Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A number of superficial structures in the extremities are well suited for sonographic imaging. This is especially true of tendons. The interfaces between internal tendon fibers produce strong specular reflections when the sound reflects off the tendon at 90 degrees. The result is referred to as a fibrillar pattern and consists of closely spaced, parallel, bright linear reflections ( Fig. 11-1 ). When imaged at less than 90 degrees, the strength of the reflections decrease, the tendons become hypoechoic, and the fibrillar pattern is lost (see Fig. 11-1 ). Variable echogenicity, depending on the relative orientation of the transducer and the structure being scanned, is referred to as anisotropy . Anisotropy is present in many parts of the body but is particularly prominent in tendons ( ![]() ). Under most circumstances, tendons should be imaged so that the fibrillar pattern is visible. However, when tendons are surrounded by echogenic tissue, it may be helpful to purposely angle the transducer so that the tendon appears hypoechoic and the contrast between the tendon and peritendinous tissues is increased. In addition, echogenic lesions and abnormal intratendinous interfaces may be best seen when the tendon is purposely made to appear hypoechoic by imaging at less than 90 degrees.
). Under most circumstances, tendons should be imaged so that the fibrillar pattern is visible. However, when tendons are surrounded by echogenic tissue, it may be helpful to purposely angle the transducer so that the tendon appears hypoechoic and the contrast between the tendon and peritendinous tissues is increased. In addition, echogenic lesions and abnormal intratendinous interfaces may be best seen when the tendon is purposely made to appear hypoechoic by imaging at less than 90 degrees.

Probably the most common reason for performing musculoskeletal examinations is to evaluate the tendons. Tendon tears are common and are relatively easy to identify and analyze with sonography. Complete tendon tears are associated with a number of sonographic findings ( Fig. 11-2 and Box 11-1 ). In many cases the end of the retracted proximal tendon will appear blunt on longitudinal views and will appear masslike on transverse views. It is useful to scan in the transverse plane from the intact portion of the tendon to the torn portion in order to visualize the thickened retracted end ( e-Fig. 11-1 ![]() and
and ![]() ). Passive or active tendon motion is also very helpful in confirming and sometimes quantifying a tear ( e-Fig. 11-2
). Passive or active tendon motion is also very helpful in confirming and sometimes quantifying a tear ( e-Fig. 11-2 ![]() and
and ![]() ). There is usually some degree of shadowing at the site of the torn proximal tendon. In most cases the shadowing is refractive in nature and does not imply underlying calcification or avulsion of bone. Another common finding is loss of the normal fibrillar architecture of the tendon or complete nonvisualization of the tendon. Fluid collections may occur at the site of torn tendons due to a hematoma (especially in the setting of a tear near or at the musculotendinous junction) or a tendon sheath effusion. Partial-thickness tears disrupt the internal fibrillar architecture in a focal region but do not cause tendon retraction ( Fig. 11-3 ).
). There is usually some degree of shadowing at the site of the torn proximal tendon. In most cases the shadowing is refractive in nature and does not imply underlying calcification or avulsion of bone. Another common finding is loss of the normal fibrillar architecture of the tendon or complete nonvisualization of the tendon. Fluid collections may occur at the site of torn tendons due to a hematoma (especially in the setting of a tear near or at the musculotendinous junction) or a tendon sheath effusion. Partial-thickness tears disrupt the internal fibrillar architecture in a focal region but do not cause tendon retraction ( Fig. 11-3 ).

Blunt tendon tip (longitudinal views)
Mass (transverse views)
Refractive shadowing
Nonvisualization
Loss of fibrillar architecture
Fluid collection


Tendonitis and tenosynovitis (inflammation of the tendon sheath) can be due to inflammatory processes (rheumatoid arthritis and other synovial-based arthritis), infection (either from penetrating trauma or blood borne), crystals (gout due to crystal precipitation), trauma (usually repetitive microtrauma), amyloidosis (chronic hemodialysis), or foreign bodies. Sonographic findings include fluid distending the tendon sheath, thickening of the synovial tendon sheath, or both ( Fig. 11-4 ). Synovial thickening may be diffuse and smooth or eccentric and nodular. Infections or hemorrhage may produce fluid with low-level echoes. Hypervascularity is often detectable when there is an active inflammatory process. Tendinopathy is a common condition that can produce pain but is not inflammatory in nature. It is generally seen as tendon thickening with decreased or heterogeneous echogenicity ( Fig. 11-5A ), and in some tendons as increased vascularity (see Fig. 11-5B and C ).


In many locations, the tendons are secured in position by ligaments or ligament-like structures. Subluxations and dislocations occur when these ligaments are torn or insufficient. The most common tendons involved are the proximal biceps tendon and the peroneal tendons ( Fig. 11-6 ). The flexor tendons of the fingers are secured to the phalanges by a set of five pulleys. Pulley rupture allows for the tendon to pull away from the bone, producing bow stringing during finger flexion ( e-Fig. 11-3 ![]() ). In some situations a subluxing or dislocating tendon will present clinically as a snapping sensation when the extremity moves in certain positions. Because this happens with movement, sonography can monitor the tendons in real time and in many cases can determine the source of the snapping (
). In some situations a subluxing or dislocating tendon will present clinically as a snapping sensation when the extremity moves in certain positions. Because this happens with movement, sonography can monitor the tendons in real time and in many cases can determine the source of the snapping ( ![]() ).
).


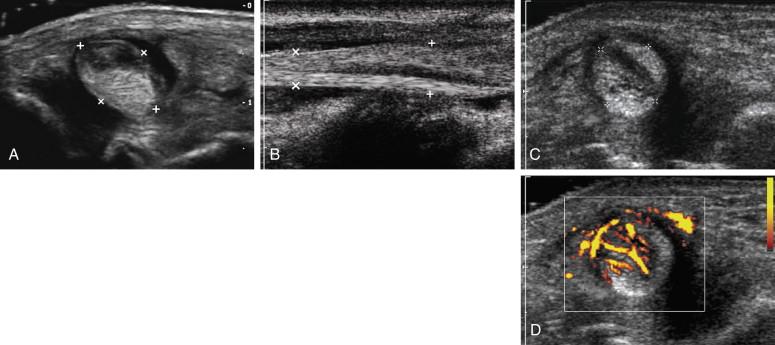
Neoplasms of the tendons are extremely rare, but giant cell tumors (GCTs) of the tendon sheath are the second most common cause of a mass in the hand. They are benign lesions that are histologically identical to pigmented villonodular synovitis. They are typically slow growing and painless and occur along the volar surface of the fingers. GCTs are solid, homogeneous, hypoechoic masses that are adjacent to the tendons and often partially surround the tendon ( Fig. 11-7 ). High-frequency color Doppler will generally show readily detectable internal blood flow and the lesions may be quite vascular. Because they arise from the sheath and not the tendon, they do not move with the tendon when the finger is flexed and extended ( e-Fig. 11-4 ![]() and
and ![]() ).
).


Non-neoplastic masslike lesions are occasionally encountered in the tendons. Trigger fingers in the digits are due to focal areas of fibrotic thickening that catch on the finger pulleys with flexion and extension. Ganglion cysts can also be intratendinous. In both cases it is important to view the tendon in motion to confirm that the lesion moves with the tendon ( e-Fig. 11-5 ![]() and
and ![]() and ).
and ).

Despite the fact that the curved, conjoined tendons of the rotator cuff are more difficult to image than straight tendons, the rotator cuff has received more attention than any other tendon. Perhaps this is because shoulder pain originating from rotator cuff disease is very common and because rotator cuff tears are difficult to diagnose and quantify clinically. Because rotator cuff sonography is among the most commonly performed musculoskeletal examinations, it is important to be familiar with its normal sonographic appearance. All four of the cuff tendons (i.e., subscapularis, supraspinatus, infraspinatus, and teres minor) appear as a band of tissue covering the humeral head. The anatomy can be thought of as a series of layers. From deep to superficial, the layers are the echogenic humeral head, the anechoic or hypoechoic articular cartilage, the relatively echogenic rotator cuff, the hypoechoic subdeltoid bursa, the hyperechoic peribursal fat, the hypoechoic deltoid muscle, and finally the subcutaneous tissues ( Fig. 11-8A ). Important normal aspects of the cuff are its outer convex contour (see Fig. 11-8B ) and the lack of compressibility with transducer pressure.

Full-thickness rotator cuff tears refer to tears that extend from the deep surface of the cuff to the superficial surface of the cuff. They may be small and only involve a tiny region of a single tendon or they may be large and involve multiple tendons. A majority of tears originate at the insertion of the cuff to the greater tuberosity near the supraspinatus/infraspinatus junction. From this point, they may extend to involve more of the supraspinatus and infraspinatus. The subscapularis tendon may also be involved with massive full-thickness rotator cuff tears. However, it is rare to have an isolated tear of the subscapularis tendon in the absence of a prior anterior shoulder dislocation or a dislocated biceps tendon, or following total shoulder arthroplasty. The teres minor is almost never involved.
The sonographic appearance of full-thickness rotator cuff tears depends on whether there is a significant amount of fluid in the joint ( Box 11-2 ). When fluid is present, the tear appears as a fluid-filled defect ( Fig. 11-9A and B ). This type of tear is very easy to identify and the appearance is easy to understand. When the defect is not filled with fluid, the overlying subdeltoid bursa and peribursal fat drop into the defect. This converts the normal convex interface between the deltoid and the cuff into a concave interface (see Fig. 11-9C and D ). In most cases this concavity is readily visible at rest. If the torn ends of the tendon have not retracted from each other, or if the defect is filled with hypertrophied synovial tissue, a concavity may not be visible at rest. In such a case compression of the shoulder with the transducer can push the bursa and peribursal fat into the defect while producing some separation of the tendon ends. As mentioned earlier, the normal rotator cuff does not compress at all. Massive tears with extensive retraction of the torn tendon produce an uncovered humeral head and no visible cuff on standard images (see Fig. 11-9E and F ). This is referred to as nonvisualization of the cuff.
Anechoic or hypoechoic defect
Focal superficial contour abnormality
Compressibility
Nonvisualization

Once a full-thickness tear has been identified, it is important to determine which tendons are involved. If the tear just involves the first 1.5 cm of cuff behind the biceps tendon, then it is isolated to the supraspinatus. If it extends to involve the cuff more than 1.5 cm behind the biceps, then the infraspinatus is also involved. These measurements are made on the short axis (transverse) views. The degree of retraction of the cuff from the greater tuberosity is measured on the long axis (longitudinal) view.
Partial-thickness tears refer to tears that do not extend all the way from the deep to the superficial surface of the cuff. They can involve the deep surface, the superficial surface, or the internal aspect of the cuff. However, the majority arise from the deep surface. The sonographic appearance of a partial-thickness tear consists of a hypoechoic defect that remains visible despite changes in the orientation of the transducer. In many cases there is also a bright reflector associated with the hypoechoic area ( Fig. 11-10 ). As with full-thickness tears, the underlying bony cortex is usually irregular. Unlike full-thickness tears, partial-thickness tears are not associated with contour changes and do not compress with transducer pressure unless they are very extensive. In this situation it can be difficult to distinguish them from a nonretracted full-thickness tear ( e-Fig. 11-6 ![]() and
and ![]() ). Both are usually treated with surgery, and therefore the distinction is not critical. Partial tears may also be associated with abnormal internal motion when the transducer is rocked back and forth in the longitudinal plane (
). Both are usually treated with surgery, and therefore the distinction is not critical. Partial tears may also be associated with abnormal internal motion when the transducer is rocked back and forth in the longitudinal plane ( ![]() ). Partial-thickness tears must be distinguished from tendon anisotropy, which normally causes the deep surface of the supraspinatus insertion to appear hypoechoic. Tendon anisotropy usually will become more echogenic when the transducer is angled upward, whereas partial tears will not change. Tendon anisotropy is usually poorly marginated, whereas partial tears are better marginated. Finally, tendon anisotropy is usually entirely hypoechoic, whereas partial tears often have at least a small hyperechoic component.
). Partial-thickness tears must be distinguished from tendon anisotropy, which normally causes the deep surface of the supraspinatus insertion to appear hypoechoic. Tendon anisotropy usually will become more echogenic when the transducer is angled upward, whereas partial tears will not change. Tendon anisotropy is usually poorly marginated, whereas partial tears are better marginated. Finally, tendon anisotropy is usually entirely hypoechoic, whereas partial tears often have at least a small hyperechoic component.


The sensitivity of sonography for full-thickness tears is approximately 95%. The sensitivity of sonography for partial-thickness tears is approximately 70% to 90%. Many studies, including well-controlled, double-blind comparisons of ultrasound and magnetic resonance imaging (MRI) using surgery as the gold standard, have shown similar sensitivity for full- and partial-thickness tears.
In addition to tears, another relatively common painful abnormality of the rotator cuff is calcific tendonitis. Calcium in the rotator cuff produces an area of increased echogenicity and in most cases an associated acoustic shadow ( Fig. 11-11 ). Sonography is the most accurate means of identifying, localizing, and quantifying rotator cuff calcification. It can also be used to guide aspiration of calcific tendonitis. MRI is excellent at detecting most soft-tissue abnormalities in the shoulder, but as elsewhere in the body, it is poor at detecting calcification.

Muscles are composed of many fascicles that are separated by fibrous tissue called the perimysium . Muscle fascicles are very hypoechoic and this produces an overall appearance of decreased echogenicity to muscles. The perimysium creates interfaces between the fascicles that on longitudinal views appear as linear, echogenic reflections and on transverse views appear as diffuse speckles within a hypoechoic background ( Fig. 11-12 ).

Muscle injuries can be the result of direct compressive trauma or distraction from sudden forceful muscle contraction. Tears of the muscle are divided into three grades: Grade 1 tears consist of tears of only a limited number of muscle fibers; Grade 2 tears are more extensive partial tears usually associated with some functional weakness; and Grade 3 tears are complete disruptions of the entire muscle. On sonography, the severity of the lesion is mirrored by the size and extent of the hematoma. Grade 3 tears are also associated with some degree of muscle retraction, usually at the myotendinous junction. The imaging characteristics of hematomas have been described in previous chapters and are similar in muscles. In the acute stage, they are relatively echogenic and solid ( Fig. 11-13A ). Over a matter of days they generally start to liquefy and convert to a complex collection (see Fig. 11-13B ). In some cases calcification may occur (see Fig. 11-13C ). Ultimately most hematomas evolve into a simple-appearing fluid collection (see Fig. 11-13D ). When they are close to completely liquefied, they can be aspirated with ultrasound guidance and this can accelerate the overall recovery time and allow for competitive athletes to return to action earlier. Intramuscular hemorrhage may dissect in between fascicles and not form a discrete collection. This is also known as a contusion and will produce thickening and increased echogenicity of the intermuscular septa. A relatively common muscle tear occurs at the aponeurosis of the gastrocnemius and soleus muscles ( Fig. 11-14 ). This is sometimes referred to as tennis leg and it causes swelling of the calf that can be clinically confused with deep venous thrombosis (DVT).


Sonography is often the initial test used to evaluate suspected masses in the extremities. In the absence of trauma, intramuscular masses should be considered tumors until proven otherwise. Findings that increase the likelihood of malignancy include large size, lobulated margins ( Fig. 11-15A ), satellite nodules, and increased blood flow (see Fig. 11-15B and C ). Primary sarcomas are often complex and metastatic tumors are usually solid and homogeneous, but there is considerable overlap in their sonographic appearance ( Fig. 11-16 ). Both lesions should be distinguished from bone lesions with associated soft-tissue components. Sonography generally plays little role in the evaluation of muscular neoplasms. However, it is an excellent method for providing guidance for biopsy.


Muscle atrophy can also be detected and quantified sonographically. Comparison of the right and left sides is easy using dual-screen acquisition ( Fig. 11-17A ). Fatty infiltration is also detectable by noting increased muscle echogenicity. This is an important part of evaluation of the shoulder in patients with rotator cuff tears (see Fig. 11-17B ).

Several anatomic structures are common to many joints. Ligaments in general have a similar sonographic appearance to tendons ( Fig. 11-18 ). However, it is more difficult to image many ligaments at 90 degrees to their long axis, and therefore it is not uncommon for ligaments to appear hypoechoic. Articular cartilage is very homogeneous and thus produces very few internal echoes. It appears as a thin, smooth, hypoechoic to anechoic layer overlying the cortical bone ( Fig. 11-19A ). It should not be confused with fluid. Fibrocartilage structures such as the glenoid labrum and the menisci of the knee can also be at least partially visualized with ultrasound. Fibrocartilage has a more complex internal architecture than articular cartilage and appears more echogenic (see Fig. 11-19B ). Sonography is not a primary means of evaluating cartilage.


One common indication for sonography of joints is to detect and guide aspiration of joint effusions. The configuration of joint fluid varies depending on the joint being scanned. In most joints real-time scanning is valuable because effusions are often compressible and will often be accentuated by certain movements. Most reactive effusions are anechoic or have few internal echoes ( Fig. 11-20A ). Septic effusions, particularly those in the superficial joints, often have detectable internal echoes (see Fig. 11-20B ) and are occasionally hyperechoic. Lipohemarthroses related to trauma can appear as multiple fluid layers (see Fig. 11-20C ).

Ganglion cysts are mucin-filled lesions that can arise from any joint. They are the most common palpable mass in the hand and wrist. Approximately 70% occur on the dorsal surface of the wrist and most of these arise from the scapholunate joint ( Fig. 11-21A ). Approximately 20% arise from the volar surface of the wrist and dissect between the flexor carpi radialis tendon and the radial artery (see Fig. 11-21B ; e-Fig. 11-7 ![]() and
and ![]() ). Approximately 10% arise from the flexor tendon sheaths of the fingers (see Fig. 11-21C ;
). Approximately 10% arise from the flexor tendon sheaths of the fingers (see Fig. 11-21C ; ![]() ). In approximately 25% of cases a neck can be seen leading toward the joint of origin (see Fig. 11-21D ).
). In approximately 25% of cases a neck can be seen leading toward the joint of origin (see Fig. 11-21D ).


Synovitis appears as thickened, hypoechoic soft tissues overlying the joint ( Fig. 11-22A ). It may be focal or diffuse and smooth or nodular. Hypervascularity generally indicates acute inflammation (see Fig. 11-22B ). Clinical and laboratory correlation is usually required to determine the etiology of synovitis.

Normal bursas are not visible with sonography. Abnormal, fluid-filled bursas can be detected around many joints. They are particularly common around the knee. The most common is the bursa between the medial head of the gastrocnemius and the semimembranosus tendon. When distended by fluid, this is referred to as a Baker's cyst. They are best identified by scanning along the medial and superior aspect of the medial head of the gastrocnemius. Baker's cysts may be simple appearing or contain internal echoes, internal septations, thick irregular walls, nodular synovial proliferation, and loose bodies ( Fig. 11-23 ). The neck that extends between the medial gastrocnemius and the semimembranosus tendon produces a beaklike appearance that is a characteristic feature. Rupture may produce a pointed margin to the inferior aspect of the cyst or fluid tracking into the calf from the inferior aspect of the cyst (see Fig. 11-23C ).

Diagnosis of bursitis in other sites depends on a thorough knowledge of the anatomic location of different bursas. This is the primary way to distinguish a fluid-filled bursa from other periarticular fluid collections ( Fig. 11-24 ).

Peripheral nerves are composed of multiple internal neuronal fascicles that appear hypoechoic on high-resolution scans ( Fig. 11-25 ). On transverse views, internal nerve fascicles are roughly round and are surrounded by the hyperechoic epineurium, a loose connective tissue composed of collagen and adipose. Peripheral nerves can simulate the tendons, but their echogenicity is less than that of the tendons; in addition, their echotexture is more fascicular, whereas the echotexture of tendons is more fibrillar. The characteristics of normal nerves and the other extremity structures described previously are reviewed in Table 11-1 .

| Tendons | Echogenic when imaged at 90 degrees to sound, otherwise hypoechoic Fibrillar architecture |
| Ligaments | Similar to tendons |
| Muscles | Hypoechoic |
| Articular cartilage | Anechoic to hypoechoic |
| Fibrocartilage | Hyperechoic |
| Peripheral nerves | Hypoechoic Fascicular architecture |
Compression and entrapment of nerves are common clinical conditions. The most common of these is compression of the median nerve in the carpal tunnel. Sonography can assist in the diagnosis of carpal tunnel syndrome by identifying swelling of the nerve proximal to the tunnel ( Fig. 11-26 ). Injured and inflamed nerves are also detected by identifying focal swelling ( e-Fig. 11-8 ![]() ).
).


Masses and cysts of the peripheral nerves can be diagnosed with sonography if continuity with the nerve is identified. This is possible when the major nerves are involved ( Fig. 11-27 ). Tumors of small nerves appear as nonspecific masses and can only be diagnosed with surgical resection. Schwannomas are usually eccentric in the nerve and neurofibromas are usually central but there is considerable overlap. Most nerve tumors are solid, hypoechoic, and vascular ( Fig. 11-28A and B ). Through transmission is common, even with completely solid nerve tumors (see Fig. 11-28A ). Heterogeneity and cystic components become more common as the tumors enlarge (see Fig. 11-28C ).


The external cortical surface of superficial bones can be visualized well with sonography as a smooth, bright reflection. Therefore abnormalities that alter the bony surface can be detected sonographically. Although sonography is generally not used as a primary technique for imaging the bones, occult bone lesions are occasionally detected during the evaluation of the overlying soft tissues. Therefore it is important to observe the bones and recognize abnormalities when they are present.
Sonography can be a valuable aid in detecting occult fractures. Nondisplaced fractures can be difficult to detect radiologically, especially in the acute period. On sonography, the area that is painful can be imaged precisely and disruptions in the surface of the bone can be readily identified ( Fig. 11-29A ). Over time, callus will form and the surface of the bone will expand and become irregular (see Fig. 11-29B ). Pathological fractures can usually be distinguished from benign fractures by detecting bone destruction and an associated soft-tissue mass (see Fig. 11-29C ). Other uses of sonography in bone disease are the detection of erosions in patients with erosive arthritis ( Fig. 11-30 ), detection of subperiosteal abscess in osteomyelitis, and guidance of percutaneous biopsies and aspirations in patients with suspected metastases and abscesses.


Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here