Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
“The eye is the light of the body; therefore, if the eye is good, then the whole body will be full of light, but if the eye is bad, then the whole body will be full of darkness.”
Clinicians should develop the ability to recognize the signs and symptoms of serious eye diseases or complications of diseases early in their course to prevent visual loss and, in some cases, preserve life. For example, early detection and treatment of congenital cataracts and congenital glaucoma are critical for visual rehabilitation. Malignant orbital tumors and intraocular tumors such as retinoblastoma are life threatening.
Ocular findings can also assist in the diagnosis of a systemic illness such as type 1 neurofibromatosis (NF1) and CHARGE syndrome ( c oloboma, h eart anomaly, a tresia choanae, r etardation, g enital anomaly, e ar anomaly).
The visual system is not completely developed at birth but progressively matures during the neonatal period. The neonatologist must recognize normal ocular findings during different stages of the infant's growth to understand what is abnormal. Some ocular and visual milestones are shown in Table 95.1 .
| Description | Age |
|---|---|
| Pupillary light reaction present | 30 weeks' gestation |
| Pupillary light reaction well developed | 1 month |
| Lid closure in response to bright light | 30 weeks' gestation |
| Blink response to visual threat | 2-5 months |
| Visual fixation present | Birth |
| Fixation well developed | 2 months |
| Visual following well developed | 3 months |
| Accommodation well developed | 4 months |
| Visual evoked potential acuity at adult level | 6 months |
| Grating acuity preferential looking at adult level | 2 years |
| Snellen letter acuity at adult level | 2 years |
| Color vision present | 2 months |
| Color vision at adult level | 6 months |
| Stereopsis developed | 6 months |
| Stereoacuity at adult level | 7 years |
| End of critical period for monocular visual deprivation | 10 years |
| Conjugate horizontal gaze well developed | Birth |
| Conjugate vertical gaze well developed | 2 months |
| Vestibular (doll's eye) rotations well developed | 34 weeks' gestation |
| Optokinetic nystagmus well developed | Birth |
| Ocular alignment stable | 4 months |
| Fusional convergence well developed | 6 months |
| Eyeball 70% of adult diameter | Birth |
| Eyeball 95% of adult diameter | 3 years |
| Cornea 80% of adult diameter | Birth |
| Cornea 95% of adult diameter | 1 year |
| Differentiation of fovea completed | 4 months |
| Myelination of optic nerve completed | 7 months-2 years |
| Iris stromal pigmentation well developed | 6 months |
A screening eye examination should be performed during the newborn physical examination and during routine well-baby checkups. The screening examination should include the assessments listed in Box 95.1 . Fortunately, the screening examination identifies most problems such as glaucoma, cataract, infection, or tumor. However, if abnormalities are suspected because of the history, presence of systemic anomalies, or abnormal result of the screening examination, a more detailed ocular evaluation is mandatory, preferably by a pediatric ophthalmologist.
Reaction to light or visual stimuli to estimate visual function
Craniofacial dysmorphism
Orbits
Eyelids
Lashes
Ocular motility
Globes
Conjunctiva
Sclera
Cornea
Iris
Pupils
Red reflex test
A screening ocular examination begins with a careful history, especially of ocular diseases in the family. For example, a family history of retinoblastoma necessitates a comprehensive ocular examination of the fundus shortly after birth. Information about maternal diseases (e.g., rubella), injuries, medications, or use of drugs or alcohol during the prenatal period should be obtained. It is also important to document the duration and abnormalities of pregnancy, labor, and delivery. Premature birth suggests potential retinopathy of prematurity (ROP), and difficult delivery with obstetric forceps can result in direct ocular trauma.
The examination should be done under comfortable circumstances and with the proper equipment ( Fig. 95.1 ). Much can be learned if the examiner takes a few moments initially to observe the infant for facial anomalies, the external ocular appearance, and ocular motility while taking the history. The examination is most easily performed with the baby in a parent's arms; the more difficult parts of an examination often can be accomplished while a baby is nursing or sucking on a bottle or pacifier. It is well documented that an oral sucrose solution can calm the baby significantly for them to allow easier examination. The screening eye examination should include an evaluation of visual function, preferably one eye at a time. For infants less than 4-6 weeks of age, visual function is assessed by withdrawal or blinking to light, or pupil constriction to light.
![Fig. 95.1, Basic equipment for ocular examination: Various types of eyelid specula to keep the eyelids open, scleral depressor to gently move the eye around, cyclomydril eye drops for dilation (1.25% phenylephrine [ophthalmic], 0.25% cyclopentolate [ophthalmic]), and a 20 or 28 diopter magnifying lens. Fig. 95.1, Basic equipment for ocular examination: Various types of eyelid specula to keep the eyelids open, scleral depressor to gently move the eye around, cyclomydril eye drops for dilation (1.25% phenylephrine [ophthalmic], 0.25% cyclopentolate [ophthalmic]), and a 20 or 28 diopter magnifying lens.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ExaminationandCommonProblemsintheNeonatalEye/0_3s20B978032356711400095X.jpg)
Maintenance of the eyes on an object is called fixation. The infant's ability to fixate and follow a target can be an approximate guide to the amount of visual function present. Beyond 4-6 weeks of age, visual function is assessed in terms of the quality of the fixation and following. By experience, the clinician must develop an age-appropriate scale that assesses this quality of fixation and following, paying attention to the intensiveness, steadiness, and maintenance of the fixation and the smoothness and duration of the following.
Visual acuity refers to the subjective response from the patient of the ability to discern images of set sizes, such as the tumbling “E” or Snellen letters. Most normally developing children can participate in some form of visual acuity testing by the age of 30 months. If additional information regarding visual function is desired, some ancillary visual tests are available and used in indicated situations. (1) Optokinetic nystagmus describes a reflex ocular response to a moving target. As a target moves across the visual field, a pursuit motion occurs, followed by a rapid return motion in the opposite direction to regain fixation. We experience this response when telephone poles or fence posts are watched from a fast-moving vehicle. With an optokinetic drum, which consists of black and white stripes on a spinning cylinder, if the examiner can elicit optokinetic nystagmus in the infant, this establishes that the child has enough visual function to discern the stripes. The stripe widths can be calibrated so as to yield a visual acuity equivalent. Optokinetic nystagmus can be evident in term newborns. (2) Another quantitative technique to measure a visual acuity equivalent in the infant older than 3 months is forced preferential looking. An infant prefers to look at black and white stripes instead of a uniformly gray target. For the test, infants are quickly shown a card that has stripes on one end and a gray target on the opposite end. The examiner watches the infant's eyes to see whether the baby looks right or left to find the stripes. If the infant's visual function is high enough to distinguish the stripes from the gray target, the infant will look consistently toward the stripes. If the infant's visual function is less than the ability to distinguish the stripes from the gray target, the infant will look randomly right or left. By varying the size of the stripes, the examiner can grade the visual acuity equivalent. The disadvantages of this procedure include the number of people necessary to administer the test, the time involved, and the need for the cooperation of an alert infant. (3) Unfortunately, most estimations of an acuity equivalent in the infant rely heavily on adequate motor responses as part of the visual evaluation. Immature or underdeveloped motor systems can reduce or interfere with eye or head movements and decrease the estimation of the visual acuity equivalent. Visual evoked potentials, which measure the electrical cortical responses to a visual stimulus, eliminate the need for patient cooperation or motor control. Electrodes are placed over the occipital cortex to monitor activity in the brain as the eyes are visually stimulated with graded stripes or checkerboard patterns, and a computer-averaged tracing is made.
Continuing with the screening eye examination, the general facial configuration and the structure of the orbits are inspected next. Note any facial dysmorphism that could affect ocular health or be part of an ocular syndrome, such as clefts or abnormal head shape. The orbits should be proportional and symmetric compared with the overall craniofacial configuration. Palpation is performed to examine the orbital margin, the contents of the upper and lower lids, and the round contour of the globes. The orbital rims should be sharply outlined. In the newborn infant, the rims are initially round and increase in vertical diameter with normal growth. The area of the lacrimal sac is palpated for abnormal masses or increased size by pressing the sac against the bones of the nose and medial orbital wall. Mucopurulent material expressed from the lacrimal puncta is a symptom of obstruction of the nasolacrimal system. The eyelids are examined grossly and are compared for symmetry of horizontal and vertical placement. Spontaneous opening and closing of the lids should be observed. The lid margins should be inspected for regularity of contour, apposition to the globe, and the presence of lacrimal puncta. The punctum, the proximal opening into the nasolacrimal drainage system, is a minute hole in each lid margin a short distance from the inner corner of the eye. A rapid up-and-down movement of the lid during nursing indicates a jaw-winking phenomenon ( Fig. 95.2 ).
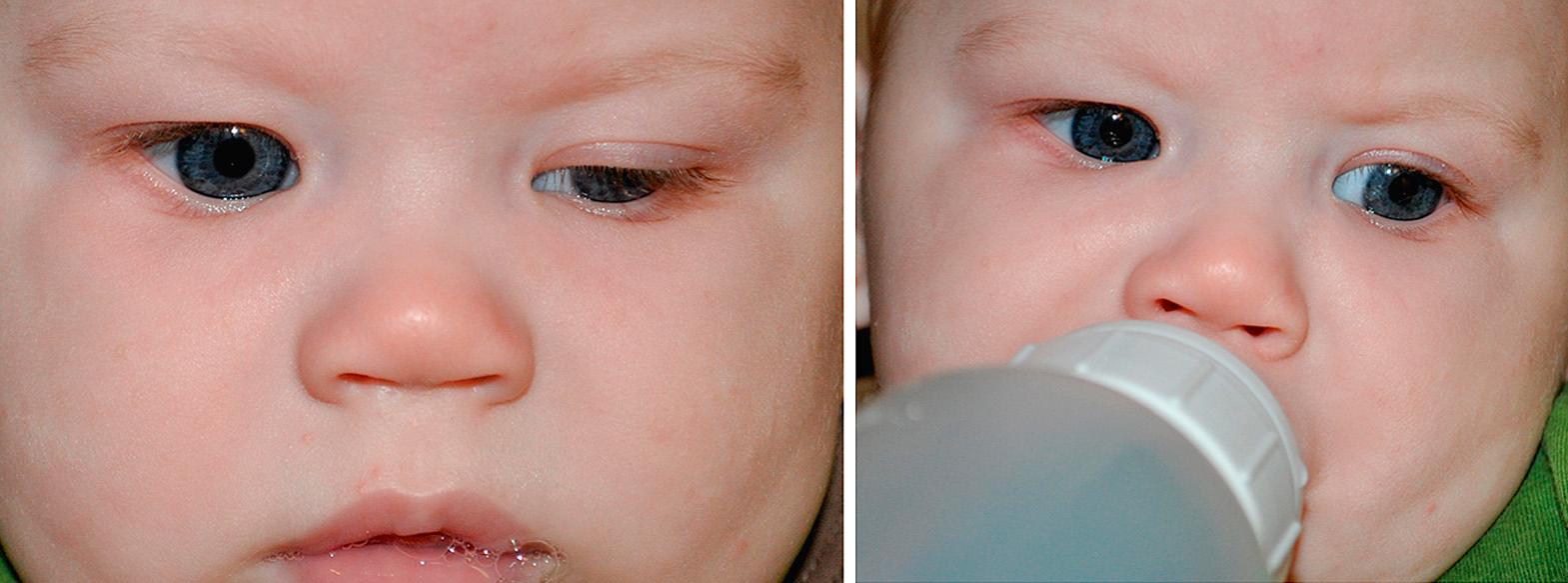
The examiner should view the lashes, which normally are directed outward in an orderly row. Abnormalities include distichiasis and trichiasis and are discussed later in this chapter. Lid position abnormalities should be checked (i.e., epiblepharon).
The ocular motor system is evaluated with a motility examination. This includes ocular alignment, conjugate ocular movements, and range of movement. Infants younger than 4 months may show small physiologic misalignments of the eyes. Misaligned eyes beyond the age of 4 months should be considered pathologic.
Alignment is tested with the corneal reflex test (Hirschberg test). The examiner shines a well-focused light at the patient's eyes and notes the spot on the cornea where the light is reflected back. If the patient is properly fixating, the light reflex should appear in the same location on each cornea, slightly nasal to the anatomic center of the cornea. If the light reflex is centered in one eye and deviated laterally in the fellow eye, esotropia is present. If the light reflex is centered in one eye and deviated nasally in the fellow eye, exotropia is present.
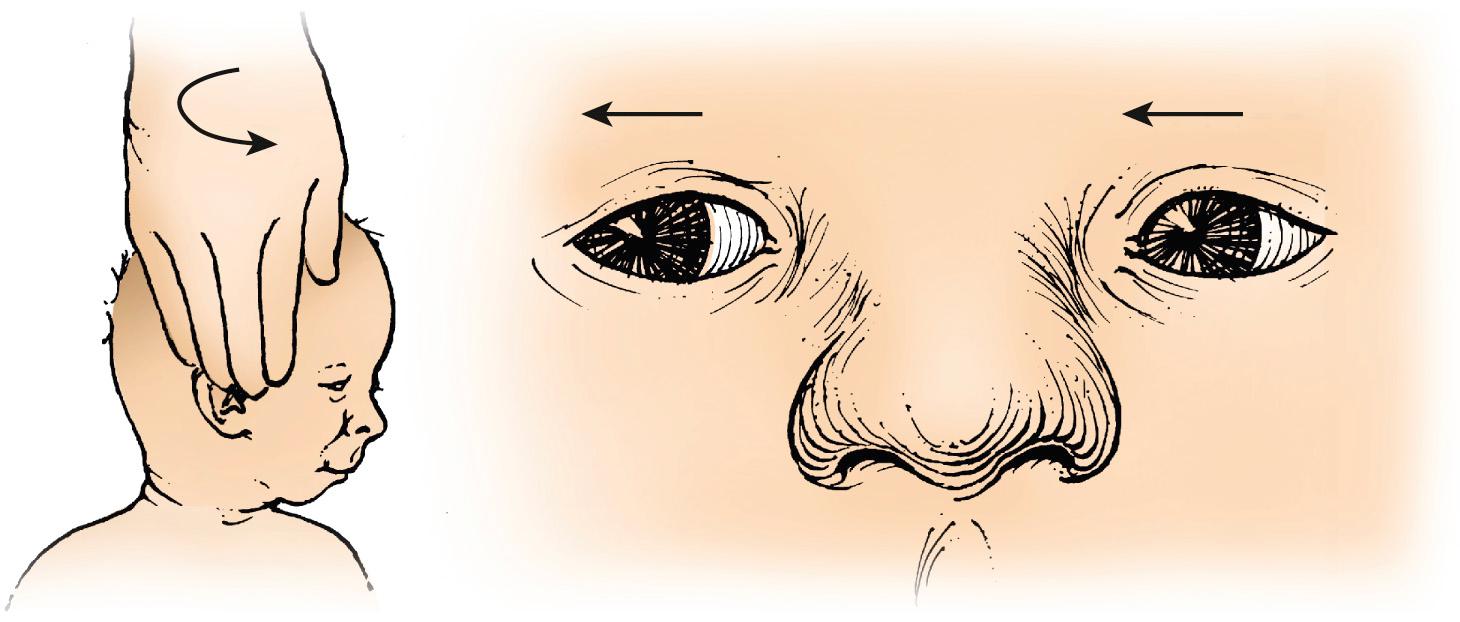
A positive cover-uncover test will support the impression of strabismus. If the patient does not appear to move the eyes well enough into the periphery, either spontaneously or by following an object, these movements can be driven by the doll's head maneuver (vestibulo-ocular reflex [VOR]). The reflex is tested by turning the infant's head to one shoulder, producing an opposite movement of the eyes ( Fig. 95.3 ). The eyes appear stationary as the head turns. This reflex may be reduced in severely brain damaged infants but normal in blind infants.
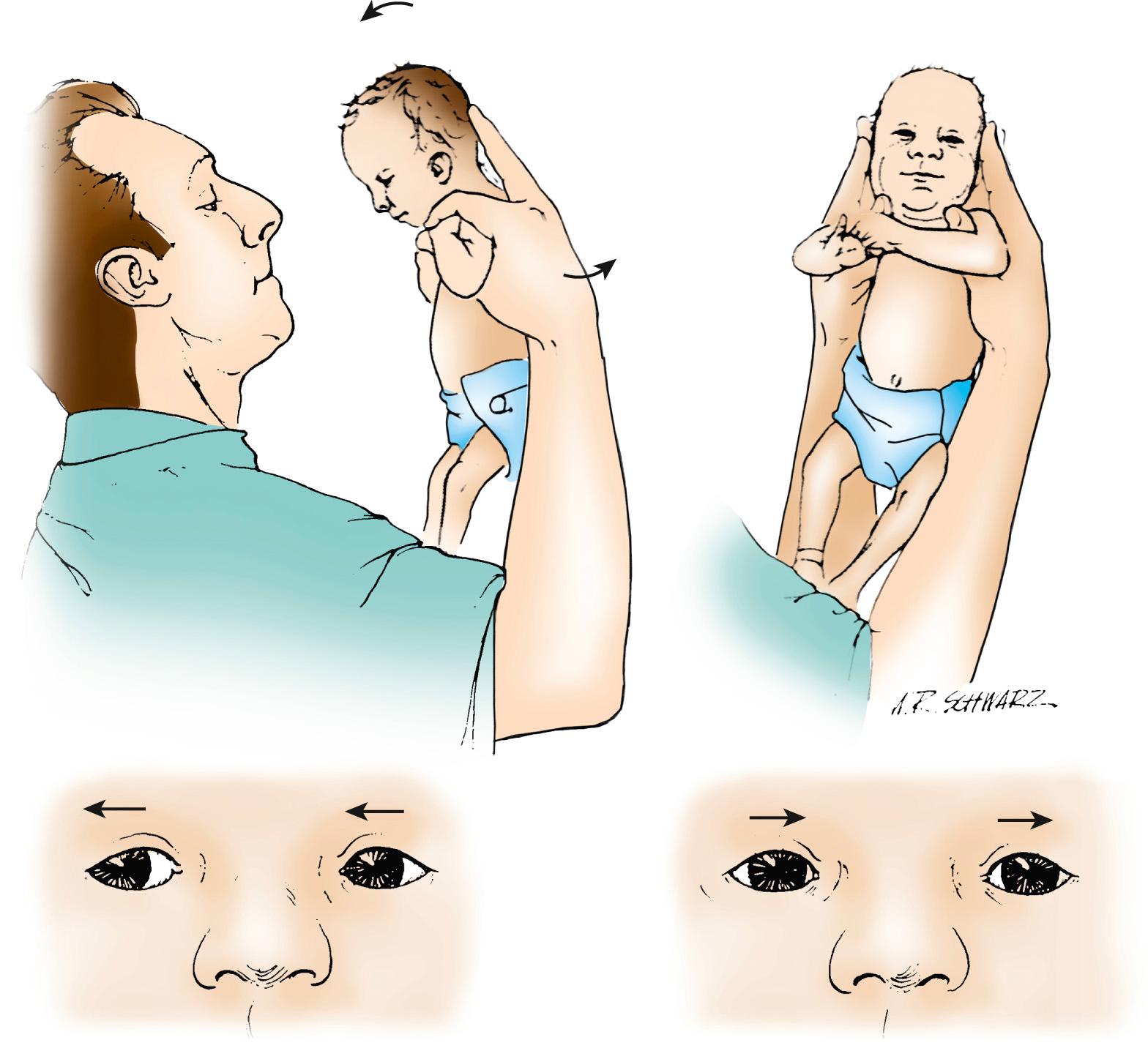
Another rotational reflex is elicited by holding the infant vertically and rotating the infant in an arc around the examiner ( Fig. 95.4 ). The infant's eyes will then tonically rotate in the direction of the spin, with short quick movements (saccades) in the direction opposite the spin. This ocular reflex may be a form of optokinetic nystagmus and is reduced in infants with major defects of the vestibular system, lower motor pathways to the extraocular muscles, visual system, or central nervous system.
Examination of the globes may be difficult owing to the inability of the examiner to open the neonates’ eyelids sufficiently. Various pediatric eye speculums, such as an Alfonso speculum (Bausch and Lomb, STORZ, San Dimas, CA), can be used to maintain the lids open and allow for adequate inspection of the ocular surface, anterior segment, or posterior segment. Before placing the eye speculum, topical ocular anesthesia can be achieved with a drop of tetracaine 0.5% eye drops. Oral sucrose can also relieve some of the discomfort of an ocular speculum. The conjunctiva is inspected after the lids are separated, with the use of a pediatric ocular speculum or fingers. The bulbar and palpebral conjunctivae are normally moist and pinkish. Redness or exudate is abnormal and can indicate infection. The conjunctivae of the lids overlying the tarsal plates should be examined after the lids are everted. Eversion is usually simple to perform with the examiner's fingers, particularly if the infant is attempting to squeeze the lids shut. The normally white sclera is evaluated for changes of color. A bluish coloration, however, is present in premature infants and in other small babies because of their very thin sclera.
The cornea is inspected with a penlight, paying attention to corneal size, shape, clarity, and luster. Magnification with loupes or an ophthalmoscope with the 20-diopter lens in place may be used. During the first few days of life, premature and term infants might demonstrate a slightly hazy cornea, which is thought to be the result of corneal edema. Thereafter, the surface of the cornea should have good luster and be absolutely transparent even to the extreme periphery. Any opacity or translucency is abnormal after the first few days of life, and referral to an ophthalmologist is indicated. Changes in transparency or opacification in the peripheral cornea may be associated with a local mesenchymal abnormality or glaucoma ( Fig. 95.5 ).
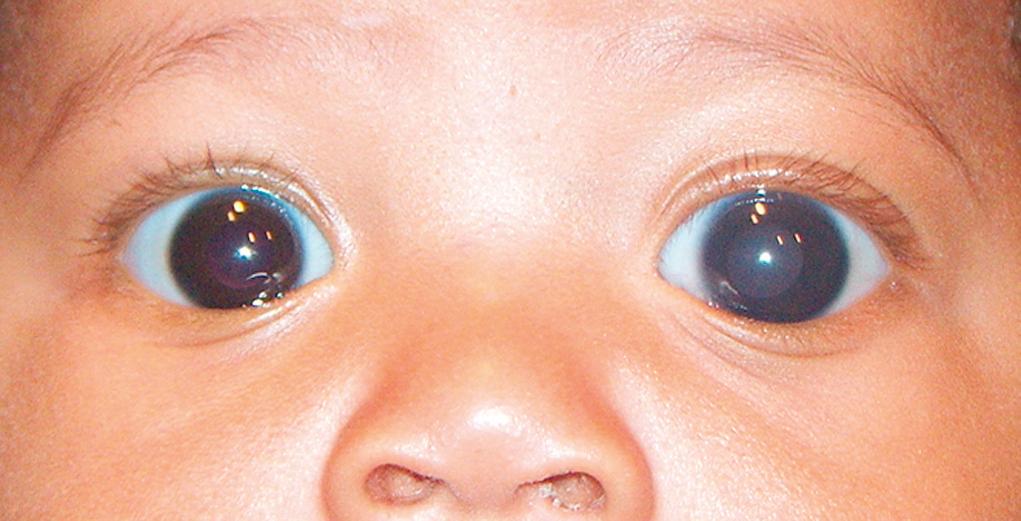
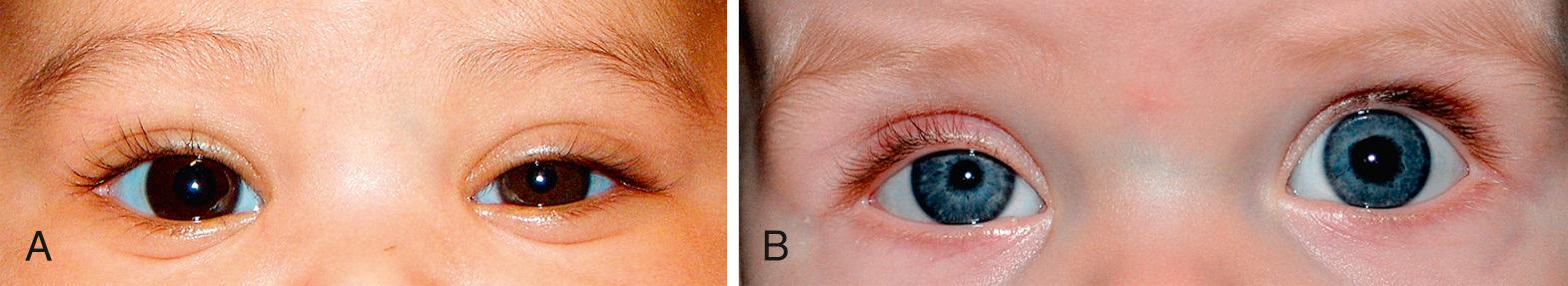
The irides are usually similar in appearance. Although normally incomplete for the first 6 months of life, pigmentation of both irides develop simultaneously. In a normal infant, the iris is often blue or blue-gray for the first few weeks or months of life. However, darkly pigmented babies can show pigmentation at birth or within the first week. Heterochromia (dissimilarity in pigmentation between the two eyes or within one eye) can indicate a normal hereditary pattern, congenital Horner syndrome ( Fig. 95.6 A, B ), or one of several syndromes (e.g., Waardenburg) discussed later in this chapter. These syndromes might not become apparent until the end of the neonatal period or even later in life when the iris is fully pigmented.
The pupils should be central, round, and equal in diameter. The pupillary space should be uniformly black. Any amount of a white reflection is abnormal and could indicate an abnormality within the lens, vitreous, or retina. The neonate's pupils are typically miotic in ambient light, perhaps related to prolonged sleep. A penlight or a transilluminator is used to shine a light at each pupil. Beyond a corrected gestational age of 30 weeks, pupils should constrict to both direct and contralateral stimulation. First, the reaction of the illuminated pupil is observed. It should constrict briskly (although the response in the neonate may be slower than in an older child) and should remain constricted as long as the illumination is maintained. If a poor response is observed, the contralateral pupil's reaction is studied. If the contralateral pupil constricts, the directly illuminated eye must have intact photoreceptors and optic nerve pathways. Failure of constriction in the directly illuminated eye in this instance could result from abnormalities in the iris. If neither pupil constricts on direct illumination to one eye, the first eye may be severely deficient in vision. The swinging flashlight test is used to check for a relative afferent pupillary defect. Normally, if the light is quickly shifted from one eye to the other, the newly illuminated eye's pupil should show an initial small constriction movement. If illumination is maintained on the eye, small rhythmic constriction and dilation movements may follow—called hippus, a normal phenomenon. If, however, the shift of light is followed by dilation of the newly stimulated eye, a Marcus Gunn (or relative afferent pupillary defect) is present. This result indicates decreased vision in the eye that inappropriately dilated. Pupillary reflexes should be completely normal in patients with central (cortical) visual impairment.
The red reflex test is an essential part of the infant screening eye examination. Examination starts with a “0” diopter setting in the direct ophthalmoscope, and the child is examined at arm's length, with both pupils illuminated ( Fig. 95.7 ). The red reflex of each eye should be clearly distinct, with no shadows or alterations. Look for any asymmetry of the intensity of the red reflex within each pupil and between the two pupils. If a red reflex cannot be seen, the pupils can be dilated with cyclomydril eye drops (Alcon Laboratories), and if a clear and equal red reflex is still not seen, the baby should be referred to an ophthalmologist. Precise viewing of the anterior chamber and crystalline lens requires a slit-lamp examination, instrumentation that is usually not available to the neonatologist or primary care physician. An indirect assessment of the clarity of the cornea, anterior chamber, lens, and vitreous is performed during the red reflex test, which measures the ability of light to enter the eye and reflect back out of the eye off the retina. Similarly, the red reflex test indirectly assesses the intactness of the retina. The adventurous neonatologist or primary care physician can attempt direct viewing of the infant's retina with a direct ophthalmoscope or a panoptic ( Fig. 95.8 ). Viewing the retina of the infant's undilated eye with a direct ophthalmoscope is a challenge, even to the pediatric ophthalmologist, but a panfundoscope can provide adequate examination with more ease. If a view of the retina is required, such as for retinopathy of prematurity screening, the consulting ophthalmologist would dilate the infant's eyes and use more sophisticated examination instrumentation such as the indirect ophthalmoscope.
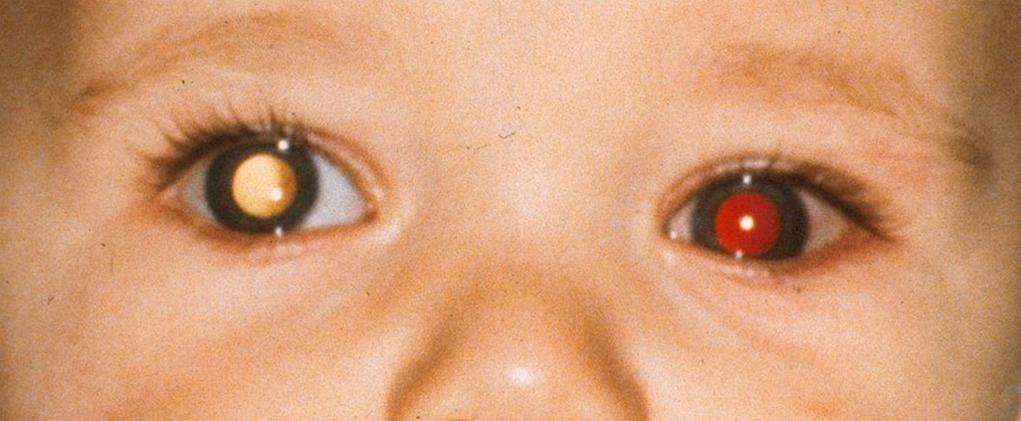

Certainly, the use of the direct ophthalmoscope to directly view the retina is not considered part of the screening eye examination in the infant. However, if a direct ophthalmoscope examination is attempted, attention should be directed to the clarity of the vitreous cavity and the appearance of the optic disc, major retinal vessels, the macula, and the surrounding retina.
The eye of the newborn infant differs from the adult eye primarily in function, although structural differences also exist. The growth of the eye, which parallels that of the brain, continues at a rapid rate for the first 3 years of life, especially during the first year. The anteroposterior dimension of the eye at birth is about 16.5 mm and grows to about 23 mm in adulthood. Normal values in the neonate fall within a very wide range. Suspected abnormalities in size often are substantiated only by comparison with measured values in the fellow eye or by ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI). The horizontal and vertical diameters of the cornea of the newborn are about 9-10 mm. Enlarged corneas suggest the diagnosis of congenital glaucoma.
The lid fissures of the term infant are usually narrow and often widely separated horizontally by prominent epicanthal folds. Normal horizontal measurement of the lid fissures in the newborn can range from 17-27 mm ( Table 95.2 ). These measurements should be symmetric. The term telecanthus indicates a disproportionate increase in the distance between the medial canthal angles. It is particularly noticeable in fetal alcohol syndrome and Waardenburg syndrome. Measurements between the two medial canthi in the term newborn vary from 18-22 mm. Hypertelorism is defined as an increase in distance between the orbits, observed clinically as a large interpupillary distance, which is often seen in many craniofacial syndromes. A secondary telecanthus is observed in patients with hypertelorism.
| Term Neonate | Premature * | |
|---|---|---|
| Intermediate canthal distance, mm | 18-22 | 12-16 |
| Medial canthus to lateral canthus, mm | 17-27 | 12-16 |
| Anteroposterior diameter of eye at birth, mm | 16 | 10-16 |
| Horizontal diameter of cornea, mm | 10 (average), 9 (lower limits) | 7.5-8 (lower limits) |
Reflex tearing to irritants is evident shortly after birth. However, emotional tearing begins at about 3 weeks of age and is developed at 2-3 months. The newborn infant possesses a strong blink reflex in response to light and to stimulation of the lids, lashes, or cornea. The reflex response to a threatening gesture does not appear until 7 or 8 weeks of age in the term infant. Repetitive eye opening is evident at birth.
After birth, the eyes should appear straight for the most part, although erratic, purposeless, and independent movements can be observed during the first few months of life. Any constant strabismus beyond the age of 4 months requires further evaluation. Conjugate horizontal gaze should be evident in the newborn; vertical conjugate gaze develops by 2 months of age. Convergence spasms are a normal transient phenomenon of infancy.
The ability to maintain a steady gaze or to fixate and follow an object is only weakly initiated at 4-6 weeks of age. At first, the eyes pursue a moving visual stimulus with short saccades. By 3 months of age, the infant can fixate and follow in both vertical and horizontal directions. At about 4 months of age, central fixation is associated with the motor activity of grasping. Binocular vision is present at about 6 months of age.
The ocular findings of the premature infant differ from those of the term neonate. At 28 weeks of gestation, the globe is only 10-14 mm in diameter. The anterior and posterior hyaloid vascular systems are usually present to some degree, although their involution continues for the next several months. Remnants of this system may be seen in the form of persistent blood vessels or fibrous strands anterior and posterior to the lens. The main hyaloid artery coursing from the optic disc to the posterior lens surface may be patent or appears as a white strand in the vitreous. A moderate amount of vitreous haze is often present at this time, interfering with visualization of the fundus. Vascularization of the retina begins with the ophthalmic artery entering the eye through the posterior edge of the eye's embryonic fissure at 4 months of gestation. Retinal vessels then grow anteriorly to vascularize the peripheral retina, a process that is not complete until near term. Thus premature infants have incomplete retinal vascularization, creating the basis for retinopathy of prematurity (see also Chapter 96 ).
Pupil constriction to light is not seen until 30 weeks of gestational age. Lack of pupil response to light should not be considered abnormal until at least 32 weeks after conception. Premature infants have a higher incidence of myopia, amblyopia, and strabismus in childhood. Careful follow-up of all children born prematurely is advisable to ensure early detection of these ocular conditions.
Routine ophthalmologic consultation for all infants in the nursery is not warranted. Pathologic ocular findings in normal neonates are sufficiently unusual that evaluation should be requested only after a screening ocular examination indicates the presence of an abnormality or for patients who for some reason are at increased risk for ocular problems. Indications for ophthalmologic consultation include a family history of congenital cataracts, retinoblastoma, congenital glaucoma, or other serious ocular diseases. Intrauterine infectious disease such as rubella, toxoplasmosis, or cytomegalovirus necessitates a thorough eye evaluation. For preterm infants, ophthalmologic consultation is necessary to exclude retinopathy of prematurity.
The contents of the orbit are confined to a conical shape by its bony walls. At the posterior apex of the orbit, the extraocular muscles originate, and the vascular and nerve structures enter the orbit. The bone structures of the lateral wall do not protect the orbital contents as far anteriorly as do the remaining sides of the orbit, which leaves the eye more susceptible to trauma on its lateral side. In the neonate, the orbital rims form a circular outline at the anterior base of the cone.
Box 95.2 lists systemic syndromes associated with abnormal orbits.
The terms proptosis, exophthalmos, and exorbitism are often used interchangeably to describe forwardly displaced eyes. In the strictest sense, proptosis results from an increase in orbital contents within a normal bony orbit, exophthalmos from Graves disease, and exorbitism from shallow bony orbits.
The diagnosis of proptosis can be confirmed if the examiner observes the infant's eyes and lids from above, over the prominence of the eyebrows. A more anterior protrusion of the orbital content is observed in comparison with the opposite side. In proptosis, the eye frequently also has a widened palpebral fissure.
Masses within the orbital cavity can expand most easily anteriorly, producing proptosis. Those located within the cone of extraocular muscles produce a symmetric anterior displacement, whereas tumors located outside the cone of extraocular muscles displace the eye outward and away from the area of origin of the tumor. A tumor in the inferior portion of the orbit displaces the eye upward and forward, whereas one located medially displaces the eye laterally and forward. A diffuse, extensive tumor can produce sufficient changes to affect the eye's movement, whereas a localized tumor often does not interfere with rotation of the eye. If a tumor is located anterior to the equator of the globe, it can extend anteriorly into the lids without producing proptosis.
An orbital encephalocele or meningocele producing proptosis may be evident at birth or may be delayed until later years. This abnormality results from a defect in the wall between the cranial cavity and the orbit, usually located at the suture lines. Pressure within the cranium causes herniation of brain tissue, meninges, or both into the orbit, most often at the inner angle of the orbit at the root of the nose.
Diagnosis is made by identifying the bone defect in association with the area of the orbital cyst. Clinically, an encephalocele is suggested by the presence of a pulsating, fluctuant cyst that can be reduced somewhat with digital pressure or that increases with coughing or crying. Excessive manipulation of the encephalocele can cause pulse and respirations to slow or can cause convulsions. Neurosurgical consultation and intervention are necessary.
Proptosis also can occur from venous engorgement of the orbital cavity such as that produced by a carotid-cavernous fistula. A cephalic bruit is often heard in the infant but is not pathognomonic of a carotid-cavernous sinus fistula. A false diagnosis of proptosis might be made when there is a slight ptosis of one eye, which gives the opposite, normal eye an appearance of a wide palpebral fissure. Marked enlargement of the eye, as in congenital glaucoma or high myopia, makes the eye appear proptotic because of the increased size of the globe. Facial abnormalities that produce shallow orbits, as in Crouzon disease, simulate proptosis because the normal amount of orbital structure appears to protrude in an abnormally shallow orbit.
Hyperthyroid exophthalmos, a rare neonatal sequela of hyperthyroidism, can occur as the result of maternal Graves disease during the last trimester of pregnancy. The infant is born with classic hyperthyroidism, including exophthalmos, upper lid retraction, and extraocular muscle involvement. Symptoms usually subside during the first 2 months of life.
Enophthalmos refers to eyes that look sunken into the orbit. Causes in infants include orbital asymmetry, microphthalmos, trauma resulting in an orbital blow-out fracture, congenital fibrosis of the extraocular muscles, and congenital Horner syndrome. Numerous syndromes, and many infants with unclassifiable facial dysmorphisms, feature deep-set eyes, such as Lowe syndrome, Cockayne syndrome, and Cornelia de Lange syndrome.
Abnormal spatial relationships between the two orbits create excessively wide or excessively narrow intraorbital distances. These abnormalities are caused by a variety of related cranial abnormalities involving the disproportionate growth or lack of development of the body and lesser wing of the sphenoid and ethmoid sinuses and of the maxillary processes. Hypotelorism (narrowing of the intraorbital distance) may be associated with central nervous system malformation.
Ocular hypertelorism is a term indicating increased separation between the bony orbits, usually greater than two standard deviations above the mean. It is an anatomic description rather than a diagnostic entity and is noted with varying severity in many syndromes such as the craniosynostosis syndromes. One condition with marked hypertelorism is frontonasal dysplasia (median facial cleft syndrome), which may be the result of morphokinetic arrest during embryogenesis.
Characteristic findings of frontonasal dysplasia are medial cleft nose, lip, and palate; widow's peak; and cranium bifidum occultum. Common other ocular abnormalities include exotropia, dacryostenosis, epibulbar dermoids, palpebral fissure changes, and optic atrophy. A phenotypically similar but probably distinct clinical entity within the frontonasal dysplasia spectrum has been reported with the features of facial midline defects, basal encephalocele, callosal agenesis, endocrine dwarfism, and morning glory disc anomaly.
Colobomas of the lids are partial-thickness or complete defects that can range from a small notching of the lid borders to involvement of the entire length of the lid. Most lid colobomas occur in the medial aspect of the upper lid. When the lower lid is involved, the defect is more often in its lateral aspects. The cause of lid colobomas is often unknown unless associated with a craniofacial syndrome. It has been suggested that these isolated colobomas arise from the localized failure of adhesion of the lid folds that results in a lag of growth or from mechanical effects of amniotic bands. Syndromes with associated lower lid colobomas are mandibulofacial dysostosis (Treacher Collins syndrome), Goldenhar syndrome (more often upper lid), amniotic band syndrome, and Burn-McKeown syndrome.
The treatment of lid colobomas is important when the lid defect prevents adequate lid closure and allows exposure of the cornea. Subsequent thickening, opacification, infection, ulceration, or perforation of the unprotected cornea can occur. Vision can be degraded by the amblyopia that develops. Early surgical correction is often required when the coloboma is greater than one-third of the eyelid margin.
The term blepharophimosis describes eyelids that are too narrow horizontally and vertically. There is usually an associated ptosis, and strabismus is frequently present. Lid fissure measurements commonly are reduced to about two-thirds of normal, whereas the space between the medial canthi is considerably widened. Surgical repair to widen the medial and lateral canthal angles, elevate the upper lid, and correct the strabismus is available.
Blepharophimosis can occur in isolation, as a feature of BPES (blepharophimosis, ptosis, and epicanthus inversus syndrome), or in multiple systemic syndromes such as fetal alcohol syndrome, Saethre-Chotzen syndrome, and van den Ende-Gupta syndrome. BPES has been mapped to the FOXL2 gene encoding a fork head transcription factor. BPES is divided into type 1 and type 2, with and without premature ovarian failure, respectively.
Epicanthus is the most commonly encountered lid abnormality. A skinfold originating in the upper lid extends over the medial end of the upper lid, the medial canthus, and the caruncle, and ends in the skin of the lower lid. It gradually disappears with the growth of the bridge of the nose as the child loses its baby face. Epicanthal folds can give a false appearance of crossed eyes (pseudostrabismus). Epicanthus inversus is similar except that the predominance of the skinfold arises in the lower lid and runs diagonally upward toward the root of the nose to overlie the medial canthus. These folds are benign and generally do not require treatment.
Congenital ectropion is an eversion of the lid margin, most commonly of the lower lid. It may be associated with other eye abnormalities or syndromes such as Duane syndrome. If the ectropion is mild, no treatment is necessary. A more severely everted lid might require surgical correction. The integrity of the cornea requires monitoring and will determine the treatment.
Often associated with other lid or ocular abnormalities, congenital entropion is an in-turning of the lid margin, most often of the lower lid. If the lashes on the lid margin rub the cornea and cause corneal abrasions, surgery is necessary.
Epiblepharon is an extra fold of skin along the lower lid that can cause lashes to turn inward. This condition usually requires no treatment and is most often seen in infants of Asian ancestry. This condition usually improves spontaneously during the first 2 years of life, but if it persists, consideration should be given to surgical correction. As the lashes are soft when the child is younger, lashes rubbing against the cornea tend not to produce symptoms. But beyond age one, as the lashes get thicker and harder, corneal irritation leading to abrasion and even ulceration not controlled with simple lubrication with ointments warrants earlier surgical intervention. 151
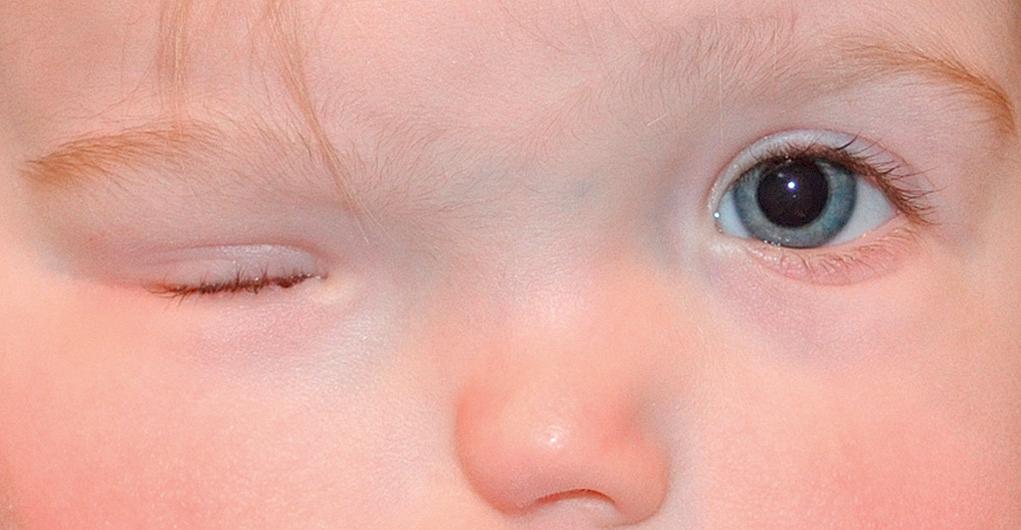
Ptosis is asymmetry in upper lid height or symmetrical lowering of both upper lids. Although both upper and lower eyelids may be ptotic, the terms blepharoptosis or ptosis generally refer to the upper lids unless otherwise specified ( Fig. 95.9 ). The condition could be idiopathic or inherited, unilateral or bilateral, congenital or acquired. When looking straight ahead, the normal lid should elevate to a point at least midway between the pupil and the upper margin of the cornea. Neonates may have a transient self-limited droopy or closed lid as a result of facial edema or lid trauma during normal vaginal delivery. A temporary, simulated ptosis (protective ptosis or guarding) can result from irritation or infection of the cornea or conjunctiva.
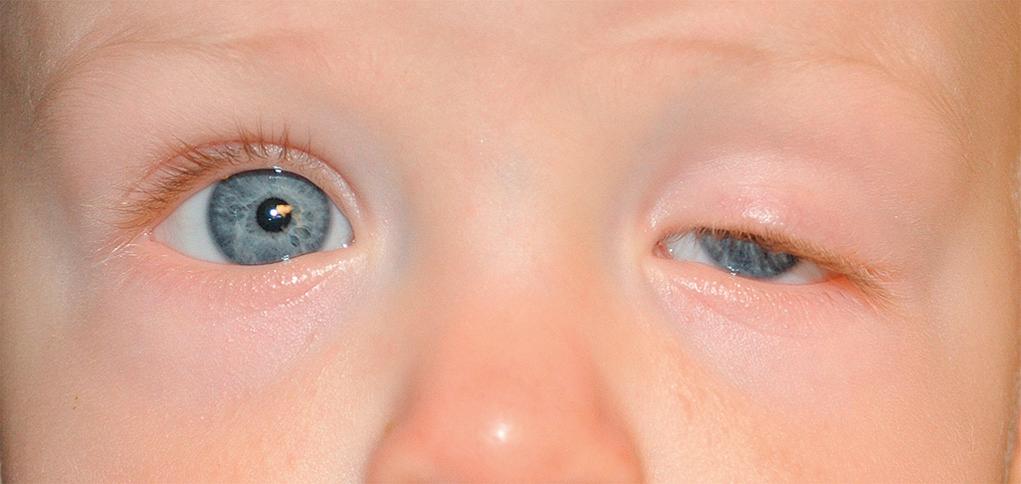
Congenital ptosis most often results from the dysfunction of the levator palpebrae muscle, mostly seen as an idiopathic or familial disorder. Although occlusion of the pupil is rare, the ptotic lid can induce a corneal astigmatism and refractive amblyopia. Occlusive patching and glasses may be needed. Mild, unilateral ptosis should prompt a comparison of pupil size to evaluate for Horner syndrome. Congenital Horner syndrome (ipsilateral ptosis, miosis, anhydrosis [see Fig. 95.6 A, B ]) is often a result of trauma at birth, although it may be associated with mediastinal disease or neuroblastoma. The involved iris may be hypopigmented.
Ptosis also may be associated with a Marcus-Gunn jaw-winking phenomenon. This syndrome is caused by anomalous motor innervation of the levator palpebrae muscle from nerve twigs to the pterygoid, masseter, or lingual muscles. The affected patient has an up-and-down rhythmic movement of the upper lid during nursing activity (see Fig. 95.2 ). The jaw-winking portion of the syndrome is thought to decrease or disappear in early adulthood, but the ptosis remains. Many surgical procedures have been designed to mitigate the ptosis.
Two forms of myasthenia gravis can produce ptosis in the neonatal period. In about 15% of neonates born to mothers with myasthenia gravis, a transient form of myasthenia occurs shortly after birth. Affected children have a weak cry and poor suck and can develop weakness in all muscle groups. Ocular symptoms are rare and most commonly involve ptosis. A variety of congenital myasthenia syndromes linked to genetic disorders of the neuromuscular junction or acetylcholine production can display ptosis and ophthalmoparesis, which are often variable and related to the level of fatigue. Pseudoptosis, or false ptosis, may be apparent when the globes are of different sizes or if enophthalmos or proptosis is present.
Microphthalmia (small eye) is a common congenital defect that can be mildly expressed. In such situations, the lid will look drooped even if it is functioning properly ( Fig. 95.10 ). A unilateral large globe caused by monocular myopia can produce a relative ptosis in the contralateral normal eye.
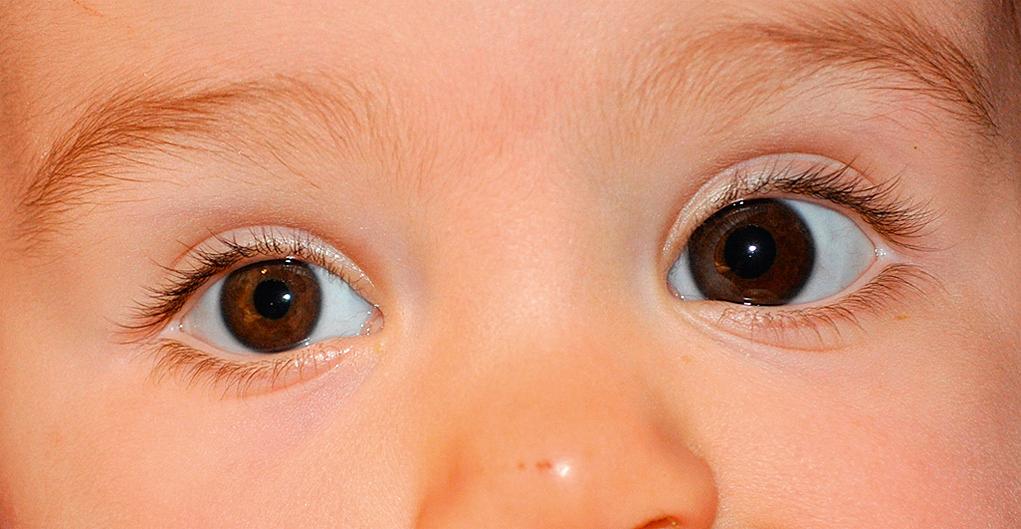
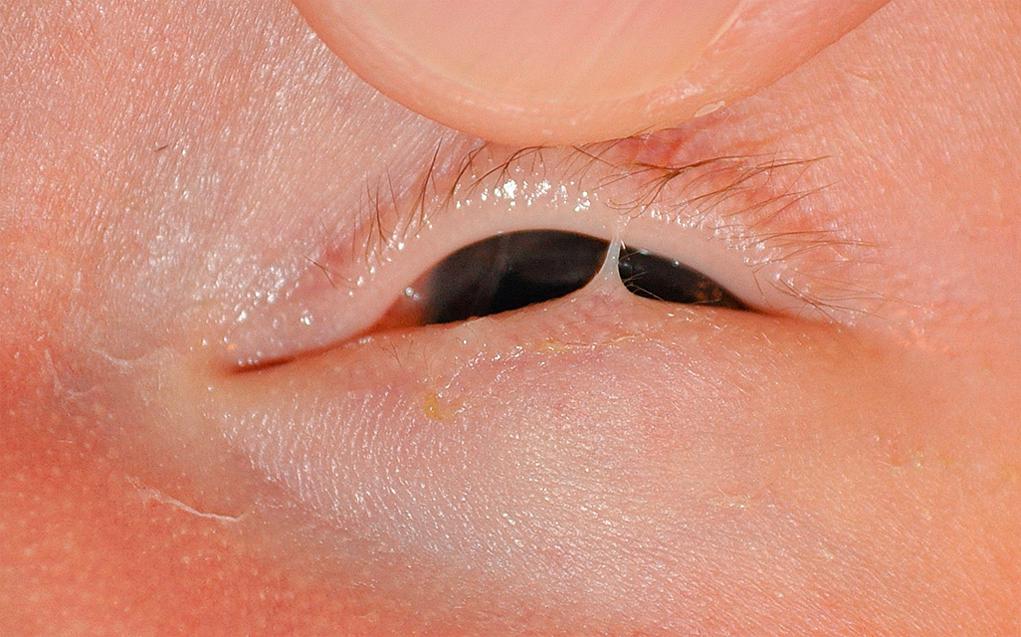
The upper and the lower lids may be fused at birth or through an inflammatory process ( Fig. 95.11 ). The term ankyloblepharon is used to designate this condition, which may be dominantly inherited. Simple excision of the bridging tissues between the eyelids is all that is required to separate the lids.
Nevi may occur anywhere on the lid margin, skin, or conjunctiva. Often a congenital nevus will not be noticed until puberty, when pigment begins to accumulate and the lesion is thought to be growing ( Fig. 95.12 ). Observation for change in size, shape, depth, or pigmentation is recommended.
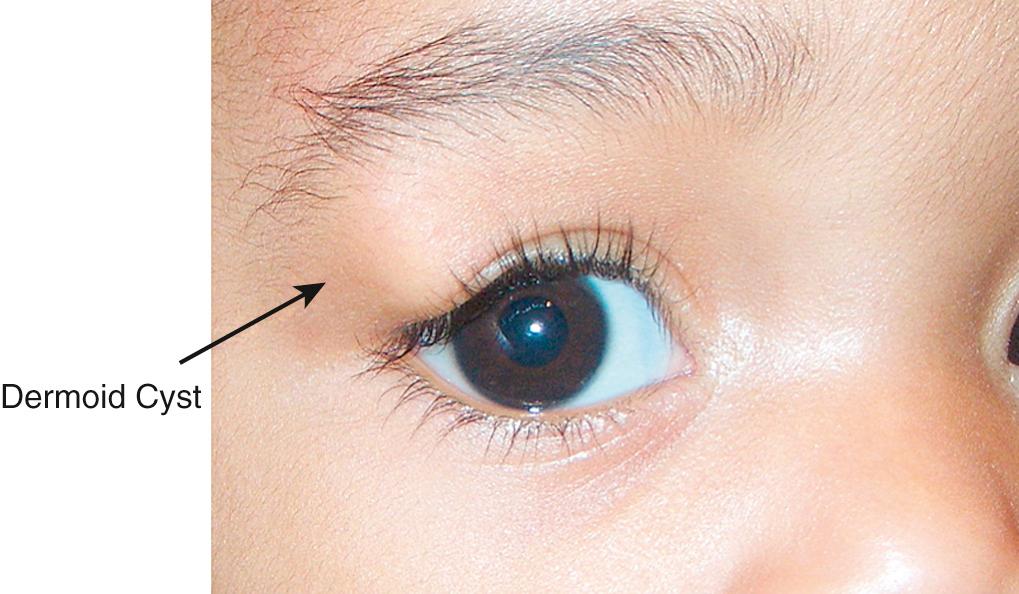
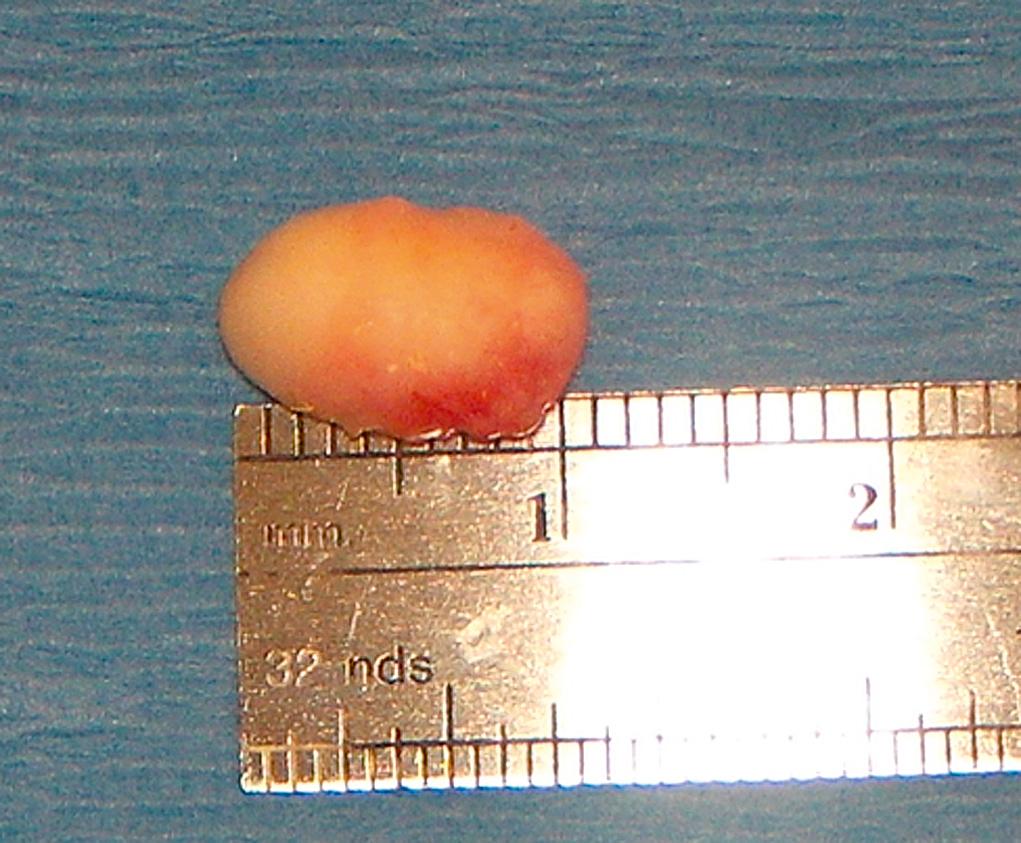
Dermoid cysts are tumors that appear at the suture lines, most commonly at the superotemporal brow or in the superior medial central area of the brow ( Fig. 95.13 ). Occasionally they appear to be free in the orbit or lid without demonstrable direct connection to a suture line. They are benign choristomata that grow slowly but will thin out the adjacent bone. They should be removed surgically without rupturing the cyst ( Fig. 95.14 ). If a dermoid cyst is ruptured by accidental trauma or surgery, severe local inflammation can result if the extruded contents are not irrigated from the wound.
Hemangiomata are quite common and occur in up to 10% of newborns. They may virtually occur in any body location including the upper or lower lids and orbit. In infants, the hemangioma usually is not well encapsulated and frequently periorbital, and orbital lesions will occur in association with similar lesions elsewhere on the body.
Although observation of lid involvement assists in making the diagnosis, a biopsy might be required to exclude other orbital tumors if the hemangioma lacks a superficial component. Crying or straining by the infant often causes the mass to increase in size and assume a bluish coloration. Digital pressure on the superficial portion of the tumor is rapidly reversed, demonstrating the high flow of capillary hemangioma. When a hemangioma involves the eyelids, amblyopia can develop. Amblyopia may be secondary to the hemangioma's obstruction of the visual axis or from pressure on the cornea that subsequently induces astigmatism and anisometropic amblyopia.
Congenital capillary hemangiomata are inconsequential at birth but grow rapidly during the first 6 months of life. Spontaneous involution occurs at about age 2-4 years. The natural history of hemangiomas is that they will regress over several years even without treatment, but local soft tissue and occasional bone deformities may remain and are common around the eye.
When threatening the sight via amblyopiogenic properties, treatment is warranted. Until recently, treatment was oral use or intralesional injections of steroids, subcutaneous interferon, or excision of the lesion. Although these modalities are still valid, they are not free of side effects. Oral propranolol or topical timolol are effective ways of treating these tumors ( Figs. 95.15 A , B ). In the past, treatment involved systemic, injectable, or topical corticosteroids with potential side effects over months of treatment. Central artery occlusion is a rare but well-described complication of steroid injection with permanent vision loss. Occasionally, surgery is indicated for well-defined, easily accessible tumors to avoid side effects of medical treatment. Oral use of propranolol (2 mg/kg/day) is found to be very effective in the hemangioma treatment. However, its use, although relatively uncommon, necessitates that side effects such as hypotension and hypoglycemia have to be monitored closely. Also, an MRI of the brain to rule out associated intracranial vascular abnormalities will prevent cerebrovascular injuries secondary to the blood flow and tension changes caused by this medication.
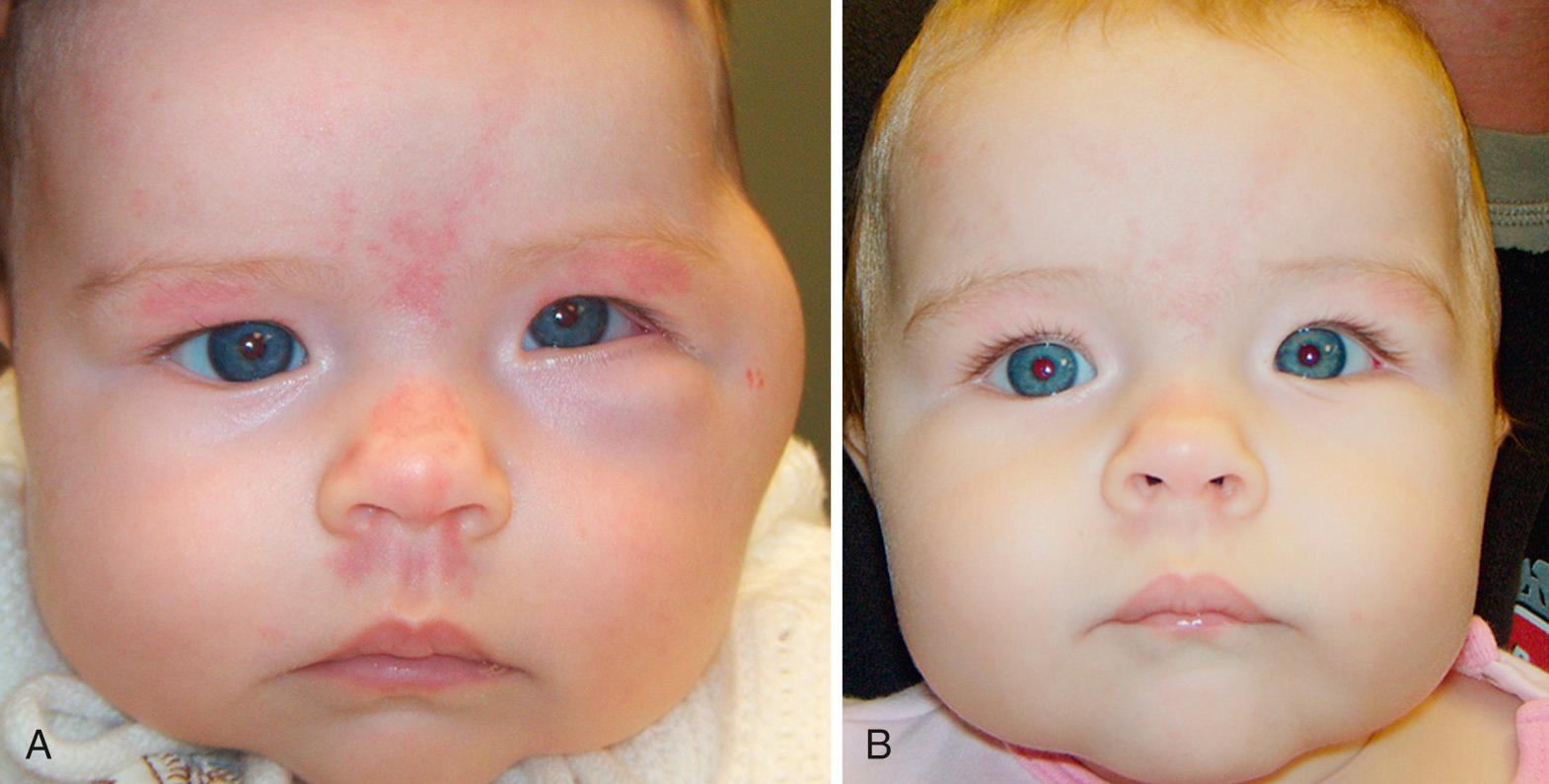
Other eyelid lesions include, but are not limited to, plexiform neuromas secondary to neurofibromatosis type-1, amyloid depositions, and juvenile xanthogranuloma.
Lashes may be redundant, absent, misdirected, or discolored. The condition may be inherited or acquired through mucous membrane diseases of the conjunctival sacs, infectious process, or trauma. Trichiasis is a lash that grows from a normal location but is misdirected toward the ocular surface. Congenital trichiasis patients should be examined for Down syndrome or signs of ectodermal dysplasia. If a second row of lashes is present in the area of the meibomian glands (metaplasia), the condition is referred as distichiasis . This condition usually results in contact of the lashes with the cornea, producing corneal irritation and abrasions. True distichiasis is rare but can easily be distinguished from trichiasis, entropion, and epiblepharon ( Fig. 95.16 ), all of which may be seen together. Excessive eyelash growth can result as a side effect from multiple medications, including topical prostaglandin analogues and epidermal growth factor receptor inhibitors.
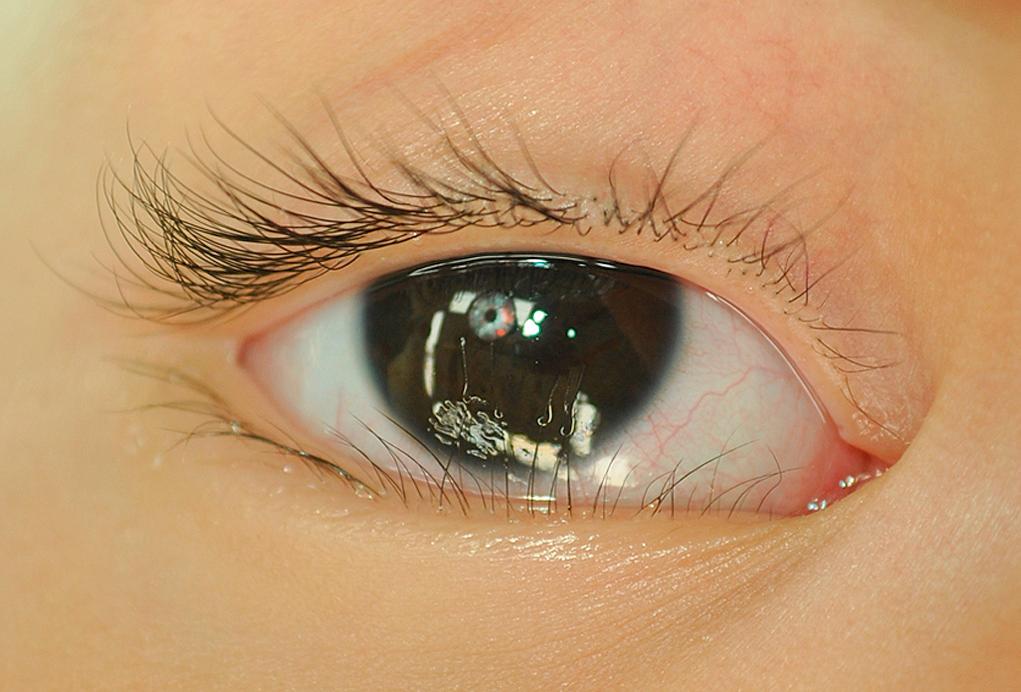
Excessive hair on the lids and forehead can occur as a dominant characteristic in male infants and, on occasion, may be extreme. Hypertrichosis involving the eyebrows, forehead, and upper lid appears in Cornelia de Lange syndrome , a pathologic dwarfism associated with multiple congenital anomalies. Hypertrichosis lanuginosa is transmitted as an autosomal dominant condition. The fetal lanugo persists into adult life, creating an abundant covering of hair on the eyebrows, forehead, eyelids, and other areas of the body.
Epiphora (excess tearing) usually does not occur until after the first 3 weeks of life, when the major portion of the lacrimal gland has become functional. Although the usual cause of epiphora is a blockage of the nasolacrimal ducts (dacryostenosis), the possibility of congenital glaucoma is the most important consideration in the differential diagnosis. Less commonly, tearing can result from an obstruction of the common canaliculus, from congenital absence of the lid puncta, or from dacryocystitis. Congenital absence of the entire lacrimal drainage apparatus is extremely rare. Reflex tearing may be produced by any stimulation of the fifth cranial nerve. Epiphora can occur as the result of corneal abrasion, corneal foreign body, or nasal and facial lesions that irritate the fifth cranial nerve. Chronic nasal congestion also may produce epiphora by mechanically blocking the nasolacrimal duct. Dacryostenosis may be present in up to 7% of neonates and creates a stagnant pooling of tears in the lacrimal sac that contributes to chronic or recurrent dacryocystitis. The inflammation is marked by a purulent exudate in the medial canthal area of the conjunctiva. Severe dacryocystitis can produce swelling and induration of the lacrimal sac medial and inferior to the medial canthus. Treatment of mild nasolacrimal infection consists of topical antibiotic drops or ointment. If surrounding cellulitis is suspected, systemic administration of medication and locally applied heat may be required. Repeated massage of the lacrimal sac at the medial canthal area serves to flush out the stagnant tears, decrease the risk for infection, and “pop” open the nasolacrimal obstruction. If the epiphora continues, a lacrimal probe passed through the nasolacrimal duct to the nose usually creates an adequate opening. Probing between 6 and 12 months of age is sometimes performed in an office setting under topical anesthesia with the baby swaddled in a sheet. In more than 90% of children with congenital dacryostenosis, the obstructions spontaneously correct during the first year of life. If persistent, treatment is then offered but requires general anesthesia because these children are too large to swaddle for an office probing. Surgical options include simple probing, silicone tube intubation, or balloon dilation of the lacrimal system.
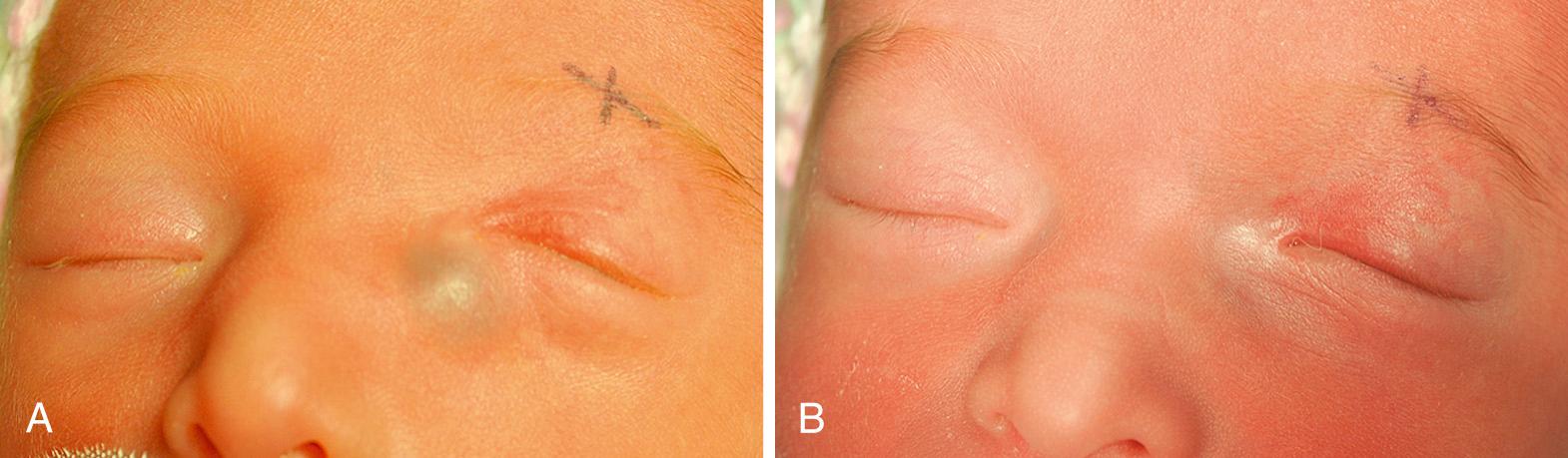
A congenital dacryocystocele presents within the first week of life as a bluish mass adjacent to but lower than the medial canthus. Similar lesions found higher than the medial canthus should be suspected to be encephalocele. The distended lacrimal sac is filled with clear fluid, feels firm or fluctuant to palpation, and does not pulsate like a frontal encephalocele. Initiating oral antibiotics is recommended as soon as a dacryocystocele is identified to prevent infection of the dacryocystocele, which can occur in up to two-thirds of these patients. If the patient presents with signs of a dacryocele infection, admission to a pediatric intensive care unit for intravenous antibiotics is required. Surgical treatment with a nasolacrimal probing should be considered within the next 2-4 days ( Figs. 95.17 A, B ).
The normal newborn will have moist eyes from basal secretion of tears. Reflex tearing from ocular irritation or psychogenic (emotional) tearing may not develop for weeks to months after birth. Infants do not usually have symptoms from dry eyes unless in rare conditions such as alacrima from a congenital lack of tear glands or Riley-Day syndrome. Recognition of alacrima in the infant is important in preventing corneal abrasions, infections, and perforations, the inevitable sequelae of dry eye. During the first month of life, an infant with alacrima from any cause might not appear different from the normal infant, because tear production is minimal during this period. The usual time of discovery is at 6-12 months of age after the lack of tears has produced changes such as scarring or ulceration of the cornea. At 1-2 months of age, early symptoms of dry eye are conjunctival hyperemia and photophobia. Instead of the ample tears expected with conjunctival irritation, a sticky mucoid secretion is produced, and the cornea shows punctuate staining with fluorescein solution application. Treatment includes frequent use of artificial tears (as often as every 15-30 minutes), punctal occlusion, or tarsorrhaphy (partial or complete temporary or permanent suturing of the upper and the lower lid together to decrease the exposed ocular surface area).
Isolated congenital lack of tears, usually bilateral, is a rare anomaly. The cause is unknown, but it has been suggested to result from hypoplasia of the lacrimal gland or from an absence of innervation of the lacrimal gland structures. The ocular findings in familial dysautonomia (Riley-Day syndrome) are characteristic and can produce the initial criteria for diagnosis. They include alacrima and corneal anesthesia. These ocular changes and constriction of the pupil by instillation of 0.125% pilocarpine should establish the diagnosis. The onset of major systemic symptoms occurs when the child is about 2 years old. An abnormal swallowing mechanism, inappropriate blood pressure and respiratory control, decreased sensitivity to pain, deficient taste perception, and abnormal ocular findings in this syndrome are the result of sympathetic, parasympathetic, and sensory neuronal abnormalities. Additional ocular findings are myopia, anisometropia (significantly different refractive error in the two eyes), exotropia, tortuosity of the retinal vessels, and occasionally ptosis. Familial dysautonomia results from a mutation in the IKBKAP gene encoding elongation protein 1, resulting in defective splicing.
Anophthalmia is a rare sporadic phenomenon in which there is total absence or a minute rudiment of the globe, and it can present either unilaterally or bilaterally ( Fig. 95.18 ). It occurs as a developmental failure of the optic vesicle. It is often accompanied by other congenital anomalies, such as central nervous system defects and mental retardation, and has been observed in isolation, in genetic defects (e.g., SOX2 , STRA6 , PAX6 genes), and in chromosomal syndromes (e.g., trisomy 13). Because lid formation does not depend on ocular formation, the lids are fully formed. However, the lids remain closed and can be partially fused, and they are smaller and sunken without the support of the eye. Treatment involves reconstruction of the orbit to improve the appearance of the face. Plastic molds of the conjunctival sac of gradually enlarging sizes are used to stretch the lids and sac sufficiently to hold an ocular prosthesis. In unilateral situations, orbital reconstruction using tissue expanders can dramatically improve the overall facial appearance.
In cryptophthalmia, the eyelids fail to cleave, and uninterrupted skin runs from the forehead to the malar area. The eyelids and eyelashes usually are absent; however, the eye can be palpated beneath the skin and might even be observed to move with the stimulation of a strong light.
The anterior segment of the eye is invariably disorganized into fibrovascular tissue adherent to the subcuticular tissue of the lids. Because the conjunctival sac is absent or small, attempts to separate the lids from the ocular structures are usually unsuccessful, and surgical correction is not advisable in most circumstances. Although a globe is present, only rarely is vision obtainable.
Cryptophthalmia is frequently associated with systemic abnormalities such as urogenital malformations (Fraser syndrome) linked to defects of the FRAS1 and FREM2 genes, and a thorough genetic evaluation is indicated.
Microphthalmia describes a variety of conditions in which the axial length of the neonatal eye is less than two-thirds of the normal 16 mm. Causes include familial, syndromic, and chromosomal abnormalities and environmental influences during gestation. Simple microphthalmia is a condition in which there is an abnormally small eye but with intact internal organization. It may be associated with other ocular features of importance: a high degree of hypermetropia, retinal folds, a tendency for choroidal effusions, and the late occurrence of glaucoma.
Complex microphthalmia describes small eyes with internal disorganization, such as anterior segment dysgenesis, cataract, coloboma, or persistent fetal vasculature. Colobomatous microphthalmia occurs when the embryonic cleft of the optic vesicle fails to close. Typically, this form is associated with other ocular anomalies such as colobomas of the iris, ciliary body, fundus, or optic nerve, or colobomatous orbital cysts (see Fig. 95.21 ).
Microphthalmia can also be associated with other ocular and systemic syndromes, including intrauterine infections such as rubella and cytomegalovirus, craniofacial anomalies, anterior segment dysgenesis syndromes, or chromosomal abnormalities. Because of the heterogenicity of associated findings, infants with microphthalmia should be evaluated by both an ophthalmologist and geneticist.
An abnormally enlarged eye in the neonatal period is rare but is extremely important to recognize. An apparently enlarged eye may be caused by colobomatous microphthalmia with an associated large cyst, congenital megalocornea (see later), or congenital glaucoma (see later). Colobomatous microphthalmia results when the embryonic optic vesicle fails to close. Tissue that originally should have become intraocular is encased in a cystic structure outside the eye in the orbit. If the cyst becomes sufficiently large that proptosis occurs, the microphthalmic eye simulates an enlarged eye.
The normal term neonate has a glistening white sclera. The overlying conjunctiva and the conjunctival vessels superimpose a filmy, vascular pattern. A generalized bluish discoloration of the underdeveloped sclera is normal in premature infants. Rarely, a congenital weakness in a small area of the sclera produces a bluish bulge called a staphyloma. The light blue color is caused by thinness of the sclera that transmits the darker color of the underlying uveal tissue. Osteogenesis imperfecta may be associated with a similar bluish discoloration of the sclera in the term neonate because of inadequately developed scleral collagen. Blue sclera may also be seen in other systemic diseases such as Marfan syndrome, Ehlers-Danlos syndrome, and Crouzon syndrome.
Pigmentation of the conjunctiva and sclera is common in darkly pigmented people. Intrascleral nerve loops appear as darkly pigmented dots about 3-4 mm from the limbus. Congenital ocular melanosis and oculodermal melanosis (nevus of Ota [see Fig. 95.12 ]) occur as a unilateral slate-blue pigmentation in infancy.
The normal premature infant might have a slightly hazy cornea for the first few weeks of life, caused by temporary excess hydration of the cornea. In the normal term infant, a similar appearance may be seen for the first 48 hours after delivery.
A persistently hazy or cloudy cornea suggests congenital glaucoma or birth injury. Anterior segment dysgenesis syndromes, corneal dystrophies, infection, or systemic disease should also be considered ( Fig. 95.19 ).
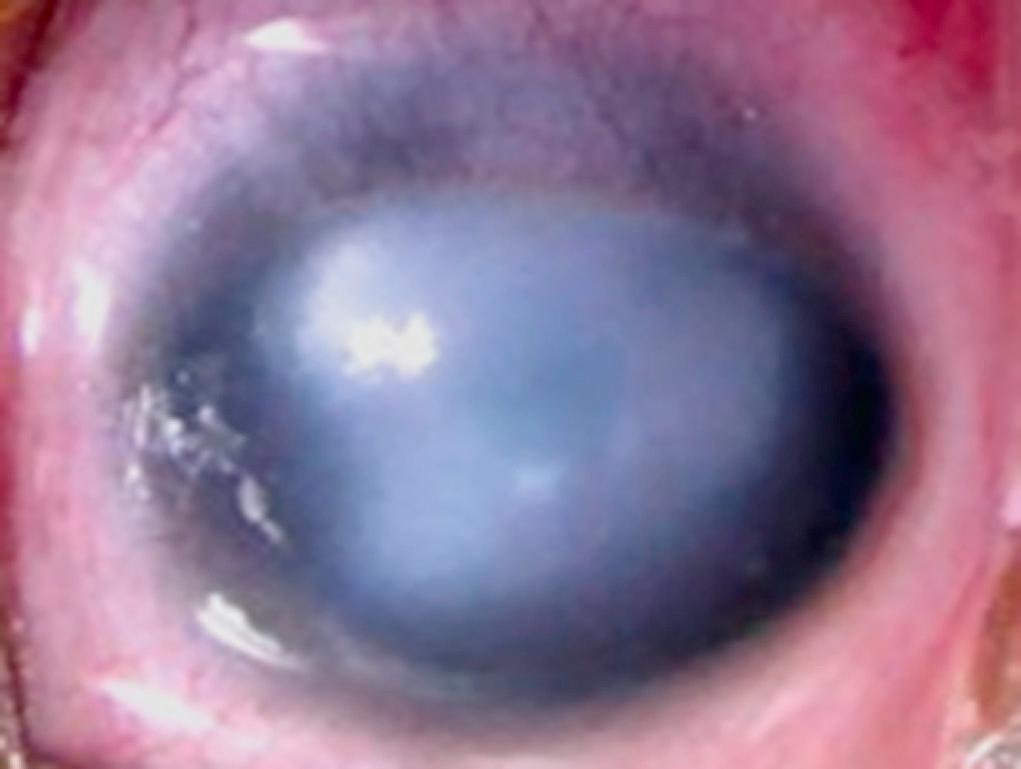
Most newborn infants have a corneal diameter of about 9-10 mm. If this measurement exceeds 12 mm, congenital glaucoma must be considered, especially if corneal haze, tearing, and photophobia are present. Megalocornea is an enlarged cornea exceeding 13 mm in diameter. The cornea is usually clear, with distinct margins and thin iris stroma. There are no other features of congenital glaucoma, such as elevated intraocular pressure, tearing, photophobia, or conjunctival injection. Megalocornea as an isolated finding is usually inherited in an autosomal dominant manner. Megalophthalmia, which is commonly inherited as an X-linked recessive trait, is characterized by deep anterior chambers, subluxation of the lens, hypoplastic iris, and cataract formation in early adult life. A corneal diameter of less than 10 mm may be an isolated finding or may be associated with other ocular anomalies. Cases of autosomal dominant and recessive inheritance of microcornea have been described.
A wide spectrum of clinical findings is observed in the anterior segment dysgenesis syndromes, including abnormalities of the cornea, anterior chamber, iris, and lens. Corneal opacities, adhesions of the iris, and glaucoma can occur. Although a continuous spectrum of findings can be evident in a family pedigree, or between the two eyes of one patient, eyes are often assigned into the distinct subgroups of posterior embryotoxon, Axenfeld anomaly, Rieger anomaly, or Peter anomaly.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here