Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The development of thoracic endovascular aortic repair (TEVAR) has dramatically revolutionized the field of cardiovascular surgery. Since Parodi's first description of an intraluminal stent graft device for the treatment of abdominal aortic aneurysms, endovascular device technology has rapidly evolved to treat the multiple pathologies seen in the thoracic aorta. In 1994, Dake first reported the initial Stanford experience with 13 patients undergoing endovascular therapy of descending thoracic aortic aneurysms. Since then, indications involving off-label use have expanded to include the treatment of aortic dissections, traumatic transections, penetrating atherosclerotic ulcers (PAUs), and intramural hematoma (IMH). Currently, there are four stent graft devices approved by the U.S. Food and Drug Administration (FDA) for the treatment of descending thoracic aortic aneurysms. Questions and concerns remain regarding the appropriate timing, indication of intervention, and durability of this fast-evolving technology. TEVAR has gained worldwide acceptance in the treatment of pathologies of the descending thoracic aorta (DTA). In the span of a decade, TEVAR has gained on-label FDA approval for the treatment of thoracic aortic aneurysm, PAU, blunt thoracic aortic injury, and type B aortic dissection. This change attests to the rapid evolution and adoption of this technology in treating thoracic aortic pathology.
As TEVAR technology evolves, recent reports have shed light on the utility of endovascular technology in treating ascending aortic and aortic arch pathology—primarily ascending aortic dissection, ascending aorta IMH/PAU, and aortic arch aneurysms in poor open surgical candidates. With improving technology, it is likely that in the future TEVAR will have a defined role in the treatment of ascending aortic and aortic arch pathology. Currently, it has become the gold standard for treatment of descending thoracic aortic pathologies.
Thoracic aortic aneurysms are abnormal dilation of the aorta, characterized by elastin fragmentation and fibrosis, resulting in medial degeneration of the aortic wall. These age-related changes likely result in the reduction of aortic integrity and strength. As the population ages, the incidence of thoracic aortic aneurysms appears to be increasing. In a recent Swedish study examining the national health care registry from 1987 to 2002, the incidence of thoracic aortic pathology rose by 52% in men and by 28% in women, reaching 16.3 per 100,000 per year and 9.1 per 100,000 per year, respectively. In the same study, the annual incidence of operations performed on the thoracic aorta increased from 0.8 per 100,000 per year in 1987 to 5.6 per 100,000 per year in 2002 for an overall sevenfold increase. In women, the increase was 15-fold, from 0.2 per 100,000 per year in 1987 to 3.0 per 100,000 per year in 2002.
The natural history of thoracoabdominal aortic aneurysms has been examined in large single-institutional series. Characterized by slow growth over time, aortic aneurysmal degeneration is an indolent pathologic process of aortic dilation leading to potential rupture or dissection. The human aorta grows generally at a rate of about 0.07 cm per year in the ascending aorta, and 0.19 cm per year in the descending thoracoabdominal aorta. If dissection is present, the thoracoabdominal aorta may grow at a slightly faster rate of 0.28 cm per year.
The major risk in thoracic aortic aneurysms pertains to the catastrophic events of rupture or dissection. The risk of rupture or dissection as a function of maximum aortic diameter has been examined in multiple studies. Clouse and coworkers examined the Olmstead County database and demonstrated that in patients with a maximum aortic diameter measuring 4.0 to 5.9 cm, the risk of rupture was 16% over a period of 5 years. When the maximum aortic diameter is greater than 6.0 cm, the risk of rupture exceeds 30% over the same period of 5 years. The Yale group have also identified “hinge points” of maximum aortic diameter that represent significant increases in the risk for rupture. In the ascending aorta, this “hinge point” appears to be at 6 cm, with a risk of rupture or dissection at 34% over the lifetime of the patients. In the DTA, the “hinge point” appears to be at 7 cm, with a 43% risk of rupture or dissection. The annual risk of rupture or dissection as a function of maximum aortic diameter has also been examined. Davies and coauthors demonstrated that in patients with a maximum aortic diameter of less than 6.0 cm, the yearly rates of rupture, dissection, or death, is less than 8%; however, the annual risk of rupture, dissection, or death dramatically increases to 15.6% when the maximum aortic diameter is greater than or equal to 6.0 cm.
General recommendation for the indication for surgical intervention has been made based on these large institutional population studies. The decision to intervene surgically must be based on a balance between the risk of surgery or intervention (in the case of TEVAR) versus the risk of rupture or dissection with medical management. The general consensus for conventional open repair is to intervene surgically at a diameter of 5.5 cm for the ascending aorta and a diameter of 6.5 cm for the DTA. Certainly, patients with significant family history of aortic disease or connective tissue disorder such as Marfan syndrome may warrant intervention at a lower threshold of aortic diameter. Furthermore, for patients with symptomatic aneurysmal disease, urgent surgical intervention is recommended regardless of size, because symptoms are an early indicator of impending rupture.
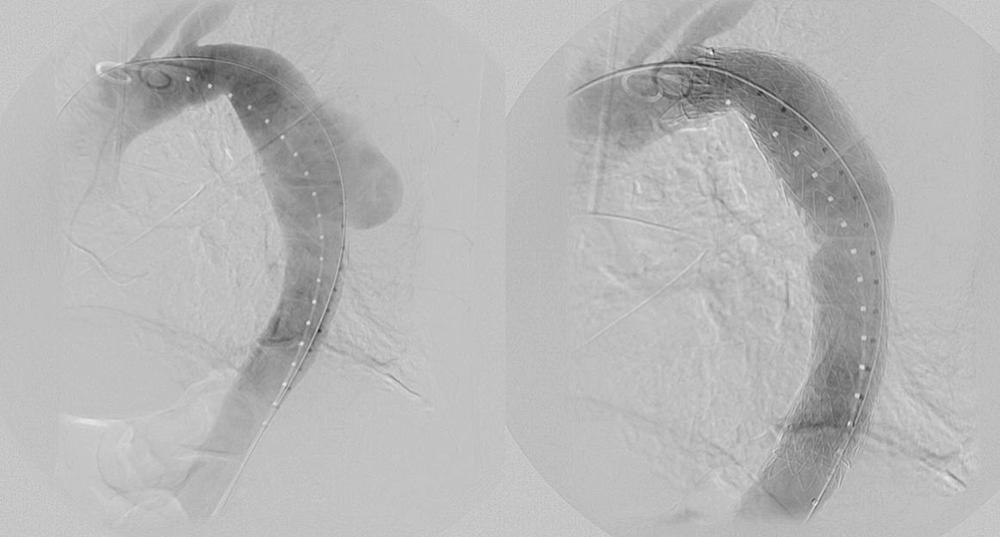
In the era of TEVAR, the perceived lower rates of morbidity and mortality have urged the question: Should the threshold for intervention in the DTA be lowered? With a mortality rate of less than 5% with TEVAR in most centers of excellence, most patients with a descending thoracic aortic diameter greater than 5.5 cm may be considered for endovascular repair ( Fig. 72-1 ). However, the final decision to intervene must be based on previously established surgical dictum: the risk of rupture must outweigh the risk of surgery, regardless of approach.
Since the early devices were first described by the Stanford group in 1994, TEVAR devices have undergone multiple modifications and clinical trials. There are four thoracic endoprostheses currently approved for the treatment of descending thoracic aortic aneurysms. Second- and third-generation devices, as well as disease-specific devices, are currently being investigated.
The Gore TAG thoracic endoprosthesis (W.L. Gore, Inc., Flagstaff, AZ) was approved by the FDA in March 2005 for the treatment of descending thoracic aortic aneurysms. The first device to be approved, the Gore TAG, is a self-expanding polytetrafluoroethylene endograft comprised of nitinol support. Concern over fracture of the longitudinal support wire led to the revision of the original Gore device to its current Gore TAG endoprosthesis. Unique to this device system is the introducer sheath, which enables the delivery of multiple devices and minimizes traumatic injury to the femoral access vessels. The introducer sheath ranges from an inner diameter of 20 Fr to 24 Fr depending on the size of the Gore TAG devices required for treatment. The Gore TAG endoprosthesis ranges from a minimal diameter of 26 mm to a maximum diameter of 40 mm, with lengths ranging from a minimum of 10 cm to a maximum of 20 cm ( Fig. 72-2 ). The mechanism of deployment involves release of the endograft from a center to peripheral fashion. Thus, precise deployment of the proximal and distal landing zones may be difficult.
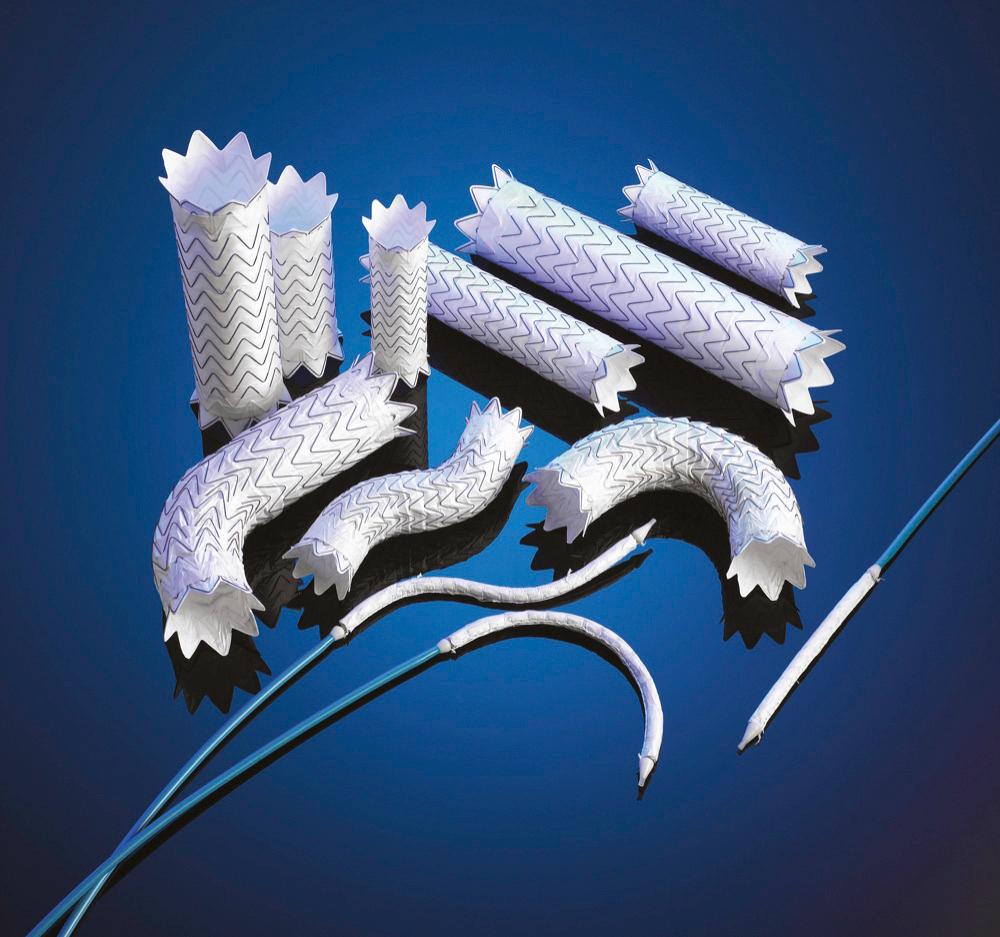
In August 2011, Gore Conformable-TAG (C-TAG) device received FDA approval. The C-TAG has essentially replaced the older-generation TAG device. It provides greater flexibility and conformability, with a broad, oversizing window (6%-33%), and is available in straight and tapered forms. The C-TAG is available in a smaller-diameter range than the TAG device (21 mm) and can be placed using an 18 Fr (inner diameter) delivery system. C-TAG is now FDA approved for use in thoracic aortic aneurysm disease, PAU, blunt thoracic aortic injury, and complicated type B dissection.
Recently approved in June 2008 by the FDA for the treatment of descending thoracic aortic aneurysms, the Talent device is a preloaded stent graft incorporated into a CoilTrac delivery system. It is a stent graft composed of a polyester graft (Dacron) sewn to a self-expanding nitinol wireframe skeleton. Radiopaque markers are sewn to the graft material to aid in visualization during fluoroscopy. The CoilTrac delivery system is a sheathless, push-rod–based delivery system. Preloaded onto an inner catheter, the Talent device is deployed by pulling back an outer catheter, allowing the device to self-expand and contour to the aorta. A balloon may be used to ensure proper apposition of the graft to the aneurysmal aorta after deployment.
The Talent device is designed as a modular system. Forty-seven different configurations ranging from a diameter of 22 to 46 mm and cover lengths from 112 to 116 mm are available. To accommodate the size differences often found between the proximal and distal portions of the aorta in thoracic aneurysms, tapered grafts are available for better aneurysmal conformability and prevention of junctional endoleaks. Four configuration categories are available: proximal main, proximal extension, distal main, and distal extension ( Fig. 72-3 ). The proximal configurations and the distal extension are offered with a bare-spring design (Free-Flo design). The bare-spring design allows for placement of the device crossing the arch vessels proximally and the celiac artery distally for suprasubclavian and infraceliac fixation, respectively.

The Valiant device is designed based on the experience with the Talent device. Similar to the Talent device, the Valiant device is also a preloaded stent graft made of the same polyester graft built onto a self-expanding nitinol skeleton. Modification has been made to improve trackability, conformability, and deployment of the Valiant device. First, device lengths have been increased to a maximum of 230 mm (130 mm for Talent). Because the device is a sheathless system, each piece requires an individual deployment through the access vessel, resulting in repeated exchange in the artery. Longer lengths have been designed to minimize device exchange during deployment. Second, the connecting bar has been removed in the Valiant device for improved conformability, especially in the arch. Third, the number of bare springs at the proximal and distal ends of the device has been increased from 5 to 8 in the Valiant device to improve circumferential force distribution and fixation along the aorta wall. Finally, the Valiant device is introduced in a new delivery system, the Xcelerant Delivery System. First available to physicians in the United States for the AneuRx AAA device, the Xcelerant Delivery System has been modified for the Valiant Device to provide a more comfortable deployment mechanism of the device, especially in tortuous anatomy of the distal arch and thoracic aorta. As opposed to a simple pullback unsheathing mechanism, the deployment of the Xcelerant Delivery System includes a gearing, ratchet-like mechanism in the handle to allow easy deployment. The amount of force required to deploy the device is reduced significantly without compromising the precision of the deployment.
Similar to the Talent device, The Valiant device is a modular design. Eighty-eight different configurations ranging from a diameter of 22 to 46 mm and cover lengths from 110 to 200 mm are available in the Valiant device. Four configurations categories are available: proximal FreeFlo straight component, proximal closed-web straight component, proximal closed-web tapered component, and distal bare-spring straight component. The proximal FreeFlo straight component is designed for the most proximal deployment zone, because the bare springs are designed to allow precise and crossing deployment of the arch vessels. In addition, it is designed as the first piece to be deployed.
In 2012, results of the VALOR II trial were reported for the use of the Valiant endoprosthesis for DTA aneurysm repair. The pivotal trial was conducted at 24 U.S. sites, assessing 30-day and 12-month outcomes. At 30 days, there were rates of 3.1% mortality, 38.1% major adverse events, 0.6% paraplegia, and 2.5% stroke. At 12 months, aneurysm-related mortality was 4%, with a stent migration rate of 2.9% and an endoleak rate of 13%.
Recently approved in 2008 by the FDA, the Cook Zenith TX2 thoracic endoprosthesis (Cook, Inc., Bloomington, IN) is designed as a modular system with a specific proximal and distal configuration ( Fig. 72-4 ). The graft consists of stainless steel Z-stents with full-thickness polyester fabric. Similar to the Medtronic delivery system, the Zenith TX2 system does not require a delivery sheath, and it is introduced as a preloaded catheter with triggers. The device sheath has a hydrophilic coating and sizes range from 18 to 22 Fr, depending on the diameter of the endoprosthesis. The diameter of the endoprosthesis ranges from 22 to 42 mm and lengths from 120 mm to 207 mm. The two components are designed to be deployed from a proximal to distal direction, and tapered devices are available.
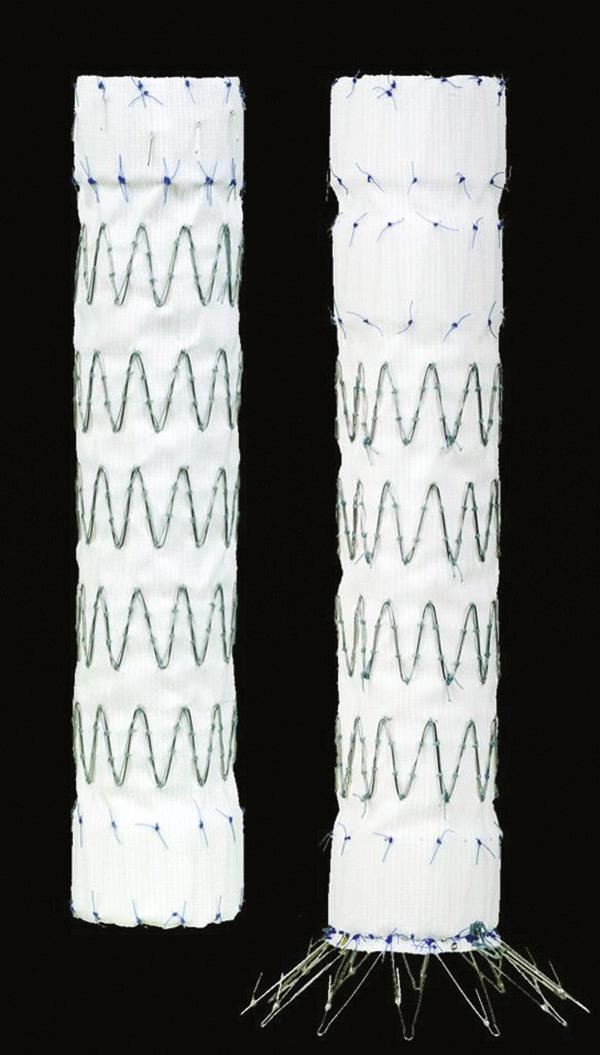
Deployment of the TX2 is achieved through a trigger system to ensure a controlled deployment. Flushed at the proximal end with barbs to prevent migration and endoleak, the device is deployed by an unsheathing mechanism. To minimize the “wind sock” effect during deployment (thus distal migration), the proximal barb component of the device is not released until the graft is deployed and the trigger released. The distal component is deployed in a similar mechanism. However, in addition to barbs, the distal component also has bare springs at its caudal portion. Proximal and distal extensions are available if additional coverage is necessary.
The Bolton Relay graft (Bolton Medical, Inc., Sunrise, FL) is the latest new company to receive FDA approval (in 2012) for TEVAR treatment of DTA aneurysmal pathology. The Relay system features a four-step sheath delivery system for modular delivery (outer diameter 22-26 Fr), with graft lengths ranging from 100 to 250 mm in straight and tapered forms; graft diameters range from 22 to 46 mm, with proximal and distal components. Proximal grafts are available in two types—Relay uncovered graft and Relay NBS covered graft. They feature S-bar (spiral support strut) technology that runs longitudinally along the stent graft, enabling improved support to the entire structure. The Relay system enables proximal capture of stent graft for precise placement, along with a secondary sealing and fixation zone that provides an extra sealing independent of proximal sealing.
Anatomic requirements and key technical considerations for successful TEVAR revolve around answering the question of what makes a patient a suitable anatomical candidate for a thoracic aortic stent graft. Initial assessment of a stent graft candidate begins with an extensive preoperative workup and evaluation. Key points of the history and physical examination should include a detailed neurologic and cardiovascular examination. Distal vascular pulses and preoperative neurologic deficiencies must be documented. Previous abdominal, pelvic, and inguinal surgeries should be noted, because these procedures may demand an alternate access route.
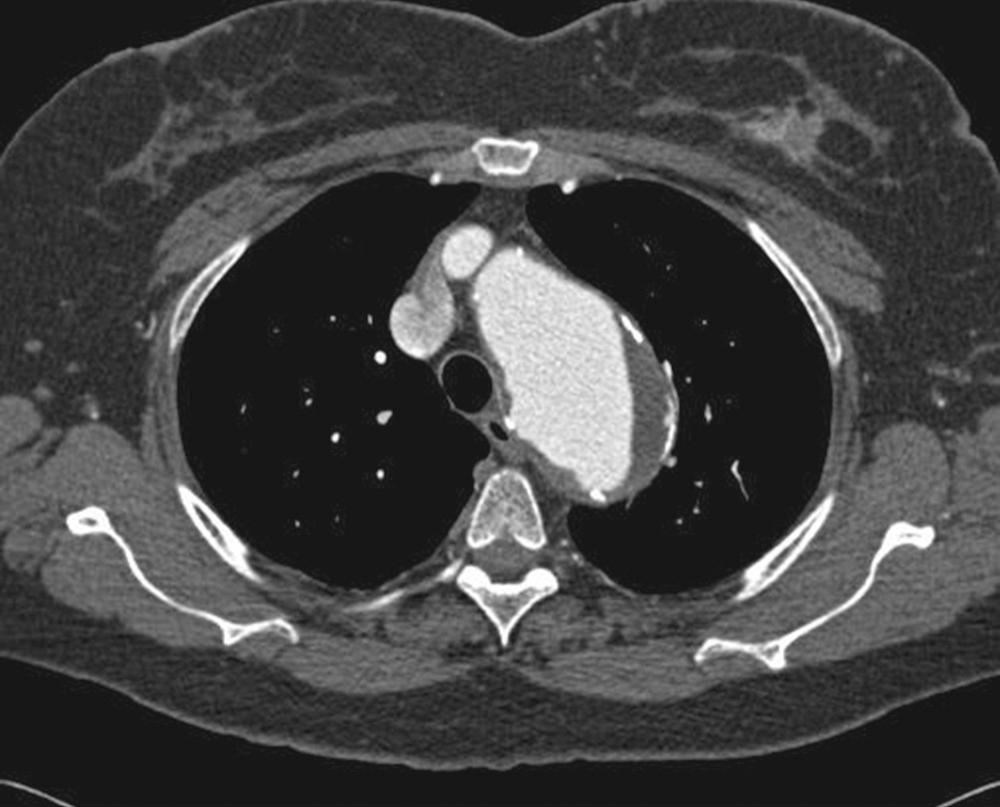
Safe vascular access for thoracic aortic device deployment is the key to thoracic aortic stent grafting. The majority of the morbidity and mortality is a direct result of arterial access complications. Extensive preoperative planning with appropriate imaging is mandatory. The “gold standard” for preoperative evaluation is a computed tomography (CT) angiogram that includes the thorax, abdomen, pelvis, and femoral arteries. Fine-cut helical CT scanning with at minimum 3-mm slices is ideal ( Fig. 72-5 ). In patients who cannot receive a CT with contrast, magnetic resonance angiography is an acceptable substitution.
All the thoracic endovascular devices take a long time to reach the descending aorta, are of large caliber to contain the thoracic aortic endoluminal graft, and are relatively stiff to allow “pushability” through the iliofemoral access points and through the abdominal aorta. The management of the delivery of the thoracic aortic stent graft is often the most challenging aspect of the case. Delivery systems of the three currently FDA-approved thoracic endoprostheses will require arterial access size of a minimum of 7.5 to 8.0 mm. Creation of a conduit to the femoral or iliac artery may be necessary to achieve adequate access. Not only the size of the arterial vessels, but also the anatomy of the iliofemoral and abdominal aorta, must be considered when planning the access route. Excessive tortuosity and atherosclerosis with occlusive disease may provide barriers to safe delivery of the endograft. In approximately 20% of patients, retroperitoneal access to the common iliac arteries will be required owing to issues of femoral or external iliac artery size and/or tortuosity.
Careful review of preoperative studies will indicate which patients will have difficult access. Patients with atherosclerotic occlusive iliac disease may be treated with standard endovascular techniques of balloon angioplasty to reduce the obstruction. Iliac stents should be avoided because of the potential interference of these stents with the thoracic aortic access devices. These procedures on the access vessels should be carried out at least 6 weeks before thoracic aortic stent grafting to allow healing of the iliac arteries post-angioplasty and manipulation. At the completion of the thoracic aortic endografting, iliac stents may be placed if appropriate.
Access to the retroperitoneum allows several options for safe device deployment. The common iliac artery may be used for device deployment. An open surgical conduit can be constructed to allow an end-to-end anastomosis or a side-to-side anastomosis. A 10-mm conduit of synthetic Dacron is commonly used and allows ample size for insertion of all necessary devices. The conduit may be brought through a separate counterincision in the groin to allow better angulation of the relatively long and stiff deployment devices. At the conclusion of the procedure, these conduits may be used to revascularize distal obstructions if needed.
Alternatively, the retroperitoneal iliac vessels, or even the distal aorta, may be accessed using direct sheath insertion. A double purse string of 4-0 Tycron is used to secure the vessel and provide hemostasis with the application of two sets of tourniquets. Direct needle puncture of the artery is followed by dilation and insertion of the device. At completion, the device is removed and the purse string sutures are tied down. Excessive tortuosity of the iliofemoral arteries requires adaptive strategies. The use of external manual manipulation provides a simple method of straightening some of the tortuosity of the aorta and iliac arteries. During fluoroscopy the operator hand can provide gentle force to the tortuous arterial segment to allow straightening and subsequent endovascular access.
In cases of iliac artery tortuosity, advanced endovascular techniques may aid in straightening these segments. The use of stiff wires or buddy wire techniques can provide some degree of straightening of the diseased arteries. In severe cases of tortuosity, brachiofemoral access may be required to perform “body flossing” with an appropriately stiff wire. Typically, a 5 Fr sheath with a long catheter is placed into the aortic arch and then into the descending aorta. A long, stiff wire, such as a 450-cm SS Guidewire (Boston Scientific, Natick, MA), is guided from the brachial artery and retrieved through the femoral artery. Gentle traction on both the brachial and femoral sites will straighten out the tortuosity. Of note, a long catheter must be placed through the brachial artery into the aorta to prevent excessive trauma by the stiff wire along the arch and innominate vessels.
At the completion of the deployment and ballooning of the thoracic stent graft, the entire route of access must be carefully examined to ensure that there has been no injury. The stiff guidewire that was used to position the thoracic endograft should be left in place as the sheaths and remaining endovascular materials are removed. A smaller sheath should be reinserted and a diagnostic aortoiliac arteriogram should be performed to evaluate for thrombus, dissection, or complete avulsion.
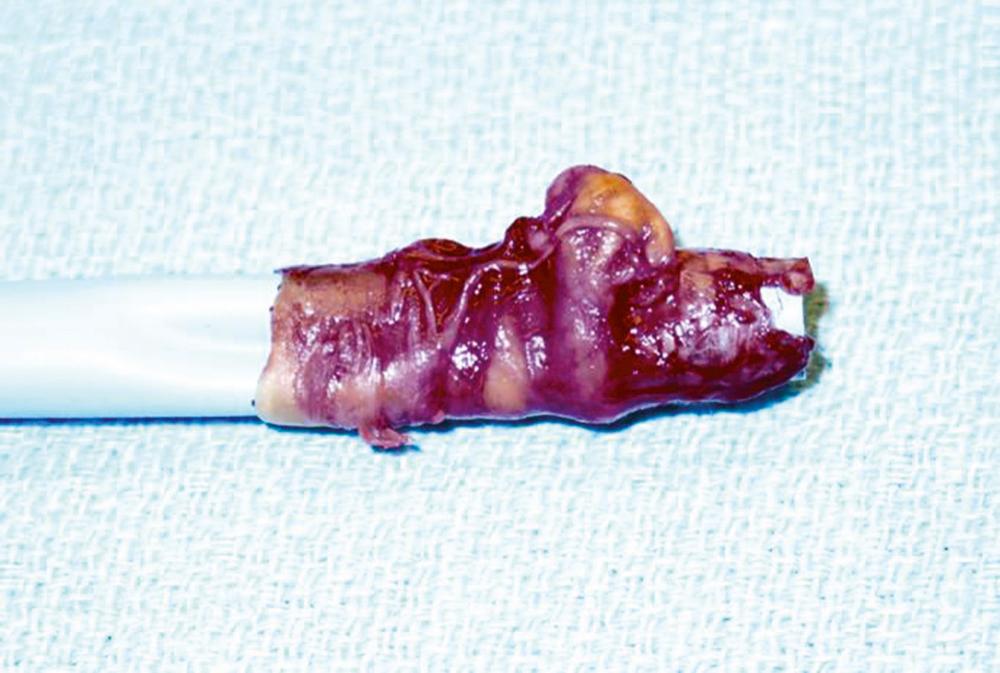
The removal of the large sheath, especially when inserted with some force and manipulation, is a particularly dangerous period when injury may occur. There have been numerous reports of successful thoracic endografting with a sheath removed with a complete iliac artery avulsed and attached ( Fig. 72-6 ). At the time of recognition, a stiff wire through this injured artery may be life-saving and allow insertion of an occluding balloon proximally to allow control of a potentially life-threatening bleed. In addition, both blood pressure and heart rate should be carefully monitored during removal and completion of the endovascular procedure for signs of an occult injury.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here