulatory mechanism is of specific interest because it implicates common molecular pathways for cystogenesis in all types of PCLD, which will be covered in greater detail in the following sections. Clinically, PCLD patients have more and larger liver cysts than ADPKD patients but have a more benign clinical course.
The discovery of PC-1 and PC-2 interactions through coiled-coil domains in their respective COOH tails and codistribution of the two proteins within primary cilia led to studies investigating the functions of the PC-1-PC-2 complex. In micro-dissected bile ducts from rat, infusion of luminal flow bends the primary cilium, triggers calcium influx into the cell, and causes the suppression of the cyclic AMP (cAMP) levels stimulated by experimental addition of forskolin. This normal response is blocked when cilia are removed by chloral hydrate or when ciliary-associated proteins (PC-1, PC-2, and Ca 2+ -inhibitable adenylyl cyclase isoform 6) are individually down-regulated by small interfering RNAs. Consistent with an impairment of calcium influx, the intracellular calcium levels of cystic cholangiocytes from a murine model of ADPKD and ARPKD and of cystic cholangiocytes from human ADPKD patients were significantly lower than those of normal cholangiocytes. In addition to flow-dependent intracellular Ca 2+ signaling, the PC-1-PC-2 complex is integrated into other signaling pathways. For example, PC-1 constitutively activates heterotrimeric G proteins to initiate downstream effects, and PC-2 antagonizes this constitutive activity. Additionally, several lines of evidence indicate that fibrocystin can bind and modify PC-2 activity. This fibrocystin-PC-2 interaction is of specific interest because it implicates a common molecular pathway for cystogenesis in ADPKD and ARPKD.
Significant research effort is currently being invested to understand how these PC-1-PC-2-fibrocystin transduction pathways result in cystogenesis and cyst growth.
Increased cell proliferation is considered a cornerstone of cyst growth, and the loss of functional PC-1-PC-2-fibrocystin complex activity is predicted to induce cell proliferation. Cystic epithelial cells can overexpress proto-oncogenes and growth factor receptors, suggesting a role for PC-1, PC-2, and fibrocystin in nuclear regulation. Distinct lines of investigation have implicated the PC-1-PC-2-fibrocystin complex in moderating cell cycle progression through the nuclear transcription factor adaptor protein complex 1 (AP-1), the JAK–signal transducer and activator of transcription (STAT) signaling pathway, the extracellular signal–regulated kinase (ERK) signaling pathway, and/or mammalian target of rapamycin (mTOR) signaling ( Fig. 64-3 ). First, PC-1 and PC-2 can independently or coordinately mediate the activity of AP-1 through pathways involving small G proteins, PKC, p38, and Jun NH2-terminal kinase 1. In cells overexpressing PC-1, the COOH terminus of PC-1 can be cleaved from the intact protein, translocate into the nucleus, and directly activate AP-1. Coexpression of PC-2 blunts the effect of cleaving of the COOH terminus of PC-1, suggesting that PC-2 can modulate the AP-1 signal by buffering the concentration of the PC-1 COOH terminus available for nuclear signaling. Second, PC-1 can bind and activate JAK2 in a PC-2-dependent manner, resulting in the phosphorylation and activation of at least two STAT transcription factors, including STAT1. Phosphorylated STAT can then enter the nucleus and arrest cell cycle progression. Loss of either PC-1 or PC-2 is predicted to diminish activated STAT levels and allow cells to reenter the cell cycle and promote cell proliferation. Third, although cAMP inhibits protein kinase A–dependent ERK signaling in normal epithelial cells, calcium restriction allows cAMP to activate ERK in cystic epithelial cells and induce hyperproliferation. Increased levels of cAMP observed in murine PCLD models (ADPKD and ARPKD) could also be the result of calcium restriction because calcium can inhibit some adenyl cyclases and activate specific cAMP phosphodiesterases. Activated ERK can promote an increase in cell proliferation both by entering the nucleus and initiating a transcriptional cascade and by moderating the tuberous sclerosis complex activity, which leads to an increase in the mTOR activity. The JAK-STAT and mTOR pathways include activation of specific cyclin-dependent kinases to drive the progression in the cell cycle. In nonorthologous cystic animal models, treatment with roscovitine, a potent cyclin-dependent kinase inhibitor, significantly blunted kidney cyst growth. Similarly, inhibition of the cell division cycle 25A (Cdc25A) messenger RNA by experimental up-regulation of miR-15A abundance reduces the disease severity in rodent models of ARPKD. Importantly, the cross-breeding of a mouse model of ARPKD with Cdc25a +/– mice (characterized by decreased Cdc25A expression but normal liver morphology) reduced the hepatorenal cystogenesis and fibrosis. Finally, pharmacologic inhibition of Cdc25A with vitamin K3 or phenyl maleimide compound 20 also decreased hepatorenal cystogenesis and fibrosis in rodent models of ADPKD and ARPKD, pointing out the potential therapeutic value of targeting Cdc25A in patients with PCLDs.
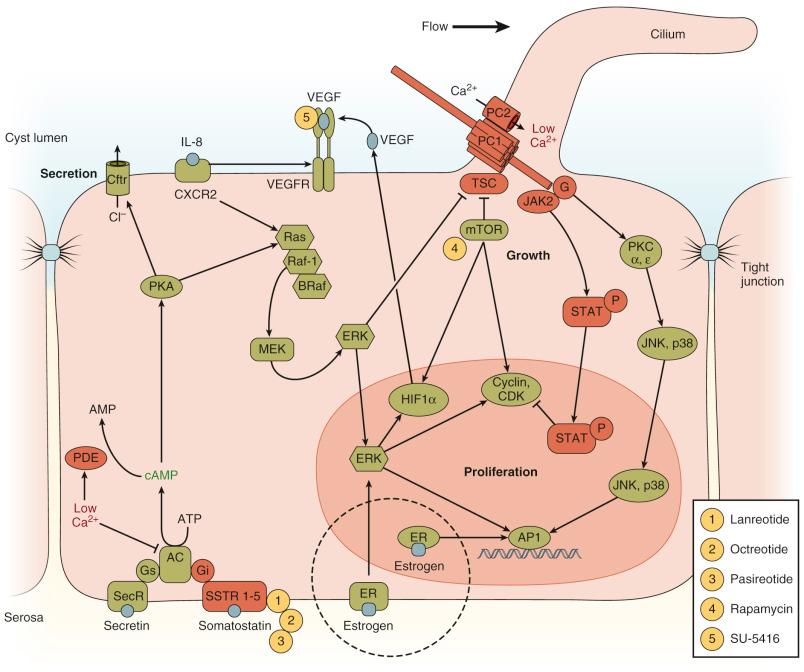
Additional lines of evidence indicate the potential significance of the mTOR pathway in driving errant proliferation of cystic epithelial cells. PC-1 can directly bind the tuberin component of the tuberous sclerosis complex, which normally inhibits the activity of mTOR. Loss of this regulation would account for the inappropriate activation of mTOR observed in ADPKD cyst epithelial cells.
Insulin-like growth factor 1 (IGF1), a promitotic factor present in the cystic liver fluid of patients with ADPKD, promotes cystic proliferation through mTOR activation. Thus mTOR was suggested as a potential therapeutic target for PCLDs. However, the use of mTOR inhibitors did not attenuate the hepatic and renal cystogenesis of animal models and patients with PCLD.
Many of the details and interrelationships of these disparate pathways for regulating transcription and growth require additional investigation. It is important to recognize, however, the emerging evidence for PC-1/PC-2/fibrocystin in regulation or coordination of cell proliferation and differentiation. These pathways can now be investigated to determine which pathways, when disrupted, give rise to fibrocystic liver disease.
The clinical course of PCLDs is markedly heterogeneous even among identical twins. Although part of this heterogeneity is likely due to differences in the specific germline and somatic mutations that occur within individuals, additional factors likely contribute to promotion of the growth of the liver cysts. Potential contributing factors include modifier genes, estrogen exposure, luminal fluid secretion, and altered expression of cytokines and growth factors.
Modifier genes are genes not directly linked to a genetic disease but influence the disease expression and severity. In ADPKD, modifier genes have been directly implicated and identified. A comprehensive list of genes that specifically modify the development of cystic liver diseases has not yet been compiled but is likely to be forthcoming and provide insight into the cellular mechanisms that moderate the disease severity.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here