Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Imaging is an essential component of the evaluation and care of the developing fetus. Ultrasound remains the core screening modality because of its availability, safety profile, and low cost. Standard of care includes a complete anatomy scan during the second trimester to assess for appropriate development. When anomalies are identified, ultrasound is critical in the assessment of fetal well-being, including interval growth, Doppler parameters, and cardiac function.
Magnetic resonance imaging (MRI) has become an important complementary modality in fetal assessment. Its large field of view and exquisite contrast and spatial resolution make it an invaluable tool in the further assessment of abnormalities. Fetal MRI has thus far been proven safe, with limited animal and human studies available that have demonstrated no firm evidence that embryos are sensitive to magnetic fields encountered from magnetic resonance systems.
When an anomaly is identified by ultrasound, fetal MRI may provide useful additional information that will help guide pregnancy management and counseling. MRI is avoided in the first trimester because of the small size of the fetus and degraded resolution as a result of movement. Determining the optimal time to scan requires a balance between the need to identify abnormalities as early as possible and the desire to obtain images of the highest quality and resolution, which would come later in pregnancy with a larger fetus whose movement is more restricted. MRI can be performed as early as 17 to 18 weeks of gestation for counseling and prenatal management. Late third-trimester studies can be useful in planning delivery and perinatal management ( ).
The American College of Radiology and Society for Pediatric Radiology have developed guidelines for the use of fetal MRI. These indications are summarized in Table 23.1 .
| Face/Neck | Cystic and solid masses |
| Masses that cause airway obstruction | |
| Thorax | Masses |
| Volumetric analysis of lung | |
| Abdomen/Pelvis | Abdominopelvic cysts |
| Complex genitourinary anomalies, such as cloacal malformations | |
| Complex renal anomalies | |
| Complex bowel anomalies | |
| Multifetal gestations | Complications of monochorionic pregnancies |
| Miscellaneous | Consider magnetic resonance imaging when assessment of fetus by ultrasound is limited by oligohydramnios or maternal obesity |
In contradistinction to the screening obstetric ultrasound, which many prospective mothers look forward to as they get a first look at their fetus, fetal MRI can be a source of great stress to a family because they are typically performed to further evaluate a known or potential abnormality. Of ultimate importance to the success of the examination is the comfort and cooperation of the patient.
The American College of Radiology does not recommend written consent from patients for fetal MRI. Nonetheless, it is important to discuss with the family potential risks and benefits. To date, there is no conclusive medical evidence that MRI at 1.5T causes either short- or long-term negative effects on the fetus. It should be acknowledged, however, that MRI effects at 3T or greater are being investigated at the time of this chapter’s writing ( ).
As with all MRI examinations, patients need to be carefully screened for metal on or in their bodies. Some centers recommend not having the mother eat immediately before the MRI in an attempt to decrease fetal motion. However, no studies have conclusively showed that this measure actually improves image quality. Patients should also be encouraged to empty their bladders before scanning to improve comfort and image quality.
MRI can be an uncomfortable examination for any patient. Care should be taken to determine whether the patient would be more comfortable on her back or side to improve her ability to lie still. Sufficient pillows should be provided to support the patient in whichever position is deemed most comfortable. Earplugs and/or headphones should be provided to distract patients from the excessive noise generated by the magnetic resonance gradients. It may be helpful to have a partner in the room with the mother to decrease anxiety.
The entire fetus, as well as large field-of-view images that include the uterus, cervix, and placenta, should be evaluated in every fetal MRI study ( ). Nonetheless, imaging protocols should be tailored to the clinical indication for the study. In case the mother cannot complete the examination because of discomfort, the earliest sequences obtained should help address the specific diagnostic question being asked.
The examination should begin with a standard three-plane localizer. Initial large field-of-view sequences of the maternal pelvis provide a global view of the uterus, placenta, and cervix and help delineate the position of the fetus relative to maternal anatomy. More directed sequences can then be acquired of specific fetal anatomy. As the fetus moves throughout the examination, subsequent sequences can be planned on the basis of earlier ones. Ideally, three planes of both the fetal brain and body should be obtained.
In the case of multiple gestations, after initial large field-of-view sequences, each fetus should be imaged separately. Distinguishing twins for purposes of imaging presents an additional challenge, and using labeling defined by ultrasound is helpful.
Single-shot techniques, which are spin-echo acquisitions in which the entirety of k-space is filled during one excitation, are the workhorses in fetal imaging because they are fast, thus limiting the deleterious effects of motion while providing excellent contrast resolution. Steady-state free precession images offer good contrast resolution with flowing blood demonstrating hyperintense signal. These display a ratio of T2/T1 contrast, thus accentuating fluid-sensitive signal, and may be performed as cine acquisitions to assess fetal movement. T1-weighted sequences are also of use, particularly to evaluate the meconium content of the bowel, subacute blood, and fat, which are hyperintense on these acquisitions. Echoplanar imaging can better delineate the fetal skeleton, providing excellent contrast between cartilage (bright) and bone (dark). Diffusion-weighted imaging can be useful in brain and renal imaging.
Intravenous contrast is not recommended for fetal MRI. The gadolinium remains within the amniotic fluid for prolonged periods. If required for maternal or placental indications, the recommendation is to deliver the fetus as quickly as possible after contrast administration in the third trimester.
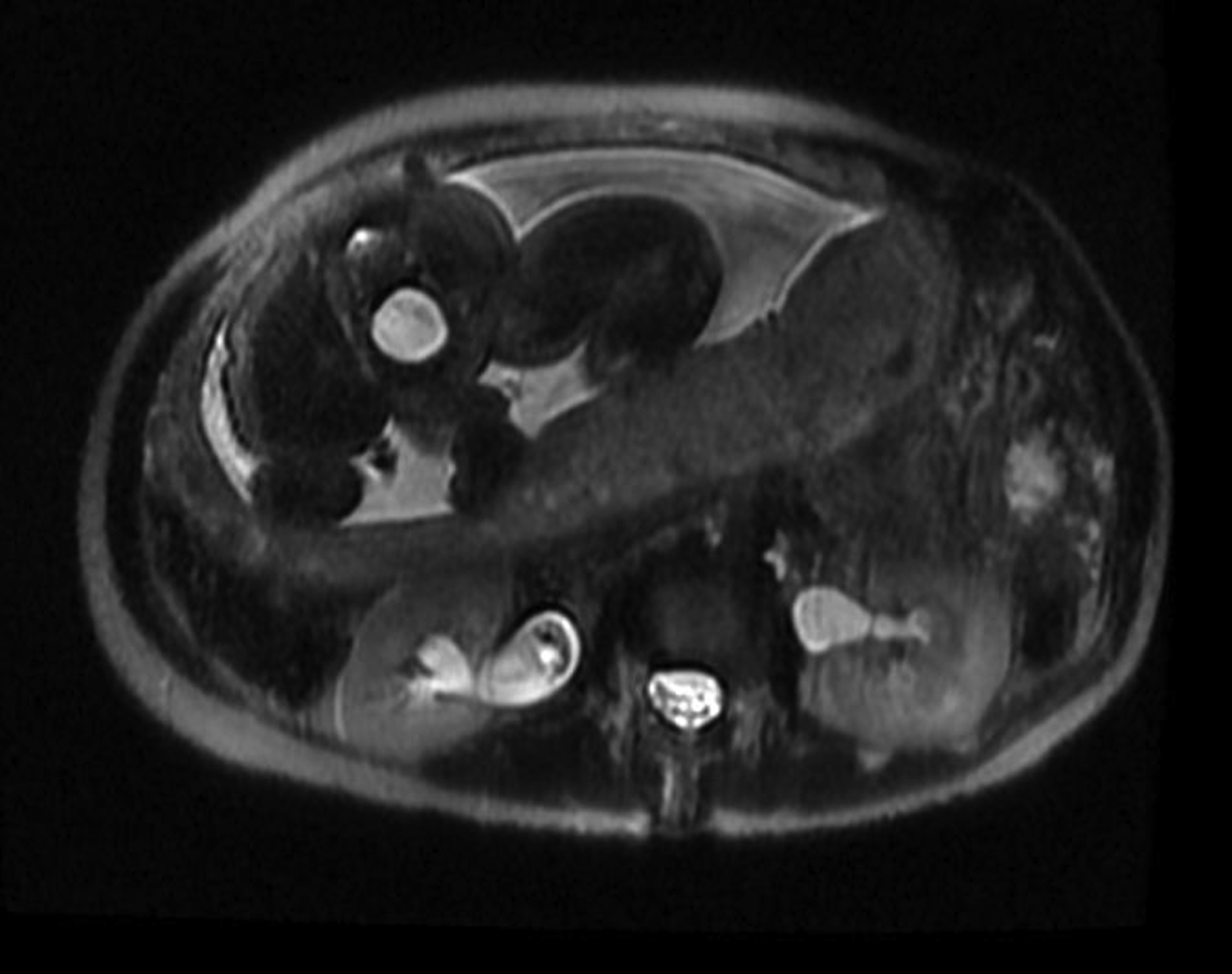
Large field-of-view images should include the mother’s abdomen and pelvis. The maternal kidneys often demonstrate some degree of collecting system dilatation because of ureteral compression by the gravid uterus, particularly late in pregnancy ( Fig. 23.1 ). Any maternal abnormalities should be described if identified.
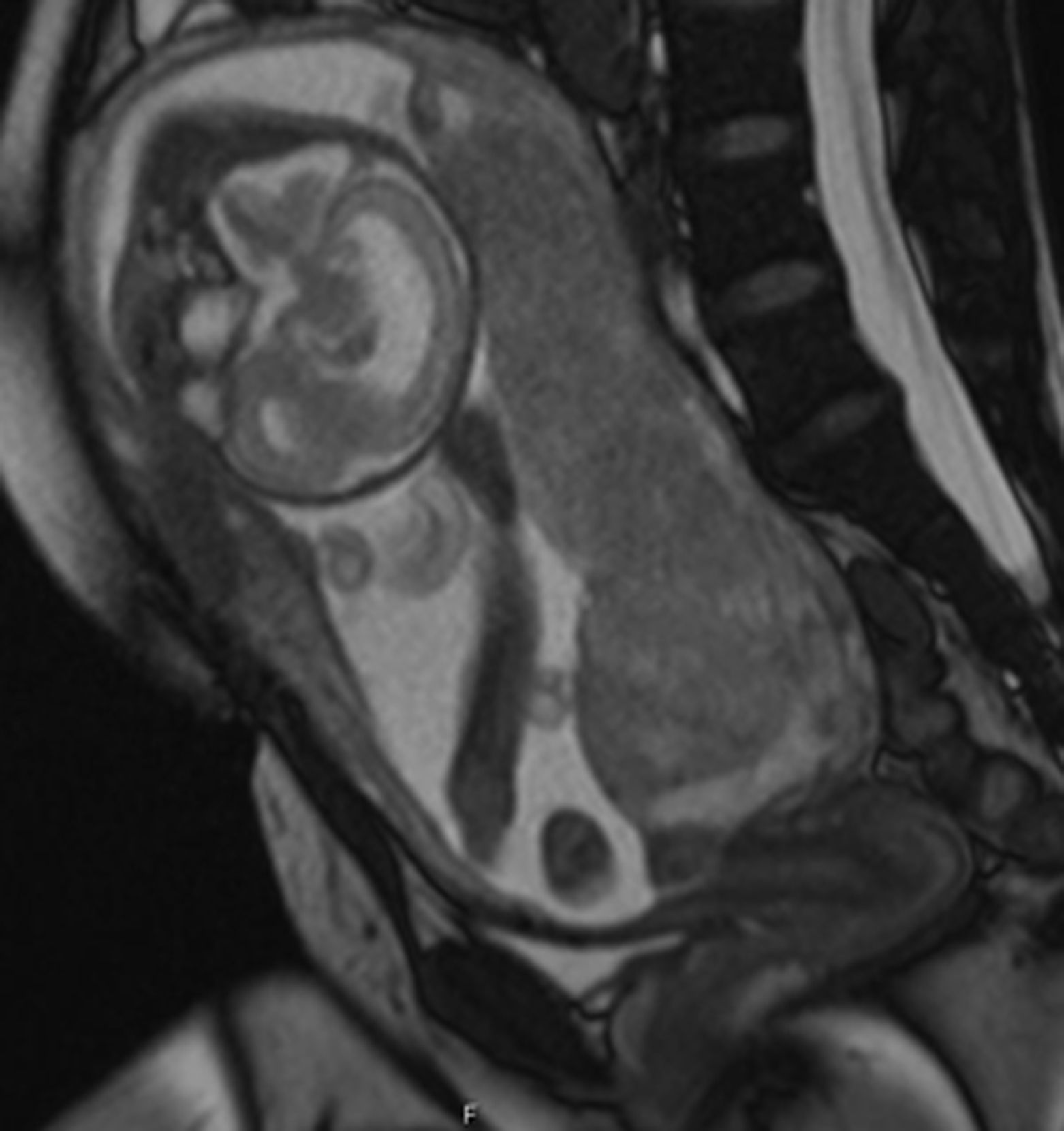
MRI also provides a global view of the pregnant uterus. The integrity of the cervix should be evaluated and cervical length measured in the sagittal plane. Any anomalies of the uterus, such as septate or bicornuate morphology, should be described, as should the size and location of fibroids ( Fig. 23.2 ).
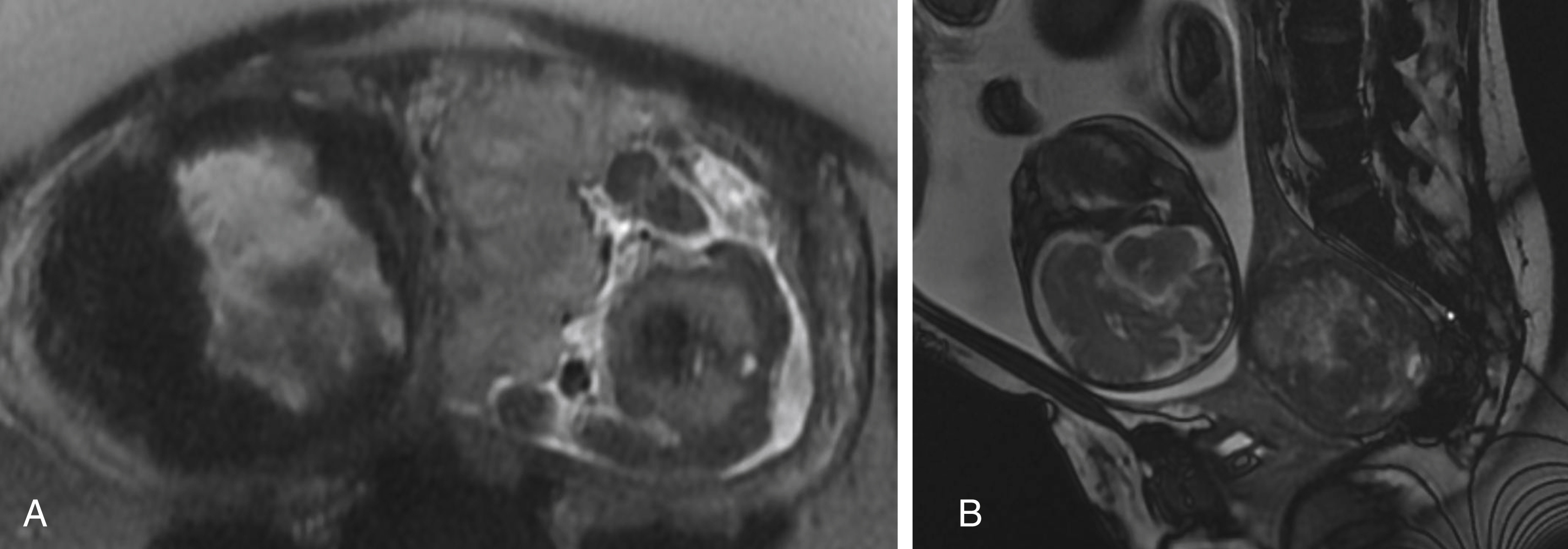
It is also important to evaluate the thickness and position of the placenta and the cord insertion. The normal placenta is discoid in configuration and homogeneously T2 hyperintense early in pregnancy, measuring 2 to 4 cm in maximal thickness, but becomes increasingly heterogeneous and lobulated later in gestation ( ). An abnormally thin placenta may indicate vascular compromise, whereas an abnormally thick placenta could be an associated finding of fetal hydrops, infection, aneuploidy, or anemia. A low-lying placenta or placenta previa, in which the placenta covers the internal cervical os, must be reported ( Fig. 23.3 ). MRI also may provide useful assessment of abnormal placentation, which is suggested by the presence of T2 dark intraplacental bands, increased placental vascularity, and focal bulging with interruption of the smooth uterine contour.
Determining situs requires a three-dimensional understanding of fetal anatomy relative to that of the mother. Simply observing that the stomach has the same laterality as the heart is not sufficient for accurate assessment of situs because situs inversus totalis may occur; a specific “left” and “right” side of the fetus must be defined. Two simple questions need be answered to confidently determine situs:
Is the fetus cephalic or breech?
Is the fetus facing the mother’s front, back, left side, or right side?
The easiest scenario to conceptualize is the front-facing breech fetus, because this position is identical to that of the mother. In this case, the fetus’s left is the mother’s left. A rear-facing cephalic fetus will also have the same left-right orientation as the mother. The front-facing cephalic fetus and the rear-facing breech fetus will have opposite left-right assignments relative to the mother.
If the fetus is facing the mother’s left or right, the side of the fetus closest to the mother’s spine will match the facing side if breech but be opposite if cephalic. For example, a breech fetus facing right will have its right side posterior relative to the mother. The same fetal left/right designation would be inferred in a cephalic fetus facing left. Some common scenarios are summarized in Table 23.2 .
| Fetal Lie | Fetus is Facing the Mother’s... | Fetal Left is Mother’s... |
|---|---|---|
| Cephalic | Front | Right |
| Cephalic | Back | Left |
| Breech | Front | Left |
| Breech | Back | Right |
| Cephalic | Left | Front |
| Cephalic | Right | Back |
| Breech | Left | Back |
| Breech | Right | Front |
Once the left/right designation has been determined, the laterality of several anatomic structures should be sought. Among the structures to note are the heart, stomach, and liver.
With regard to the heart, efforts should also be made to determine the side on which the apex lies; the apex pointing leftward defines levocardia, whereas the apex pointing rightward signifies dextrocardia. A right-sided heart with apex pointing left is not dextrocardia but rather levocardia with dextroposition.
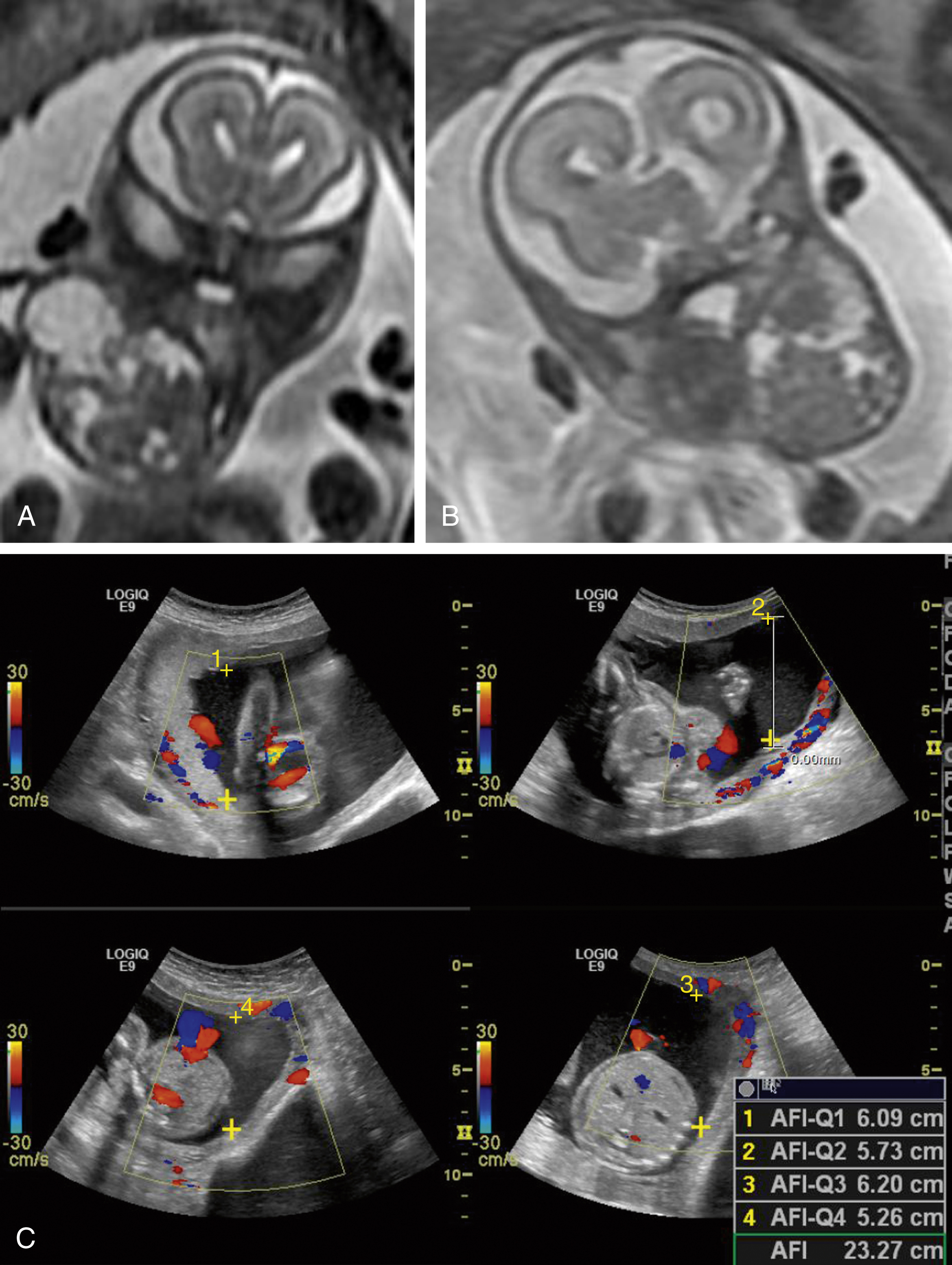
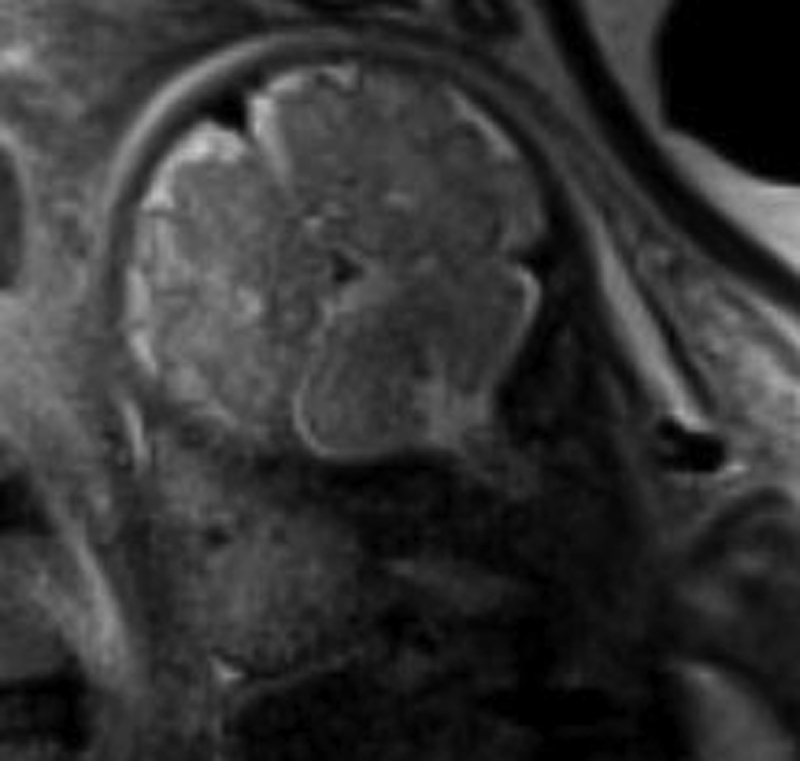
Normal anatomic positioning of abdominal structures is known as situs solitus, and the presence of dextrocardia in this setting portends a high association with congenital heart disease (>95%). Abdominal situs inversus describes the scenario in which anatomic structures are reversed in orientation but otherwise maintain normal anatomic structure and relationships. Levocardia in this setting guarantees congenital heart malformation, whereas dextrocardia with abdominal situs inversus portends a slightly higher incidence of congenital heart disease than the general population (3%–5%). Any situs that is not solitus or inversus is classified as situs ambiguous, or heterotaxy, in which the anatomic relationships of major organs are altered. In the case of left isomerism, multiple spleens may be present. In fetuses with right isomerism, there may be no spleen ( ). The liver is midline in either case ( Fig. 23.4A–C ).
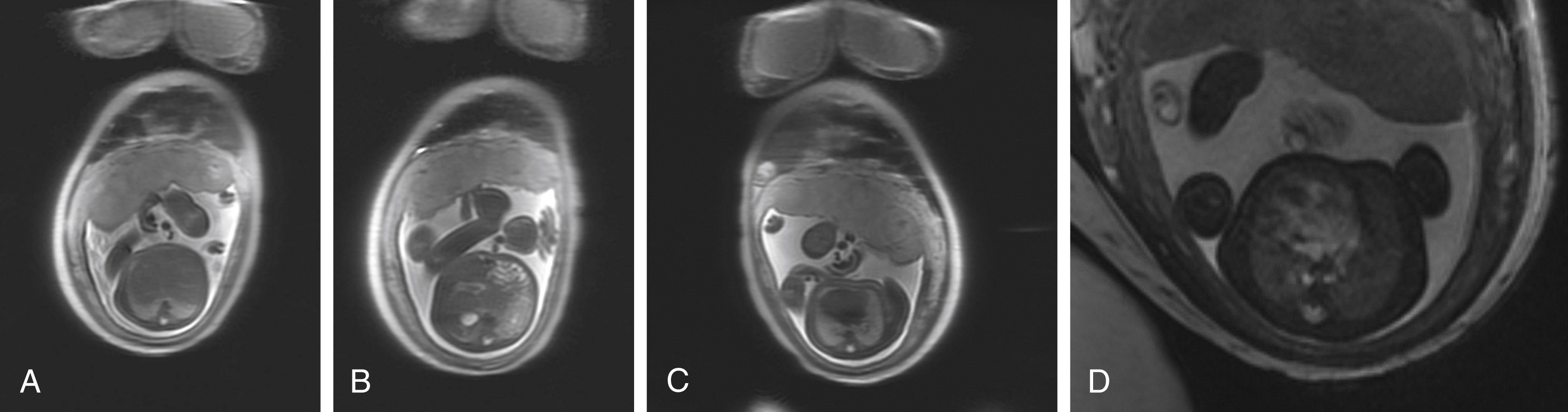
Multiple other anomalies that have a high association with heterotaxy should also be sought, including intestinal malrotation, gallbladder absence, inferior vena cava interruption with azygous continuation (see Fig. 23.4D ), and anomalous pulmonary venous return. Fetal echocardiogram should be performed because of the high risk for complex congenital heart disease.
A top-down approach may be used to survey the normal fetal face and neck. Both globes should be present, equal in size, and normally seated in the orbits ( Fig. 23.5A ). Asymmetry in globe size may indicate a genetic or chromosomal abnormality that can have important prognostic implications. Proptosis could signal the presence of an intraorbital abnormality or craniosynostosis. The interocular diameter should be roughly equal to orbital width; hypotelorism and hypertelorism can both indicate chromosomal abnormalities or syndromic conditions of the fetus.
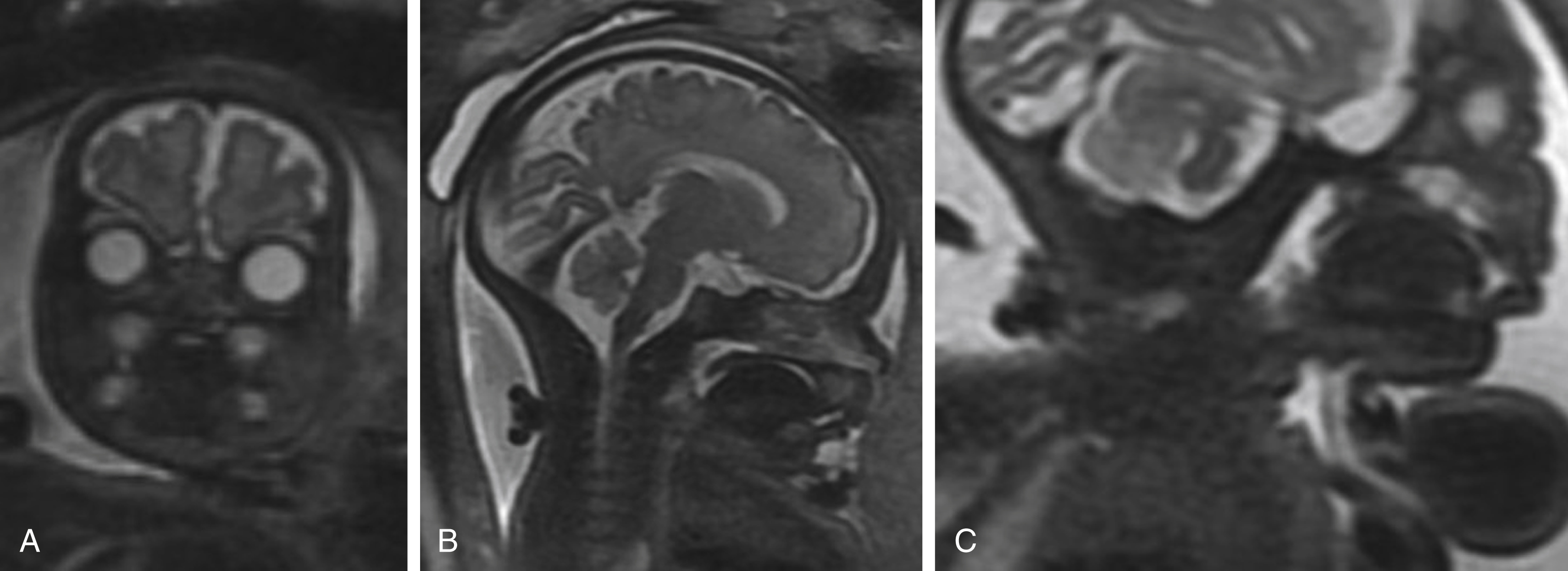
The maxilla, mandible, lip, and palate should be symmetrical and intact ( ). Abnormally small mandible, or micrognathia, is a finding associated with a plethora of syndromes, best noted on sagittal images. Nasal bone hypoplasia is better assessed with ultrasound. The tongue should not protrude beyond the confines of the oral cavity ( Fig. 23.5B ); macroglossia can impede the airway postnatally and has syndromic associations, such as with Beckwith-Wiedemann syndrome (BWS).
The fetal airway is well delineated by MRI. Although the fetus does not require pharyngeal patency for growth and survival, because it draws oxygen from the maternofetal circulation, any airway impedance will have serious implications at delivery. The airway should be uniformly T2 hyperintense because of the presence of amniotic fluid ( Fig. 23.5C ). Any narrowing or frank obstruction of the pharynx must be identified.
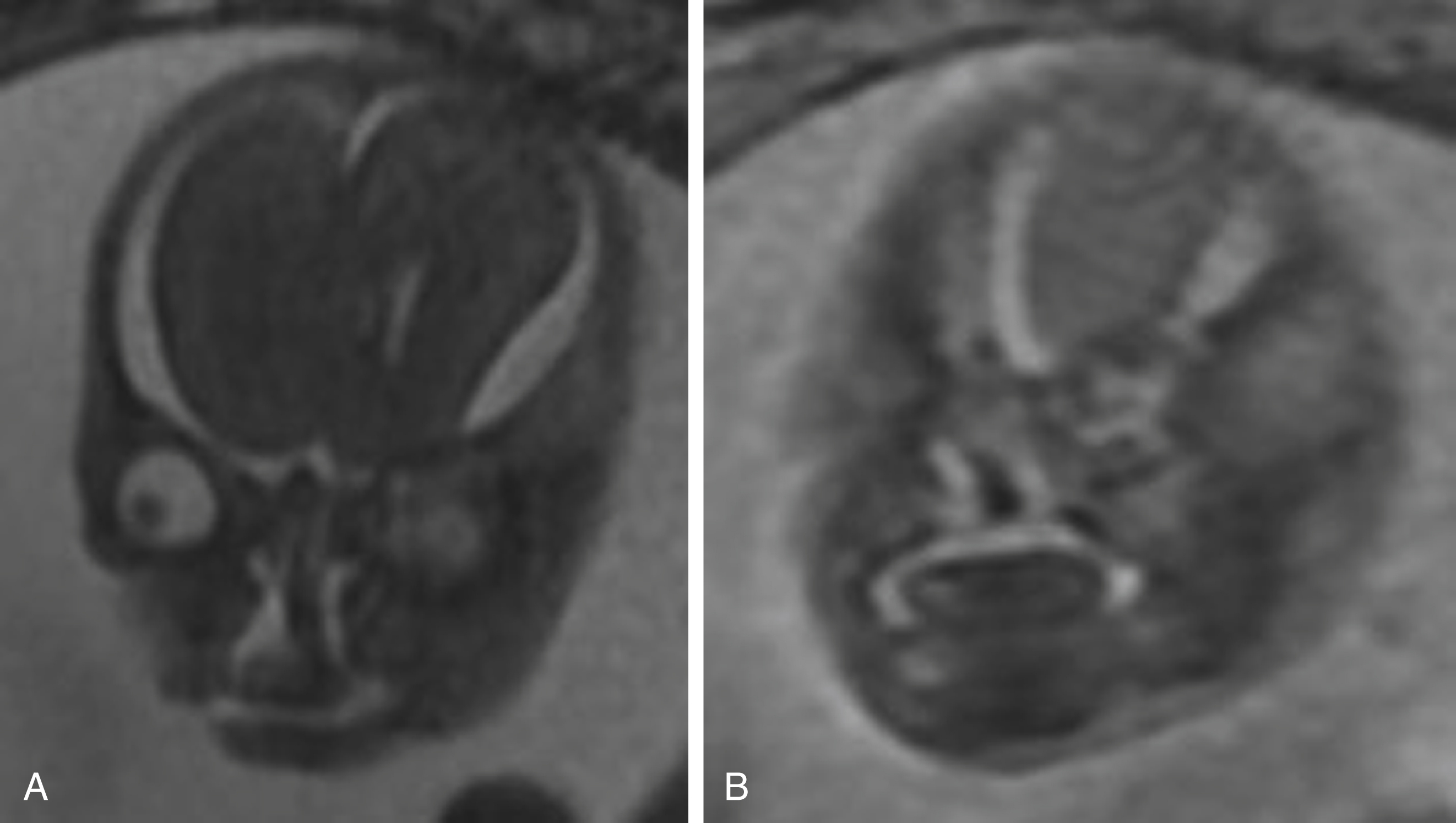
Cleft lip and/or palate are relatively common and typically treatable craniofacial defects. Ultrasound is a reliable tool in the evaluation of cleft lip and primary palate, although sonographic evaluation of the fetal face can be somewhat limited depending on maternal body habitus and the lie of the fetal head at the time of scanning. MRI is better in assessing the secondary palate, which forms the majority of the hard palate ( ).
In up to 11% of cases, there is an association between cleft lip and/or palate and chromosomal abnormalities. MRI is particularly useful for complex clefts and central clefts, which have a high incidence of associated central nervous system anomalies. This association increases in frequency as the size and extent of the cleft increases. The two most important features of cleft lip and palate for defining the surgical approach are the laterality and anteroposterior extents of the defect(s), which have been shown to be more precisely assessed by MRI ( Fig. 23.6 ).
Micrognathia may be missed in the second trimester because rapid mandibular growth occurs later in gestation. Research by ( ) proposed quantitative parameters to assess micrognathia, including measurement of the inferior facial angle (IFA) and jaw index. The IFA is drawn on a midline sagittal image of the face with a line through the nasal root perpendicular to the plane of section and another parallel to the outer surface of the mandible. An IFA less than 50 degrees is concerning for micrognathia ( Fig. 23.7A–B ). The jaw index is the mandibular anteroposterior diameter normalized to biparietal diameter, which helps eliminate some of the effects of gestational age. A jaw index of less than the fifth percentile for gestational age suggests micrognathia. These calculations can be made with ultrasound, but MRI may provide greater accuracy.
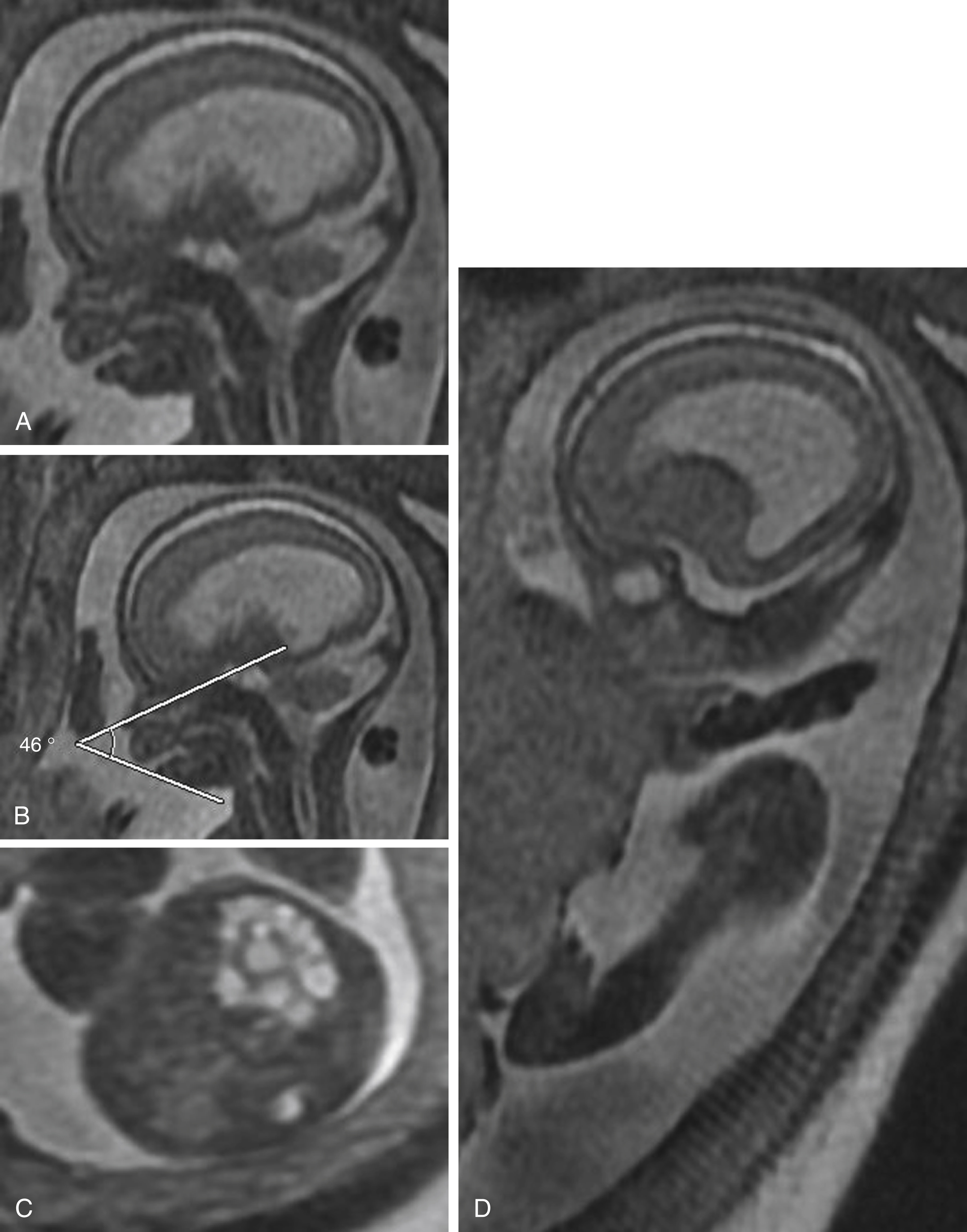
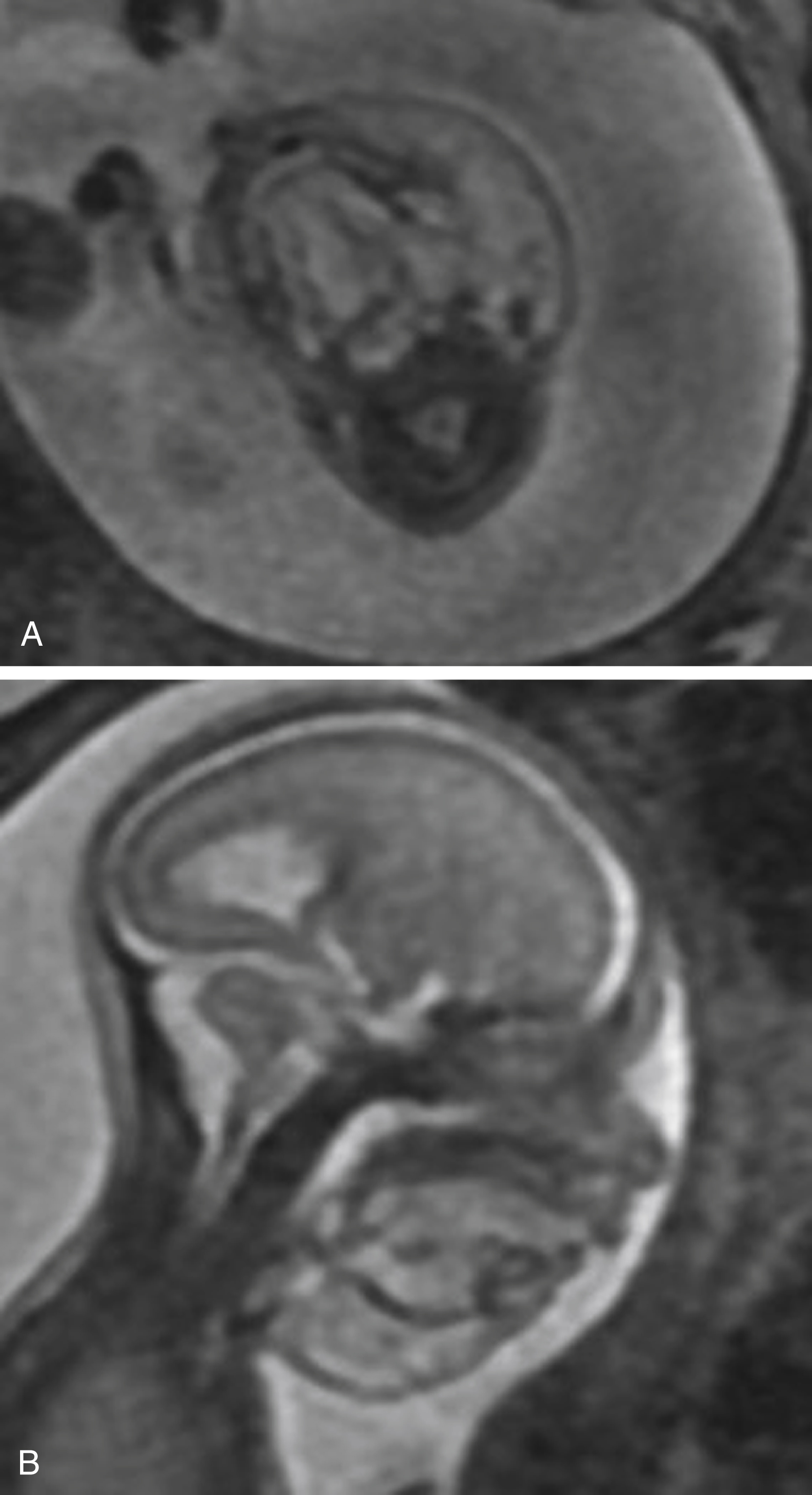
Once the diagnosis of micrognathia has been made, there is a strong association with chromosomal abnormalities, necessitating comprehensive assessment of the fetal body for anomalies ( Fig. 23.7C–D ). Furthermore, true micrognathia may be associated with a small oral cavity, leading to posterior displacement of the tongue, potentially compromising airway integrity and affecting delivery planning.
Several masses can develop in the fetal face and neck. These can be cystic and/or solid. The most critical feature of the mass is its location in relation to the airway. Regardless of the cause of the mass, airway obstruction can lead to rapid decompensation in the delivery room. Various fetal and early perinatal interventions are available but require advance planning, including ex utero intrapartum treatment, in which a surgical airway is established with the fetus outside of the uterus but with maintenance of maternofetal circulation as the source of oxygen for the baby.
The majority of fetal neck masses are one of four entities: lymphatic malformation, teratoma, congenital hemangioma, and goiter. Lymphatic malformations result from failure of connection between the lymphatic and venous systems, resulting in isolated collections of lymph. In the neck, they tend to be posterior or posterolateral T2-hyperintense multiseptate masses that insinuate among normal structures and involve multiple anatomic spaces ( Fig. 23.8 ). Fluid–fluid levels are not uncommon and are the result of internal hemorrhage. Cervical teratomas have a variable imaging appearance because of their composition of tissues from multiple germ cell layers. They may contain solid and cystic components, and calcifications are nearly pathognomonic, although not well seen by MRI ( Fig. 23.9 ). Hemangiomas are vascular tumors that typically contain prominent vascular flow voids and are T2 hyperintense. Follow-up postnatal imaging may demonstrate decreased size if the lesion is of the rapidly involuting variety versus the noninvoluting hemangioma, which will not regress spontaneously after birth ( Fig. 23.10 ). Fetal goiter may result from maternal thyroid dysfunction, such as autoimmune thyroid diseases in which thyroid-stimulating antibodies can cross to the fetal circulation and stimulate the fetal thyroid gland. Imaging appearance is unique, as the homogeneously T1-hyperintense bilobed thyroid gland is significantly enlarged ( Fig. 23.11 ).
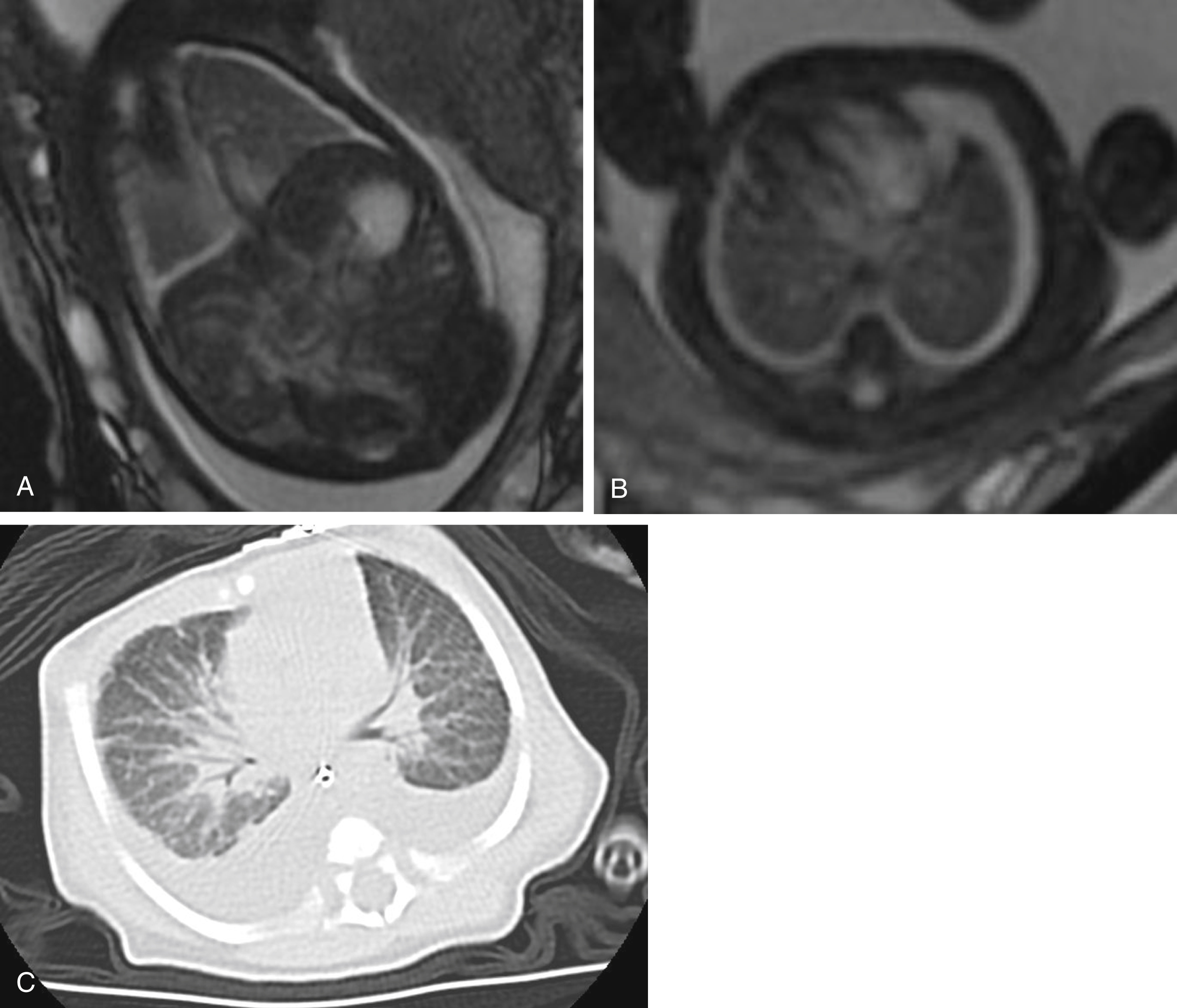
A central-to-peripheral approach is recommended for comprehensive evaluation of the fetal thorax. The intrathoracic trachea should be patent and filled with fluid, similar to the upper airway in the neck. The heart should be located in the midline with leftward-pointing apex. The lungs should be relatively symmetrical in size, homogeneous in signal intensity, and slightly hyperintense on T2-weighted images because of the presence of amniotic fluid in the lungs ( Fig. 23.12 ). A fluid-filled esophagus is typically not seen; if the proximal esophagus is particularly conspicuous, tracheoesophageal fistula should be considered. In some cases the great vessel branching pattern can be assessed.

Diffuse hyperintense T2 signal throughout the lung parenchyma may be secondary to impedance of the outflow of amniotic fluid from the lungs ( Table 23.3 ). This can be caused by tracheal obstruction. Cervical masses that compress the pharynx and thoracic masses, native to either the lung or mediastinum, also can cause airway compression with similar fluid retention in the lungs. At times, amniotic fluid can dilate the more distal airways. Unrecognized and untreated, high airway obstruction has a mortality rate of 80% to 100%. An obstructed airway as a result of intrinsic atresia or stenosis is known as congenital high airway obstruction syndrome. The most severe airway obstruction should be managed perinatally with the ex utero intrapartum treatment procedure.
| Diffuse | Focal |
|---|---|
| Airway compression by mass | Congenital pulmonary airway malformation |
| Congenital high airway obstruction syndrome | Pulmonary sequestration |
| Pulmonary lymphangiectasia | Congenital lobar overinflation |
| Bronchial atresia | |
| Bronchogenic cyst | |
| Congenital diaphragmatic hernia |
Other causes of diffusely heterogeneous lung signal include primary and secondary lymphangiectasia. Primary lymphangiectasia is due to obstruction of the pulmonary lymphatics, whereas secondary lymphangiectasia is the sequela of cardiac dysfunction related to congenital heart disease ( Fig. 23.13 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here