Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Eosinophil infiltration of the skin is commonly observed and therefore clinicopathologic correlation is required to determine a correct diagnosis
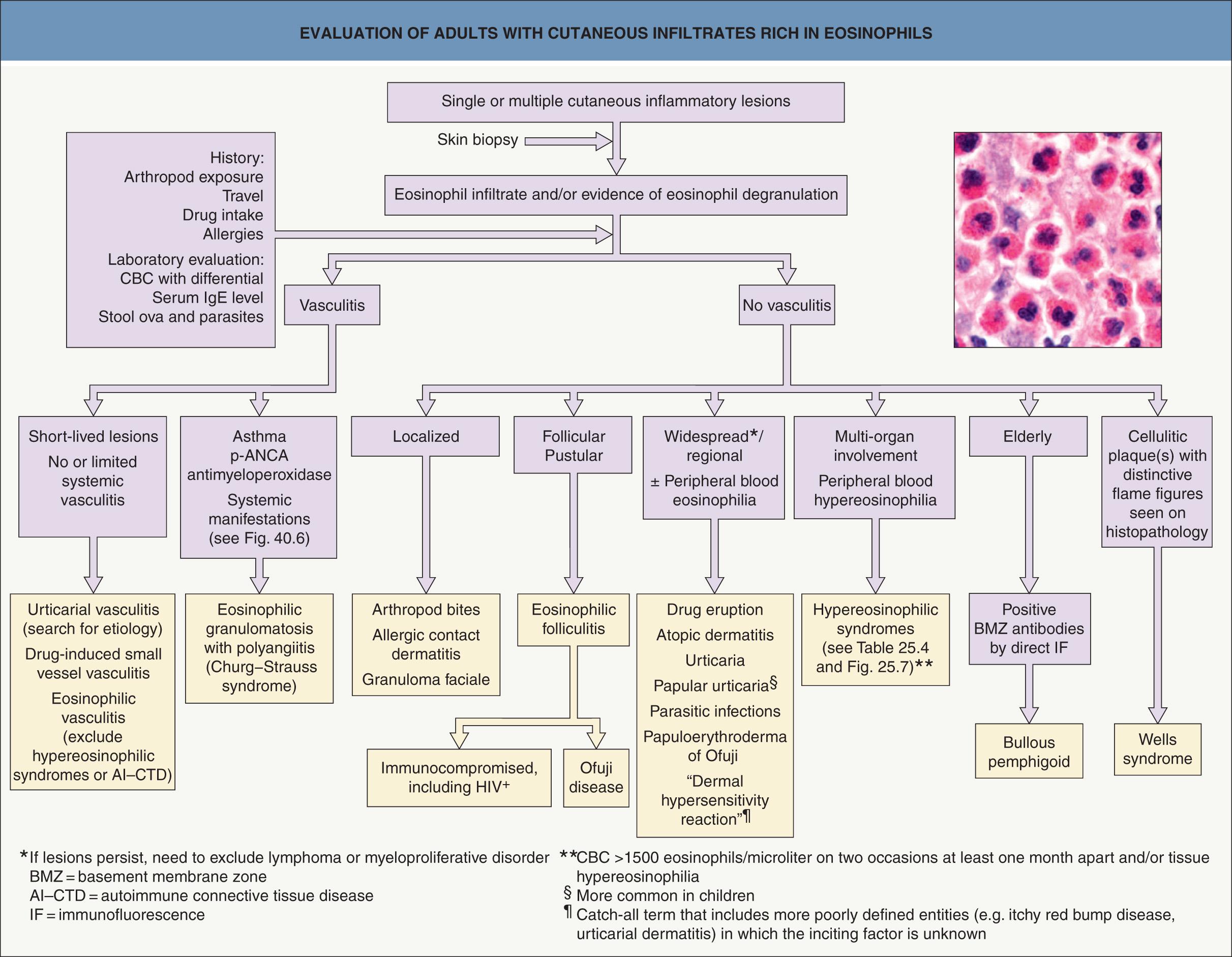
Eosinophil-associated dermatoses include arthropod bite reactions and drug eruptions (“bugs and drugs”), as well as parasitic infections, autoimmune blistering diseases, and Wells syndrome
Eosinophils commonly disrupt and lose their morphologic integrity as they deposit toxic granule proteins and other inflammatory mediators in tissues; therefore, the presence or absence of intact eosinophils histopathologically may not accurately reflect their pathogenic role
In patients with persistent peripheral blood eosinophilia, including those with the hypereosinophilic syndromes, tissue infiltration and eosinophil-derived effector molecules may cause significant end-organ damage
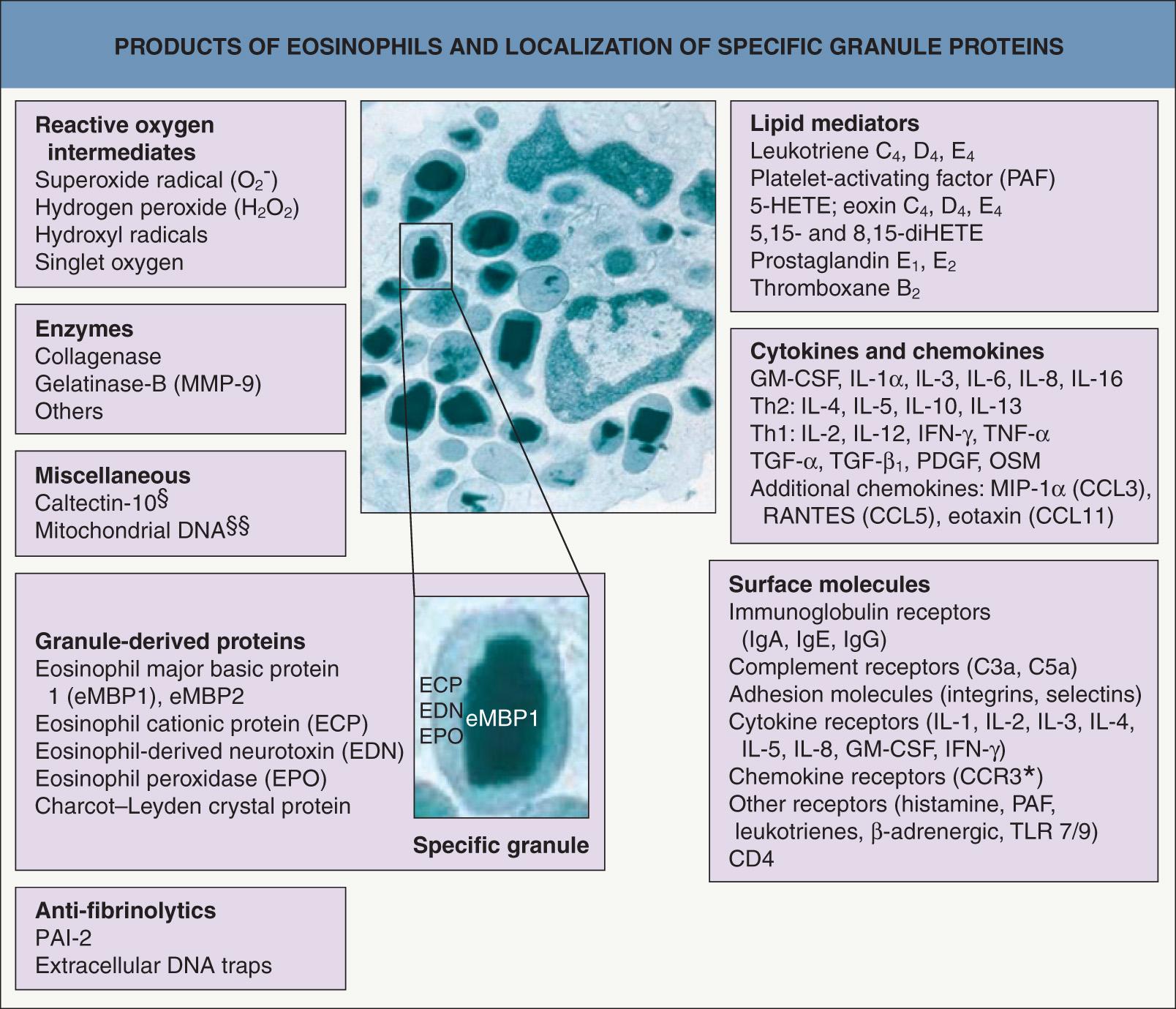
The eosinophil is produced in the bone marrow and circulates transiently (8–18 hours) in the peripheral blood. Except for the bone marrow, gastrointestinal tract (distal to the esophagus), and lymphoid tissues (including spleen, thymus and lymph nodes), eosinophils found within tissues are classically associated with allergic inflammation or extra-gastrointestinal parasitic disease. In 1879, Paul Ehrlich named the “eosinophil” because of the intense staining of its distinctive cytoplasmic granules with the acidic dye, eosin. Ultrastructurally, membrane-bound, specific eosinophil granules have an electron-dense core and less dense matrix ( Fig. 25.1 ). The eosinophil produces multiple factors (e.g. toxic cationic proteins, oxidative products) that contribute to its role in inflammation . Surface molecule expression is important in activation and/or priming and further directs eosinophil involvement in inflammation (see Fig. 25.1 ) .
Eosinophils are attracted into tissues and activated by at least three interrelated signals: (1) chemokines; (2) other cytokines; and (3) adhesion molecules. Several members of the C-C chemokine gene superfamily are chemotactic for eosinophils, and these chemoattractants include the eotaxin family and RANTES ( r egulated on a ctivation, n ormal T cell e xpressed and s ecreted). Eosinophil-active chemokines signal primarily through C-C chemokine receptor (CCR)-3, which is expressed by eosinophils. Eotaxins 1–3 are specifically chemotactic for eosinophils, while RANTES is chemotactic not only for eosinophils but also for monocytes, T lymphocytes, natural killer cells, and basophils (but not neutrophils). In addition to their chemotactic properties, the eotaxins and RANTES induce production of reactive oxygen species by eosinophils, indicating that they have both chemotactic and functional activation effects. As eosinophil chemoattractants, the eotaxins are stronger than RANTES, and eotaxins 1 and 2 are also stronger in inducing reactive oxygen species by eosinophils than are eotaxin 3 and RANTES. Eotaxins 1, 2 and 3 and RANTES are produced by dermal fibroblasts, while RANTES is also produced by keratinocytes, positioning them well for participation in cutaneous inflammation .
Eosinophils enter tissues by traversing blood vessels. Similar to other leukocytes, selectin, integrin and immunoglobulin gene superfamily members (see Ch. 102 ) contribute to the signaling needed for eosinophil transmigration . In particular, eosinophils constitutively express the integrin very late antigen (VLA)-4, a ligand for vascular cell adhesion molecule (VCAM)-1, which is induced on endothelial cells by chemokines and cytokines. After entering the extracellular matrix, eosinophil activity is influenced by interactions between cell-surface integrins and fibrous proteins (in particular, fibronectin, laminin and collagen) and glycosaminoglycans (especially hyaluronic acid and chondroitin sulfate). Integrin expression, specifically CD11b/CD18 (MAC-1), is also critical for eosinophil effector functions, including degranulation.
Activating cytokines such as granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-3, and IL-5 are also important for the effector functions of eosinophils . Several lines of investigation indicate that eosinophils are recruited to and activated in tissues by cytokine activity from the Th2 subset of T cells, which produces IL-4, IL-5, IL-10 and IL-13, in addition to cytokines common to Th1 cells. Natural killer cells also produce IL-5, and mast cell-derived cytokines contribute indirectly to eosinophil activation via induction of IL-5 and GM-CSF. Eosinophils themselves elaborate important inflammatory and regulatory cytokines, including IL-1α, TGF-α and TGF-β 1 , GM-CSF, IL-3, IL-5, IL-6, IL-8, TNF-α, and macrophage inflammatory protein 1α (see Fig. 25.1 ). As a result, eosinophil activation also occurs in an autocrine manner. In cytotoxicity assays, eosinophils are maximally activated by GM-CSF, followed by IL-3, IL-5, TNF-α and IL-4, in order of potency.
Upon activation, eosinophils release the contents of their granules into extracellular spaces via three mechanisms : cytolytic degranulation, piecemeal degranulation, and regulated secretion. Cytolytic degranulation is characterized by organelle rupture, chromatolysis of nuclei with loss of morphologic integrity and identity of eosinophils, and extensive deposition of eosinophil granules and granule products within tissue ; this process occurs in many inflammatory disorders, including skin diseases (e.g. atopic dermatitis), as well as within affected organs in the hypereosinophilic syndromes (HES).
Eosinophil major basic protein 1 (eMBP1), the constituent of the granules' crystalline cores (see Fig. 25.1 ), directly damages helminths, mammalian cells and tissues, exemplified by its ability to cause exfoliation of bronchial epithelial cells. eMBP1, but none of the other eosinophil granule proteins, stimulates histamine release from human basophils. Furthermore, eMBP1 stimulates neutrophils, inducing release of superoxide and lysozyme. Eosinophil cationic protein (ECP or RNase3) and eosinophil-derived neurotoxin (EDN or RNase2) are members of the RNase family. ECP is a potent toxin for parasites through a different mechanism than eMBP1 and is more effective at killing certain helminths than eMBP1. EDN, as its name implies, is a neurotoxin and also has antiviral activity against RNA viruses.
Eosinophil peroxidase (EPO) kills numerous microorganisms in the presence of hydrogen peroxide (generated by eosinophils and other phagocytes) and halide. This combination of products also initiates mast cell secretion. Binding of EPO to microbes, including Staphylococcus aureus , greatly potentiates their killing by phagocytes. EPO-coated tumor cells are spontaneously lysed by activated macrophages, and eMBP1 is also toxic to tumor cells. EPO and eMBP1 are potent platelet agonists that lead to release of 5-hydroxytryptamine (serotonin) and promote clotting.
The presence of eosinophils in certain normal organs (see above) suggests a role in homeostasis. In addition, eosinophil infiltration is associated with tissue remodeling and fibrosis. Although cytokines, including IL-5 and eotaxins, are known to mediate eosinophil development and survival, mechanisms underlying the basal regulation of eosinophils, including their circadian cycling, remained largely unknown until the identification of innate lymphoid cells (ILCs). Long-lived type 2 ILCs, also known as ILC2s, are resident in peripheral tissues and regulate basal eosinophilopoiesis and tissue eosinophil accumulation via constitutive and stimulated cytokine expression . ILC2s secrete IL-5 constitutively and are induced to coexpress IL-13 during type 2 inflammation, resulting in localized eotaxin production and eosinophil accumulation.
Studies of the interactions of skin ILCs with other cell types demonstrated that dermal ILCs interact selectively and strongly with mast cells . Type 2 innate lymphocytes have been designated part of the “Th2 franchise” , and a broad role for ILC2s is emerging for both eosinophils and metabolic homeostasis, e.g. in atopic dermatitis , in glucose metabolism within adipose tissue , and in allergic respiratory disease , including associated tissue remodeling . Interactions of eosinophils with nerves may in part explain pruritus and other neurophysiological changes (e.g. white dermographism) observed in eosinophil-infiltrated tissues .
Eosinophils may act as antigen-presenting cells for microbial (including staphylococcal superantigens), viral and parasitic antigens, promoting T-cell proliferation, and they play a role in the regulation of mast cell function. Activation of eosinophils via toll-like receptors (TLR) 7 and TLR9 affects several eosinophil functions, with the overall response influenced by a Th2-like cytokine milieu . In addition, eosinophils can generate mitochondrial DNA traps that contain ECP and eMBP1 and display antimicrobial activity and can promote thrombosis. In summary, eosinophils participate in innate and acquired immunity and their functional diversity has numerous ramifications for both homeostasis and disease pathogenesis .
“Eosinophil-associated dermatoses” encompass a wide range of diseases characterized by the presence of a few to many eosinophils and/or evidence of eosinophil degranulation in skin and/or mucous membranes. Disorders traditionally associated with eosinophil infiltration include arthropod bite reactions and ectoparasite infestations (see Chs 84 & 85 ), drug eruptions, helminth infections (see Ch. 83 ), and Wells syndrome ( Fig. 25.2 ). In addition, there are a number of other skin diseases in which tissue eosinophilia is often present, including bullous pemphigoid, urticarial dermatitis, and eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome) ( Table 25.1 ). However, it must be emphasized that tissue eosinophilia can be observed in a plethora of skin disorders, from inflammatory and infectious to neoplastic. Lastly, mild to moderate eosinophil infiltration is a key feature of IgG4-related disease (IgG4-RD), in which fibrosis can also be a major finding . Initially described as a pancreatic disorder with increased serum IgG4 levels and numerous IgG4-positive plasma cells in affected tissues, the spectrum of IgG4-RD has been expanded to include any organ, including the skin. Proposed cutaneous manifestations are granuloma faciale and lesions that resemble angiolymphoid hyperplasia with eosinophilia ( Table 25.2 ).
| EOSINOPHIL-ASSOCIATED DERMATOSES | |
|---|---|
| Diagnosis | Clinical clues |
| Common entities | |
| Allergic contact dermatitis ( Ch. 14 ) | History of exposure; suggestive distribution; positive patch test |
| Arthropod bite/sting reactions ( Ch. 85 ) | Primarily involves exposed skin; mosquitoes, fleas, spiders, ticks, and mites; exaggerated reactions in patients with CLL |
| Atopic dermatitis ( Ch. 12 ) | History of atopy; pruritus; flexural and extremity accentuation |
| Drug eruptions ( Ch. 21 ) | Drug history, especially new drugs within the previous 2 weeks or up to 6 weeks for DRESS/DIHS Histopathologically, eosinophils are present in ~50% of cutaneous drug reactions |
| Erythema toxicum neonatorum ( Ch. 34 ) | Newborn with erythematous macules, papules and pustules, often with prominent erythematous flare |
| Parasitic infections, particularly helminths ( Ch. 83 ) | Cysticercosis, dirofilariasis, larva migrans, onchocerciasis, schistosomiasis, and others |
| Scabies ( Ch. 84 ) | Marked nocturnal pruritus; web-space, umbilical and groin involvement |
| Urticaria ( Ch. 18 ) | Pruritic, migratory, transient (lasting <24 hours) wheals |
| Less common entities | |
| Annular erythema of infancy ( Ch. 19 ) | Annular or serpiginous non-pruritic plaques in infants |
| Autoimmune bullous dermatoses ( 29 , 30 , 31 , 32 ; see Table 29.4 for differential diagnosis of eosinophilic spongiosis) | Pemphigoid (particularly bullous pemphigoid, pemphigoid gestationis) > pemphigus, linear IgA bullous dermatosis, dermatitis herpetiformis, epidermolysis bullosa acquisita |
| Eosinophilic dermatosis associated with hematological disorders (eosinophilic dermatosis of hematologic malignancy; Ch. 33 ) | Most common in patients with CLL, but reported in patients with other hematologic malignancies |
| Eosinophilic fasciitis (Shulman syndrome; Ch. 43 ) | Sudden onset of symmetrical induration of skin and subcutaneous tissues of the limbs; peripheral blood eosinophilia |
| Eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome; Ch. 24 ) | Palpable purpura and tender papulonodules; peripheral blood eosinophilia; respiratory tract (e.g. asthma), neurologic (e.g. mononeuritis multiplex), and cardiac involvement; prominent IgG4 and IgE responses |
| Eosinophilic (pustular) folliculitis ( Ch. 38 ) | Immunosuppression, including HIV infection and post-transplant ^ : severe pruritus and papules on the face and upper trunk |
| Ofuji disease: typically a Japanese patient with chronic, recurrent follicular pustules in a seborrheic distribution with tendency to form circinate plaques | |
| Pediatric: follicular pustules on the scalp of an infant | |
| Eosinophilic, polymorphic, and pruritic eruption associated with radiotherapy (EPPER) | Local and generalized pruritus, erythematous papules and sometimes vesicles |
| Eosinophilic vasculitis (histopathologic reaction pattern rather than specific dermatosis) | Pruritic urticarial and purpuric papules, angioedema; juvenile temporal arteritis (see below) |
| Eosinophilic ulcer of the oral mucosa (may be within spectrum of CD30 + lymphoproliferative disorders; Ch. 72 ) | Rapidly enlarging nodule that develops ulceration; tongue most common site |
| Epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia; Ch. 114 ) | Single or multiple nodules of the face, scalp and/or ears |
| Granuloma faciale | See text |
| Hypereosinophilic syndromes (HESs) | See text |
| Incontinentia pigmenti ( Ch. 62 ) | Vesicles and bullae along the lines of Blaschko in neonate (stage I); peripheral blood eosinophilia |
| Juvenile temporal arteritis | Nodule in the temporal region in an older child or young adult due to eosinophilic vasculitis |
| Juvenile xanthogranuloma ( Ch. 91 ) | Yellow to red–brown papules or nodules on the head and neck, upper trunk or proximal extremities; early lesions may have numerous eosinophils |
| Kimura disease ( Ch. 114 ) | Subcutaneous masses and lymphadenopathy of the head and neck region; peripheral blood eosinophilia, increased serum IgE levels |
| Langerhans cell histiocytosis (especially eosinophilic granuloma; Ch. 91 ) | Disseminated pink and yellow–brown papules (often with crusts); intertriginous involvement; occasionally, nodules |
| Lymphoproliferative disorders of the skin, benign and malignant ( 119 , 120 , 121 ) | Presentation varies depending upon specific type; variable number of eosinophils seen in entire spectrum of lymphoproliferative disorders and commonly observed in LyP and cutaneous ALCL |
| Mastocytosis ( Ch. 118 ) | Pink–tan to red–brown macules, papules and plaques that urticate with stroking (Darier sign) |
| Papuloerythroderma of Ofuji | See text |
| Polymorphic eruption of pregnancy (PEP; also referred to as pruritic urticarial papules and plaques of pregnancy [PUPPP]; Ch. 27 ) | Primigravida in third trimester; urticarial papules and plaques, especially in striae |
| Pruritic papular eruption of HIV disease ( Ch. 78 ) | Pruritic non-follicular papules in a symmetric distribution; HIV infection; may be an exaggerated reaction to arthropod antigens but without a recognized history of bites |
| Seabather's eruption (e.g. larvae of Linuche unguiculata and Edwardsiella lineata ; Ch. 85 ) | Pruritic papules of skin covered by swimsuit; occurs after ocean swimming |
| Small vessel vasculitis (leukocytoclastic vasculitis; Ch. 24 ) | Palpable purpura that favors the lower extremities; eosinophils more commonly observed in drug-induced variant |
| Swimmer's itch or cercarial dermatitis (larvae of avian and mammalian schistosome species; Ch. 83 ) | Pruritic papules on uncovered skin; occurs after fresh-water swimming |
| Urticarial allergic eruption | Annular or gyrate urticarial plaques that persist >24 hours |
| Wells syndrome | See text |
| Less well-defined entities, with overlapping features | |
| Hypereosinophilic dermatitis of Nir–Westfried | Pruritic, papular eruption with peripheral blood eosinophilia; often dapsone-responsive |
| Itchy red bump disease (papular dermatitis) | Markedly pruritic pink to red papules; may be associated with eczematous patches and/or dermographism |
| Oid-oid disease (exudative discoid and lichenoid chronic dermatosis of Sulzberger and Garbe) | Middle-aged, predominantly Jewish, men; pruritic lesions commonly involving genitals; progressive stages: discoid → exudative → lichenified; peripheral blood eosinophilia |
| Pachydermatous eosinophilic dermatitis | South African black teenage girls with generalized pruritic papules, hypertrophic genital lesions and peripheral blood eosinophilia; dapsone-responsive; possibly a variant of hypereosinophilic dermatitis of Nir–Westfried |
| Papular eruption of blacks | Young, black men with intensely pruritic papules on the trunk and upper arms |
| Urticarial dermatitis | Very pruritic; eczematous and/or urticarial lesions involving the trunk and proximal extremities; often occurs in the elderly and may reflect immunosenescence; minimally responsive to topical corticosteroids and oral antihistamines but can improve with UVB phototherapy or dapsone |
| IgG4-RELATED DISEASE (IgG4-RD) | |
| Characteristic findings (not present in all patients) | |
|
|
| Affected organs/tissues and/or manifestations – emerging spectrum | |
|
|
|
|
|
|
|
|
|
|
| Cutaneous disorders (or subsets thereof) that may fall within this spectrum | |
|
|
|
|
In most patients, a solitary or few red–brown, firm plaques or nodules appear on the face; involvement of extrafacial sites is uncommon
Histopathologically, vasculitis is seen focally, with dense dermal infiltrates of neutrophils, lymphocytes and plasma cells, admixed with numerous eosinophils; the inflammation spares the upper papillary dermis (grenz zone) and fibrosis also may be present
A chronic cutaneous vasculitis in which IgG4-producing plasma cells are usually found
Initially described by Wigley as “eosinophilic granuloma” (not related to Langerhans cell histiocytosis), Pinkus later coined “facial granuloma with eosinophilia” . Granuloma faciale has been included by some authors in the spectrum of IgG4-related disease (IgG4-RD; see Table 25.2 ) .
Granuloma faciale occurs predominantly in middle-aged white men, but has been observed in black and Asian men as well as women.
While the precise pathogenesis remains unknown, interferon-γ and increased local IL-5 production are implicated as important mediators . Direct immunofluorescence (DIF) microscopy demonstrates granular deposition of IgG, IgA, IgM, and/or C3 in blood vessel walls, a nonspecific finding that suggests a role for immune complexes. An increased ratio of IgG4- : IgG-bearing circulating plasma cells and/or relative increases of IgG4-positive plasma cells in skin lesions has been observed, but it is unclear if this is a specific finding.
Granuloma faciale usually presents as a solitary, asymptomatic, red–brown plaque on the face, with a predilection for the forehead, cheek, and preauricular area ( Fig. 25.3A,B ) . Less often, multiple papules or plaques may be present ( Fig. 25.3C ) ; in one retrospective analysis of 66 patients, one-third had more than one lesion . Uncommon locations for granuloma faciale include the ears, scalp, and trunk or extremities ; in the previously cited retrospective study, 7% of the patients had extrafacial involvement . The individual lesions tend to persist and only occasionally resolve spontaneously. Granuloma faciale has not been associated with systemic disease.

In the dermis, there are perivascular and interstitial infiltrates of neutrophils, lymphocytes and plasma cells, admixed with numerous eosinophils. Characteristically, the inflammation spares the upper papillary dermis, creating a “grenz zone” ( Fig. 25.4 ). Features of leukocytoclastic vasculitis are most prominent early on, with older lesions tending to have fewer neutrophils and more eosinophils and plasma cells as well as fibrosis. Because of the presence of eosinophils, IgG4-bearing plasma cells and lamellar fibrosis, it has been proposed that at least a proportion of cases of granuloma faciale may represent a cutaneous manifestation of IgG4-RD (see Table 25.2 ) .
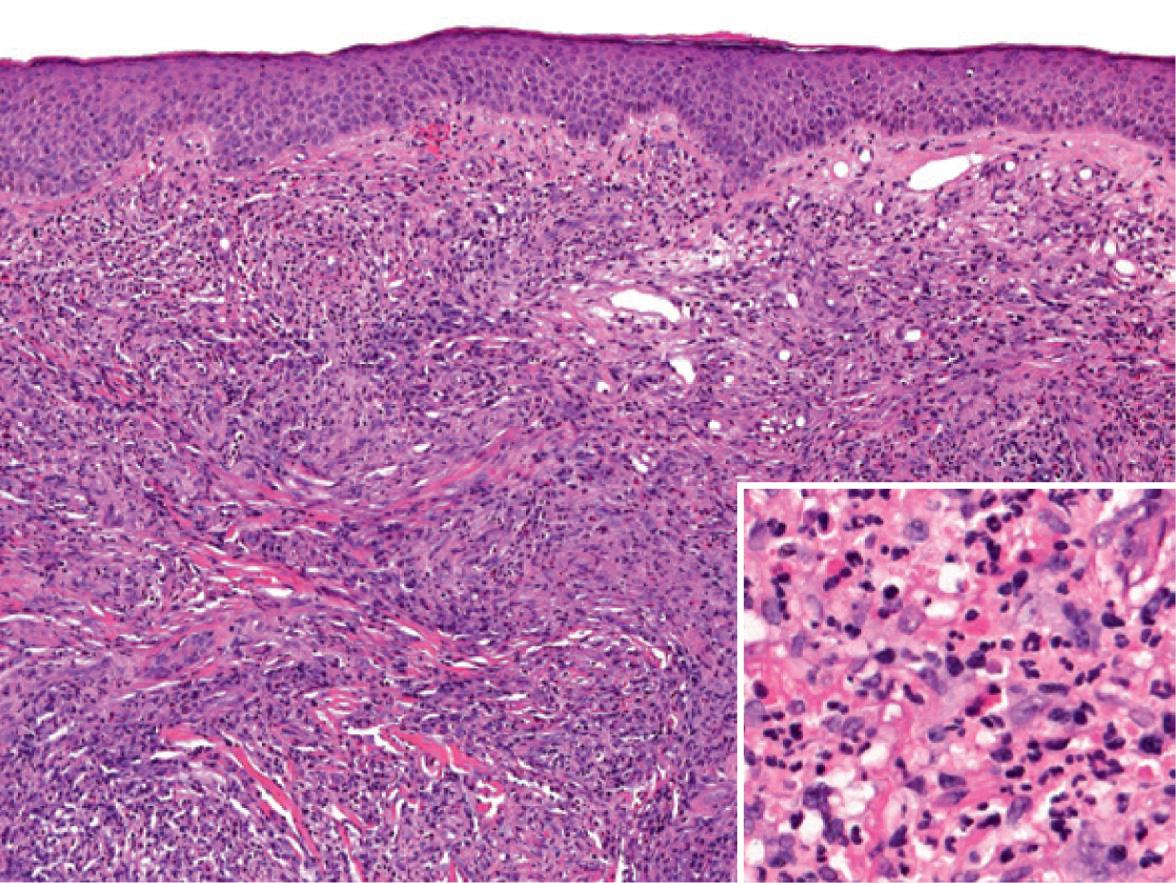
The clinical appearance of granuloma faciale is distinctive, but the differential diagnosis can include lymphoma, persistent arthropod bite reactions, angiolymphoid hyperplasia with eosinophilia, tumid lupus erythematosus, and several granulomatous disorders (e.g. sarcoidosis, leprosy, granulomatous rosacea). The histopathology of granuloma faciale may have features in common with rheumatoid neutrophilic dermatitis, neutrophilic dermatosis associated with lupus erythematosus, epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia), or persistent arthropod bite reactions. Granuloma faciale may also resemble erythema elevatum diutinum (EED), both clinically and histopathologically. However, EED presents as multiple red–brown papules, plaques or nodules in a symmetric distribution on the extensor aspects of the extremities, with a predilection for the skin overlying joints. Fibrosis also tends to be more pronounced and lipid-laden macrophages may be seen in EED, but not in granuloma faciale.
Because of the facial location, treatment is often desired. Unfortunately, granuloma faciale is frequently resistant to therapy. Intralesional triamcinolone suspension (2.5–5 mg/ml) is often a first-line therapeutic option. As with other recalcitrant dermatoses, there are many anecdotal alternatives with reported efficacy, including oral and topical dapsone (50–150 mg daily), oral clofazimine (300 mg daily), topical PUVA, and topical calcineurin inhibitors (pimecrolimus, tacrolimus).
Surgical excision, cryosurgery, dermabrasion, electrosurgery, and CO 2 or pulsed dye laser therapy all have been advocated, but each carries a significant risk of scarring given the depth of inflammation. In addition, recurrences after excision have been reported. Laser therapy that targets the prominent vascular component, e.g. 595 nm pulsed dye laser, 532 nm potassium titanyl phosphate laser, has led to improvement. With recognition that granuloma faciale has features in common with IgG4-RD, additional therapies such as prednisone may be considered, although the risk–benefit ratio will need to be addressed for this localized cutaneous disease.
Occurs most commonly in elderly men
Widespread, pruritic, red–brown papules that may evolve into a confluent erythroderma with characteristic sparing of the skin folds (“deck-chair” sign)
Chronic course, with periodic exacerbations
Histopathologically, a nonspecific pattern consisting of lymphohistiocytic inflammation with a variable number of eosinophils
Peripheral blood eosinophilia, lymphopenia, and increased serum IgE level in more than two-thirds of patients
Association with malignancy (especially T-cell lymphoma and gastric carcinoma), infections (including HIV and hepatitis C virus), and drugs
Often responds to oral corticosteroids or phototherapy, alone or in combination with a systemic retinoid
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here