Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Q24.1 What are the advantages of extracorporeal photopheresis (ECP) systems in which the psoralen is released directly into the treatment mix (vs oral administration)? (Pg. 272)
Q24.2 What are the potential cardiovascular and thromboembolic risks of ECP? (Pg. 272)
Q24.3 What are the proposed mechanisms by which ECP may benefit patients with cutaneous T-cell lymphoma (CTCL)? (Pg. 273)
Q24.4 What are proposed mechanisms by which ECP may benefit patients with graft-versus-host disease (GVHD) and autoimmune dermatoses such as scleroderma? (Pg. 273)
Q24.5 What are the components of a typical ECP regimen for CTCL patients? (Pg. 274)
Q24.6 What are four prognostic factors for a favorable outcome with ECP in patients with erythrodermic CTCL? (Pg. 274)
Q24.7 On flow cytometry of peripheral blood, which T-cell markers are most useful in monitoring the patient’s response to ECP? (Pg. 275)
Q24.8 What multiagent regimens that include ECP show promise for CTCL patients? (Pg. 275)
Q24.9 Aside from ECP for CTCL, what is the most promising additional dermatologic indication for ECP therapy? (Pg. 276)
Q24.10 Which organ systems affected by chronic GVHD (cGVHD) benefit most from ECP? (Pg. 276)
Q24.11 Overall, which subsets of scleroderma patients are most likely to respond to ECP? (Pg. 277)
8-Methoxypsoralen
Adverse effect
Antigen-presenting cell
American Society for Apheresis
Chronic graft-versus-host disease
Complete response
Corticosteroid
Cutaneous T-cell lymphoma
Cytotoxic T lymphocyte
Dendritic cell
Epidermolysis bullosa acquisita
Extracorporeal photopheresis
US Food and Drug Administration
Granulocyte–macrophage colony-stimulating factor
Graft-versus-host disease
Interferon
Narrow-band ultraviolet B
Natural killer
Oral lichen planus
Polymerase chain reaction
Positron emission tomography–computed tomography
Psoralen plus ultraviolet A
Retinoid X receptor
T-cell receptor
Tumor necrosis factor-α
Regulatory T cells
Ultraviolet A radiation
The authors wish to acknowledge Dr. Jaehyuk Choi and Dr. Peter W. Heald for their contributions to the previous edition of this chapter.
Extracorporeal photopheresis (ECP), also known as extracorporeal photochemotherapy and extracorporeal photoimmunotherapy, initially demonstrated a therapeutic impact on cutaneous T-cell lymphoma (CTCL) in the trial of monotherapy for erythrodermic disease in association with leukemic involvement (also known as Sézary syndrome). The US Food and Drug Administration (FDA) approved ECP for treatment of this condition in 1988. Since then, it has been applied in the management of several other immune-mediated cutaneous disorders in addition to CTCL. ECP for noncutaneous disease, such as organ transplant rejection, is not fully addressed in this chapter (see section on FDA-Approved Indications). ECP is regarded as an immunotherapy because of its well-documented effects on immune cells. In addition, this is supported by its therapeutic effects extending well beyond that expected by the deletion of the small portion of circulating leukocytes, which are isolated, photochemically altered, and then reinfused during the procedure.
Several factors influence the decision to initiate ECP therapy. ECP devices are available in over 150 centers worldwide. The newer Therakos Cellex Photopheresis System machine is currently the only version produced and supported for the palliative treatment of the skin manifestations of CTCL that are unresponsive to other forms of treatment. The Cellex has several advantages over the immediate predecessor device, the UVAR-XTS. The Cellex can be more readily and safely used in the treatment of lower body weight patients (<40 kg) and significantly shortens the treatment time by allowing double need access. Furthermore, the Cellex can use either discontinuous or continuous cycles and the continuous cycles lead to less fluid shifts (although requires dual intravenous access). For these reasons, the Cellex induces fewer patient-related complications such as hypotension and the need for red cell transfusions in pediatric patients. Because most ECP therapy protocols typically require treatment courses over months to years, geographic constraints may limit the availability of therapy. Most treatment centers perform ECP in an outpatient setting, and an individual treatment session typically 1.5 to 2 hours with the Cellex. Such centers require a highly skilled nursing staff trained to perform such treatments.
Previously, the ECP procedure was performed after ingestion of 8-methoxypsoralen (8-MOP). (For the pharmacology of 8-MOP, see Chapter 23 .) Q24.1 Currently, the ECP device automatically injects the appropriate amount of 8-MOP (UVADEX, Therakos, was approved by the FDA in 1999 for use with ECP) directly into the treatment mix before ultraviolet (UV) irradiation within the cassette. Because this approach results in a much smaller net systemic dose of 8-MOP than with previous oral administration, the potential for adverse effects (AE) of cutaneous photosensitivity after ECP treatment is substantially lower.
The ECP procedure ( Fig. 24.1 ) can be performed through a peripheral vein, preferably with a 16- to 18-gauge needle, which may limit therapy to those patients with accessible veins. The placement of a catheter, such as a double-lumen hemodialysis catheter, for treatment purposes should be considered temporary, for example, of the order of approximately 3 months, and weighed against the increased risk of infection associated with such lines. Specialized subcutaneous ports (e.g., Vortex AngioDynamics) may also be used. Q24.2 After venous access is established, the patient undergoes discontinuous or continuous pheresis cycles to harvest the leukocyte-rich buffy coat. Because of the time-lag between extraction and reinfusion, these cycles may temporarily deplete the patient of 200–400 mL of intravenous volume. Thus, if the patient’s cardiovascular system cannot tolerate such a rapid volume depletion, ECP is contraindicated. The separated leukocytes are held in a sterile bag, from which they are pumped through a plate and exposed to ultraviolet A (UVA) radiation. At the completion of the treatment cycle the reinfusion phase leaves the patient at a net gain of approximately 500 mL of fluid. In patients with mild intolerance of the fluid loading, diuretics can be simultaneously administered to avoid hypervolemia. However, the newer Cellex can use a continuous flow system, in which the blood is simultaneously returned with extraction, thereby processing less extracorporeal volume; in a study of Cellex system in patients with CTCL, extracorporeal volumes were 216 mL and 266 mL for double-needle and single-needle treatments, respectively.
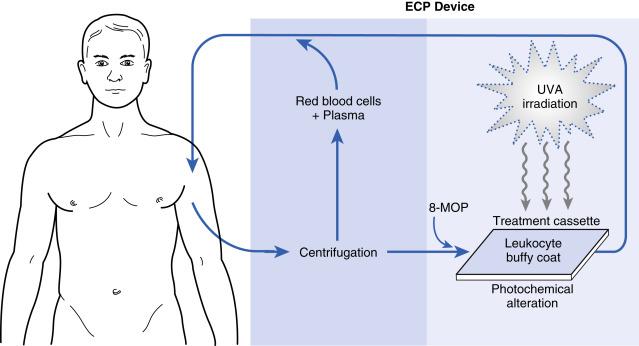
Heparin is a typical anticoagulant for ECP and for patients who cannot tolerate heparin, such as those with a history of heparin-induced thrombocytopenia and those with high risk of bleeding, acid citrate dextrose can be used.
For a complete discussion on the pharmacology of 8-MOP, see Chapter 23 .
Although the precise mechanism of ECP has yet to be fully elucidated, crucial components of ECP have been identified from clinical studies and laboratory models ( Box 24.1 ). Mechanisms of action have been best studied for ECP in the treatment of CTCL, which likely have some relevance for therapeutic responses observed in the T-cell-mediated autoimmune diseases (such as scleroderma) for which ECP has also been used. ECP has also been used with some success in graft-versus-host disease (GVHD) and solid organ transplant rejection. In these conditions, ECP is thought to cause immune tolerance through induction of regulatory T cells (Treg).
Dendritic cell differentiation followed by internalization and presentation of tumor antigens
Stimulation of anti-T-cell (tumor cell) immune responses
Induction of apoptosis of activated (autoreactive) T cells
Induction of immunoregulatory cytokine shifts
Q24.3 An immunological mechanism for ECP has been hypothesized. In support of this, there were clinically significant persistent responses in CTCL patients in the original phase I/II study, despite the fact that only about 5% of the body’s circulating malignant T cells were extracorporeally exposed to activated 8-MOP/UVA. Furthermore, the effects of ECP on circulating leukocytes appears to selectively target lymphoma cells. CTCL patients treated with ECP maintain their absolute number of normal T cells with a disproportionately greater decrease in their total body burden of malignant T cells. Completely responding patients selectively exhibit loss of all detectable signs of malignant cells. Lastly, response to ECP is improved in individuals with normal or near normal natural killer (NK) and CD8+ cytotoxic T lymphocyte (CTL) numbers and function. Taken together, these findings suggest that a potential mechanism of action of ECP may be the stimulation of NK and CTL-mediated antitumor responses.
As a possible explanation for its immunological effects, treatment with photopheresis induces large numbers of monocytes to express dendritic cell (DC) differentiation markers by flow cytometry as well as by deoxyribonucleic acid (DNA) microarray analysis. Furthermore, these induced DC are functional, with the capacity in vitro to stimulate allogeneic CD4+ T cells to proliferate as well as differentiate CD8+ T cells into cytotoxic cells. During ECP, isolated leukocytes are passaged through the exposure plate that is coated with the patient’s plasma proteins and platelets. Monocytes transiently adhering to the surface are activated and a portion of these will undergo monocyte-to-dendritic cell maturation. Simultaneously, lymphocytes from CTCL patients undergo programmed cell death (i.e., apoptosis) during ECP caused by the exposure to UVA in the presence of 8-MOP. ECP-generated DC therefore have the ability and the opportunity to ingest the pathogenic apoptotic T cells, ultimately leading to the potential production and reinfusion of putative T-cell-loaded antigen-presenting cells (APC) capable of stimulating immunity against CTCL cells. The recent development and study of a miniature ECP device in a mouse tumor model demonstrated that monocyte-to-dendritic cell differentiation and the resulting tumor-loaded DC were important for the selective antitumor effects of ECP.
Alternative mechanisms reviewed elsewhere emphasize that the primary effect of ECP is to induce apoptosis in the target pathogenic cell, such as the circulating CTCL malignant cells, or to induce cytokine shifts from increased T-helper type (Th) 2 cytokines (e.g., interleukin [IL]-4, IL-5, IL-13, IL-31) and T-regulatory cytokines (e.g., TGFβ), to increased Th1 cytokines that favor an antitumor milieu including interferon (IFN)-γ.
Q24.4 The reinfusion of syngeneic apoptotic cells by photopheresis appears to induce tolerogenic effects, which may explain the utility of ECP in autoimmune disease, GVHD, and organ transplant rejection. Based on animal models and clinical experience, this effect appears to be orchestrated by DC and Treg as well as immunomodulatory cytokines. In a mouse model of contact hypersensitivity, the extracorporeal treatment of splenocytes and lymph node cells led to the induction of tolerance mediated by CD4+ CD25+ T cells, the putative regulatory T-cell population, in an antigen-specific manner. Underscoring the importance of DC, the tolerance was lost with depletion of CD11c+ cells during reinfusion. Similar tolerizing effects of ECP were seen in mouse models of lupus-like GVHD and skin transplant. In line with this, the use of prophylactic ECP in lethal murine GVHD models resulted in immune tolerance in the host, as measured by reduced DC activation and increased Treg cells. How and why ECP seems to induce stimulatory DC, such as in the setting of CTCL, and inhibitory DC, such as in the setting of chronic GVHD (cGVHD), remain unclear.
Studies performed in human patients further supports the importance of Treg cells to the success of ECP in treating these diseases. In patients with lung transplantation, graft survival after ECP was correlated with increasing levels of circulating CD4+ CD25+ cells induced by ECP. In addition, in patients with cGVHD, a clinically significant cutaneous response was seen exclusively in patients who developed clonal populations of alloreactive T lymphocytes; because these cells expanded in the absence of clinical disease, these cells presumably represent Treg. Similar studies have shown normalization of inverted ratios of CD4 to CD8 cells after ECP treatment, suggesting a reduction in cytotoxic effector cells and potentially the expansion of the CD4+ regulatory T-cell component.
In addition, several studies have demonstrated that ECP has the ability to influence leukocyte cytokine production, including the production of tumor necrosis factor-α (TNF-α) and IL-6 after ECP along with increased IFN-γ and decreased IL-4 from peripheral blood mononuclear cells. Such shifts in T-helper cytokine profiles may have a definite role in the pathogenesis of autoimmune diseases, and therefore may partially explain the beneficial responses observed in patients treated for autoimmune disease with ECP.
Studies show favorable results of ECP in erythrodermic CTCL. The United States Cutaneous Lymphoma Consortium (USCLC) recommends ECP as a first-line monotherapy in Sézary syndrome, with IFN-α, bexarotene, and/or methotrexate being the alternative or combination options. A recent consensus statement from the UK Photopheresis Society recommends ECP for all patients with erythrodermic CTCL (stage III or IVA) and with one or more of the following: peripheral blood T-cell clone, 10% or more circulating Sézary cells, or CD4:CD8 ratio >10. Q24.5 The current regimens for using ECP ( Box 24.2 ) reflect the schedules used in the initial multicenter clinical trials. The first version of the leukocyte photoactivating device took an entire day to treat a patient, and treatments were given on 2 consecutive day cycles, which were repeated at 4-week intervals. Based on the success of this schedule, most patients have subsequently followed the 2-day, every 4-week regimen. There have been modifications in the scheduling of ECP treatments that have also met with success. Bowen and colleagues reported using an every-2-week regimen at the initiation of therapy. The CTCL patient in that report had hyperleukocytic disease, and after the leukemia improved, the patient was put on a 4-week cycle. Several other centers have used this accelerated delivery schedule at the initiation of therapy. The results of the initial clinical trials and follow-up studies showed that as monotherapy, ECP can induce a remission in approximately one-fourth of treated patients. One-half of the patients with erythrodermic CTCL had a significant partial response and the remaining one-fourth had no response or progressive disease. A more recent review of the literature demonstrated that this same proportion of responses was observed in the smaller series of CTCL patients as reported by Zic and colleagues, and Armus and colleagues. Duration of response should also be noted. A recent retrospective study evaluating long-term response in patients in the original clinical trial in 1987 showed duration of response of 14 and 9 months for partial and near-complete response (CR), respectively, after initiating ECP. Q24.6 Good prognostic factors include (1) absence of tumor-stage skin lesions, (2) short disease course (<2 years), (3) absence of significant internal organ involvement or bulky lymphadenopathy, and (4) minimal pretreatment with chemotherapy.
Cutaneous T-cell lymphoma (American Society for Apheresis category I)
Scleroderma (category III)
Pemphigus vulgaris (category III)
Pemphigus foliaceous (no recommendations)
Epidermolysis bullosa acquisita (no recommendations)
Acute GVHD (category II)
Chronic GVHD (category II)
Prevention of GVHD
Atopic dermatitis (category III)
Nephrogenic systemic fibrosis (category III)
Oral erosive lichen planus (no recommendations)
History idiosyncratic reactions to psoralen compounds
Pregnancy and lactation
Severe cardiac disease
Poor venous access (may need central line)
Rapidly progressing disease (such as ‘tumor eruptive CTCL’)
Hematocrit <25% (should be transfused with red blood cell to hematocrit of at least 29%)
Platelets <20 000/uL
Diastolic blood pressure <70 mm Hg (may need saline infusion and/or red blood cell transfusion)
Congestive heart failure
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here