Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The endocrine system is finely integrated—the hypothalamus, pituitary gland, and target glands continually communicate through feedback inhibition and stimulation to control all aspects of metabolism, growth, and reproduction. By understanding this interplay and carefully manipulating these systems via provocative or suppressive stimuli, it is possible to characterize an underlying abnormality and provide directed treatment.
Prolactin levels can be elevated as a result of a variety of pharmacologic and physiologic stimuli; values greater than 200 ng/mL are almost always associated with the presence of a pituitary tumor.
The initial screening test for acromegaly is measurement of the serum insulin-like growth factor-1 (IGF-1).
It is often unnecessary to perform provocative stimulation tests to document growth hormone deficiency in patients with a known history of pituitary disease or in those with evidence of three or more pituitary hormone deficiencies.
Provided that the hypothalamic-pituitary-thyroid axis is intact, the ultrasensitive thyroid-stimulating hormone test is the best method for detecting clinically significant thyroid dysfunction.
When measuring thyroglobulin as a tumor marker for thyroid cancer, always simultaneously check for thyroglobulin antibodies.
The best screening test for pheochromocytoma is either the plasma free metanephrine or 24-hour urinary free metanephrines. The patient should avoid caffeine, alcohol, acetaminophen, monoamine oxidase inhibitors, and tricyclic antidepressants for at least 5 days before testing.
It is frequently unnecessary to perform an adrenocorticotropic hormone (ACTH) stimulation test in critically ill patients. A random cortisol of greater than 25 μg/dL (700 nmol/L) during stress makes it highly unlikely that the patient is adrenally insufficient.
Midnight salivary cortisol (MSC) is a highly sensitive, highly specific, and very simple way to screen for Cushing disease.
The endocrine system is a finely tuned system in which the hypothalamus, pituitary gland, and various endocrine glands communicate through an intricate scheme of hormone-mediated feedback inhibition and stimulation stimuli. Hormones are classically defined as substances that act at sites distant from their place of origin. Included under the rubric of hormones are moieties that act in an autocrine (act directly on themselves), paracrine (act adjacent to the cells of origin), or intracrine (act within the cells of origin without ever exiting the cells) fashion. Through this intimate interplay of signals, the endocrine system serves to control metabolism, growth, fertility, electrolyte and water homeostasis, and responses to stress.
The pituitary gland, also known as the hypophysis , is located within the confines of the sella turcica. It is connected to the median eminence of the hypothalamus by the infundibular stalk and is divided into an anterior lobe (adenohypophysis) and a posterior lobe (neurohypophysis). It weighs about 0.6 g and measures about 12 mm in transverse and 8 mm in anteroposterior diameter. The anterior pituitary gland possesses five distinct hormone-synthesizing and hormone-secreting populations of cells. These cell groups include somatotrophs, which secrete growth hormone (GH); lactotrophs, which secrete prolactin (PRL); thyrotrophs, which secrete thyroid-stimulating hormone (TSH); gonadotrophs, which secrete the α and β subunits of follicle-stimulating hormone (FSH) and luteinizing hormone (LH); and corticotrophs, which secrete proopiomelanocortin (POMC). POMC is cleaved within the pituitary gland to form adrenocorticotropin (ACTH), β-endorphin, and β-lipotropin (β-LPH). The hypothalamus communicates with the anterior pituitary gland by secreting its own set of trophic hormones that are specific for each of the cell populations within the pituitary gland ( Fig. 25.1 ). These trophic hormones travel along the infundibular stalk to the adenohypophysis through a system of portal vessels.
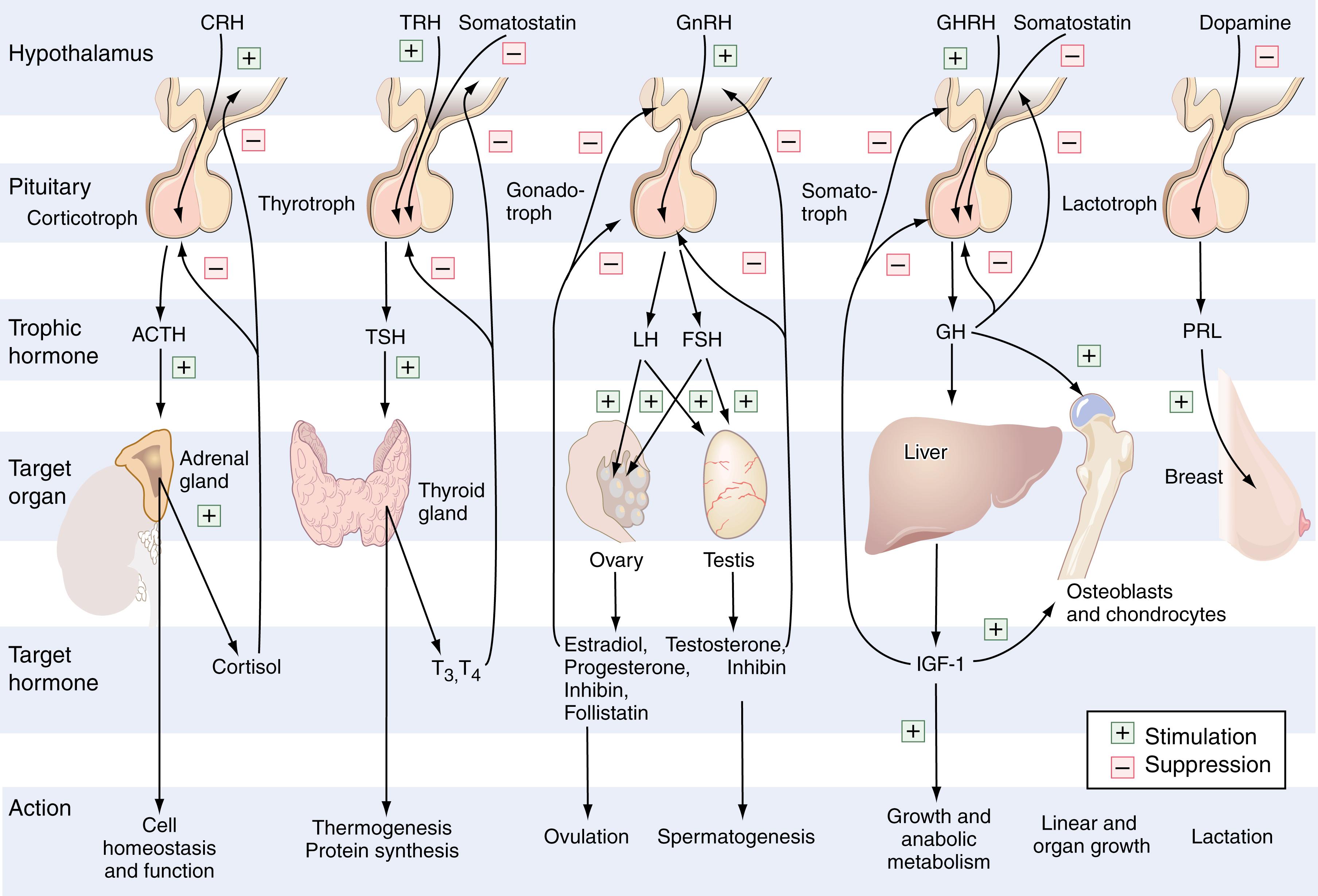
In contrast to the anterior pituitary gland, the posterior pituitary gland (neurohypophysis) does not synthesize hormones. The hormones that it does secrete, arginine vasopressin (AVP; also known as antidiuretic hormone [ADH]) and oxytocin, are synthesized within the magnicellular neurons of the paraventricular and supraoptic nuclei of the hypothalamus, transported along the axons, and stored in the nerve terminals that end in the neurohypophysis. A summary of the different hormones secreted by the pituitary gland can be found in Box 25.1 .
Growth hormone (GH; somatotropin)
Thyroid-stimulating hormone (TSH; thyrotropin)
Gonadotropins
Follicle-stimulating hormone (FSH)
Luteinizing hormone (LH)
Proopiomelanocortin (POMC)
Adrenocorticotropin (ACTH)
β-lipotropin
β-endorphin
Prolactin (PRL)
Arginine vasopressin (AVP) = Antidiuretic hormone (ADH)
Oxytocin
Abnormalities of pituitary function fall within two broad categories: hormonal excess and hormonal deficiency. Hormonal excess usually occurs as the result of clonal expansion of a distinct population of cells. However, it can result from an increase in trophic hormones from the hypothalamus or ectopic sites. The causes of hormonal deficiency are more varied ( Box 25.2 ) and can result in the deficiency of one or more hormones, often with continued and progressive loss of other hormones over time.
Pituitary neoplasm
Pituitary adenoma
Craniopharyngioma
Metastases or rarely primary carcinoma
Iatrogenic
Radiation
Hypophysectomy
Stalk resection
Granulomatous disease
Sarcoidosis
Infection
Tuberculosis
Syphilis
Fungi
Hemorrhage and infarction
Postpartum necrosis (Sheehan syndrome)
Head trauma
Apoplexy
Aneurysms of the internal carotid artery
Autoimmune lymphocytic hypophysitis
Hemochromatosis
Primary hypothalamic disorders
Tumor
Granulomas
Idiopathic or genetic deficiencies of hormones within the pituitary gland or hypothalamus
Pituitary tumors may be classified as microadenomas (<1 cm in greatest diameter and confined to the sella) or macroadenomas (≥ 1 cm in greatest diameter). They are further subcategorized into secretory and nonsecretory varieties ( Table 25.1 ). All tumors have the potential to grow; in doing so, they can compress the optic chiasm, resulting in visual field defects, of which bitemporal hemianopia is the most frequent presentation. Invasion into the cavernous sinus can lead to compression of cranial nerves III, IV, VI, V1, and V2 and the intracavernous portion of the internal carotid artery. It can also lead to hydrocephalus caused by obstruction of the third ventricle. Aside from oversecretion of a particular hormone and extension into surrounding regions, these tumors can also cause hormonal deficiency due to compression of other cell lineages within the pituitary gland.
| Lactotroph (PRL) | 30% |
| Somatotroph (GH) | 15% |
| Combined GH/PRL | 8% |
| Corticotroph (ACTH) | 15% |
| Thyrotroph (TRH) | 1% |
| Pleurihormonal | 4% |
| Nonfunctioning | 27% |
PRL is a polypeptide produced by the lactotrophs of the pituitary gland; it is responsible for the initiation and maintenance of lactation. Its secretion is normally kept at low levels by the inhibitory actions of dopamine produced by the hypothalamus. Similar to several pituitary hormones, PRL is secreted in a circadian fashion, with the highest levels attained during sleep and a nadir occurring between 10 am and noon ( ). PRL is secreted in a pulsatile fashion, the amplitude and frequency of which not only vary throughout the day but are influenced by a variety of physiologic stimuli (e.g., stress, pregnancy, exercise). Because of these factors and a serum half-life of 26 to 47 minutes, it had been recommended that when screening for hyperprolactinemia three specimens be obtained at 20- to 30-minute intervals. Each sample could either be analyzed separately and their results averaged or an equal aliquot from each sample could be pooled into a single specimen for analysis. Current Endocrine Society guidelines recommend screening using a single determination, collected at any time of day, reserving the option to follow the aforementioned recommendations if the diagnosis is in doubt ( ). A single PRL level above the sex-matched upper limit of normal is diagnostic for hyperprolactinemia, with values above 250 μg/L usually indicating the presence of a prolactinoma. PRL is measured by immunometric assay.
The major circulating form of PRL is the nonglycosylated monomer. A number of other forms can occur, including “big” PRL and macroprolactin (“big, big” PRL), which are considered to be PRL coupled with immunoglobulin ( ; ). Believed to be clinically inert, these forms can react with commonly utilized immunoassays, resulting in falsely elevated PRL levels in patients in whom pathologic elevation of PRL is not supported clinically. There are two laboratory approaches by which to detect macroprolactin. The gold standard—gel filtration chromatography (GFC)—separates and quantifies prolactin isoforms by size. The second method—precipitation by polyethylene glycol (PPEG)—removes macroprolactin from serum before quantification of monomeric PRL using a standard immunoassay ( ; ; ; ; ; ; ; ). Macroprolactinemia is considered significant if immunoreactive monomeric prolactin is <40% ( ). The existence of macroprolactinemia should be entertained when the clinical picture is not concordant with the PRL level.
The degree of PRL elevation usually correlates well with tumor size. Under certain circumstances, the PRL may be falsely low or minimally elevated considering the size of the tumor. This discordance can be explained by the “hook effect,” and it may result in the misdiagnosis of a patient having a nonfunctioning chromophobe adenoma instead of a prolactin-secreting adenoma.
This effect results when two antibodies to an antigen—in the current case, PRL—are used in an enzyme-linked immunosorbent assay (ELISA; see Chapter 45 ). One of these antibodies, called the capture antibody , is embedded on a solid surface, while the second, called the secondary antibody , is conjugated to a probe that is either an enzyme or a fluorescent or chemiluminescent moiety. If both antibodies are present in a so-called one-step ELISA , at high concentrations of antigen, excess antigen will bind to the secondary antibody in solution, blocking it from binding to any antigen captured by the capture antibody on the solid surface. Thus, the enzyme or fluorescent signal on the solid surface will be diminished, making it appear as if there is a low concentration of antigen ( ; ).
This effect does not occur if a wash step is performed prior to adding the secondary antibody; the wash step removes all excess antigen. Also, performance of homogeneous assays for PRL avoids the hook effect. If a hook effect is suspected, the sample should be diluted, often to levels of 1:1000, and then assayed. Typically, only a single dilution is performed when assaying for PRL in single-step ELISAs. It is preferable to utilize assays for PRL that avoid the hook effect.
If the pretest probability of the patient having a PRL-secreting tumor is high, it is recommended that the serum sample be subjected to serial dilutions or, at the least, a 1:100 dilution.
PRL acts on breast tissue where, in the setting of estrogen priming, it stimulates lactation. PRL also acts at the hypothalamus to inhibit the secretion of gonadotropin-releasing hormone (GnRH). Inhibition of GnRH results in a decrease in the release of LH and FSH from the anterior pituitary gland. In females, this leads to a decrease in estrogen and progesterone synthesis and secretion by the ovaries, along with failure of ovarian follicular maturation (ovulation). In males, a deficiency of FSH and LH causes a decrease in testicular production and synthesis of testosterone, along with a halt in spermatogenesis. It has also been suggested that hyperprolactinemia may stimulate adrenal androgen production and have an effect on immune responsiveness ( ; ).
The reference value for serum PRL is 1 to 25 ng/mL (1–25 μg/L) for women and 1 to 20 ng/mL (1–20 μg/L) for men. The higher PRL levels seen in females begin postpuberty and are presumably due to the stimulatory effect of estrogen ( ). During pregnancy, a progressive rise in serum PRL is observed, with levels reportedly reaching as high as 500 ng/mL by the third trimester ( ). This is largely due to an increase in the number of PRL-secreting cells and can be associated with a doubling or even greater increase in pituitary gland size ( ). PRL levels fall back to baseline about 3 weeks postpartum in women who are not breastfeeding. In nursing mothers, basal PRL levels remain moderately elevated, with episodic bursts in secretion in response to suckling.
PRL levels are increased by many physiologic and pathologic factors, as well as by a wide variety of medications ( Box 25.3 ). Elevations in PRL resulting from physiologic and pharmacologic stimuli rarely exceed 200 ng/mL.
Physiologic
Sleep, stress, postprandially, pain
Coitus, pregnancy, nipple stimulation or nursing
Systemic disorders
Chest wall or thoracic spinal cord lesions
Primary or secondary hypothyroidism
Adrenal insufficiency
Chronic renal failure
Cirrhosis
Psychiatric medications: Phenothiazines, haloperidol, thioxanthenes, buspirone, olanzapine, risperidone, domperidone, monoamine oxidase inhibitors, fluoxetine, amitriptyline
Metoclopramide
Antihypertensives: labetalol, α-methyldopa, reserpine, verapamil
Antihistamines H 2 : cimetidine, ranitidine
Estrogens, oral contraceptives, oral contraceptive withdrawal
Opiates: heroin, methadone, morphine, apomorphine
Thyrotroph (TRH)
Prolactin-secreting pituitary tumor: prolactinoma, acromegaly
Macroadenoma (compressing the pituitary stalk)
Macroprolactinemia
Pressure on or transection of the pituitary stalk, interrupting the transmission of dopamine to D 2 receptors on the lactotrophs: Surgery, traumatic transection, granulomas, metastases, meningioma, irradiation, histiocytosis X
Ectopic secretion of prolactin by nonpituitary tumors
Idiopathic
Polycystic ovarian disease
Epileptic seizures
PRL deficiency can be seen with pituitary necrosis or infarction and in some cases of pseudohypoparathyroidism. In women with complete PRL deficiency, menstrual disorders and infertility have been found ( ). It is PRL excess that is associated with clinical pathology. Hyperprolactinemia leads to inhibition of GnRH secretion, which typically manifests as sexual dysfunction and infertility in both men and women. Women may present with luteal phase abnormalities, oligomenorrhea, or frank amenorrhea, with or without galactorrhea. Men will present with hypoandrogenemia, decreased libido, and impotence. Pituitary adenomas are an important cause of hyperprolactinemia; however, any sellar or parasellar process that compresses the pituitary stalk and interrupts the tonic delivery of dopamine can lead to disinhibition of PRL secretion. Usually, the height of elevation of serum PRL levels correlates with the likelihood of the presence of a pituitary tumor; levels of PRL in excess of 200 ng/mL almost always signify the presence of a prolactinoma ( ; ; ). Unlike other functioning pituitary tumors, the degree of elevation of PRL correlates fairly well with the size of the tumor.
Hyperprolactinemia exists in 20% to 40% of patients with acromegaly. This may be due to the presence of a mixed tumor (containing both lactotrophs and somatotrophs) or to interference with the normally active, PRL-inhibitory mechanisms (e.g., interruption of dopamine delivery owing to stalk compression by a tumor, resulting in disinhibition of PRL secretion). Another important cause of hyperprolactinemia is hypothyroidism. Thyrotropin-releasing hormone (TRH) not only stimulates TSH secretion but it also stimulates PRL secretion, thus explaining the mild hyperprolactinemia seen in both primary (thyroid) and secondary (pituitary) hypothyroidism. Therefore, thyroid function tests (free thyroxine [FT 4 ] and TSH) are always indicated to rule out hypothyroidism when a patient with hyperprolactinemia is evaluated. Thyroid hormone replacement therapy will usually return the PRL level to normal.
It is important to evaluate all patients discovered to have an abnormally elevated PRL. Since hyperprolactinemia can be found in upward of 40% of cases of acromegaly, it is appropriate to measure insulin-like growth factor (IGF)-1. Other hormones that may be assayed include FSH, LH, free testosterone, estradiol, and, if clinically indicated, tests of adrenal axis function. Rarely, hyperprolactinemia may be caused by ectopic hormone production. All patients should undergo computed tomography (CT) or magnetic resonance imaging (MRI) of the sella, performed with and without contrast. If there are no contraindications, MRI is preferred, as it provides better contrast and anatomic detail, is better for visualizing microadenomas, and is safer for serially monitoring patients since there is no exposure to radiation. A formal visual field examination is also a key monitoring tool in managing patients with pituitary tumors and should be done at least yearly in patients with stable disease.
GH is a single-chain polypeptide of 191 amino acids synthesized, stored, and secreted by the somatotrophs of the pituitary gland in response to the secretion of growth hormone–releasing hormone (GHRH) by the hypothalamus. Somatostatin, also produced by the hypothalamus, inhibits GH synthesis and release. Although evidence exists for the direct action of GH on long bone growth in children, most of its anabolic and metabolic actions are mediated indirectly through an intermediary, IGF-1 (also called somatomedin C ). IGF-1 is synthesized in the liver and in certain target tissues in response to stimulation by GH. IGF-1 circulates in the blood complexed to IGF-binding proteins (IGF-BPs); IGF-BP3 is the predominant circulating species. As with all hormones, it is the free, unbound form that is biologically active. Like somatostatin, IGF-1 negatively feeds back on the pituitary gland to inhibit GH secretion.
GH is secreted in a pulsatile fashion; the frequency and amplitude of the peaks are greatest during puberty, exhibiting a steady decline with increasing age ( ). As up to 70% of GH secretion occurs during stage 4 (slow-wave) sleep, it has been suggested that the age-related decline in stage 4 sleep may account for the decline in GH seen with aging ( ; ). In addition to GHRH and somatostatin, several other factors regularly mediate GH secretion ( Fig. 25.2 ). Major stress (e.g., surgery, sepsis), fasting, sex steroids, chronic malnutrition, apomorphine, levodopa, uncontrolled diabetes mellitus, and high-protein meals all stimulate GH secretion. Women tend to have higher GH levels than men, perhaps because of estrogen sensitization of the hypothalamus to other GH stimuli ( ).
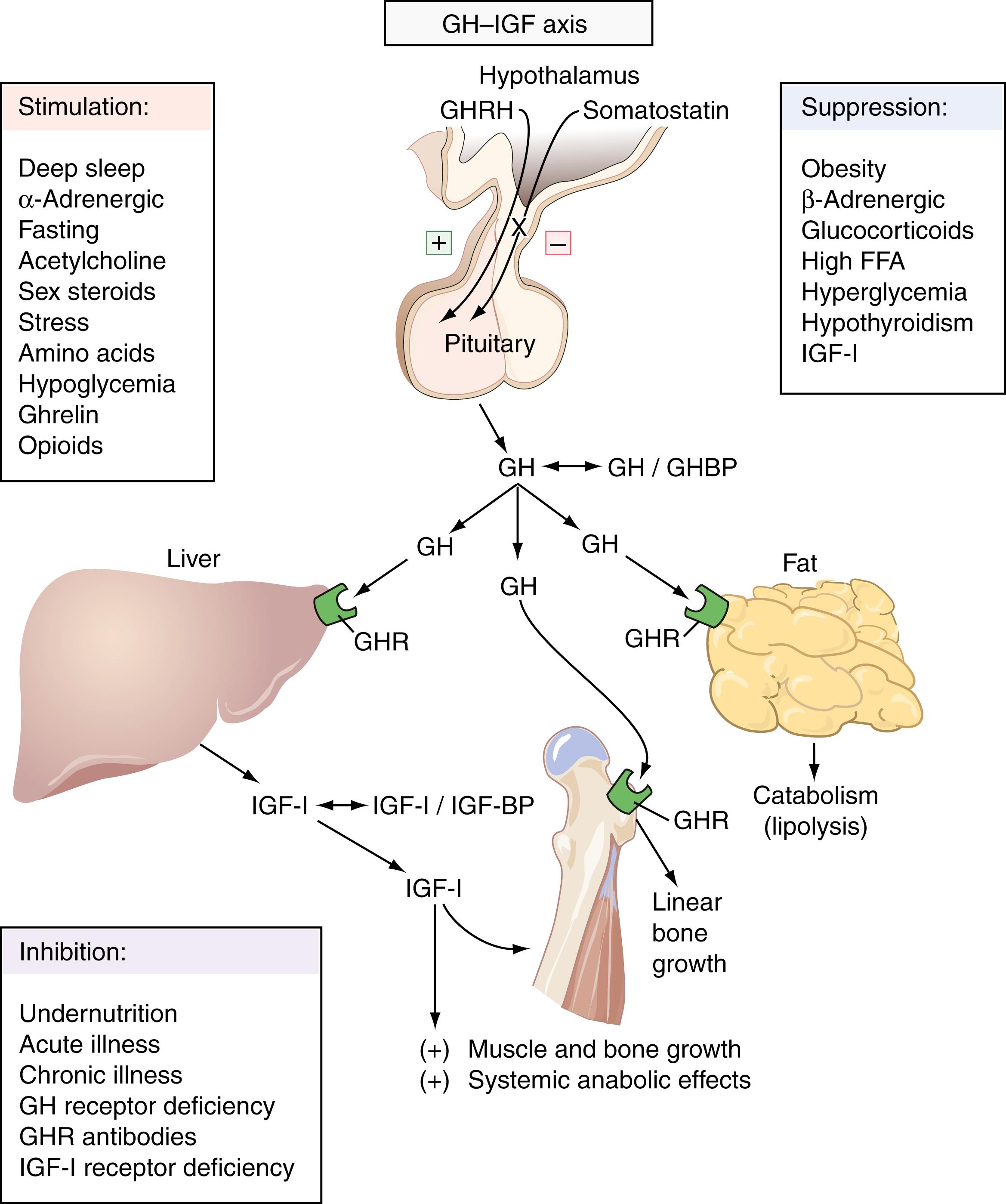
Serum GH is undetectable for most of the day in healthy, nonstressed individuals. This fact, along with the episodic nature of GH secretion, makes a single sampling difficult to interpret. As a result, the diagnosis of GH deficiency is made using GH measurements following pharmacologic stimulation, and GH excess is confirmed by failure of GH suppression following an oral glucose load. GH is commonly measured by chemiluminescent immunoassay.
Somatostatin is synthesized in the paraventricular and arcuate nuclei of the hypothalamus. It inhibits GH and TSH secretion. It also inhibits the secretion of insulin and several gut hormones (e.g., motilin, secretin, gastrin). Ghrelin, a 28–amino acid peptide produced by the gastric neuroendocrine cells and the hypothalamus, binds to the GH secretagogue receptor to stimulate the secretion of GHRH and GH.
Idiopathic growth hormone deficiency is the most common cause of GH deficiency (GHD) in children, whereas a pituitary adenoma is the most common etiology in adult-onset GHD. No simple reproducible method for determining abnormal GH secretory patterns exists. In healthy normal individuals, 70% to 80% of GH results are below 1 ng/mL (<1 μg/L), and secretory peaks typically reach 20 to 40 ng/mL (20–40 g/L) ( ). Thus, in a child with decreased growth velocity, a low or nondetectable GH does not necessarily indicate GHD. Similar to GH, IGF-1 declines with age. IGF-1 is more diagnostically useful in patients younger than 40 years of age; however, it is still not sensitive enough to be used as a standalone test to make the diagnosis of GHD ( ). Manipulation of the endocrine system through stimulation and suppression of the various axes is often required for diagnosing conditions of hormonal deficiency and surfeit. In this vein, GHD is diagnosed by showing failure of GH to increase adequately in response to pharmacologic stimulation. Several endocrine organizations have published guidelines on GH deficiency, detailing which patients to test, which test to utilize, and what cutoffs to apply ( ; ). The insulin tolerance test (ITT) has long been considered the gold standard for diagnosing GHD. However, it is most unpleasant for the patient, requires the attendance of a physician throughout the testing period, and is contraindicated in those with a history of seizures or cardiac or cerebrovascular disease. Failure of GH to rise above 3 to 5 ng/mL in adults when glucose drops to <40 mg/dL ( , ) and above 10 ng/mL in children is considered to be abnormal. In patients unable to undergo an ITT and in those needing a second confirmatory test, arginine stimulation alone or in combination with GHRH is usually the next step. Combination testing with GHRH plus arginine was preferred, as many normal adults failed stimulation with arginine alone ( ), however since GHRH is no longer commercially available in the United States, the glucagon stimulation test (GST) has become the accepted alternative. The mechanism by which glucagon stimulates GH release is not well understood. In this test, glucagon is administered IM to the patient, and GH serum levels are determined every 30 minutes over a 4-hour period. GH usually peaks after 120 to 180 minutes. The initial dose given depends on the BMI. For BMI ≤25 mg/M 2 , a dose of 1 mg is given; for BMI values >25 mg/M 2 , a dose of 1.5 mg is given. GHD is diagnosed by failure of GH to rise above 3 μg/L (a lower cut-point of 1 μg/L in overweight/obese patients improves sensitivity and specificity) ( ). Two caveats about this test should be noted: (1) glucagon is contraindicated in patients with pheochromocytoma, and (2) no studies have been performed in patients with diabetes or pre-diabetes (see Chapter 17 ), so the diagnostic accuracy of this test in these patients has not been established ( ). Common side effects of GST included nausea, vomiting, and delayed hypoglycemia. Glucagon is contraindicated in persons with pheochromocytoma. Other pharmacologic stimuli such as L -dopa, clonidine, and arginine are weak GH secretagogues requiring even lower cutoff points for diagnosis and are not widely recommended for use in the United States ( ). No studies have been performed in persons with diabetes or prediabetes, and therefore the diagnostic accuracy in these populations remains unclear (Yuen, 2011). Other methods to assess for GHD include 24-hour or nighttime monitoring of GH, and provocative tests using clonidine or L -dopa. All of these tests carry significant individual variability. They have not been systematically studied and are used mainly in the pediatric population ( ).
Because body mass index (BMI) affects the GH response to stimulation with GHRH plus arginine, newer guidelines recommend the use of GH cutoffs based on BMI for interpretation of results ( ). There are two settings in which an argument can be made against the need to test for GHD. The first is in patients with known pituitary disease who have a low IGF-1 level. The other scenario is in patients with evidence of three or more pituitary hormone deficiencies; studies have shown these patients to have a greater than 96% chance of having GHD ( ).
Because of perceived and actual deficiencies in these tests, the ghrelin-receptor agonist test is a current avenue of investigation. Ghrelin, sometimes called the “hunger hormone,” is produced by the stomach. Release of ghrelin increases appetite, stimulates the release of GH, and regulates glucose metabolism. Ghrelin has been shown to stimulate growth hormone release through the growth hormone secretagogue receptor (GHSR)1a in the anterior pituitary and hypothalamus (Muller et al., 2015). Macimorelin acetate is an oral ghrelin receptor agonist. When administered in an oral form at 0.5 mg/kg, macimorelin is readily absorbed and stimulates GH secretion with good tolerability ( ). It has been shown to be a reproducible safe diagnostic test for adult growth hormone deficiency, with accuracy comparable to that of the ITT ( ). In a post hoc analysis, using a GH cutoff of 5.1 ng/mL, it showed 92% sensitivity and 96% specificity ( ). A GH cutoff of ≤2.8 μg/L is suggested by the AACE 2019 guidelines ( ).
For children, it is common to use exercise testing in screening for GHD. Further testing is not indicated if the results are normal. However, if GH fails to increase adequately, pharmacologic testing is warranted ( ). Healthy children may not respond to any single GH provocative test; therefore, GHD in childhood is defined by failure of serum GH to reach defined levels when at least two different pharmacologic stimuli are used. IGF-1 measurements have been used to screen for GHD in children; however, since a variety of factors can decrease IGF-1, it is not used to confirm the diagnosis of GHD. IGF-1 levels decline in malnutrition, hypothyroidism, hepatic disease, uncontrolled diabetes mellitus, renal disease, and with age. Levels of IGF-BP3 usually parallel those of IGF-1 and are often low in GHD. However, results falling within the reference range do not exclude the diagnosis of GHD. Up to 18% of subjects with GHD may have normal IGF-1 levels. Care should be taken when interpreting values for IGF-1, IGF-BP3, and GH because the assays themselves and the cutoffs used to define normal differ greatly between laboratories. To address these issues, there is a move to have laboratories utilize a World Health Organization international standard for GH and IGF-1 and to create a normative range from a statistically valid control group ( ).
It is important to understand the difference in the physiology behind testing with GHRH as compared to the other secretagogues. ITT and arginine stimulate the production of GHRH; clonidine increases GH secretion by inhibiting the release of somatostatin. For these stimuli to elicit a rise in GH, the hypothalamus must be intact. When GHRH is used for testing, the hypothalamus is bypassed and the pituitary gland is directly stimulated; therefore, it is possible to miss up to 50% of patients who have tertiary (hypothalamic) GHD.
Individuals with childhood-onset idiopathic GHD are less likely to have permanent GHD. It is recommended that unless they have a known genetic mutation, embryopathic lesion, or irreversible lesion/damage to the pituitary gland/hypothalamus, they should be taken off GH replacement and retested when they reach early adulthood ( ).
Growth hormone overproduction can result in the condition called acromegaly . If the condition develops before closure of the epiphyses, these individuals may be exceedingly tall ( gigantism ). More commonly, it presents during adulthood, causing diffuse enlargement of soft tissues and organs throughout the body. Characteristic features include prognathism, frontal bossing, and spade-like hands. The screening test for clinically suspected acromegaly is a randomly collected IGF-1. A normal IGF-1 with respect to the appropriate age- and gender-matched reference ranges rules out the diagnosis of acromegaly ( ). However, it should be noted that a physiologic increase in IGF-1 may be seen during pregnancy and late-stage adolescence ( ). If the level of IGF-1 is elevated or borderline with respect to the appropriate age- and gender-related reference range, it becomes necessary to confirm the diagnosis using an oral glucose tolerance test (OGTT). The OGTT is performed by obtaining baseline blood samples for glucose and GH, administering 75 g of glucose orally, and then obtaining blood for glucose and GH every 30 minutes over the next 2 hours. A normal response is suppression of GH to <1 ng/mL (1 μg/L) at any time during the test. If GH fails to drop to below 1 ng/mL (1 μg/L), the patient is diagnosed as having acromegaly ( ; , ). GH may fail to adequately suppress in the setting of puberty, uncontrolled diabetes mellitus, malnutrition, hepatic disease, and renal disease. However, all but puberty are associated with a normal serum IGF-1 ( ).
The difficulty comes in diagnosing mild disease. Freda had studied 60 postoperative patients with acromegaly, 22 patients with active disease (elevated IGF-1), 38 patients in remission (normal IGF-1), and 25 healthy controls. The highest nadir GH was 0.13 μg/L in the controls and 0.3 μg/L in those with active disease. Of those with active disease, 50% had GH values <1 μg/L, leading to misclassification of these individuals as being normal when the criterion of a GH value <1 μg/L was applied ( ; ). In a more recent study of 16 patients with mild disease (the majority having microadenomas), Dimaraki and colleagues (2002) found that 50% of patients with acromegaly were able to suppress their GH levels to <1 μg/L following an OGTT. A proposed reason for these patients with active disease showing normal suppression is that they may have subtle changes in GH secretion that are not elicitable using this short suppression test. To address this issue, the following suggestion has been proffered ( ). If the initial random IGF-1 is normal and GH is <0.3 μg/L, then acromegaly is excluded. If either test is abnormal, repeat the IGF-1 and proceed to an OGTT. Failure of GH to be suppressed below 0.3 μg/L, accompanied by an elevated IGF-1, is diagnostic of acromegaly. Suppression of GH below 0.3 μg/L with normal IGF-1 excludes acromegaly. For those who adequately suppress their GH but have an elevated IGF-1, close follow-up is recommended ( ). Oral, but not transdermal, estrogen can reduce IGF-1 concentrations ( ); therefore, temporarily withholding oral estrogen therapy during testing may be prudent.
Acromegalic patients may show a paradoxical rise, an absent response, or a partial decline in GH in response to an OGTT ( ). Random GH sampling is not adequate to establish the diagnosis of acromegaly because considerable overlap in GH exists between healthy persons and patients with the disease. In addition, GH can be increased in patients with a number of disorders, including renal disease, cirrhosis, malnutrition, and uncontrolled diabetes mellitus, as well as in patients under physical or emotional stress. Although larger adenomas are generally associated with higher GH levels, the correlation between GH levels and acromegalic manifestations is poor. In most patients with untreated acromegaly, GH levels remain above basal levels throughout the day, ranging from 10 to 100 ng/mL (10–100 μg/L). Serum GH levels in excess of 50 to 100 ng/mL (50–100 μg/L) are virtually never seen in nonacromegalic persons ( ). Other diagnostic maneuvers using TRH, GnRH, and GHRH yield discordant results in about 50% of patients with acromegaly. Therefore, their use for diagnostic purposes or for monitoring disease activity is not recommended ( ). In patients with documented acromegaly but no evidence of a pituitary tumor, GHRH should be measured to detect a hypothalamic or ectopic source for acromegaly.
Patients treated for acromegaly require lifelong monitoring for disease activity. Patients treated with surgery or radiation can be monitored by the GH response to OGTT and a random IGF-1 measurement. The goal of therapy is to normalize IGF-1 and suppress the GH response to OGTT to <1 ng/mL (<1 μg/L). Several studies have shown that when these goals are attained, mortality rates become comparable with those of the normal population ( ; ; ; ). If an ultrasensitive assay is available, an OGTT GH nadir of <0.4 μg/L can be used. However, this lower cutoff has not been demonstrated to improve metabolic outcome or better diagnose remission (Melmed, 2018; Ku, 2016). As mentioned previously, caution is required in interpreting IGF-1 levels in women on oral but not topical estrogen treatment, as oral estrogens lower IGF-1 concentrations ( ). For patients on somatostatin receptor analogs, monitoring is performed by measuring GF-1 and GH just prior to the next treatment dose. Patients on GH receptor blockers are monitored by a random IGF-1 alone. In rare instances, acromegaly may be due to ectopic secretion of GHRH (e.g., bronchial carcinoid, pancreatic islet cell tumors, small cell lung cancer), in which case the measurement of GHRH may be useful.
Oxytocin and vasopressin (see Chapter 15, Chapter 9 ) are the two major hormones of the posterior pituitary gland. They each have distinct physiologic roles, with partially overlapping effects in stimulating smooth muscle contraction and in maintaining water homeostasis. They are small oligopeptides, each composed of nine amino acid residues with a total mass of about 1 kDa. They are synthesized in the nerve cell bodies within the hypothalamus and are transported along axons to the nerve terminals within the posterior pituitary gland, where they are stored in secretory granules. Neuronal action potentials originating in the hypothalamus are conducted along the axons, resulting in degranulation of the vesicles and release of oxytocin and vasopressin into the perivascular space and ultimately into the circulation. Unlike the anterior pituitary gland, the posterior pituitary gland is little more than a repository where hormones are held until they are secreted. Accordingly, disruption of the infundibular stalk or destruction or removal of the posterior pituitary gland does not necessarily result in complete loss of these hormones, as they are produced in the hypothalamus.
Oxytocin secretion is stimulated by stretching of the cervix and vagina during parturition; this is known as the Fergusson reflex. Oxytocin contributes to uterine contractions late in labor both by direct action on the myometrium and by stimulation of prostaglandin secretion by the decidua ( ). It also plays a role in hemostasis at the placental site following delivery. Throughout pregnancy, estrogen upregulates the numbers of oxytocin receptors on the uterus and decidua, resulting in increased sensitivity to oxytocin toward the end of the term.
Oxytocin stimulates the myoepithelial cells surrounding the mammary glands and lactiferous ducts to contract, resulting in milk ejection. In response to suckling, neurogenic stimuli are transmitted from the nipple to the third, fourth, and fifth thoracic nerves, to the spinal cord, to the midbrain, and on to the hypothalamus, where oxytocin release is triggered. Psychological stimuli can bypass this pathway, with bursts of oxytocin secretion occurring with anticipation of nursing or on hearing a baby cry ( ; ). Stressful situations can inhibit its secretion, resulting in decreased milk ejection.
Pathologic conditions associated with oxytocin excess or deficiency are rare and are limited to case reports. Its function in males remains unknown. Clinical demand for the measurement of oxytocin levels is extremely rare. Because it has a half-life of 3 to 5 minutes and is subject to rapid degradation by oxytocinase, individual reference laboratory procedures for its collection must be strictly adhered to if meaningful results are to be obtained.
AVP (ADH) is synthesized within the paraventricular and supraoptic nuclei of the hypothalamus. The major function of ADH is to maintain osmotic homeostasis by regulating water balance. It does this by stimulating the V2 receptors on principal epithelial cells that line the cortical collecting ducts of the kidney. As discussed in Chapter 15 , ADH induces an increase in the production of cyclic adenosine monophosphate (cAMP), which, in turn, causes the water channels (aquaporins) to fuse with the apical membrane of the principal cells, increasing water permeability and reabsorption. At much higher blood concentrations, AVP also acts as a potent pressor by causing vasoconstriction (V1 receptor), stimulates ACTH secretion from the anterior pituitary gland, and stimulates the production of clotting factor VIII.
ADH secretion is modulated by changes in serum osmolality and by alterations in intravascular volume. Changes in plasma osmolality are detected by osmoreceptors located within the anterior hypothalamus. Plasma ADH rises and falls in direct proportion to the serum osmolality. A concordantly linear relationship exists between ADH and the urine osmolality (Uosm). The Uosm is capped at about 1200 mOsm/kg, the maximum Uosm allowed by the renal medullary concentration gradient.
ADH secretion is maximally stimulated at a serum osmolality greater than 295 mOsm/kg and is suppressed when the osmolality falls below 284 mOsm/kg. Thirst can be considered the fail-safe mechanism for fluid and osmotic homeostasis, as it is initiated at a greater plasma osmolality (Posm) than is needed to stimulate ADH secretion. The thirst receptors are located within the hypothalamus and are distinct from the osmoreceptors that stimulate ADH secretion.
Changes in intravascular volume are detected by baroreceptors. There are two sets of baroreceptors: low-pressure volume receptors located in the right atrium and pulmonary venous system, and high-pressure arterial baroreceptors located in the carotid sinus and aortic arch. These receptors are normally under tonic inhibition; a drop in intravascular volume removes the inhibition and results in a rise in ADH, leading to increased water reabsorption. ADH secretion is much more sensitive to changes in osmolality than to changes in intravascular volume. A 1% to 2% increase in osmolality will cause a rise in ADH secretion. A much greater stimulus, such as a 5% to 10% drop in blood volume or blood pressure, is needed before the baroreceptors trigger the release of ADH.
In addition to changes in osmolality and intravascular volume, other physiologic factors that can stimulate ADH secretion include nausea, cytokines, interleukin (IL)-6, hypoglycemia, hypercarbia, and nicotine ( ; ).
Basal plasma vasopressin normally ranges from 0.5 to 2 pg/μL. In concert with atrial natriuretic hormone, thirst, and the renin-angiotensin-aldosterone axis, ADH works to maintain blood pressure, volume, and tonicity. Accordingly, when a patient is evaluated for a disorder of water homeostasis, these multiple interactive factors must be taken into consideration. Normal plasma osmolality ranges from 280 to 295 mOsm/kg and plasma sodium from 135 to 145 mmol/L. It can be determined directly by measuring the plasma freezing-point depression or the plasma vapor pressure. It can also be determined indirectly, by measuring the plasma sodium, blood urea nitrogen (BUN), and glucose, and applying the following formula (also, see Chapter 9 ):
This equation is discussed at length in Chapter 15, Chapter 9 .
Because sodium is the major plasma solute, hyponatremia is tantamount to hypoosmolality. There is usually excellent agreement between the directly measured and calculated osmolality. However, there are two situations when this does not hold true: in pseudohyponatremia (also termed factitious hyponatremia ), and in the presence of high concentrations of other effective solutes. In pseudohyponatremia, the plasma volume is displaced by the presence of high concentrations of lipids or proteins (e.g., hyperglobulinemia), resulting in a low plasma concentration of Na+, although the activity of Na+ in water is normal. In these instances, if Na+ is directly measured with an ion-specific electrode (ISE), the activity reading is normal. In order to extend electrode life, laboratories using high-throughput analyzers generally perform ISE measurements on diluted plasma (so-called indirect ISE measurement), making the resultant plasma concentration measurement vulnerable to pseudohyponatremia. The measured Posm is unaffected by the presence of hyperlipidemia or paraproteinemia, as the concentration of solute particles per volume of fluid is unchanged.
The presence of high concentrations of osmotically active solutes—such as glucose, radiographic contrast agents, mannitol, and ethylene glycol—causes a shift of water from the intracellular to the extracellular compartment, leading to dilutional hyponatremia (this situation, though quite different from that described earlier, is also sometimes referred to as pseudohyponatremia ). Similar translocational hyponatremia occurs with the absorption of glycine during transurethral prostate resection as well as in gynecologic and orthopedic procedures ( ; ). The calculated Posm is unreliable in these situations; Posm must be measured directly instead. A difference between the calculated and measured Posm of greater than 10 mOsm/kg supports the presence of pseudohyponatremia or other osmolar solutes ( ). The most common situation causing a large “osmolal gap” is ethanol intoxication.
In hyperglycemia, the measured Na+ should be corrected by the addition of 1.6 mmol/L for each 100-mg/dL increase in serum glucose above normal (100 mg/dL). A more recent analysis suggested that this formula may underestimate the decrease in Na+ caused by hyperglycemia and supported the use of a 2.4-mmol/L correction factor ( ). True hyponatremia is present if, after the correction formula is applied, the serum Na+ is still <135 mmol/L.
During pregnancy, there is a resetting of the osmostat, with a drop in osmolality of approximately 10 mOsm/kg ( ). The precise mechanism for this is not known; however, studies have suggested a role for the hormone relaxin and for the enzyme oxytocinase. During pregnancy, there is a progressive increase in placental production of oxytocinase, which, in addition to breaking down oxytocin, breaks down vasopressin, possibly resulting in a change in the hypothalamic AVP osmolality feedback loop.
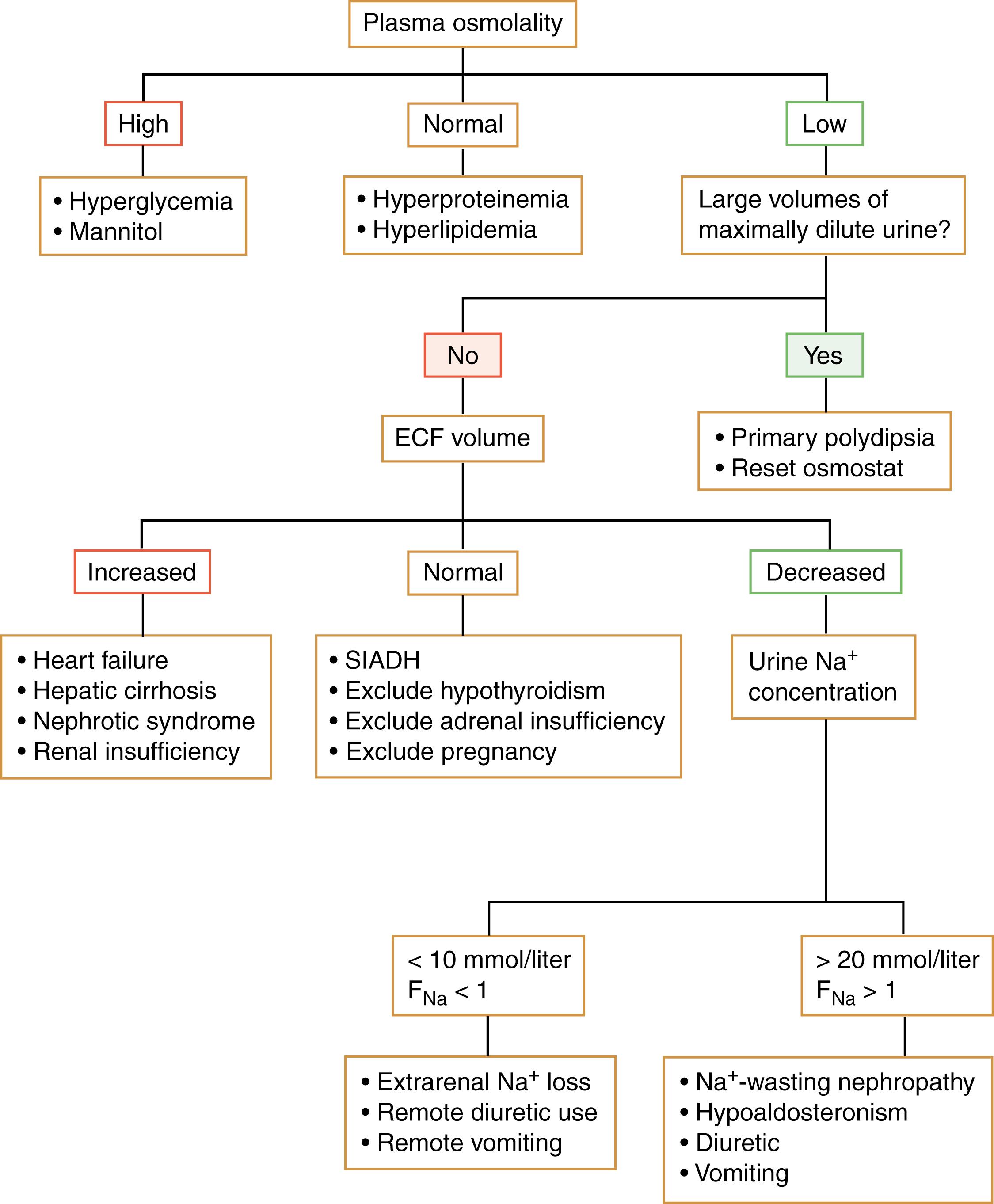
Diabetes insipidus (DI) is classified as being either central (due to absent or decreased ADH secretion from the hypothalamus or neurohypophysis) or nephrogenic (due to renal resistance to the actions of ADH). Each is further subcategorized as being complete or partial. All variants are characterized by the passage of large volumes of dilute urine (>2.5 L/day) in the face of an inappropriately elevated Posm. Provided that the thirst mechanism is intact and there is free access to water, most individuals can avoid dehydration and maintain a normal Posm and serum sodium levels.
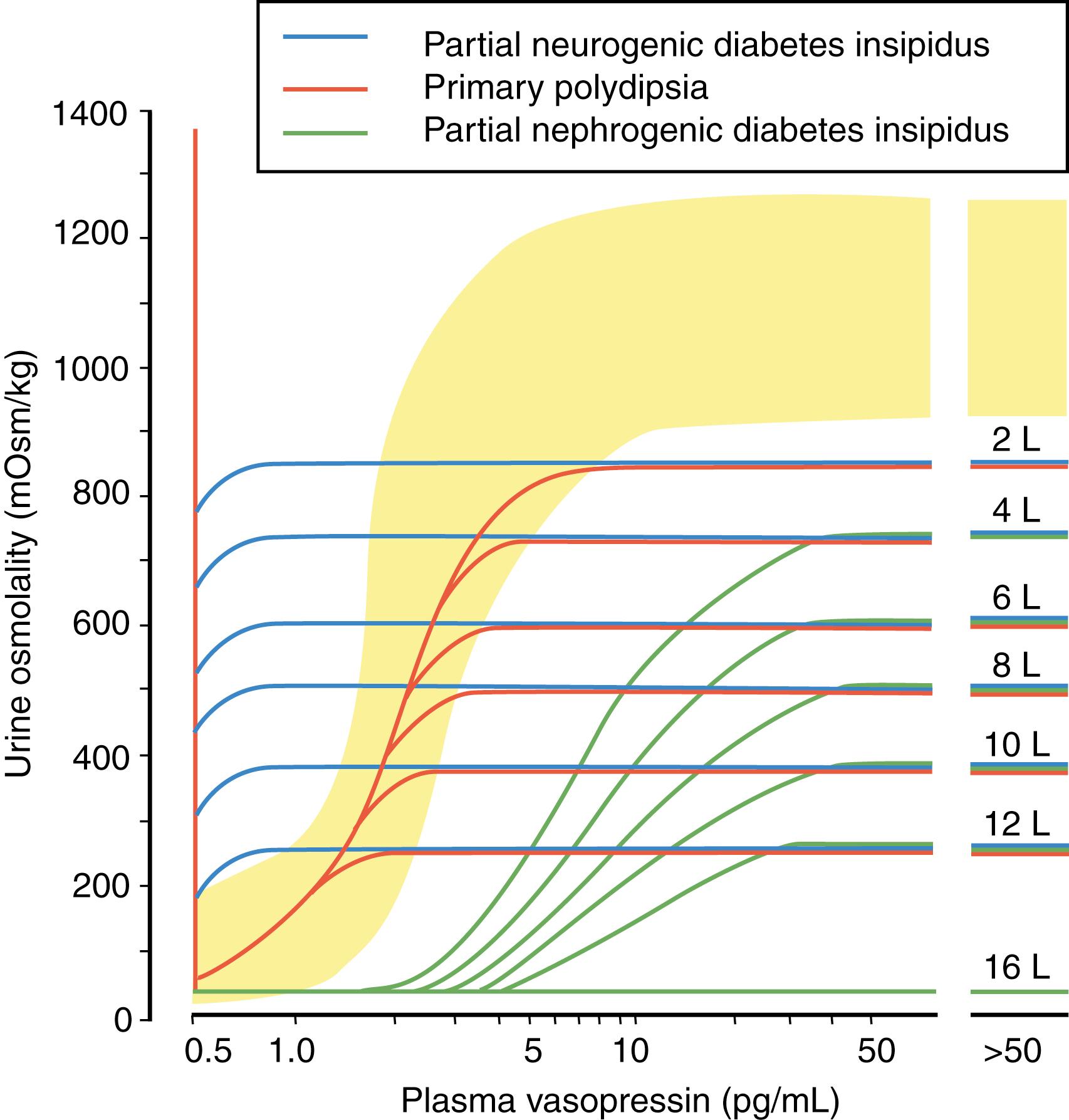
The water deprivation test is the preferred diagnostic test for identifying the presence of DI ( Table 25.2 ). Central and nephrogenic DI can be distinguished by the response to either endogenous or exogenous vasopressin ( Table 25.3 ). In neurogenic DI, ADH levels are low and the kidney rapidly acts to conserve water in response to exogenous ADH administration. In contrast, nephrogenic DI is associated with normal or increased levels of ADH, and administration of additional ADH has little or no effect on renal water reabsorption. Differentiating partial nephrogenic DI from primary polydipsia can be more difficult. Unlike DI, in which the underlying problem is difficulty in holding on to free water, in primary polydipsia the pathophysiology arises from increased intake of fluids. Primary polydipsia (PP) can be due to either dipsogenic DI or psychogenic polydipsia. In dipsogenic DI, the setpoint for ADH secretion is normal; however, a resetting of the thirst threshold occurs so that it is now below the threshold for ADH secretion. In psychogenic polydipsia, the osmostat for ADH secretion is normal. However, because of underlying psychiatric illness, the subject has a compulsion to drink excessive volumes of liquid. This intake of copious volumes of fluid as seen in PP leads to a washout in the medullary concentrating gradient within the kidney, resulting in a decreased ability to concentrate the urine in response to dehydration. Differentiating between nephrogenic DI and PP, as well as among those who have an equivocal response to ADH, is best achieved by plotting the basal and postdehydration Uosm and plasma ADH on nomograms created by Zerbe and Robertson ( Figs. 25.3 and 25.4 ) ( , ). Direct measurement of plasma ADH may be helpful in confirming the diagnosis of a pathologic ADH deficiency or excess in the presence of a known or suspected disorder of renal function or fluid and electrolyte homeostasis, or when a patient is unable to tolerate physiologic testing. In cases of congenital DI, family members who are heterozygote carriers of ADH gene mutations may be identified by demonstrating decreased ADH levels, which may be the only subclinical manifestation of ADH deficiency.
| Patients with mild polyuria may be instructed to withhold all fluid intake from 10 pm onward. For those with more severe polyuria (8 to 10 L/day), water deprivation should be started early in the morning under close observation. | |
|
|
| Precautions | |
| When possible, discontinue any medications that can influence ADH secretion. Observe for hypotension and nausea, which may stimulate ADH secretion. The patient should not be permitted to smoke during the test. | |
| Interpretation | |
| Normal | Final Uosm before AVP challenge is higher than Posm. Following AVP challenge, the Uosm is less than 10% higher than the maximal Uosm achieved with water restriction alone. |
| Neurogenic DI | Final Uosm before AVP challenge is less than Posm. Following AVP administration, there is a greater than 50% increase in Uosm. |
| Nephrogenic DI | Final Uosm before AVP challenge is less than Posm. Following AVP administration, there is a less than 10% increase in Uosm. |
| Partial central DI | Uosm may be higher than Posm following dehydration; however, there is only a 10% to 50% increase in Uosm following administration of AVP. |
| Partial nephrogenic DI | Uosm may be higher than Posm following dehydration; however, there is a greater than 10% increase in Uosm following administration of AVP. |
| Plotting basal and postdehydration Uosm and plasma ADH on the nomograms from Zerbe and Robertson will permit further distinction between partial nephrogenic DI, partial central DI, and primary polydipsia (see Figs. 25.3 and 25.4 ). | |
| Disorder | BASELINE | AFTER 12-HOUR FLUID RESTRICTION | Urine Osmolality Post-AVP Challenge | ||||
|---|---|---|---|---|---|---|---|
| Serum Na+ and Osmolality | Urine Na+ and Osmolality | Serum ADH | Serum Na+ and Osmolality | Urine Na+ and Osmolality | Serum ADH | ||
| Normal control | N | N | N | N | High | High | Same |
| SIADH | Low | N–High | High | Low–N | High | High | — |
| Neurogenic DI | N-High | Low | Low | High | Low–N | Low | Increased |
| Nephrogenic DI | N–High | Low | N–High | High | Low–N | High | Same |
| Psychogenic polydipsia | Low–N | Low | Low | N | N–High | N–High | Same |
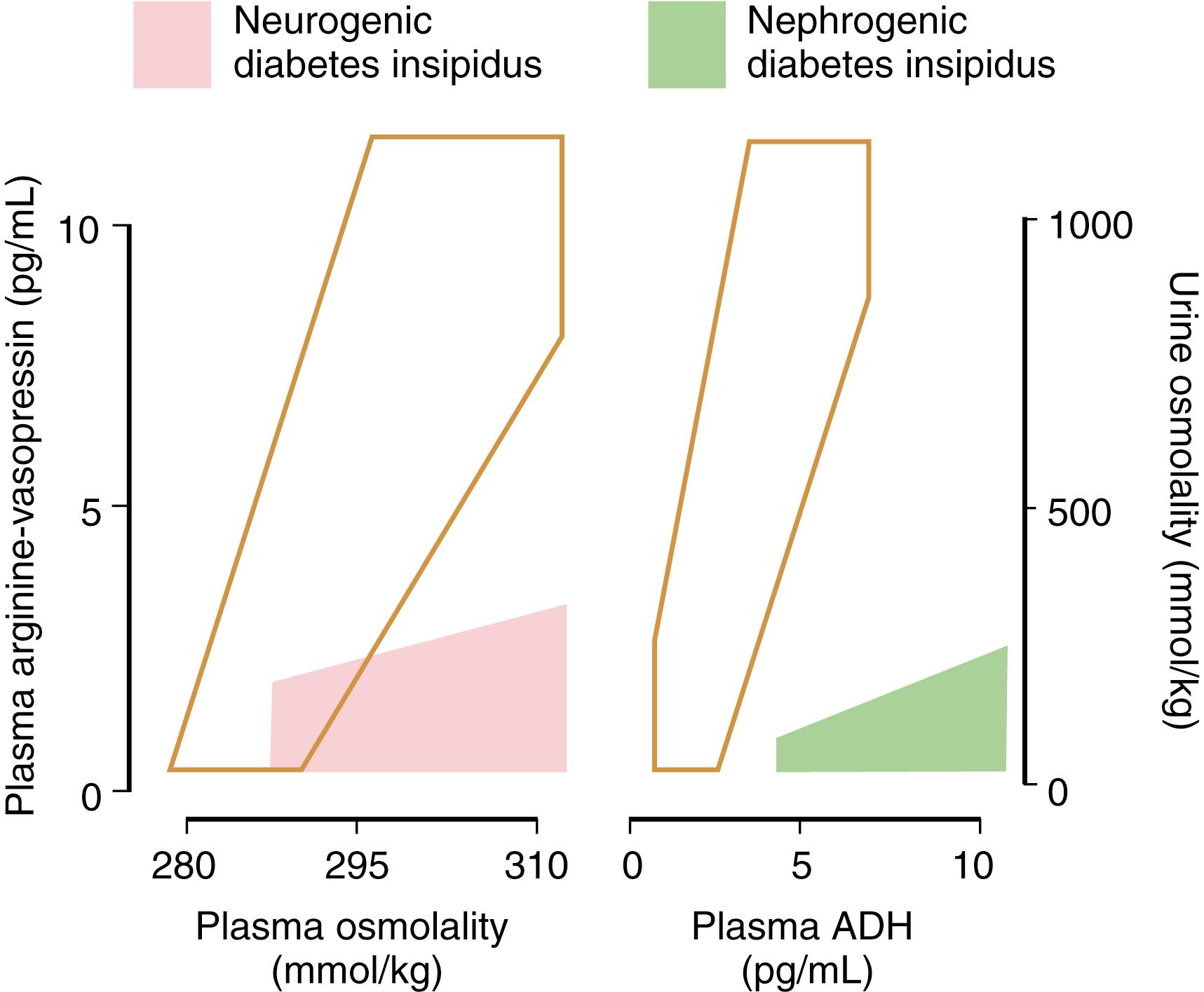
Due to instability of ADH (Morgenthaler, 2006), low accuracy (38%) in differentiation between DI and PP (Fenske, 2012), and the lack of a clinically useful description of the relationship between plasma AVP levels and osmolality ( ), other avenues for diagnosing DI vs. PP have been sought. Among ADH biomarkers is copeptin, the 39 amino-acid C-terminal segment of pre-pro-arginine vaspression. It is secreted in response to the same stimuli as AVP and in equimolar amounts to AVP. Its high ex-vivo stability and ease of measurement make it an excellent surrogate for AVP. The normal range for copeptin is 1.0-13.8 pmol/L, with a higher median value seen in men ( ). A baseline copeptin level of >21.4 pmol/L (without pre-thirsting) is considered diagnostic of nephrogenic DI ( ). Measurement of copeptin in conjunction with the hypertonic infusion test has been shown to be superior to the standard water deprivation test in distinguishing PP from central DI, with a diagnostic accuracy of 97% when using a cutoff of >4.9pmol/L. In complete and partial DI the copeptin is ≤4.9 pmol/L and >4.9 pmol/L in PP with a sensitivity of 93.2% and specificity of 100% (Fenske, 2018). In CKD, P(cop) and P(vp) both increased with decreasing estimated glomerular filtration rate (eGFR), but P(cop) increased much faster than P(vp) ( ). Copeptin also rises in acute illness, myocardial infarction, and stroke. Another proposed approach is copeptin measurement following stimulation by arginine infusion, which has the advantage of already being a standard clinical test used to evaluate GHD and has been shown to differentiate between DI and PP with high accuracy (95%) (Winzeler 2019).
Nephrogenic DI is either congenital or acquired. An X-linked receptor defect is the most common etiology for congenital nephrogenic DI; medications are the most common cause of acquired nephrogenic DI ( ). Medications known to be associated with acquired nephrogenic DI include lithium, demeclocycline, and methoxyflurane anesthetics. Other causes of acquired nephrogenic DI include hypercalcemia, hypokalemia, sickle cell disease, and postobstructive uropathy ( Box 25.4 ).
Primary
Familial
Idiopathic
Acquired
Tumors—craniopharyngioma, pituitary tumors, metastases (i.e., lung, breast), Rathke cleft cyst, nonlymphocytic leukemia
Granulomatous disorders—sarcoidosis, Langerhans cell histiocytosis, Wegener granulomatosis
Traumatic—head trauma, surgery
Infectious—tuberculosis, meningitis, encephalitis
Vascular—cerebral aneurysm, sickle cell anemia, Sheehan syndrome
Drugs—alcohol, diphenylhydantoin, chlorpromazine, and β-adrenergic agonists
Other—lymphocytic hypophysitis, hypoxic encephalopathy
Hereditary
Mutation in the V 2 receptor
Mutation in the aquaporin gene
Acquired
Drugs—lithium, phenytoin, demeclocycline, vinblastine, cisplatin, propoxyphene, colchicine, gentamicin, amphotericin, ethanol, atrial natriuretic hormone, norepinephrine, methoxyflurane anesthetics, furosemide
Electrolyte disorders—hypercalcemia, hypokalemia
Systemic illnesses—sickle cell, multiple myeloma, amyloidosis, sarcoidosis, Sjögren syndrome, polycystic kidney disease
Other—low-protein diet, postobstructive uropathy
Neurogenic DI may be congenital, the result of an autosomal-dominant mutation in the ADH signal peptide or in the exons that code for neurophysins ( ; ). More commonly, neurogenic DI is induced by lesions affecting the hypothalamus (metastatic tumors, trauma, granulomatous disorders) or medications. Drugs that may cause neurogenic DI by suppressing ADH release include phenytoin (Dilantin), chlorpromazine, and α-adrenergic agonists. It has been suspected that autoantibodies directed against the ADH-producing cells of the hypothalamus may be responsible for some cases of idiopathic neurogenic DI ( , ; ). These antibodies are detectable by indirect immunofluorescence antibody.
Another common condition in which decreased ADH levels have been found is primary nocturnal enuresis in children. Although these patients have ADH levels only slightly lower than controls (2.9 ng/L vs. 3.6 ng/L), the clinical symptoms are reliably ameliorated by vasopressin therapy ( ). The determination of ADH levels in diagnosing this condition is not always helpful because of moderate overlap between enuretic and normal children. Measuring the specific gravity in the first morning urine is more reliable for identifying children who may benefit from ADH supplementation; they will have a urine specific gravity below 1.015 ( ).
The syndrome of inappropriate secretion of ADH (SIADH) is characterized by a euvolemic hypoosmolar hyponatremia associated with hyperosmolar urine (the result of continued inappropriate natriuresis). By definition, SIADH cannot be diagnosed until nonosmotic stimuli for ADH secretion and other pathologies that interfere with free water clearance have been excluded ( ). Physiologic triggers of ADH secretion include nausea, pregnancy, hypoglycemia, intracranial hypertension, mechanical ventilation, and hypoxia. Hypothyroidism and glucocorticoid deficiency cause a decrease in free water clearance, leading to a dilutional hyponatremia. Mineralocorticoid deficiency can lead to hyponatremia caused by increased renal sodium loss. It is therefore extremely important to test for deficiencies of these axes and to institute appropriate hormonal replacement therapy before making a diagnosis of SIADH. Other confounding factors that can hamper the diagnosis of SIADH include renal disease, cardiac disease, and medications such as diuretics. In these situations, one can cautiously perform a water load test ( Table 25.4 ). In the absence of medications or other conditions that may impair diuresis, failure to excrete 80% to 90% of the administered water load within 4 hours and to suppress the Uosm to <100 mOsm/kg is consistent with the diagnosis of SIADH.
|
|
| Interpretation | |
| Normals | Excrete 80% to 90% of the administered water load within 4 hours and suppress the Uosm to <100 mOsm/kg. |
| SIADH | Failure to meet these criteria in the absence of medications or other conditions that may impair diuresis is consistent with the diagnosis of SIADH. |
In the right clinical setting, the diagnosis of SIADH often can be confidently made on the basis of serum and urine electrolyte determinations alone. A spot urine Na+ less than 30 mmol/L can generally distinguish those with hyponatremia due to volume depletion from those with SIADH, in whom urine Na+ is greater than 30 mmol/L ( ). In contrast, differentiating the patient with euvolemia and SIADH from the one with hypovolemia and concomitant renal salt wasting (e.g., salt-losing nephropathy, diuretic use) can be much more difficult. In addition to hyponatremia, both have a spot UNa+ greater than 30 mmol/L and a fractional excretion of Na+ greater than 1. The fractional excretion of sodium (FENa [%]) is calculated as follows:
where U [Na + ] = urine sodium concentration, P [Na + ] = plasma sodium concentration, U [Cr] = urine creatinine concentration, and P [Cr] = plasma creatinine concentration.
One way to distinguish between the two is to administer 1 L of 0.9% NaCl intravenously over 24 hours for 2 days. A rise in serum Na+ by more than 5 mmol/L is suggestive of hypovolemia. In SIADH, no change or an increase of less than 5 mmol/L occurs. Alternatively, fluid restriction to 600 to 800 mL/day for 2 to 3 days will lead to improvement in hyponatremia in SIADH but not in renal salt wasting (see Table 25.3 ).
The cause of renal salt wasting in SIADH is twofold. At the onset of the disease, there is volume expansion, which inhibits the renin-aldosterone axis and leads to greater improved delivery of sodium to the distal tubules. The continued sodium loss is due to secretion of atrial natriuretic protein ( ), which also is stimulated by increased intravascular volume.
Because of hyperfiltration and the local actions of ADH on the renal V2 receptor, SIADH is one of the two states in which some of the lowest uric acid levels occur; the other is pregnancy. In SIADH, the serum levels of ADH may be variably increased, usually out of proportion to Posm. However, approximately 20% of cases meeting the physiologic diagnosis of SIADH will not have detectable levels of ADH ( ). This may be due to insensitivity of the assay to low levels of ADH, increased renal sensitivity to ADH, or perhaps the presence of another hormone with antidiuretic activity ( ).
The diagnosis of SIADH is largely a diagnosis of exclusion, with the clinician having ruled out other causes of hyponatremia ( Fig. 25.5 ). It most often occurs as a manifestation of the paraneoplastic syndrome. However, it may also occur in central nervous system trauma or infection, in lung disease, or as the result of medications (vinca alkaloids, tricyclic antidepressants, selective serotonin reuptake inhibitors; Box 25.5 ).
CNS disease
Neoplasm, infection, trauma, cerebrovascular accident
Oat cell carcinoma and adenocarcinoma of the lung, pancreatic cancer, lymphoma
Pulmonary infection
TB, pneumonia, positive-pressure ventilation
Idiopathic
Oral hypoglycemics—chlorpropamide, tolbutamide
Antineoplastics—vincristine, cyclophosphamide
Diuretics—hydrochlorothiazide
Psychotropics—amitriptyline, phenothiazines, SSRIs, monoamine oxidase inhibitors
Other—morphine, barbiturates, clofibrate, nicotine, acetylcholine, anesthetic agents, β-adrenergic stimulants, metoclopramide, desmopressin
CNS, Central nervous system; SIADH, syndrome of inappropriate secretion of antidiuretic hormone; SSRIs, selective serotonin reuptake inhibitors.
The normal thyroid gland weighs about 15 to 25 g. It consists of two lobes connected in the middle by a narrow isthmus. It is divided into lobules, each composed of 20 to 40 follicles, separated by highly vascular connective tissue. The follicles are the site of thyroid hormone synthesis and storage. They are ring-shaped structures, formed by a single-cell band of follicular cells which surrounds a cavity containing colloid, thyroid hormone, thyroglobulin (Tg), and a variety of other glycoproteins. The follicular cells rest on a glycoprotein-rich basement membrane that separates the cells from surrounding capillaries. Microvilli at the apex of the follicular cells extend into the colloid, where iodination, exocytosis, and the initial phase of hormone secretion occur. The thyroid gland also contains parafollicular cells, or C cells, which are responsible for the synthesis and secretion of calcitonin, a hormone important in calcium metabolism ( ; ).
An intact hypothalamic-pituitary-thyroid (HPT) axis and a ready source of iodide are required for normal thyroid hormone synthesis. The hypothalamus secretes TRH, which, in turn, stimulates the thyrotrophs of the anterior pituitary gland to secrete TSH (thyrotropin, thyroid stimulating hormone). As implied by the name, TSH stimulates thyroid hormone synthesis and secretion by the thyroid gland. Thyroid hormone exerts negative feedback on both the hypothalamus and pituitary gland to maintain a TSH concentration within narrow limits; it also acts peripherally to mediate numerous metabolic activities.
Under TSH stimulation, iodine enters the follicular cells as inorganic iodide and through a series of metabolic steps is integrated, forming thyroid hormones, thyroxine (T 4 ), and 3,5,3′-triiodothyronine (T 3 ). The sequence of events as depicted in Figure 25.6 can be broken down into (1) active transport of iodide into the cell; (2) iodination of the tyrosyl residues on Tg (organification); (3) coupling of iodotyrosine molecules within Tg to form T 4 and T 3 ; (4) proteolysis of Tg with release of free iodotyrosine, T 4 , and T 3 , and secretion of iodothyronine into the circulation; (5) deiodination of iodotyrosines within the thyroid and reuse of liberated iodide; and (6) deiodination of T 4 to T 3 .
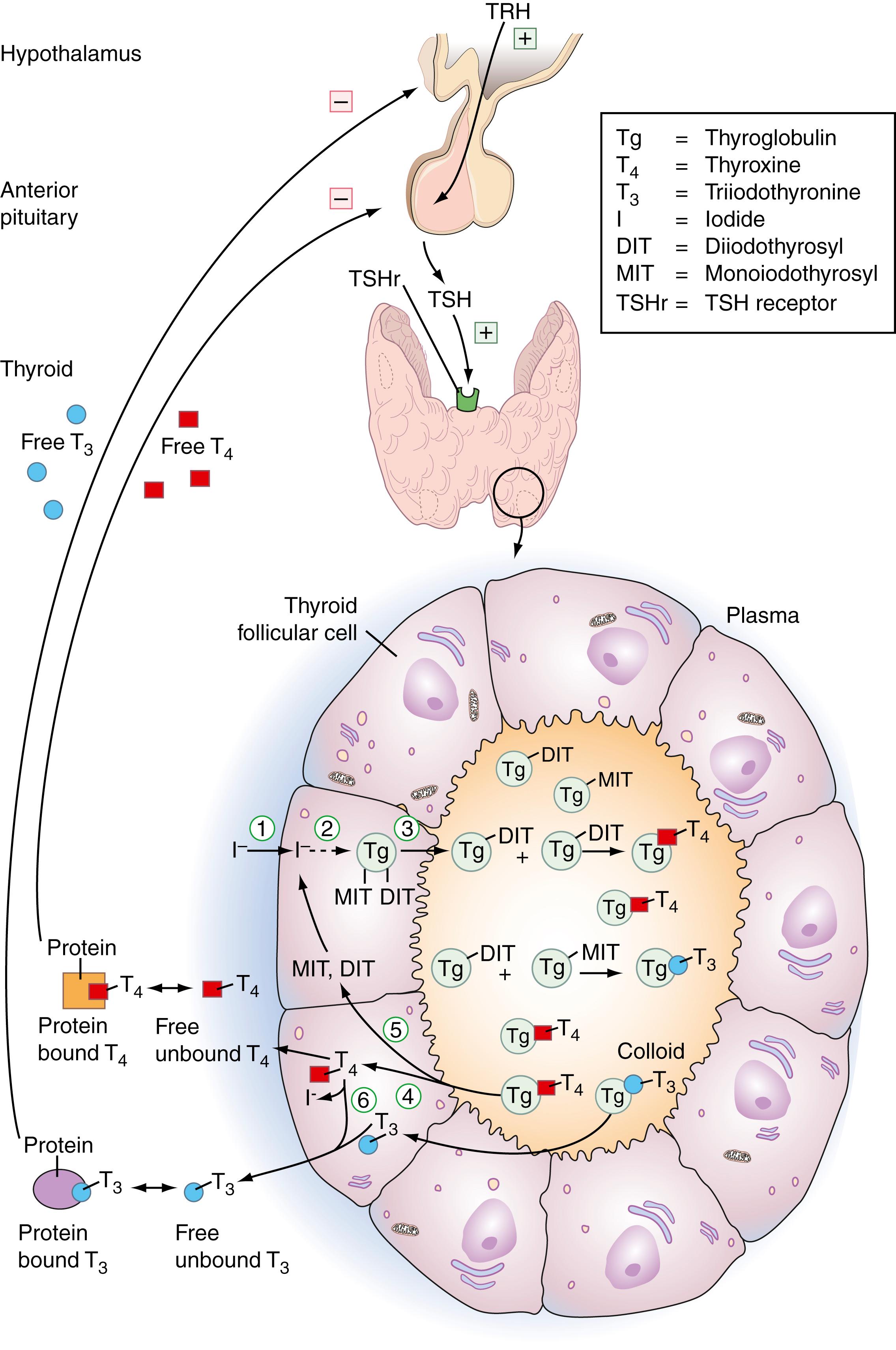
One hundred percent of circulating T 4 is of thyroidal origin, whereas only 20% of T 3 is of thyroidal origin; 80% of T 3 is produced enzymatically in nonthyroidal tissues by 5′-monodeiodination of T 4 ( ). Approximately 110 nmol (85 μg) of T 4 and 10 nmol (8.5 μg) of T 3 are produced daily by the thyroid gland. The thyroid hormones circulate while attached to plasma proteins. About 70% of T 4 is bound to thyroxine-binding globulin (TBG), 20% to transthyretin (formerly called thyroxine-binding prealbumin ), and 10% to albumin. Although most of the circulating T 3 is bound to TBG, it does so with a 10-fold reduced affinity as compared with that of T 4 ( ; ). Small percentages of T 4 and T 3 remain unbound to protein—about 0.03% and 0.3%, respectively. As with other hormones, it is the free component that is metabolically active.
Thyroid disease may be classified functionally into hyperthyroidism, hypothyroidism, and euthyroidism. Signs and symptoms of hyperthyroidism include heat intolerance, tachycardia, weight loss, weakness, emotional lability, and tremor. The most common clinical syndrome associated with hyperthyroidism is Graves disease, caused by circulating antibodies to the TSH receptor. Other disorders that lead to hyperthyroidism include toxic multinodular goiter, toxic adenoma and, rarely, TSH-secreting pituitary tumors and thyroid carcinoma.
Hypothyroidism can result in hoarseness, cold sensitivity, dry skin, constipation, bradycardia, and muscle weakness. Myxedema coma is an advanced stage of thyroid hormone deficiency characterized by progressive stupor, hypothermia, and hypoventilation. Failure of the thyroid gland to secrete an adequate amount of thyroid hormone is called primary hypothyroidism . This is most commonly iatrogenic in origin, the result of ablation with radioactive iodine or thyroidectomy to treat hyperthyroidism, or radiation to the head and neck for malignancy. Central hypothyroidism occurs when TSH secretion is decreased as a result of a pituitary disorder. Tertiary hypothyroidism is the result of hypothalamic dysfunction.
Thyroid diseases such as goiter, thyroid adenoma, and thyroid carcinoma typically occur in individuals who are euthyroid (clinically and biochemically). Accurate TSH assays permit the diagnosis of subclinical hyperthyroidism and hypothyroidism. These patients appear clinically euthyroid; however, their TSH values are respectively suppressed or elevated. A number of clinical situations can lead to difficulties in the interpretation of thyroid function tests: the presence of abnormal protein–binding proteins (congenital or drug induced); alterations in thyroid hormone metabolism (as seen in those hospitalized with acute psychiatric illness); and alterations due to medications that affect thyroid hormone binding or the HPT axis directly and with substances causing assay interference (biotin).The most important and most common problem with thyroid function tests probably occurs in those patients with various illnesses that do not directly involve the thyroid gland, so-called nonthyroidal illness .
Iodine is a major component of thyroid hormone; the main source of iodine is dietary intake. Deiodination of organic iodine-containing moieties within the gland serves as another source. Inorganic iodide is transported into the follicular cell by the sodium-iodide symporter (NIS) located in the basolateral membrane ( ). TSH modulates NIS activity: an increase in TSH secretion augments the uptake of iodide into the follicular cell. Iodide transport into the thyroid gland is also influenced by the serum iodide level; iodide deficiency increases pump activity, and iodide excess inhibits iodide uptake.
Thyroglobulin (Tg) is a glycoprotein synthesized by the rough endoplasmic reticulum in the basal and perinuclear regions of the follicular cell. It is acted on by the Golgi apparatus in the apical portion of the follicular cell, where iodination of the tyrosyl residues leads to the formation of monoiodotyrosyls and diiodotyrosyls (MITs and DITs). Tg is then transferred into the colloid for storage ( ; ). The transformation of Tg into thyroid hormone requires two separate oxidative reactions, both catalyzed by thyroid peroxidase (TPO): the binding of iodide to Tg tyrosyl residues (iodination) to form the iodotyrosyls MIT and DIT, and the subsequent coupling of MIT and DIT to produce T 4 and T 3 ( ; ). T 4 and T 3 are released from Tg via lysosomal degradation and secreted into the circulation at the basal membrane. All of these reactions are under the control of TSH.
About half of all T 4 is monodeiodinated in at the 5′ position to form T 3 , and about 40% undergoes deiodination of the inner ring of T 4 to form reverse T 3 (rT 3 ). The formation of rT 3 , the third major circulating form of thyroid hormone, is catalyzed by the enzyme 5-deiodinase. Reverse T 3 has no biological activity, has a short half-life of 4 hours, and circulates bound to TBG; its formation is considered a disposal pathway in the peripheral metabolism of T 4 ( ).
The Tg DIT/MIT ratio is influenced by iodide intake. DIT is the preferential iodotyrosine formed; thus, when iodide is abundant, T 4 is the predominant form of hormone synthesized and secreted, but when iodide sources are diminished, MIT is produced in greater quantities, leading to increased T 3 formation and release. In addition, the thyroid gland has a 5′-deiodinase that converts T 4 to T 3 . This process is under the control of TSH, so that T 3 secretion is enhanced during periods of TSH stimulation (i.e., primary hypothyroidism) or the presence of TSH-stimulating immunoglobulin [TSI]) ( ).
The monodeiodination of T 4 to T 3 and rT 3 accounts for 70% of the peripheral metabolism of T 4 ( ); the remainder of T 4 metabolism occurs by conjugation of T 4 to sulfate, deamination, and decarboxylation to form the acetic acid analog tetrac, and by ether link cleavage ( ) ( Table 25.5 ). T 3 but not rT 3 can be conjugated with sulfate to form T 3 -sulfate, and can be converted to its acetic acid analog, triac ( ). The enzymes responsible for these reactions are L -aminotransferase, located in the liver, and phenolsulfotransferases, which are present in many tissues throughout the body ( ). After their formation from T 4 , both T 3 and rT 3 can also undergo deiodination to form 3,3′-diiodothyronine (T 2 ).
| T 4 | T 3 | |
|---|---|---|
| Production rate, nmol/day | 110 | 50 |
| Fraction of circulating hormone of thyroid origin | 100% | 20% ∗ |
| Serum concentration | ||
| Total, nmol/L | 100 | 1.8 |
| Free, pmol/L | 20 | 5 |
| Fraction of total hormone in free form | 0.0002 | 0.003 |
| Half-life, days | ≈7 | 0.75 |
| Relative metabolic potency | 0.3 | 1.0 |
∗ 80% of circulating T 3 comes from the peripheral deiodination of T 4 . To convert total T 4 from nmol/L to μg/dL or free T 4 from pmol/L to ng/dL, divide by 12.87. To convert total T 3 from nmol/L to ng/dL or free T 3 from pmol/L to pg/dL, multiply by 65.1.
Protein-bound thyroid hormone fractions are considered biologically inert, functioning as reservoirs for the formation of free thyroid hormone. Unbound T 3 is responsible for all of the metabolic effects of thyroid hormone. T 3 binds to the thyroid hormone receptor located on the nuclear membrane. This complex is transported to the thyroid hormone response elements (TREs) situated in the promoter regions of target genes, leading to increases in mRNA and protein synthesis. Four isoforms of the T 3 receptor have been described: α 1 , α 2 , β 1 , and β 2 ( ). α1 and β1 receptors are present in most tissues. The β 2 receptor is unique to the pituitary gland and is central in the negative-feedback regulation of TSH by thyroid hormone. The α 2 receptor is inhibitory and acts as a negative regulator of thyroid hormone action.
Mutations in the β receptors, which diminish the ability of T 3 to bind to the nucleus, have been described in the syndrome of impaired sensitivity to thyroid hormone (previously termed thyroid hormone resistance syndrome ). Individuals with this syndrome have growth retardation and mental disabilities of varying degrees, as well as hypothyroidism ( ).
The physiologic regulators responsible for integrating thyroid gland function with the periphery include hypothalamic hormone, TRH; pituitary hormone, TSH; thyroid hormones free T 4 (FT 4 ) and free T 3 (FT 3 ). TRH enhances TSH synthesis, stimulates the secretion of preformed TSH from the thyrotrophs, and modulates the bioactivity of TSH, resulting in the secretion of bioactive TSH ( ; ). TRH itself is under the negative-feedback influence of circulating thyroid hormones (TRH mRNA levels are inversely related to the circulating T 3 values) ( ). The same feedback inhibition occurs at the level of the thyrotrophs.
TRH is a modified tripeptide (pyroglutamyl-histidyl-proline-amide), released from the prepro molecule by a peptidase ( ). TRH is found in the hypothalamus, brain, C cells of the thyroid gland, δ cells of the pancreas, myocardium, prostate, testis, and spinal cord. The neuron bodies producing TRH are innervated by catecholamines, leptin, and somatostatin-containing axons, which ultimately influence the rate of TRH synthesis.
Thyroid hormone production is self-regulated through feedback inhibition at the level of the hypothalamus (TRH) and pituitary (TSH). TRH also affects the production of other pituitary hormones, for example, it stimulates the release of prolactin from the lactotrophs. Leptin plays a significant role in the regulation of the TRH gene ( ), affecting the individual’s appetite for food intake. The precise interaction of leptin and the thyroid axis remains to be elucidated.
It is difficult to develop a specific antibody for TRH, and assays are not clinically useful.
TSH is a glycoprotein consisting of two mono-covalently linked α and β subunits. The α subunit shares the same amino acid sequences as LH, FSH, and human chorionic gonadotropin (hCG). It is the β subunit that carries specific information to the binding receptors for expression of hormonal activities.
The radioimmunoassay for measuring TSH was first developed by Odell and colleagues in 1965. This first-generation assay did not have sufficient sensitivity to distinguish normal TSH levels from the suppressed levels seen in primary hyperthyroidism. By the mid-1980s, a “sensitive” or second-generation immunometric TSH method using monoclonal or polyclonal antibodies was developed; sensitivity was improved to 0.1 to 0.2 mIU/L. Refinements in the immunometric method led to the third-generation assays now in common use, which allow measurement down to about 0.005 mIU/L. Fourth-generation TSH assays have been developed displaying sensitivities as low as 0.0004 mIU/L. However, they are not widely available and they fail to provide clinically useful information above and beyond that offered by third-generation assays. The American Thyroid Association (ATA) recommendations state that third-generation assays should be able to quantitate TSH in the 0.010- to 0.020-mIU/L range on an interassay basis with a coefficient of variation of 20% or less. In reporting assay results, the ATA recommends using functional sensitivity, defined as the point at which the interassay precision has a coefficient of variation equal to or less than 20% ( ).
Owing to improvements in the sensitivity of the TSH assay and the fact that TSH has an inverse log/linear relationship with FT 4 (e.g., small changes in FT 4 lead to large changes in TSH), this test alone can identify virtually all instances of hyperthyroidism and hypothyroidism. Exceptions exist in individuals with structural hypothalamic or pituitary disease, thyroid hormone resistance, disruption in HPT axis function due to medications, or when results are factitious as a result of substances causing assay interference (e.g., biotin, heterophile antibodies). Improved sensitivity of the third-generation assays facilitates distinguishing profound TSH suppression typical of frank Graves thyrotoxicosis (TSH <0.01 mIU/L) from modest degrees of TSH suppression (0.01–0.1 mIU/L) observed with mild (subclinical) primary hyperthyroidism and some cases of nonthyroidal illness. Although assay sensitivity has improved, the normal range has remained unchanged at approximately 0.35 and 5.0 mIU/L for most laboratories.
Serum TSH levels can be assayed using immunoassays and by mass spectroscopy, the latter being considered to be the gold standard with which all assays are compared. Reference intervals (RI) are specific for each methodology and manufacturer; this can prove problematic as patients move between doctors and different labs. To that end, the International Federation of Clinical Chemistry (IFCC) has formed a Committee for Standardization of Thyroid Function Tests. They are in the later stages of creating a process that harmonizes and standardizes TSH and FT 4 measurements. Until this is accomplished, it is recommended, when possible, to longitudinally adhere to using one particular lab for monitoring patients with thyroid disease ( ; ).
In hyperthyroidism, TSH is suppressed while FT 4 and/or FT 3 are elevated, while in hyperthyroidism caused by a TSH-producing tumor or pituitary resistance, the elevated FT 4 and/or FT 3 are accompanied by an inappropriately normal to slightly elevated TSH. In subclinical hyperthyroidism, the TSH is low, while both the FT 4 and FT 3 are in the normal range ( Table 25.6 ).
| Disorder | TSH | T 4 | T 3 | FT 4 | Tg | TBG | rT 3 | aTPO | ATG | TBII | TSI | TBA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary hypothyroidism | ↑ | ↓ | N or ↓ | ↓ | N or ↓ | N | ↓ | N or ↑ | N or ↑ | N or ↑ | n | n or ↑ |
| Transient neonatal hypothyroidism | ↑ | ↓ | ↓ | ↓ | N or ↓ | N | ↓ | N | N | ↑ | n | ↑ |
| Hashimoto thyroiditis/hypothyroidism | ↑ | N or ↓ | N or ↓ | N or ↓ | N or ↓ | N | ↓ | ↑ | ↑ | n or ↑ | n | n or ↑ |
| Graves disease | ↓ | ↑ | ↑ | ↑ | ↑ | N | ↑ | ↑ | ↑ | ↑ | ↑ | n or ↑ |
| Neonatal Graves disease | ↓ | ↑ | ↑ | ↑ | ↑ | N | ↑ | n or ↑ | n or ↑ | ↑ | ↑ | n or ↑ |
| TSH deficiency | N or ↓ | ↓ | ↓ | ↓ | ↓ | N | ↓ | n | n | N | n | n |
| Thyroid dyshormonogenesis | ↑ | ↓ | ↓ | ↓ | N, ↓ or ↑ | N | ↑ | n | n | N | n | n |
| Thyroid hormone resistance | N or ↑ | ↑ | ↑ | ↑ | ↑ | N | ↑ | n | n | N | n | n |
| TSH-dependent hyperthyroidism | ↑ | ↑ | ↑ | ↑ | ↑ | N | ↑ | n | n | n | n | n |
| T 4 protein-binding abnormalities ∗ | N | V | V | N | N | V+ | V | n | n | N | n | n |
| Nonthyroidal illness | V | N or ↓ | ↓ | V | N | N | N or ↑ | n | n | N | n | n |
| Subacute thyroiditis † | ↓ or ↑ | ↑ or ↓ | ↑ or ↓ | ↑ or ↓ | ↑ or ↓ | N | ↑ or ↓ | n | n | N | n | n |
∗ The spectrum of binding protein abnormalities includes increased or decreased TBG binding, increased or decreased transthyretin binding, and ↑ albumin binding.
† Subacute thyroiditis involves a transient period of hyperthyroidism followed by a transient hypothyroid state.
In primary hypothyroidism, in which the problem lies with the thyroid gland itself, the TSH elevation is accompanied by low FT 4 . In the setting of secondary (pituitary) and tertiary (hypothalamic) hypothyroidism the TSH is inappropriately normal for the low levels of T 4 and T 3 . As the thyroid gland fails, the hypothalamus and pituitary compensate by increasing the synthesis and secretion of their respective hormones. This results in a state termed subclinical hypothyroidism in which the TSH is elevated while the T 4 , T 3 , and FT 4 remain normal.
An important cause of both increased and decreased TSH is nonthyroidal illness (NTI). Patients with NTI tend to have low TSH levels during the acute phase of their illness, rising to within or above the reference range with resolution of the underlying process and returning to normal once the acute illness has resolved. The interpretation of thyroid function tests (TFTs) in this setting is often confounded by the use of medications such as opioids, glucocorticoids, and dopamine, which suppress TSH secretion. New to this list of medications are checkpoint and tyrosine kinase inhibitors, which can cause secondary hypothyroidism (hypophysitis) as well as primary thyroid dysfunction.
The TSH-centered strategy has two primary limitations. First, it assumes that hypothalamic-pituitary function is intact and normal. Second, it assumes that the patient is stable (i.e., the patient has not had recent adjustments in therapy for hyperthyroidism or hypothyroidism) ( ). If either of these criteria is not met, the serum TSH result can be misleading (see Table 25.6 ). In these instances, measurement of FT 4 can often help clarify the diagnosis. In situations in which the TSH is low and the FT 4 or TT 4 is normal, measurement of the total T 3 (TT 3 ) or FT 3 is fitting, as the patient may have T 3 thyrotoxicosis.
After release from the follicles, T 4 binds to various proteins in the blood (thyroid-binding globulin, albumin, thyretin). T 4 can be measured by immunoassay after the hormone is separated from the carrier protein. The reference range is 5 to 12.5 μg/dL in adults, with slightly lower results for certain pediatric age groups. Although TSH is the principal test for assessing thyroid function, T 4 measurements are often used along with TSH and can be of importance in interpreting TSH results. The combination of a low T 4 and increased TSH is indicative of primary hypothyroidism, whereas elevated T 4 and/or T 3 combined with a decreased TSH is characteristic of primary hyperthyroidism. Hyperthyroidism, in which the T 4 is elevated while the serum T 3 is at or below the reference range, is termed T 4 thyrotoxicosis . This can occur in patients with iodine-induced thyrotoxicosis (e.g., patients on amiodarone or following iodine contrast media). A suppressed TSH in association with a normal to low-normal T 4 and a high T 3 characterizes T 3 thyrotoxicosis . This is more common in a toxic nodule and the elderly (see Table 25.6 ).
Severe NTI is associated with low T 4 and T 3 levels. This so-called low T 4 and T 3 syndrome, or euthyroid sick syndrome, is felt to be an adaptive response to reduce metabolic demands and conserve protein stores ( ) and is associated with a poor prognosis ( ). It is thought to arise from a maladjusted central inhibition of TRH ( ; ) ( Fig. 25.7 ).
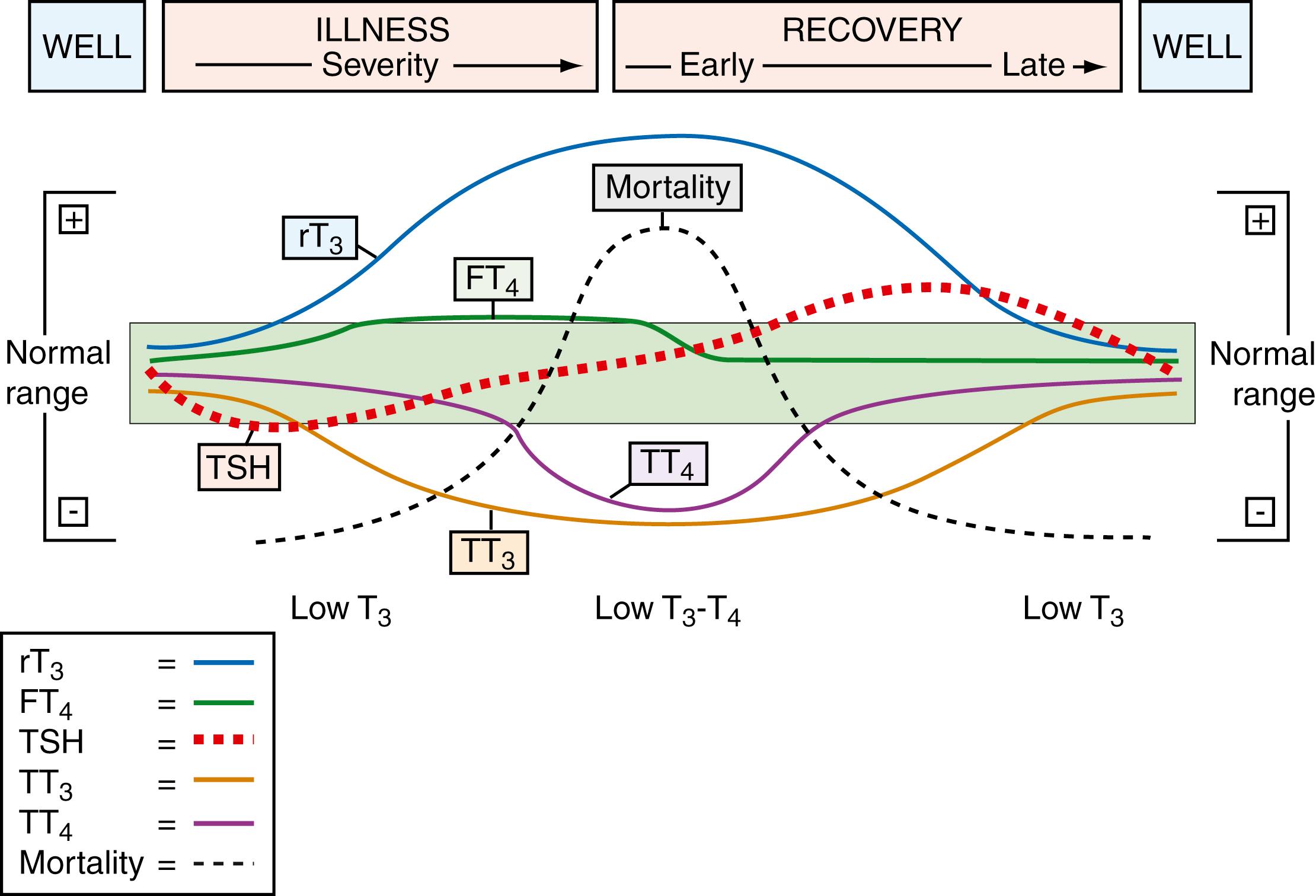
Euthyroid hyperthyroxinemia is distinguished by the presence of an increased serum T 4 in association with a normal TSH in an otherwise euthyroid individual. This entity has a variety of causes, including increased synthesis of binding proteins, as seen with certain drugs (e.g., estrogen) or medical conditions (e.g., liver disease), and decreased conversion of T 4 and T 3 (e.g., high doses of β blockers or steroids). It is also seen in patients who are acutely hospitalized for psychiatric illness, in those with familial dysalbuminemia, and in thyrotoxic patients with concomitant NTI. When medications or other factors cause increased protein binding, there is a consequent increase in serum total T 4 ; conversely, a decrease in binding capacity leads to a decrease in serum total T4. These perturbations do not have any physiologic effect in persons with a normal thyroid axis, as the bioactive, free hormone fraction remains unaffected. In these situations, free T 4 correlates better with thyroid functional status than does serum total T 4 .
The T 3 uptake (T 3 -UP) test is a classical method of adjusting the total T 4 measurement for alterations in binding protein. The T 3 -UP test yields a relative measurement of unoccupied T 4 binding sites. It was performed by saturating available binding sites with radiolabeled T 3 , then measuring the unbound T 3 by adsorbing it onto a resin (hence the term T 3 resin uptake). Procedures giving analogous results can be performed via nonisotopic methods on automated analyzers. The free thyroxine index (FTI) is then calculated as (T 3 -UP of the patient)/(mean T 3 -UP of the reference population) multiplied by the patient’s total T 4 . The FTI (reference range, 5.4–9.7) is only a relative estimate of the free T 4 concentration. In general, during thyrotoxicosis, both total T 4 and T 3 -UP are elevated. Conversely, in hypothyroidism, both total T 4 and T 3 -UP are decreased and, when there is a binding globulin abnormality, these two values diverge (e.g., total T 4 is elevated and the T 3 -UP is decreased or the total T 4 is decreased and the T 3 -UP is increased). The FTI has been largely replaced by the direct measurement of free T 4 by immunoassay, dialysis, and ultrafiltration.
FT 4 is the biologically active fraction of T 4 . Equilibrium dialysis is the traditional reference technique for measuring FT 4 . It is unaffected by changes in binding protein concentration. It is carried out by dialyzing serum against a nonprotein buffer using a membrane impermeable to proteins so that FT 4 will achieve equal concentrations on both sides of the membrane. The protein-free solution is then collected and analyzed for FT 4 . Even this laborious method does not give a true measure of FT 4 in all cases because small molecules that may affect T 4 binding are diluted in the procedure. Refinement of this technique to include tandem mass spectroscopy (ED/MS-MS), has made this the new gold standard for measuring several hormones, including FT 4 .
ED/MS-MS is costly, time-consuming, and ill-suited to meet the high-volume demands of clinical laboratories. Immunoassays performed on automated clinical chemistry platforms can rapidly provide appropriate results in most clinical circumstances and have become the mainstay for measuring all thyroid hormones. There are various circumstances in which immunoassays can be unreliable. Spurious results can occur with abnormal variants of the binding proteins (e.g., familial dysalbuminemic hyperthyroxinemia, a condition with high T 4 but normal FT 4 , caused by an albumin variant that binds T 4 abnormally) or in the presence of T 4 or T 3 autoantibodies ( ). Increases or decreases in serum FT 4 without concomitant changes in metabolic state have been reported in pregnancy, NTI, and in patients being treated with certain medications. For example, prolonged administration of phenytoin or carbamazepine results in a 15% to 30% decrease in both serum T 4 and FT 4 . In these circumstances, dialysis or ultrafiltration methods are indicated.
Biotin, a commonly used supplement, has recently been documented to interfere with immunoassays that use streptavidin and biotinylated antibodies. Depending on whether a competitive or sandwich technique is used, the measured FT 4 or TSH will be falsely elevated or falsely low, respectively. Options to remedy this include having the patient stop biotin-containing supplements 3 to 5 days prior to testing or referring to a laboratory that does not use a streptavidin-biotin methodology ( ).
The RI for FT 4 is highly assay specific. In addition, RIs vary throughout pregnancy, infancy and childhood. It is therefore important to use the proper RI corresponding to each trimester of pregnancy and age range for infants and children. As with TSH, a process to harmonize FT 4 assays using the FT 4 obtained by ED/MS-MS as the universal standard, is currently under development ( ). Until this standardization is finalized, it is recommended that one maintain consistency in the commercial lab and methodology utilized for measuring FT 4 and TSH.
T 3 , generally measured by immunoassay, has a reference interval typically in the range of 60 to 160 μg/dL (0.9–2.46 nmol/L). T 3 is much less bound to protein than is T 4 ; thus, a relatively greater proportion of T 3 than T 4 exists in a free, diffusible state. Since assays for FT 3 are less widely available and less widely standardized, the current practice is to measure total T 3 as opposed to FT 3 . Total T 3 concentrations are measured by competitive immunoassay methods that are now mostly nonisotopic and use enzymes, fluorescence, or chemiluminescence molecules as signals ( ). The measurement of T 3 is useful in confirming the diagnosis of hyperthyroidism when TSH is suppressed but there is only minimal to no elevation in T 4 .
In 90% of patients with hyperthyroidism, both T 4 and T 3 are increased, with the increase in T 3 usually greater than that of T 4 . Serum T 3 can be normal or low in patients with hyperthyroidism in the setting of NTI or if they are taking medications that decrease the conversion of T 4 to T 3 (e.g., high-dose propranolol, amiodarone). Hyperthyroidism in association with an elevated T 3 but normal T 4 and FT 4 is termed T 3 thyrotoxicosis ( ). Patients with this syndrome are a heterogeneous group with no distinctive signs or symptoms. Although most patients with T 3 thyrotoxicosis have Graves disease, it may also be seen with toxic multinodular goiter or toxic adenoma. T 3 thyrotoxicosis has been reported to occur in ≈1% to 4% of all thyrotoxic patients, with a much higher incidence in regions with iodine deficiency.
The measurement of T 3 is generally not useful in evaluating patients suspected of having hypothyroidism as levels fall within the RI in 15% to 30% of patients ( ). However, decreased T 3 levels are seen in patients with severe hypothyroidism, whose T 4 < 2 μg/dL (32 nmol/L) ( ). In acute NTI, such as myocardial infarction, T 3 declines rapidly to about 50% of the reference value within a matter of days ( ). T 3 concentrations are also low in cord blood but increase rapidly during the first few hours of life.
Blood concentrations of T 3 rise rapidly following the ingestion of T 3 -containing preparations (e.g. Cytomel, Amour thyroid), peaking within 2 to 4 hours. Clearance is also rapid, with a half-life of 0.68 to 1.38 days ( ). As a result, knowing both the time of hormone administration and consistency of blood test timing in relation to hormone ingestion is essential for proper interpretation of serum T 3 results. In contrast, the T 3 level is stable in patients treated with T 4 (levothyroxine). As in this situation, T 3 is derived from the peripheral conversion of T 4 ; stable levels are reached after only a few weeks of therapy.
A number of pharmacologic agents interfere with the conversion of T 4 to T 3 (i.e., glucocorticoids, amiodarone, large dosages of propranolol), causing low levels of T 3 , low FT 3 , and normal or even high levels of serum T 4 and FT 4 , with normal TSH.
rT 3 is a biologically inactive metabolite of thyroxine that is produced through 5-deiodination of T 4 . T 3 and rT 3 tend to vary reciprocally. In NTI, rT 3 is elevated while T 3 is diminished. rT 3 is increased in healthy newborns, in patients with hyperthyroidism (including factitious hyperthyroidism), and in patients taking certain drugs, such as amiodarone and propranolol. Measurements of rT 3 are of little clinical utility and rarely if ever indicated.
Tg is synthesized and secreted by thyroid follicular cells. It is present in the serum of normal individuals in the range of up to about 30 ng/mL (45 pmol/L). The serum Tg concentration reflects thyroid mass, thyroid injury, and TSH receptor stimulation ( ). Tg is increased in a variety of disorders, including Graves disease, thyroiditis, and nodular goiter. The routine measurement of Tg is never indicated. Its utility resides in monitoring for recurrence of certain variants of thyroid cancer, in the diagnosis of thyroid dysgenesis in congenital hypothyroidism, and in helping to distinguish thyrotoxicosis factitia from other forms of thyrotoxicosis.
While Tg is helpful in monitoring for recurrence/persistence of well-differentiated thyroid carcinoma, it is of no utility in patients with undifferentiated thyroid malignancies or medullary thyroid cancer. The clinical value of Tg measurement is limited in the presence of Tg antibodies (Tg Abs), found in 20% to 25% of patients with thyroid carcinoma. Tg Abs can interfere with Tg measurements by immunoassay, resulting in falsely low serum Tg concentrations ( ). It is therefore extremely important to always test for Tg Abs whenever measuring Tg. LC/MS-MS may eventually allow for accurate measurement of Tg in the presence of Tg Abs ( ). Even though the presence of Tg Abs may invalidate the value of Tg in monitoring recurrence and response to treatment in differentiated thyroid cancer, because Tg Ab reflects thyroid tissue mass, Tg Ab itself may be used as a surrogate marker ( ; ; ).
Recombinant human TSH (rhTSH) is commonly used to test patients with thyroid cancer for the presence of residual/recurrent disease. This is more practical, more convenient, and markedly less unpleasant for the patient than stopping thyroid hormone suppression over the span of several weeks to increase the TSH level; an elevated TSH is required for radioactive iodine (RAI) uptake to occur in remaining remnant or recurrent tumor. Measurement of Tg 72 hours following the last dose of rhTSH correlates highly with the presence of recurrence; some authorities support the use of this test alone without a concomitant whole-body scan (WBS) for iodine for those with “low-risk” tumors (<40 years of age, no distant metastases, size <1 cm, well differentiated). All other patients with thyroid cancer need to undergo WBS and measurement of Tg, which can either be accomplished using rhTSH or thyroid hormone withdrawal. Using a highly sensitive assay for Tg in patients who have undergone remnant ablation, a post-rhTSH Tg > 0.5 to 1.0 ng/mL, or Tg > 0.1 ng/mL while on suppression is suspicious for recurrence/persistence of disease and needs to be worked up accordingly (i.e., full restaging while hypothyroid off of suppressive therapy) ( ). Poorly differentiated tumors can cease production of Tg, in which case other modalities for monitoring—such as positron emission tomography scan and thyroid ultrasound—may be utilized.
In the early weeks post-thyroidectomy, serum Tg levels typically fall, with a half-life of 2 to 4 days. The pattern of change in serum Tg while on T 4 suppression is a better indicator of a change in tumor burden than any single serum Tg value ( ). Because of inter-assay variability, it is extremely important to use the same laboratory for monitoring TSH, Tg, and Tg Ab.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here