Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The fibroblastic and fibrohistiocytic tumors of bone represent a heterogeneous group of lesions ranging from purely benign to highly malignant neoplasms. Non-ossifying fibroma at one end of the spectrum is a common and self-limiting tumor developing during childhood and usually not requiring treatment. Desmoplastic fibroma is a locally aggressive neoplasm that can show morphologic overlap with central low-grade osteosarcoma. Sclerosing epithelioid fibrosarcoma rarely arises within bone but can mimic osteosarcoma due to a densely hyalinized collagenous matrix that can be mistaken for bone. Fibrosarcoma of bone is also rare and comprises low-, intermediate, and high-grade tumors that differ significantly in clinical outcome. At the end of the malignant spectrum is undifferentiated pleomorphic sarcoma , which is a highly aggressive neoplasm of bone characterized by diverse morphologic patterns and a lack of specific differentiation or matrix formation.
Non-ossifying fibroma is a common benign and generally self-limiting spindle cell tumor that typically occurs in the metaphyseal region of long bones (reflecting the former name, metaphyseal fibrous defect).
Non-ossifying fibromas most commonly involve the distal femur and the proximal tibia, sites that account for 80% of lesions. The distal tibia, both ends of the fibula, and the distal radius may also be involved. The flat bones, the small bones of the hands and feet, the spine, and the craniofacial skeleton are generally not affected. Most non-ossifying fibromas are asymptomatic and discovered incidentally on radiographs taken for other reasons. Undoubtedly, many are never diagnosed, and the WHO estimates that 30%–40% of children develop one or even multiple lesions during skeletal maturation. Large non-ossifying fibromas may cause pain, and some patients present with pathologic fracture. Symptoms, when present, usually develop in patients in their mid-teens.
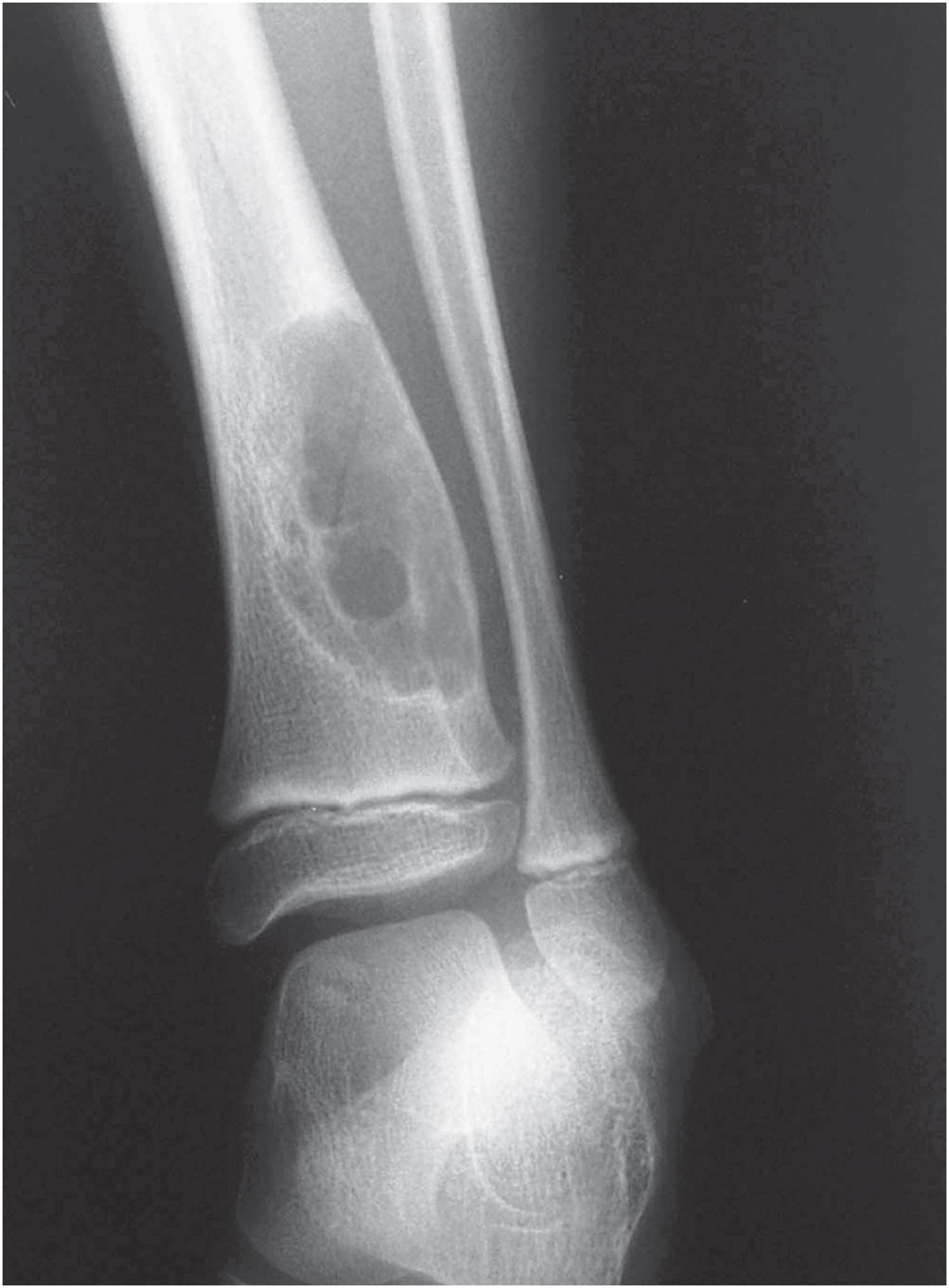
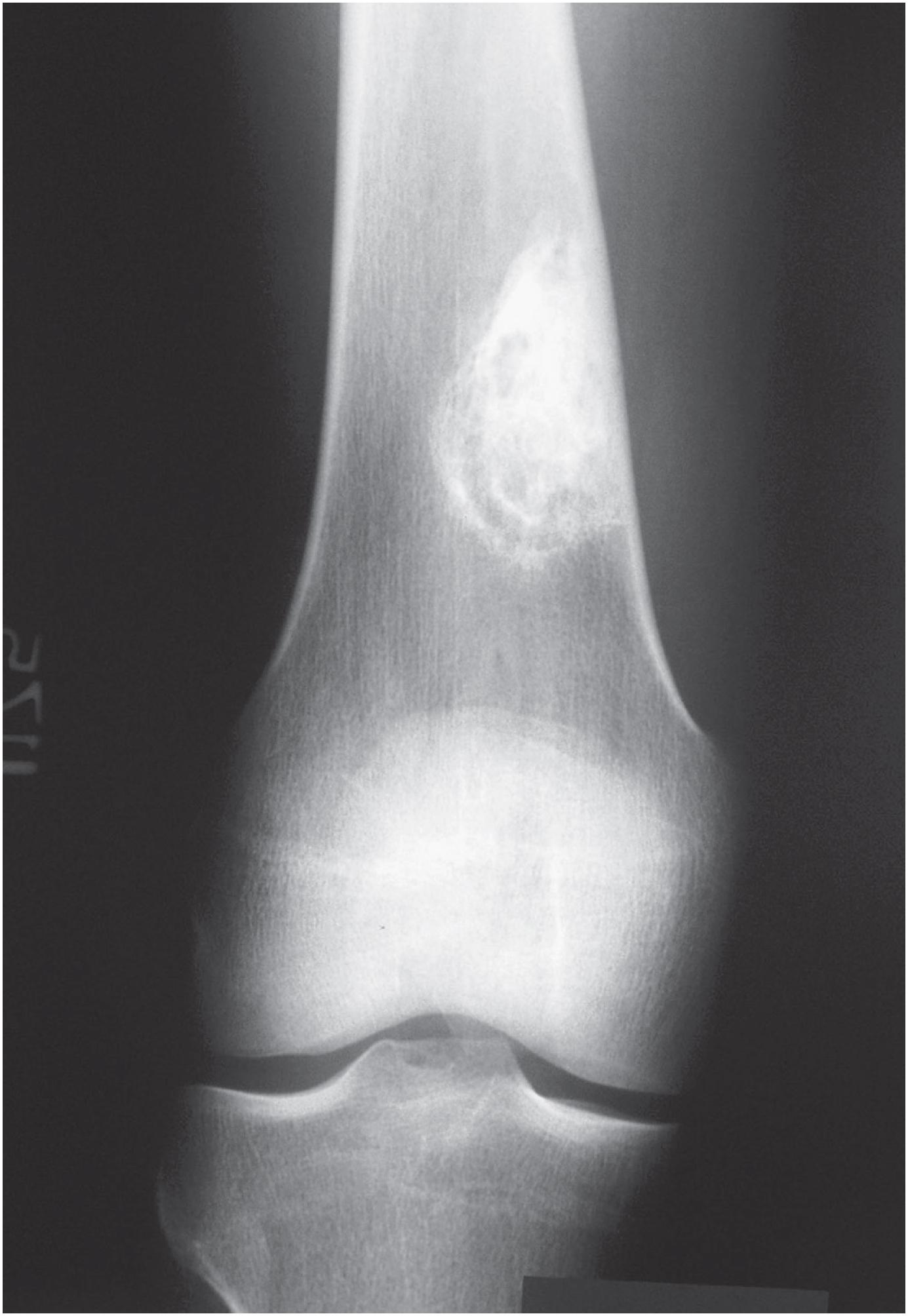
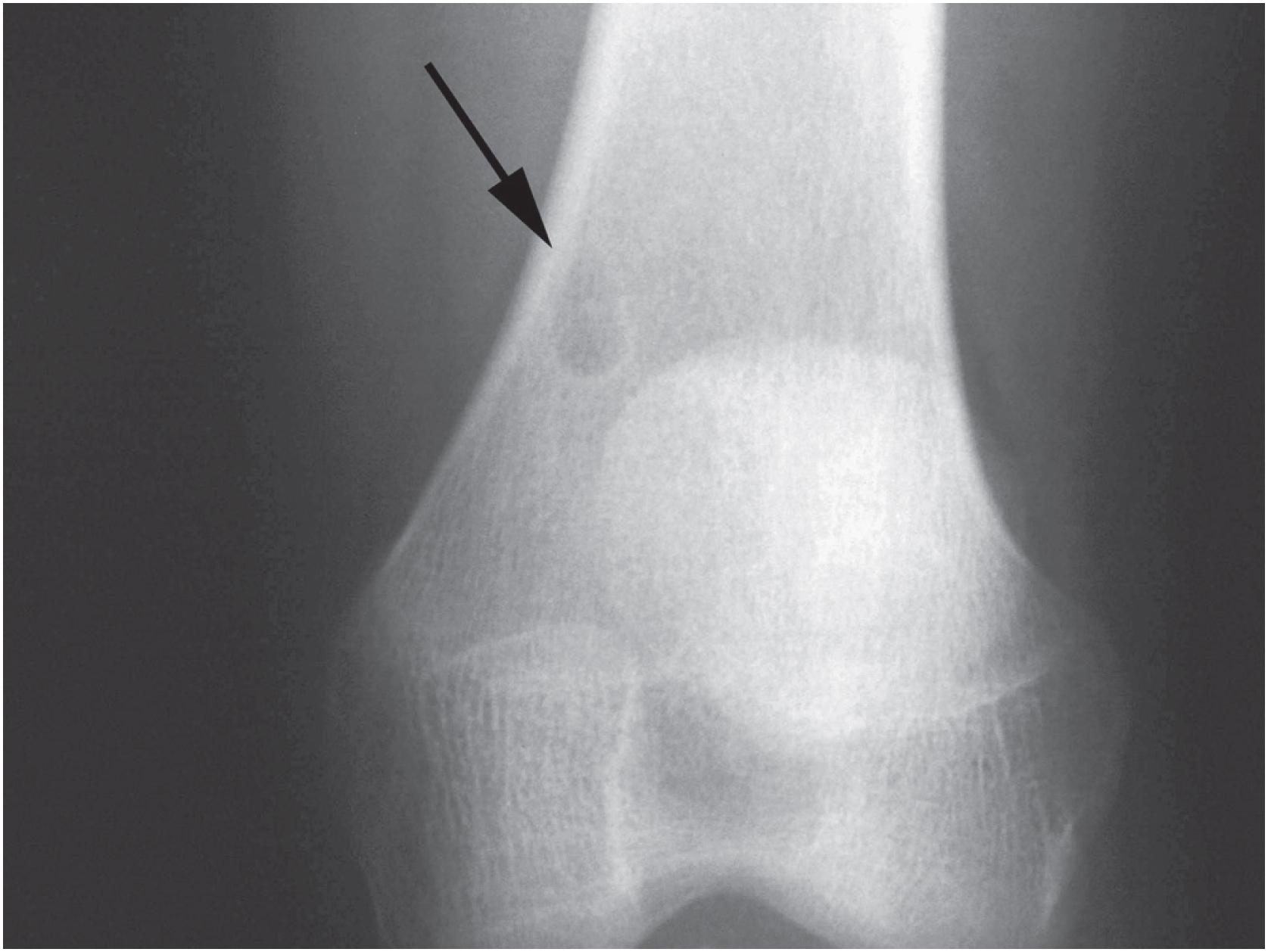
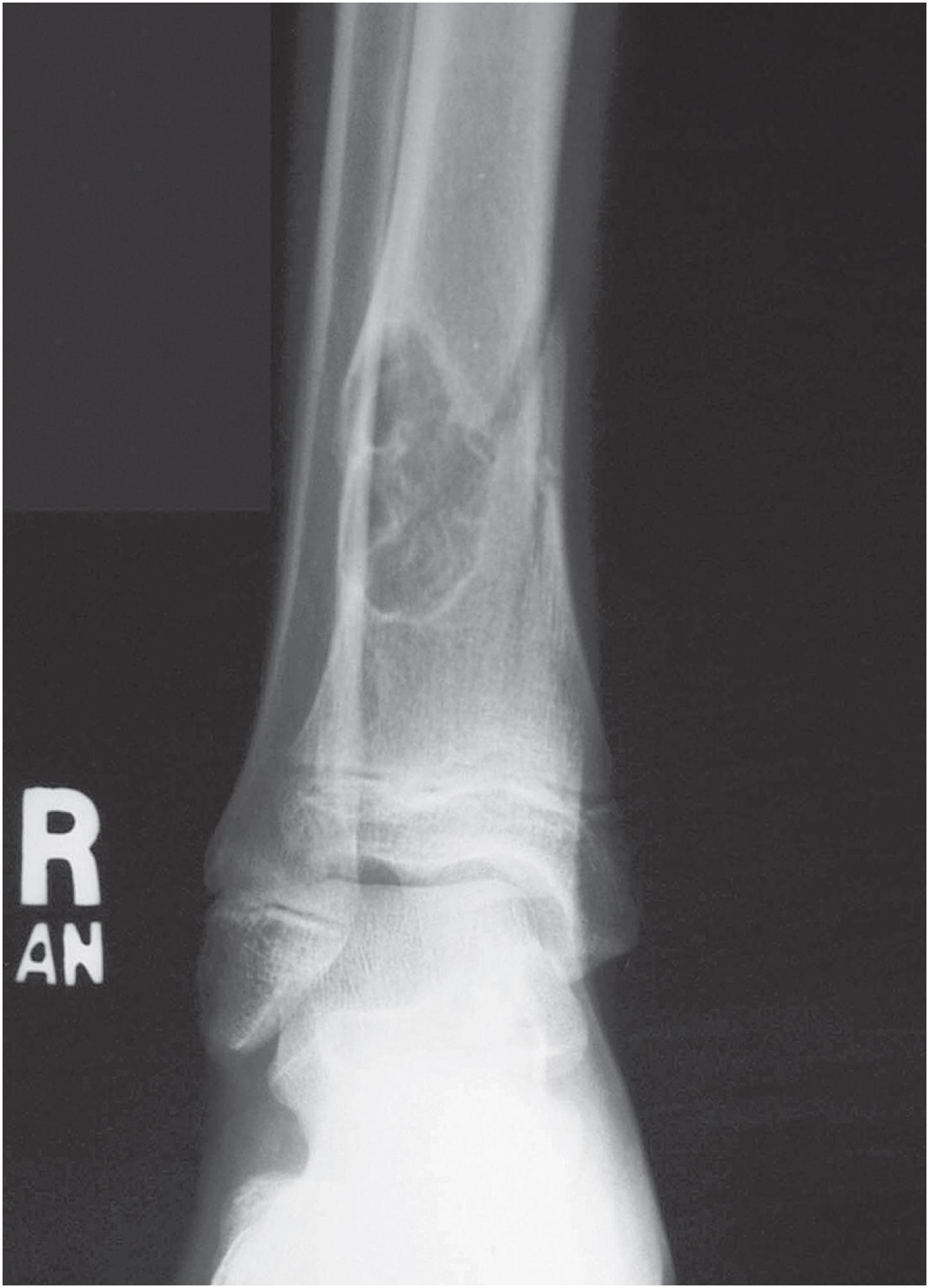
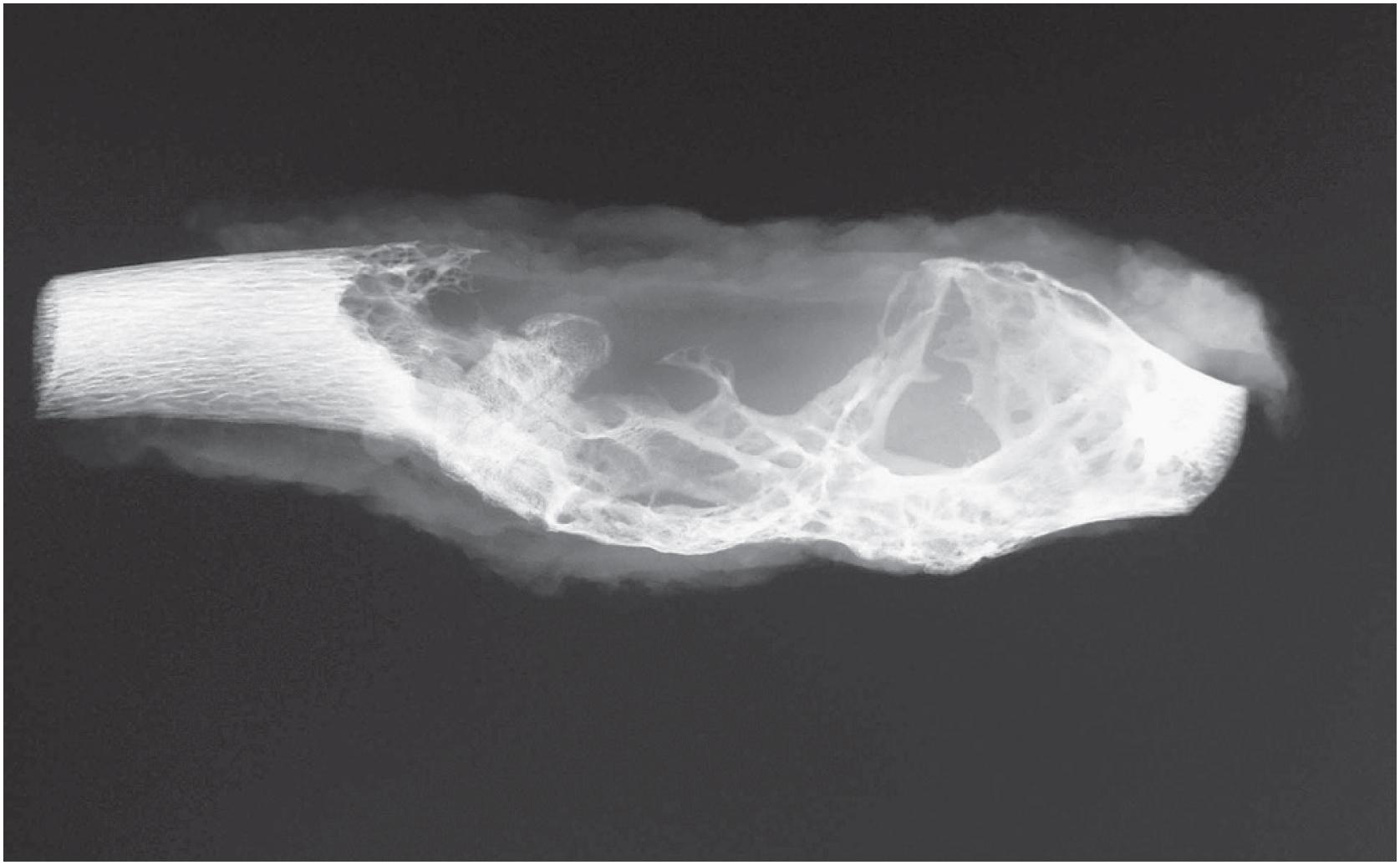
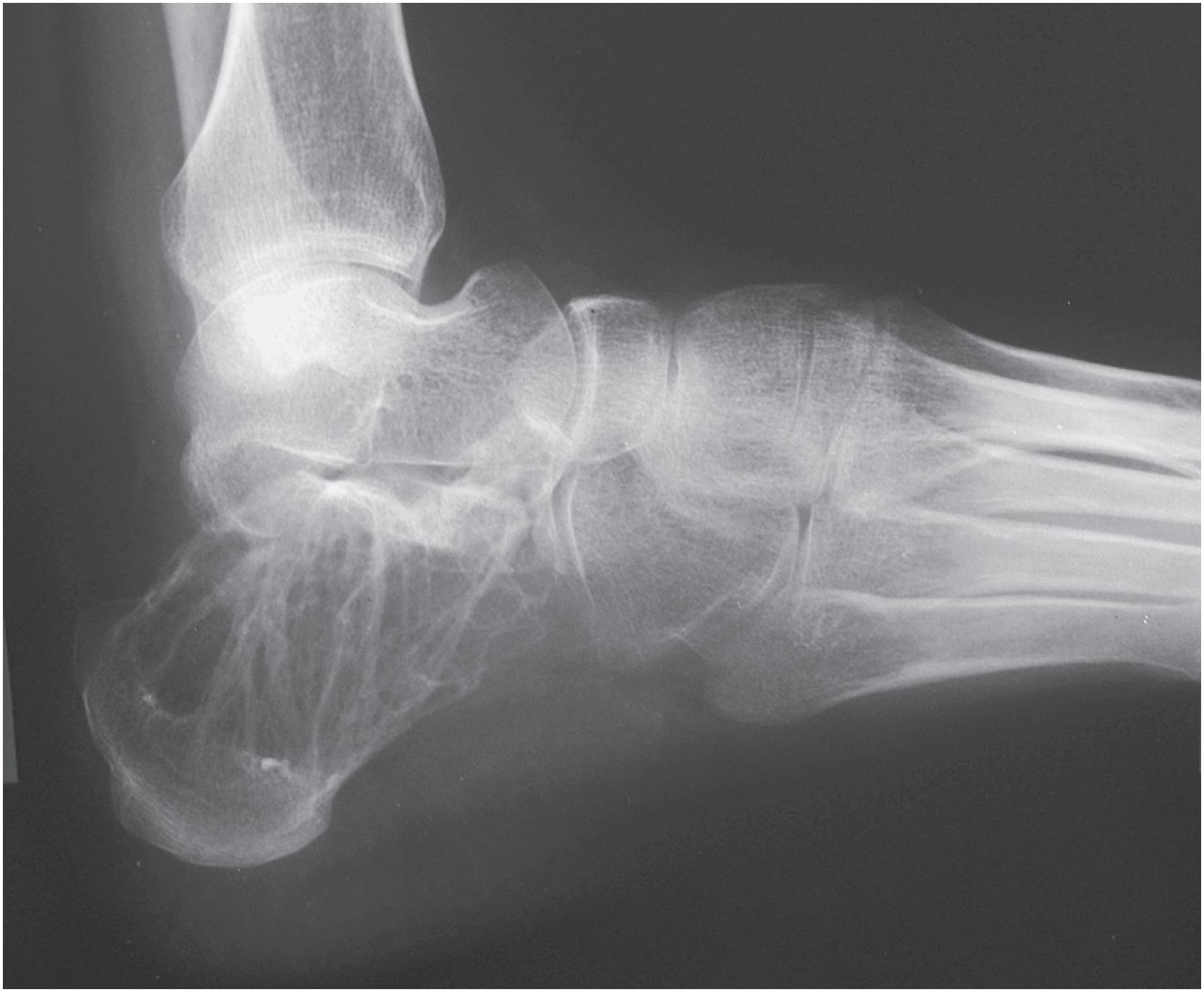
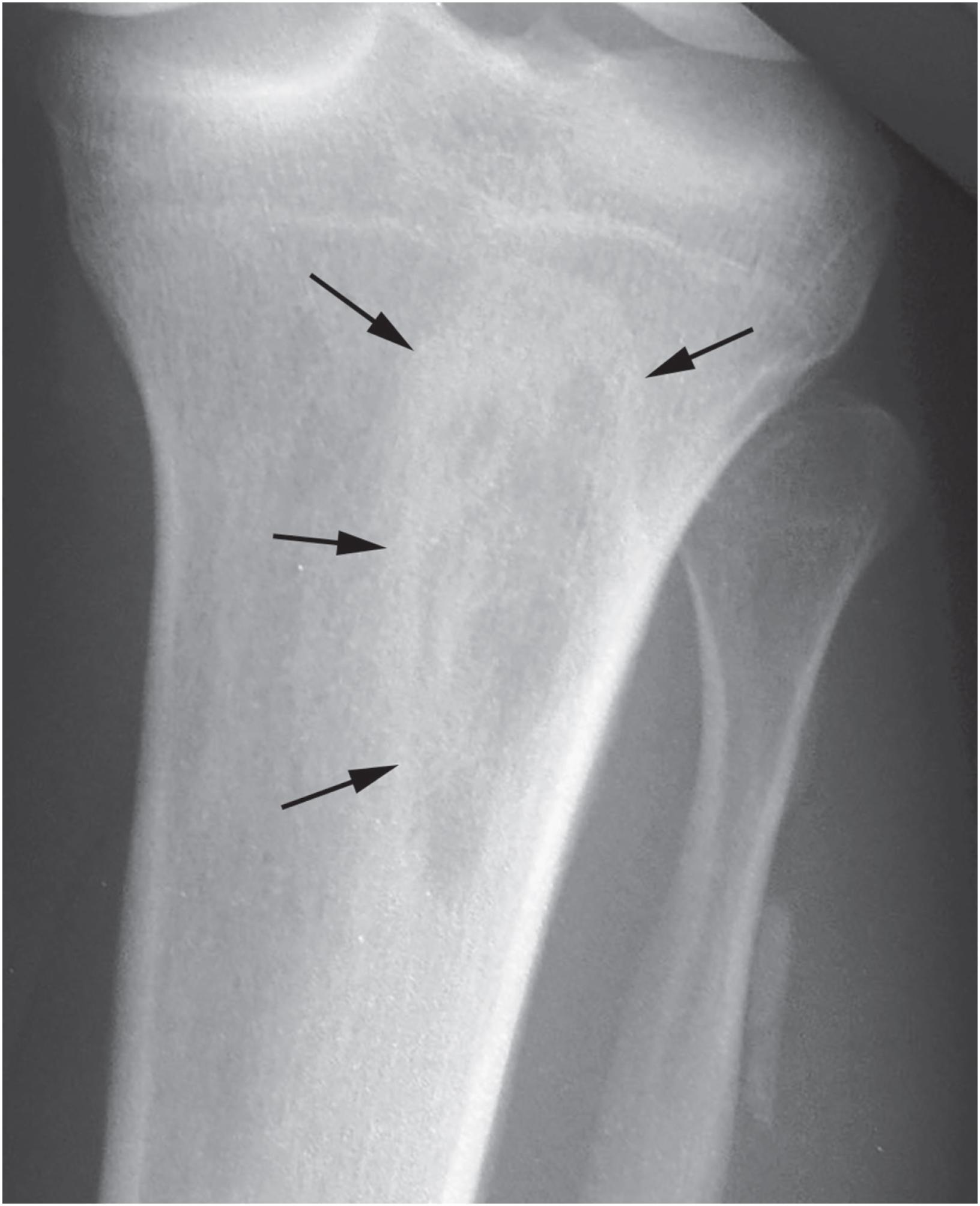
Non-ossifying fibromas are lobulated and lytic metaphyseal lesions. Characteristically, they are eccentric in the medullary cavity and juxtaposed to one cortex. In addition, the margins are scalloped, often grape-shaped, and well corticated ( Fig. 18.1 ). Non-ossifying fibromas in older patients often show intralesional radiodensities, a manifestation of remodeling ( Fig. 18.2 ). Small intracortical lesions (1–2 cm) have traditionally been termed fibrous cortical defects , a term that is no longer recommended by the current WHO classification ( Fig. 18.3 ). Some non-ossifying fibromas can reach >5 cm in maximum diameter and cause cortical expansion and, eventually, pathologic fracture ( Fig. 18.4 ).
Curettings from non-ossifying fibromas consist of friable, yellow-brown soft tissue. A resected specimen shows a well-defined tumor that may erode the cortex.
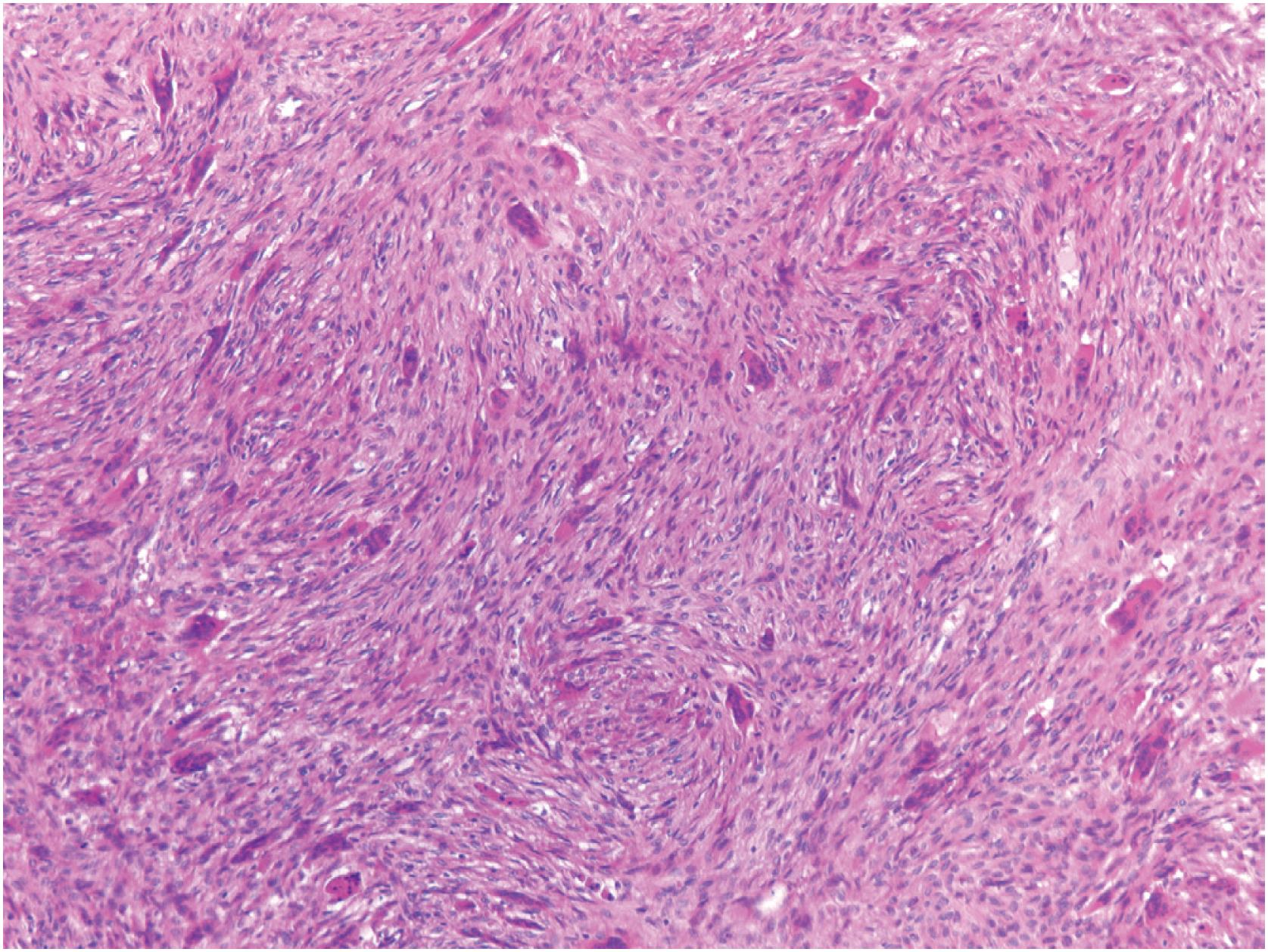
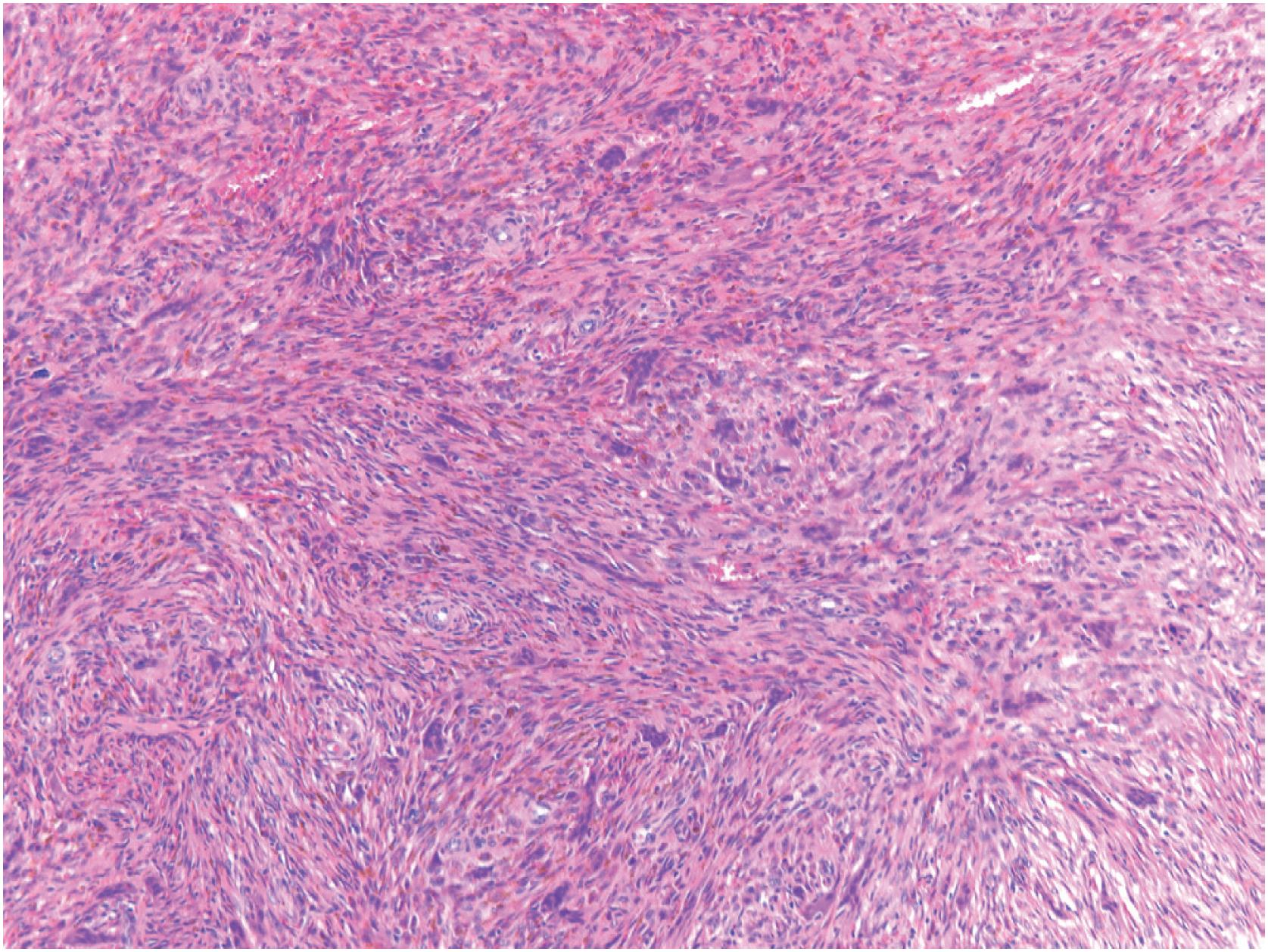
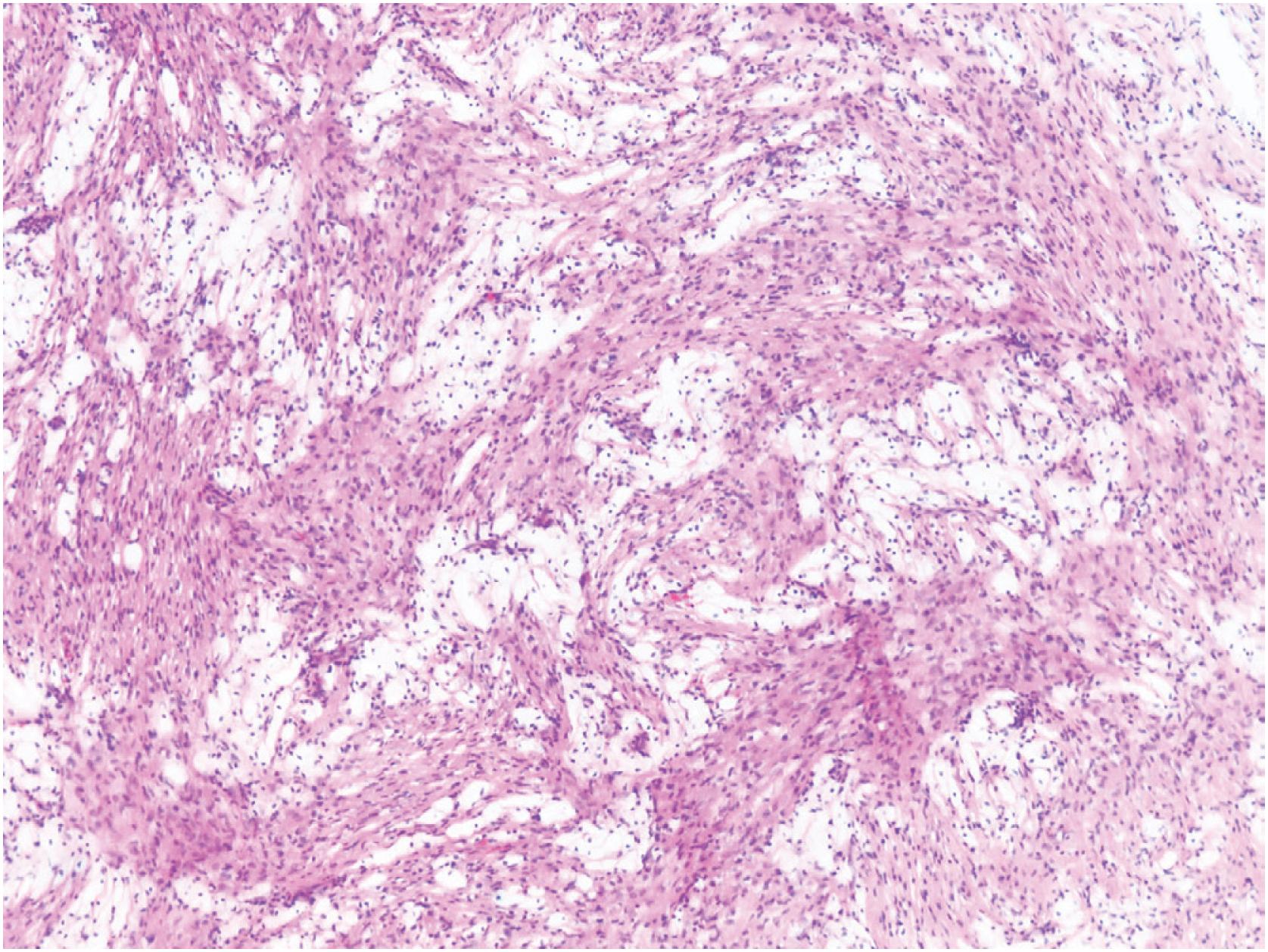
Non-ossifying fibromas consist of a monomorphic spindle cell proliferation with scattered small multinucleated giant cells, although the giant cells may be scarce. Characteristically, the spindle cells are arranged in a whorled or storiform pattern ( Fig. 18.5 ). Occasionally, the stroma is cellular, and the spindle cells often have plump uniform nuclei. In some larger lesions, a moderate number of typical mitotic figures are present. In addition to the giant and stromal cells, foam cells and occasional hemosiderin-laden macrophages are seen ( Figs. 18.6 and 18.7 ). Reactive bone formation is common, especially in older lesions. In the presence of a pathologic fracture, the tumor may show extensive areas of necrosis. Non-ossifying fibroma has no specific immunohistochemical profile.
Non-ossifying fibroma form during skeletal growth and, in the majority of cases, are driven by activated MAP kinase signaling caused by KRAS and FGFR1 mutations (>80% of cases). Whereas formerly believed to represent reactive lesions caused by exaggerated subperiosteal osteoclastic resorption during metaphyseal modelling, these pathogenic hotspot mutations strongly indicate that non-ossifying fibroma is a neoplasm. The same mutations have also been detected in giant cell granulomas of the jaws, which show similar morphology and can co-occur together with non-ossifying fibroma in RAS-related tumor syndromes (so-called RA-Sopathies). Multiple lesions are well known to develop in neurofibromatosis type 1 and Jaffe-Campanacci syndrome (both caused by NF1 mutations), as well as in the rare oculoectodermal syndrome (caused by KRAS mutations).
Because non-ossifying fibromas consist of spindle cells admixed with multinucleated giant cells, giant cell tumor of bone may be considered in the differential diagnosis. However, giant cell tumors typically involve the epiphyseal portions of a bone. In contrast, non-ossifying fibromas are confined to the metaphases and shift towards the diaphysis during skeletal growth. Genetically, giant cell tumors harbor pathogenic H3-3A ( H3F3A ) mutations, of which the most common variant (p.G34W) can be detected immunohistochemically. This mutation is not present in non-ossifying fibroma.
The fibrous stroma of a non-ossifying fibroma frequently contains reactive woven bone formation. This combination of new bone and a fibrous stroma may lead to the misdiagnosis of fibrous dysplasia . However, the radiographic presentation of non-ossifying fibroma and fibrous dysplasia are very characteristic and distinct; fibrous dysplasia furthermore does not typically show storiform spindle cell proliferation or multinucleated giant cells.
Some non-ossifying fibromas may show increased cellularity and contain numerous mitotic figures. These features, in addition to tumors demonstrating areas of necrosis, may lead to the misdiagnosis of a malignant tumor . However, nuclear pleomorphism and atypical mitotic figures are never present in a non-ossifying fibroma. Moreover, the radiographic features are those of a benign, well-circumscribed lesion with a sclerotic rim, features not seen in malignant neoplasms.
Non-ossifying fibroma is a self-limited lesion. Although rarely it may reach >5 cm in maximum diameter, tumors usually measure between 2 and 4 cm. Small, asymptomatic, non-ossifying fibromas require no treatment; most regress spontaneously during skeletal maturation, which is why non-ossifying fibromas are rarely discovered in adults. Larger lesions, particularly those that expand the cortex or those that present with pathologic fracture, are treated with curettage and bone grafting. Recurrence after treatment is rare.
Non-ossifying fibromas are benign and self-limited spindle cell tumors that harbor mutations in the MAP kinase signaling pathway
Eighty percent of lesions occur in the metaphyses of the distal femur or the proximal tibia; the exact incidence is unknown, but up to 30%–40% of children and adolescents might be affected during skeletal growth
Occasionally, lesions present with pathologic fracture
Lesions form in childhood during skeletal growth
Most lesions are asymptomatic; rarely, larger lesions cause pain
Lesions may present with pathologic fracture
A well-defined eccentric lytic lesion in the metaphyseal portion of a long bone
A sclerotic rim is present
Most lesions can be left untreated. Larger lesions or lesions with pathologic fracture may need to be curetted
Lesions consist of friable, yellow-brown tissue
Cellular but uniform spindle cells arranged in a storiform pattern; often, multinucleated giant cells, foam cells, and hemosiderin-laden macrophages are present
Giant cell tumor of bone
Fibrous dysplasia
Desmoplastic fibroma is an extremely rare, locally aggressive, and incompletely understood bone tumor. Due to morphologic similarity, it has been considered the intraosseous counterpart of desmoid type fibromatosis. However, the vast majority lack CTNNB1 mutations and only show weak and cytoplasmic beta-catenin staining, rendering a relation between the two lesions rather unlikely. Extremely rare fibroblastic tumors of bone showing CTNNB1 mutations more likely represent intraosseous desmoid type fibromatosis.
Desmoplastic fibroma has been reported in patients between the ages of 20 months and 60 years. However, most lesions occur in adolescents and young adults with peak incidence being between the ages of 15 years and 25 years. Patients complain of pain, often for several years, which suggests slow growth. On rare occasions, a desmoplastic fibroma may be an incidental finding. Any bone may be affected, but most lesions occur in the long bones (particularly the distal femur and proximal tibia), mandible and the pelvis.
Desmoplastic fibromas present as purely lytic lesions that characteristically show a multilobulated, expansile pattern ( Fig. 18.8 ). They are usually well defined and often have a bubbly appearance ( Fig. 18.9 ). When they occur in a long bone, they are centered in the metaphysis ( Fig. 18.10 ), occasionally extending into the epiphysis. The cortex is often focally eroded or disrupted, and a soft tissue mass, best visualized on magnetic resonance imaging (MRI), may be present. Approximately 10% of patients present with pathologic fracture. A helpful clue to the diagnosis of desmoplastic fibroma on MRI is that most lesions have a low to intermediate signal on T2-weighted images.
Desmoplastic fibromas are extremely dense fibrous lesions. They are grey-white and range in size from 3 to 10 cm, although lesions as large as 20 cm have been reported.
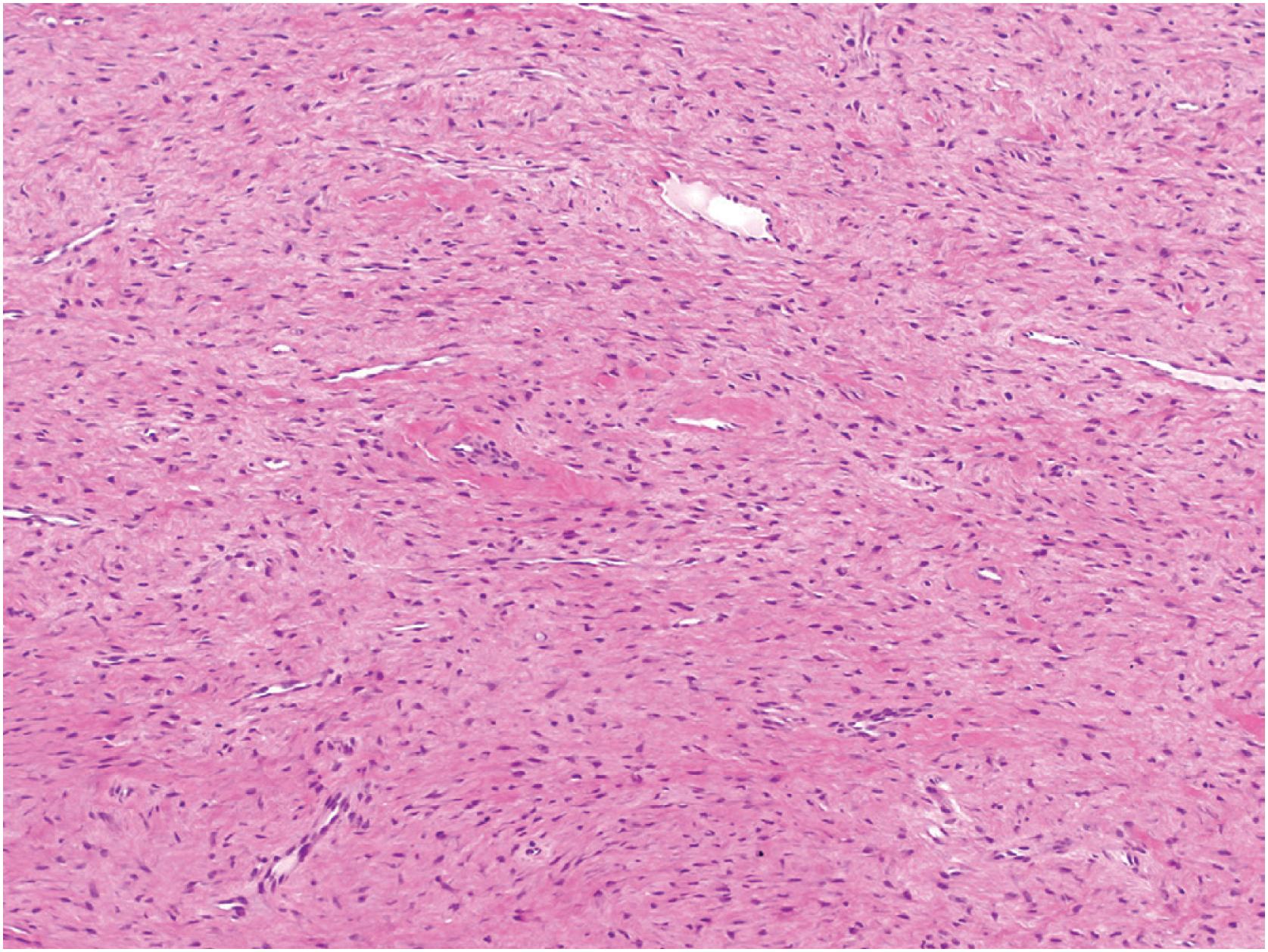
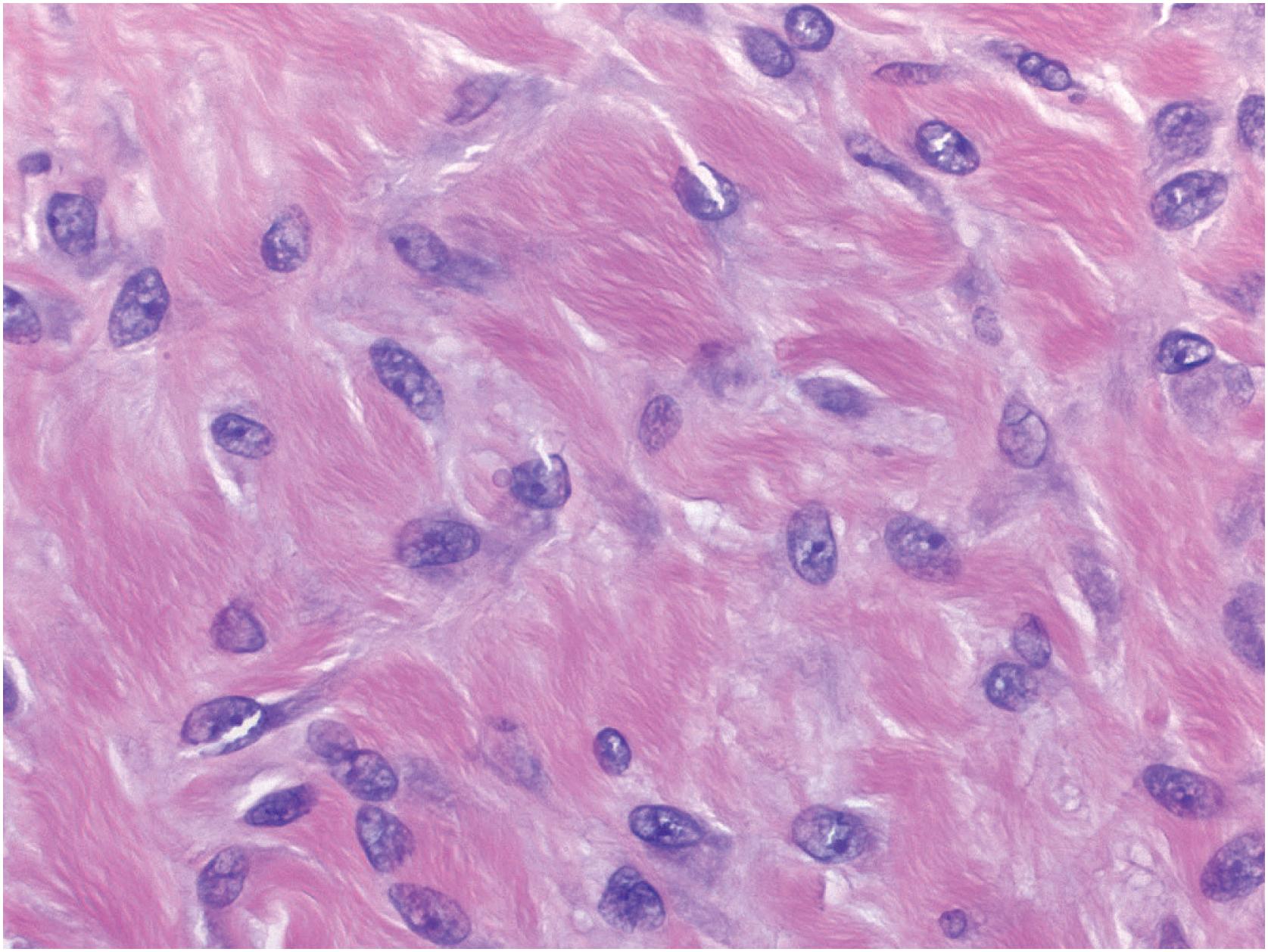
Desmoplastic fibromas show a patternless growth pattern and are composed of benign-appearing spindle cells embedded within a densely collagenized stroma ( Fig. 18.11 ). If infiltration is seen, it is usually limited and at the periphery of the tumor. The lesional cells show bland, oval, or elongated nuclei, usually without nucleoli and resemble fibroblasts or myofibroblasts ( Fig. 18.12 ). Mitotic figures are rare; necrosis is typically absent. There can be morphologic overlap with desmoid type fibromatosis. Lesional bone formation is not a feature of desmoplastic fibroma.
Immunohistochemistry is not helpful in the diagnosis of desmoplastic fibroma of bone. Lesions are occasionally positive for smooth muscle actin, but there is no specific marker to facilitate the diagnosis. Beta-catenin immunoreactivity is variable but generally confined to the cytoplasm. MDM2 and CDK4 are negative.
Fibrous dysplasia and central low-grade osteosarcoma can both present with only little matrix formation and may be confused with desmoplastic fibroma. However, careful attention to imaging studies can be helpful since desmoplastic fibroma is generally purely lytic as opposed to the mixed lytic/sclerotic or ground glass patterns of both differential diagnoses. The background of fibrous dysplasia appears less dense in collagen fibers; in equivocal cases, testing for GNAS mutations usually allows a definitive diagnosis. FISH analysis can identify MDM2 amplification in central low-grade osteosarcoma, but since only one-third of cases are positive, the differential diagnosis can remain challenging in selected cases.
Desmoplastic fibroma of bone is a benign but locally aggressive proliferation of fibroblast-like spindle cells without matrix formation
Desmoplastic fibroma is rare; it most commonly occurs in the long bones; the mandible is also a frequently involved site
Desmoplastic fibroma is a benign tumor; morbidity results mostly from local recurrence if incompletely removed
Desmoplastic fibroma usually occurs in adolescents and young adults; however, cases have been reported between the ages of 20 months and 60 years
Pain is the most frequent presenting complaint; often, the pain has been present for many years
Lesions are lytic with a destructive lobulated pattern; the cortex is often eroded; with MRI, lesions show low to intermediate signal intensity on T2-weighted images
Excision with clear margins is the treatment of choice; simple curettage is associated with an increased rate of local recurrence
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here