Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
As detailed in the Introduction to this book, the last 60 years have been witness to truly remarkable progress in the treatment of childhood cancer. With current therapies, over 80% of children diagnosed with cancer in the United States can be cured of their disease. As a result of this progress, there are now over 500,000 survivors of childhood cancer (SCC) living in the United States. Despite these successes, SCC experience unacceptable morbidity and mortality. In a St. Jude LIFE study of 1713 adult SCC, by age 45 the cumulative prevalence of any chronic health condition and a serious/disabling or life-threatening chronic health condition were 95.5% and 80.5%, respectively. These data validate the very high rates of morbidity and mortality previously reported by the Childhood Cancer Survivor Study ( https://ccss.stjude.org/ ). In addition to physical health conditions, SCC face emotional, psychological, and sociological impairment, such as learning challenges, mental illness, and financial toxicity. Given the complex, multisystem morbidities they experience, the large and growing population of SCC require intricate, long-term medical and psychological follow-up to maintain their health and quality of life. Meeting this need, the field of “survivorship” has developed as a medical subspecialty that provides tailored and focused long-term follow-up for SCC while conducting research to better understand, address, and prevent the long-term complications of cancer therapy. Any center that treats children for cancer should have some form of structured long-term follow-up for SCC. Various models exist for how to provide this follow-up care.
The care of SCC often requires the involvement of multiple disciplines. As the complexity of their care negatively impacts SCC’s adherence to medical care and screening recommendations, models of care are required that facilitate the coordinated, streamlined delivery of health services. This is particularly important as SCC’s follow-up decreases over time, while the risk for developing late effects increases. There are a variety of models that can be effective in supporting SCC as outlined in Table 33.1 . Several considerations factor into which model is most appropriate for a given setting, including:
the availability and expertise of medical personnel in the area;
the specific risk for late effects in the survivor population being followed; and
the ability of SCC to self-manage their care, considering social, financial, and cognitive barriers.
| Name | Description | Benefits | Shortcomings |
|---|---|---|---|
| Specialized survivorship clinics a | Provide a personalized, coordinated care system led by a multidisciplinary team of specialists to support, educate, and manage survivors of childhood cancer |
|
|
| Primary care doctor-led management | Primary care doctors support survivors in learning the signs and symptoms of potential late effects and directing them to specialist as needed. Primary care doctors must be in contact with oncologists in order to provide proper care and facilitate referrals back to oncology as needed |
|
Primary care doctors are often less knowledgeable than specialists regarding late effects |
| Shared care |
|
Gives family doctors an avenue through which to communicate with specialists regarding late effects, implementable in areas without a large number of specialists | Requires careful coordination of care by both the family doctor and the specialist |
a Within the category of survivorship clinics, there are various types that exist, specializing in different age-groups, some for pediatrics, and some for adults. Additionally, there are survivorship clinics that follow patients lifelong. This model may be particularly advantageous because it allows pediatric survivors to be consistently followed by the same program without transition.
While there is no consensus regarding the optimal frequency or method of follow-up, complete survivorship care should include at least the following elements:
educating and counseling SCC and their families regarding the risk of late effects;
facilitating screening for late effects following guidelines (discussed next);
providing referrals to specialists as needed; and
providing psychosocial support services to SCC and their families as needed.
Whichever model is employed for follow-up, there are multiple points in the cancer timeline at which interventions can reduce the burden of late effects (shown schematically in Fig. 33.1 ). These include:
cancer prevention (e.g., smoking cessation and sun protection),
exposure reduction (e.g., elimination of radiation therapy for many patients with Hodgkin lymphoma—see Chapter 20: Hodgkin Lymphoma ),
primary prevention (e.g., the use of dexrazoxane to reduce cardiac toxicity associated with anthracyclines),
secondary prevention (e.g., the use of carvedilol or lisinopril to reduce the risk of cardiac morbidity in those already exposed to anthracyclines),
lifestyle modifications (e.g., encouraging exercise to modify the risk of cardiac toxicity in those exposed to chest radiation), and
screening and early detection [e.g., mammography and breast magnetic resonance imaging (MRI) starting at a young age for women exposed to chest radiation].
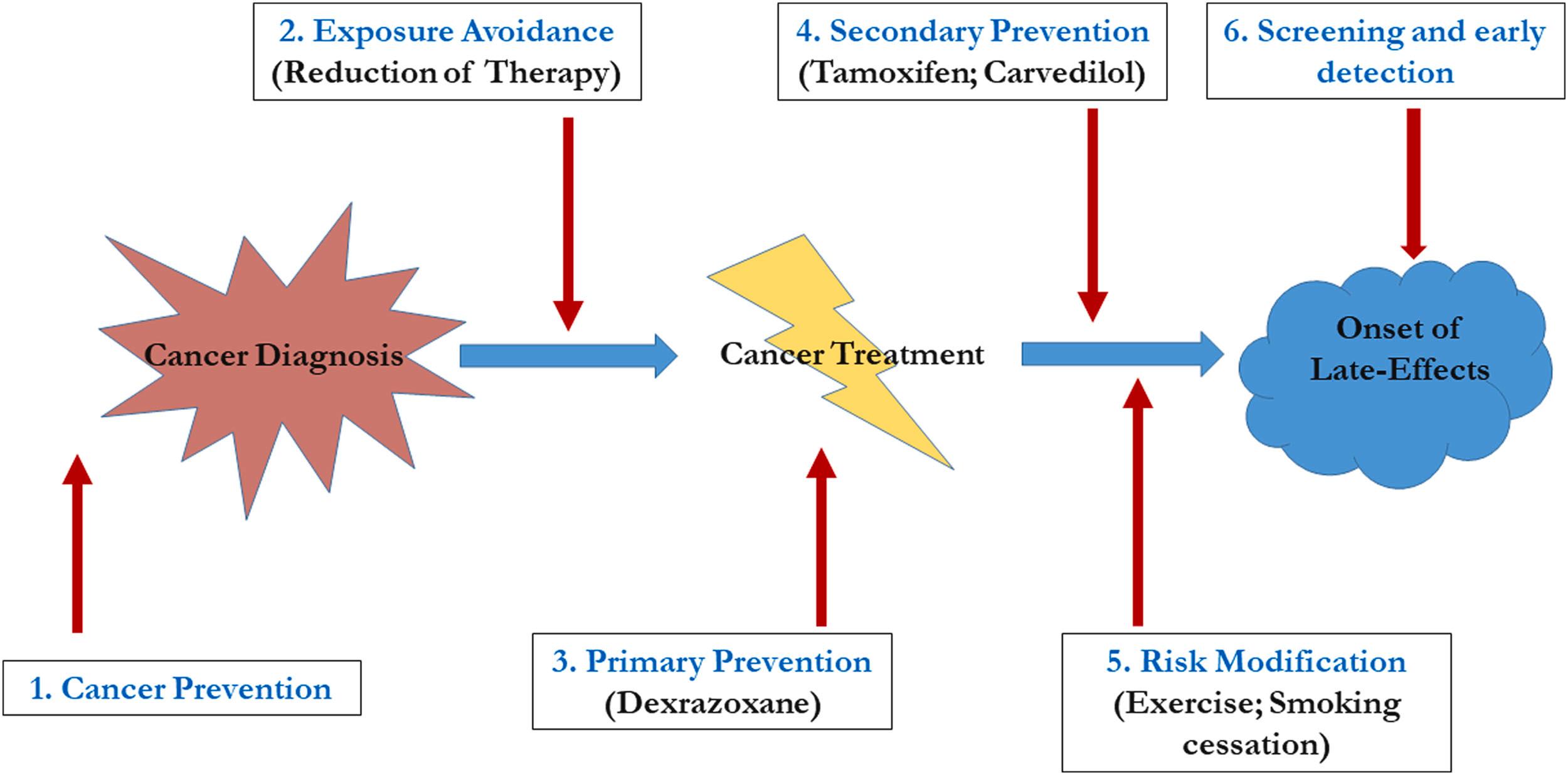
Approaches to exposure reduction and primary and secondary prevention have come about as a result of survivorship research and a better understanding of the consequences of cure. The primary focus of survivorship care to date, however, has been on reducing the burden of late effects in those already exposed to chemotherapy through lifestyle modifications and screening and early detection. The ability to predict the risk for specific late effects based on exposures has resulted in the development of screening guidelines that represent a combination of evidence-based approaches and expert opinion. These include the Children’s Oncology Group guidelines ( www.survivorshipguidelines.org ) and those of the International Guidelines Harmonization Group for Late Effects of Childhood Cancer ( www.ighg.org ). In order to optimally utilize these guidelines, it is necessary to know the SCC’s treatment exposures and to be able to elicit relevant information from the history. Important information to be obtained in follow-up of SCC is listed in Table 33.2 . Table 33.3 lists selected late effects associated with common chemotherapeutic exposures and suggested screening. Table 33.4 lists selected late effects associated with organ exposure to radiation and suggested screening.
|
| Exposure | Late effect | Suggested screening |
|---|---|---|
| Any chemotherapy exposure |
|
|
| Anthracyclines |
|
|
| Alkylators |
|
|
| Bleomycin | Pulmonary toxicity |
|
| Corticosteroids |
|
|
| Epipodophyllotoxins | Secondary leukemia |
|
| Heavy metals (cisplatin/carboplatin) |
|
|
| Methotrexate/cytarabine (high dose and intrathecal) | Neurocognitive deficits |
|
| Plant alkaloids |
|
|
| Radiation exposure | Late effect | Suggested screening |
|---|---|---|
| Any site |
|
|
| Abdomen |
|
|
| Brain |
|
|
| Breast tissue |
|
|
| Heart |
|
|
| Lungs |
|
|
| Ovaries |
|
|
| Pituitary (>1800 cGy) |
|
|
| Testicles |
|
|
Surgery remains the primary therapy for many musculoskeletal tumors and the most visible late effect is amputation. As internal prostheses have become more refined, the ability to perform limb-salvage procedures has dramatically improved. Limb-sparing procedures are now performed in over 90% of osteosarcoma cases, without a deleterious effect on outcome. Although there are many advantages to a limb-salvage approach to extremity tumors, there are significant disadvantages as well. In particular, the prostheses generally cannot tolerate extreme stress, so sport participation is limited. Further, prosthesis infection, deterioration, and failure occur in as many as 20% of patients, which may require multiple additional surgeries.
Corticosteroids such as dexamethasone and prednisone used in cancer therapy [especially for acute lymphoblastic leukemia (ALL)] have resulted in avascular necrosis (AVN), with adolescents at the highest risk. Nearly 3% of children treated for ALL will develop AVN. Protocols have attempted to decrease this incidence by decreasing the number of continuous weeks of exposure to dexamethasone and by preferentially utilizing prednisone over dexamethasone. The incidence of reduced bone mineral density in SCC is also elevated, likely due to a combination of chemotherapeutic exposures [corticosteroids and high-dose methotrexate (HDMTX)] and reduced weight bearing during treatment. Routine recommendations include adequate daily intake of calcium (1000–1500 mg) and vitamin D (400 IU daily) and weight-bearing exercise. The use of bisphosphonates and other bone absorption-reducing therapies in childhood cancer survivors remains investigational.
For many musculoskeletal tumors, especially those not surgically resectable, radiation remains a keystone of therapy. Despite its effectiveness, radiation therapy carries with it the risk of significant consequences, which vary by site. Spine and extremity radiation can cause scoliosis, atrophy, or hypoplasia of muscles, AVN, reduced bone mineral density, discrepancy in extremity length, and alteration in sitting-to-standing height ratio. Brain and head radiation may affect facial structure, tooth enamel, and formation, as well as cause learning challenges, memory loss, and personality changes. Secondary neoplasms are a risk of radiation to any site.
Women treated with chest radiation experience an incidence of breast cancer similar to those of women carrying BRCA mutations. By the age of 50, survivors treated with chest radiation have a 30% incidence of breast cancer, with the highest risk being in those exposed to >20 Gy. Breast cancer screening with mammography and bilateral breast MRI is recommended for women who received >20 Gy and should be considered in those who received 10–19 Gy.
These late consequences are related to dose, site, volume, and age of the child at the time of radiation. The higher the dose and the younger the child, the more pronounced the late effects. Some protocols have been designed to reduce potential radiation-associated late effects through the use of:
low-volume, low-dose radiation combined with effective systemic therapy;
improvements in the delivery of radiation, including proton radiation; and
dental prophylaxis prior to radiotherapy in maxillofacial sites.
Regular physical examinations with appropriate imaging (e.g., MRI for suspected AVN) can identify musculoskeletal problems early and potentially reduce their impact on a survivor’s quality of life. Survivors who have been exposed to high doses of steroids, especially in conjunction with radiation or HDMTX, benefit from an evaluation of bone mineral density at entry into long-term follow-up. Survivors should be counseled to engage in routine weight-bearing exercise and to consume the recommended daily allowance of vitamin D and calcium.
Chemotherapy, radiotherapy to fields that involve the heart or major vessels, and especially their combined use, have the potential to cause both early and late cardiac complications. After cancer recurrence and secondary neoplasms, the leading cause of morbidity and mortality in long-term SCC is cardiac-related disease. Cardiac late effects include the development of cardiomyopathy and congestive heart failure, valvular heart disease, and the onset of coronary artery disease at a younger age. Despite a focus on identification of risk factors and monitoring, cardiac toxicity remains somewhat of an idiosyncratic event. Recent publications have begun to examine the role of genetic polymorphisms (e.g., those involved in anthracycline metabolism) in the development of late cardiovascular disease.
Anthracycline-induced myocyte death results in hypertrophy of existing myocytes, reduced thickness of the wall of the heart, ischemia, and interstitial fibrosis. The heart is unable to compensate adequately to meet the demands of growth, pregnancy, or other cardiac stress, which results in late-onset anthracycline-induced cardiac failure. The incidence of cardiomyopathy is related to the cumulative dose of anthracyclines, occurring in ~10% of survivors after a cumulative dose of <400 mg/m 2 , ~20% after 400–599 mg/m 2 , ~50% after 600–799 mg/m 2 , and almost 100% after 800 mg/m 2 . While toxicity with higher doses of anthracyclines is well documented and studied, studies suggest that even patients exposed to <100 mg/m 2 may have long-term cardiac dysfunction.
Anthracycline-induced cardiomyopathy is a progressive disorder. It ultimately manifests with signs of congestive heart failure, including exercise intolerance, dyspnea, peripheral edema, pulmonary rales, S3 and S4 heart sounds, and hepatomegaly. Rapid progression of symptoms may occur with pregnancy, anesthesia, the use of illicit drugs (e.g., cocaine), prescription drugs, or alcohol. Early cardiomyopathy may be influenced by pharmacotherapy, such as with angiotensin-converting enzyme (ACE) inhibitors. Once it progresses to florid cardiac dysfunction, the only definitive treatment remains cardiac transplant that exposes survivors to a new set of health risks and long-term consequences.
Anthracycline exposure is also associated with prolonged QT c intervals, sinus node dysfunction, and premature ventricular contractions. While arrhythmias and conduction abnormalities may be self-limited, some survivors may require pacemakers for persistent heart blocks.
Cyclophosphamide-induced cardiac effects occur primarily with high-dose preparatory regimens for stem cell transplantation. Cyclophosphamide causes intramyocardial edema and hemorrhage, often in association with serosanguineous pericardial effusion and fibrous pericarditis. This cardiotoxicity is usually reversible.
Radiation is toxic to cardiomyocytes through multiple mechanisms, including ischemia, chronic inflammation, and fibrosis. In addition to acting in concert with anthracyclines to increase the risk of cardiomyopathy, radiation to the heart can also induce valvular damage, pericarditis, or coronary vessel damage, increasing the risk of ischemic heart disease. Cardiac doses over 20 Gy confer the highest risk.
Radiation fields with the potential to impact the heart include:
chest,
abdomen,
spine (thoracic or while spine), and
total body irradiation.
Radiation to the neck can damage the carotid vessels, increasing the risk of stroke.
In addition to a good interval history and physical examination, important screening tools for evaluating cardiovascular function following exposure to anthracyclines or radiation include:
electrocardiogram (EKG),
echocardiogram (ECHO),
cardiac stress tests,
radionuclide cardiac cineangiocardiography, and
carotid ultrasound.
EKG findings include prolonged QT c (0.45 or longer), second-degree atrioventricular block, complete heart block, ventricular ectopy, ST elevation or depression, and T-wave changes. An association between prolongation of QT c interval and anthracycline dose over 300 mg/m 2 has been described in childhood cancer survivors.
ECHO-based screening indices include shortening fraction (SF) and velocity of circumferential fiber shortening for the measurement of left ventricular contractility.
Radionuclide cardiac cineangiocardiography [multigated acquisition (MUGA)] determines the ejection fraction and is useful for those patients in whom a high-quality ECHO cannot be obtained. A left ventricular ejection fraction (LVEF) of 55% or more indicates normal systolic function.
The following criteria define progressively deteriorating cardiac function:
a decrease in the SF by an absolute value of 10% from the previous test,
SF <29%,
a decrease in the MUGA LVEF by an absolute value of 10% from the previous test,
MUGA LVEF <55%, and
a decrease in the MUGA LVEF with stress.
Management of anthracycline- and radiation-induced cardiomyopathy may include:
digoxin to improve ventricular contractility,
diuretics to decrease sodium and water retention, and
ACE-inhibiting agents (e.g., enalapril) to decrease sodium and water retention and decrease afterload.
Prognosis of progressive late-onset heart failure is poor.
The Children’s Oncology Group provides guidelines for long-term follow-up of cardiac function via ECHO following anthracycline and radiation exposure, the frequency of which is determined by the cumulative anthracycline doses and exposure to radiation:
No screening:
<15 Gy and no anthracyclines
Every 5 years:
15 Gy but <35 Gy and no anthracyclines
<15 Gy+<250 mg/m 2 cumulative anthracyclines
Every 2 years:
35 Gy and no anthracyclines
15 Gy+< 250 mg/m 2 cumulative anthracyclines
250 mg/m 2 cumulative anthracyclines
After completion of therapy, all patients with any amount of anthracycline exposure or thoracic radiation therapy should have an EKG and ECHO or MUGA. Those with normal studies at that point should be followed as per the recommended guidelines, while patients with an abnormal study either at the end of therapy or at the time of initial long-term follow-up should have more frequent evaluations. It is important to counsel patients to avoid tobacco smoke and to encourage the maintenance of a healthy weight and physical fitness. In addition to the ECHO and EKG screening, cholesterol and blood pressure should be checked annually and aggressively managed as each is an independent risk factor for cardiac disease. Patients who received >40 Gy to the neck should undergo color Doppler ultrasound of the carotids 10 years after completion of treatment as a baseline.
Both chemotherapy and radiation therapy can cause acute and chronic lung injury. Younger children are at more risk than adolescents or adults for the development of chronic respiratory damage.
Unilateral or partial lung resections performed in the management of pulmonary metastases can lead to scarring and reduced lung capacity.
The primary offending agents include bleomycin and nitrosourea, with clinical manifestations usually occurring months after a critical cumulative dose is reached or exceeded. Busulfan is also associated with pulmonary fibrosis, with the greatest risk in patients who have received >500 mg. Pulmonary toxicity is increased when any of these agents are given in combination with radiation therapy to the lungs or chest. Children who have undergone transplant are at risk for chronic pulmonary issues such as chronic graft-versus-host disease of the lungs and bronchiolitis obliterans syndrome.
Precisely 10% of SCC experience fibrosis when their cumulative dose of bleomycin exceeds 400 units, although lung injury has been observed in children receiving 60–100 units/m 2 . Bleomycin pulmonary toxicity manifests as dyspnea, dry cough, and rales. Radiographic findings include interstitial pneumonitis with reticular or nodular pattern and pulmonary function tests (PFTs) show a restrictive ventilatory defect with hypoxia, hypercapnia, and chronic hyperventilation. Assessment includes respiratory examination and chest radiography; however, diffusion capacity of carbon monoxide is considered to be the most sensitive test. Radiation therapy, renal insufficiency, cisplatin, cyclophosphamide, exposure to high levels of oxygen, and pulmonary infections can exacerbate the effects of bleomycin.
The greatest risk for those exposed to carmustine and lomustine occurs at doses >600 mg/m 2 . The clinical manifestations of nitrosourea toxicity are the same as bleomycin, although pulmonary fibrosis is more commonly associated with carmustine.
While bleomycin and nitrosourea are most commonly associated with long-term pulmonary toxicity, other agents such as MTX, 6-mercaptopurine, and procarbazine have been associated with an acute hypersensitivity reaction, which can result in long-term pulmonary function changes.
Cytosine arabinoside, MTX, ifosfamide, and cyclophosphamide have been associated with noncardiogenic pulmonary edema. This complication occurs within days of the beginning of treatment and can also result in long-term pulmonary function changes.
Other host factors that can contribute to chronic pulmonary toxicity include asthma, infection, smoking, and having had a history of assisted ventilation.
Radiation therapy in children younger than 3 years of age results in increased pulmonary toxicity. Radiation therapy to the lungs can cause impairment of the proliferation and maturation of alveoli, resulting in chronic respiratory insufficiency. This is thought to be consistent with a proportionate interference with the growth of both the lungs and chest wall. Radiation also causes damage to the type II pneumocyte, which is responsible for the production of surfactant and the maintenance of patency and surface tension of the alveoli. As a result of changes in surfactant production, there is a decrease in alveolar surface tension and compliance. These children exhibit decreased mean total lung volumes and DLCO (diffusion capacity of carbon monoxide) that is approximately 60% of predicted values.
The effects of direct radiation to the lungs also include damage to the endothelial cells of the capillaries resulting in alterations of perfusion and permeability of the vessel wall, likely mediated by cytokine production that stimulates septal fibroblasts increasing collagen production and pulmonary fibrosis. Pulmonary toxicity for radiation can manifest as pulmonary fibrosis, interstitial pneumonitis, restrictive lung disease, or obstructive lung disease.
The late radiation injury to the lung is characterized by the presence of progressive fibrosis of alveolar septa and obliteration of collapsed alveoli with connective tissue. In asymptomatic adolescents treated for Hodgkin lymphoma, chest radiographic findings or PFTs consistent with fibrosis have been found in over 30% of patients and these changes have been detected months to years after radiation therapy. The incidence of radiation-induced fibrosis has decreased over time due to refinements in radiation therapy.
Children who receive craniospinal radiation (either for leukemia or malignant brain tumors) also have a risk of developing late restrictive lung disease.
Radiation to the chest, thorax, axilla, mantle, or mediastinum puts patients at risk for pulmonary toxicity, with the greatest risk seen in those who received ≥15 Gy to these fields, or TBI with ≥6 Gy in a single fraction or ≥12 Gy in fractionated doses.
It is important to quickly identify patients developing pulmonary toxicity, obtain appropriate diagnostic tests, and implement interventions to prevent the toxicity from worsening. Concerning symptoms include cough, shortness of breath, and dyspnea on exertion. In addition to a good interval history and physical examination, important diagnostic tests for possible pulmonary toxicity include:
PFTs: these should be obtained immediately following completion of therapy, at entry to long-term follow-up and as needed based on symptoms.
Chest radiograph.
Computed tomography (CT) scan of the chest based on symptoms and findings on PFTs and chest radiograph.
Any patient with symptoms or diagnostic testing consistent with pulmonary toxicity should be referred to a pulmonologist. In the interim, bronchodilators, expectorants, antibiotics, and oxygen can be used for symptomatic relief. It is important to counsel at-risk SCC to avoid tobacco smoke and on the importance of receiving the annual influenza vaccination and SARS-CoV-2 vaccine.
Many survivors experience learning difficulties in school, which can be ameliorated with an appropriate individualized education plan (IEP) implemented. In the United States, SCC qualify for school accommodations through Section 504 of the federal Rehabilitation Act of 1973. A neuropsychologist familiar with the CNS effects of treatment for childhood cancer can perform a neurocognitive assessment and identify specific strengths and weaknesses in the SCC. These can be used to formulate interventions that can compensate for any weaknesses. Such interventions may include extra time on tests to compensate for reduced processing speed, sitting at the front of the class, and having tests taken in isolation to compensate for attention deficits, or having tutoring to bolster a specific weakness. Without the knowledge of the risks of therapy, subtle learning problems may be easily overlooked, potentially leading to poor school performance, reduced self-confidence, lower education achievement, lower earning potential, and a lower quality of life. Children treated before 5–6 years of age, especially those treated before 3 years of age, are at a higher risk for developing cognitive impairments than those treated after the age of 8–10 years.
Survivors of childhood brain tumors usually have had surgery to remove the tumor. Depending on the location of the tumor and the degree of resection, almost any aspect of neurological function can be impaired. It is critically important to understand the location and extent of the surgery, as well as any perisurgical deficits that have persisted beyond therapy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here