Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Percutaneous endovenous stenting has emerged as a powerful technique for treating chronic venous obstructions. Stenosis and chronic total occlusions (CTOs) are amenable to endovascular correction. The technique is minimally invasive, safe, and effective and does not preclude open correction in case of failure. Endovenous procedures can be applied in geriatric patients and in those who have significant comorbidities that would otherwise preclude open procedures. Intravascular ultrasound (IVUS) diagnostics have revealed that obstruction is present in a broader spectrum of chronic venous disease (CVD) than previously imagined. Furthermore, stent correction of obstruction alone in combined obstruction-reflux appears to yield good symptom relief even when the reflux component remains uncorrected ; this suggests that the pathophysiologic importance of obstruction has been underestimated. These observations point to a broad, dominant role for stent usage in highly symptomatic CVD patients, signifying a major therapeutic paradigm shift in the management of advanced CVD.
Postthrombotic etiology for chronic venous obstruction is well established. With the use of IVUS, it has become clear that nonthrombotic iliac vein lesions (NIVLs) are also a common feature among “primary” CVD patients with advanced symptoms. The lesions occur at arterial crossover points over the vein ( Fig. 52.1 ). Intraluminal webs and strictures are often present and are thought to result from pulsatile trauma in an intimately associated artery. Other less common causes of chronic venous obstruction include tumors, retroperitoneal fibrosis, and radiation injury. Inferior vena cava (IVC) filters are also emerging as a significant cause of iatrogenic iliocaval obstruction; some filters appear to be more prone to this development than others.
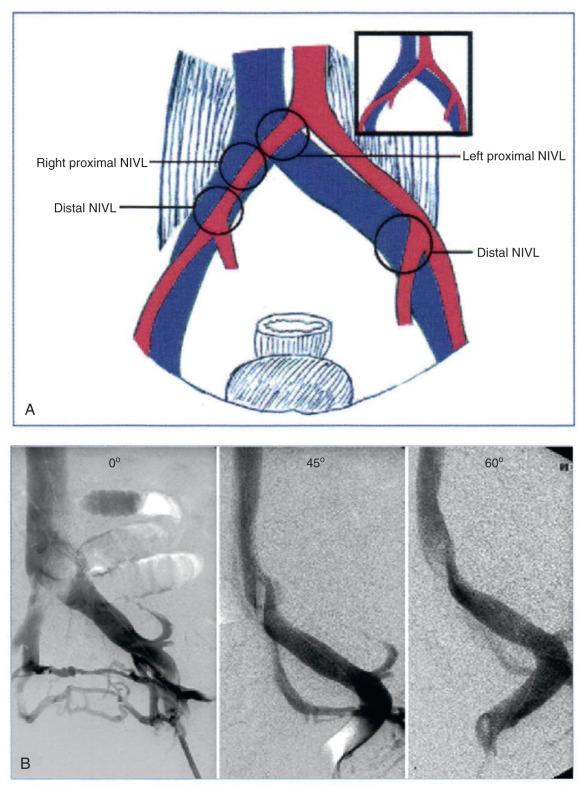
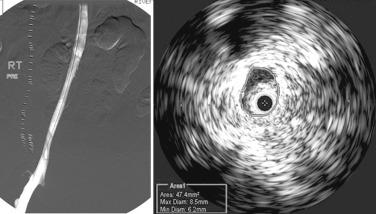
Postthrombotic disease often involves multiple venous segments, but symptom production appears to be mainly related to the iliac vein segment because of its poor collateral potential. Natural collateral pathways based on embryology appear to develop rapidly in obstructions of other venous segments, particularly in femoral vein obstructions and to a lesser extent in caval obstructions. These collateral pathways may already exist in putative form, becoming functional when straight-line flow faces higher resistance than in the quiescent collaterals. The process is just the reverse of that seen in venous stenting, when collaterals disappear instantaneously with clearance of obstruction. The profunda femoris vein is an embryologic collateral and often becomes visible on venography within a few hours after the onset of femoral vein thrombosis. Lesions causing CTO of the IVC are sometimes found incidentally in asymptomatic patients. In contrast, pelvic vein collaterals that appear impressive on venography are often functionally inadequate, and such patients continue to be symptomatic. Some postthrombotic iliac vein lesions develop a tough perivenous sheath that restricts the vein and retards collateral development. Such diffuse iliac vein stenosis, first noted by Rokitanski in autopsy studies, appears as a patent but smaller-caliber iliac vein on venography. The reduction in luminal size is clearly evident on IVUS examination but is easily missed on venography ( Fig. 52.2 ). Focal iliac vein lesions may be impervious to routine venography as well. For these reasons a symptomatic iliac vein lesion may remain occult, whereas the more obvious femoral vein occlusions are readily apparent. The latter are seldom the main source of symptoms, however, and a diligent search for the culprit lesion in the iliac veins is in order.
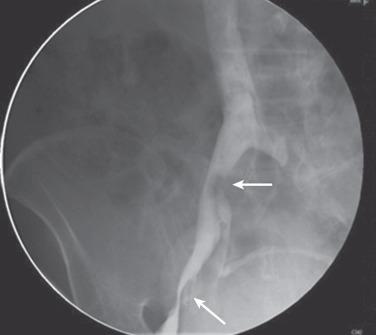
The discovery through IVUS of a high incidence of NIVLs in patients with symptomatic CVD and relief of symptoms with venous stenting has highlighted the pathophysiologic peculiarities of these lesions. First, these lesions are widely prevalent in the asymptomatic population, with a gross prevalence of up to 30% in autopsy studies and up to 66% with more sensitive modern imaging techniques. With IVUS, the lesions are found in greater than 90% of patients with advanced symptoms. These are characteristics of permissive pathology, which is widely prevalent in human disease. Patent foramen ovale (PFO), with an incidence of approximately 25% in the asymptomatic general population, becomes symptomatic in a small fraction, with onset of paradoxic embolus. Not surprisingly, PFO is invariably found in patients with paradoxic embolus. Other examples of permissive or secondary pathologies include obesity and diabetes, diabetes and neuropathy, carotid plaque and transient ischemic attack, ureteric reflux and pyelonephritis, Helicobacter pylori and peptic ulcer, and acid reflux and asthma. In case of silent iliac vein obstructions, a number of secondary events may trigger the onset of symptoms ( Box 52.1 ). A postthrombotic iliac vein lesion related to a remote thrombotic event can also remain silent for years or decades before symptoms are precipitated by a later trigger event ( Fig. 52.3 ).
Trauma
Infection (cellulitis)
Reflux
Deep venous thrombosis
Joint surgery
Orthostasis and poor calf pump (in the elderly)
Obesity
Edematogenic medications (many cardiovascular drugs)
Postmenopausal hormonal changes
Lymphatic damage and venous disease itself or from cancer, chemotherapy, radiation, or surgery
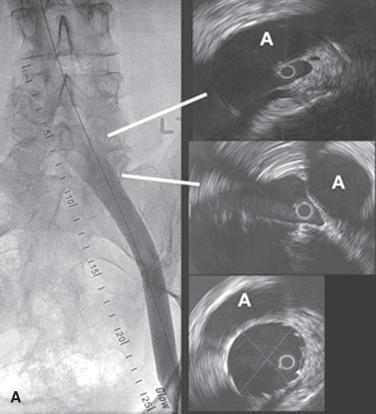
A general principle in treating these complex pathologies is to address the permissive pathology first, which usually provides definitive cure. The experience with iliac vein obstructions has been similar. In cases of combined obstruction and reflux, iliac vein stenting alone has resulted in effective symptom remission even when associated reflux was quite severe and remained untreated. In an analysis of 528 limbs treated by iliac vein stenting for obstruction, severe reflux (reflux segment score ≥3) was present in 58% and axial reflux in 42% that remained uncorrected. Cumulative ulcer healing and clearance of dermatitis were noted in 54% and 81% of the limbs, respectively, at 5 years. No difference was noted between limbs with severe residual reflux and those with no reflux or less severe reflux. Venous pathologies uniquely respond to partial correction with symptom remission. For example, in combined superficial and deep reflux, the correction of superficial reflux alone may provide symptom relief. In axial reflux, the repair of a single valve may suffice. Valve reconstruction of a refluxive valve below an obstructed venous segment has been shown to be effective. With the advent of stent technology, repair of obstruction has become much easier than correction of reflux, which still requires open surgery. The satisfactory therapeutic response to partial pathologic correction means that a stepwise treatment paradigm starting with the simplest and minimally invasive techniques can be pursued; more complex or open treatment modalities can be reserved until later if there is recurrence of symptoms. The satisfactory clinical relief obtained with correction of chronic venous obstruction has suggested that in terms of pathophysiology, obstruction is as important as reflux, which has been the focus for the last 3 centuries. IVUS-proven iliac vein obstruction occurs in isolation without associated reflux in 27% to 36% of CVD patients who present with advanced symptoms. Venous ulceration appears to occur most commonly with combined obstruction and reflux, and only rarely with a single pathology of either obstruction or reflux.
There is an intimate relationship between the venous and lymphatic systems in genesis as well as in function. Lymphatic dysfunction can be demonstrated in approximately 30% of patients with advanced CVD. Lymphoscintigraphic delay in node visualization appears to be a defect in the precollectors at the microcirculatory level; the form and function of large collector lymphatics are apparently preserved. The precise nature of the damage to the precollectors is unknown but presumably related to the overloaded lymphatic transport imposed by venous dysfunction.
The clinical presentation of chronic venous obstruction is often indistinguishable from that attributed to reflux alone or in combination. Venous obstruction can manifest in the entire spectrum of clinical classes represented in the Clinical Etiology Anatomy Pathophysiology (CEAP) classification. All age groups, both sexes (women more often), and both sides (left more often) are affected. Advanced clinical manifestations of pain, swelling, or skin changes have a high likelihood of being associated with venous obstruction with or without reflux, as already outlined. These clinical features can occur alone or in combination. Some aspects of clinical presentation associated with iliac vein obstruction deserve special commentary.
Limb pain usually has strong orthostatic (standing or seated) features. It is typically diffuse over the limb (differing from pain localized to varices), although pain may be predominant in one region, such as the calf or shin. The character of the pain may vary from “bursting,” to “shooting,” to “heaviness.” In some patients, no overt pain is present, but there is soreness to touch or contact. Restless legs and cramps are common. Pain is relieved by elevation or ambulation, both of which reduce venous pressure. Compression stockings, when properly used, provide relief. Many of these pain-relieving maneuvers have become habitual in long-standing disease, and leading questions are necessary to elicit the information. Many patients routinely sleep with their legs on pillows, for example, and may not offer this information unless specifically asked. Atypical pain presentation is not uncommon, such as pain occurring at night while the patient is recumbent. Patients often get up and “walk off” the nocturnal pain. In some patients, venous limb pain can mimic claudication, with difficulty going long distances or climbing stairs without intermittent rest. A special form of orthostatic venous pain (venous hypertension syndrome) occurs in approximately 10% of patients and is often missed because the limb looks entirely healthy, with no venous signs. Venous pain disproportionate to clinical signs of venous disease is usually due to iliac vein obstruction.
Limb swelling occurs more commonly and more extensively with iliac vein obstruction than with superficial or deep reflux alone. With saphenous reflux, swelling is usually intermittent and confined to the ankles. With extensive deep venous reflux, swelling may advance to involve the leg but is confined to below the knee and is generally only mild to moderate in severity. Massive limb swelling is invariably due to iliac vein obstruction. Recurrent cellulitis of the limb usually indicates the presence of iliac vein obstruction.
Venous dermatitis and ulceration most commonly result from a combination of deep venous reflux and obstruction. However, in a small subset of patients with isolated saphenous reflux, venous dermatitis or ulceration can occur if the saphenous vein is large (often >10 mm). Venous swelling can mimic all the “classic” features of lymphedema, including humping of the dorsum of the foot, squaring of the toes, and Stemmer's sign. A diagnosis of lymphedema should not be made without lymphoscintigraphy. Because of the high prevalence of chronic venous disease and frequently associated lymphatic damage, it is probably the most common cause of secondary lymphedema dwarfing the incidence of primary lymphedema. Therefore, it is worthwhile to do an IVUS examination to rule out iliac vein obstruction in patients presenting with lymphedema.
Iliac vein thrombosis is a special form that can propagate distally, prompted by an underlying NIVL type of lesion. Recurrent venous thrombosis is a recognized cause of symptoms in postthrombotic syndrome. The etiology of iliac vein obstruction (i.e., NIVL vs. postthrombotic) cannot be determined in most cases by clinical features alone except when there is a clear history of prior deep venous thrombosis.
Standard investigative techniques have poor sensitivity to detect iliac vein obstruction. Routine duplex ultrasound does not provide adequate color-flow images of the iliac vein. The technique described by Labropoulos and colleagues offers better visualization and velocity measurements, but its accuracy has not been determined. The presence of reflux, even severe reflux (multisegmental or axial), does not rule out the presence of iliac vein obstruction or the possibility of symptom remission with stent correction. Absence of reflux or trivial reflux (reflux in only one or two segments, nonaxial reflux, or reflux in a small saphenous vein) in the context of severe symptoms does suggest iliac vein obstruction. It is well established that routine venography in frontal projections, even through the transfemoral route, is approximately only 50% sensitive to iliac vein obstructive lesions. Recent data have demonstrated that the location of the iliocaval bifurcation varies substantially, averaging almost one full vertebral level between IVUS and venography. This review demonstrated that relying on venography alone may result in a missed proximal lesion of the common iliac vein (up to 78% incidence) and potential inadvertent jailing of the contralateral limb (up to 12% of patients). In addition, the anatomic characteristics of venous lesions, including degree and location of maximal disease, were not accurately identified with venography. The accuracy of modern imaging techniques, such as high-resolution computed tomography or magnetic resonance imaging, in detecting iliac vein lesions remains undetermined. No hemodynamic tests have adequate sensitivity to detect iliac vein obstruction. Exercise femoral vein pressures, outflow fraction measurement, and arm/foot pressure measurement can be useful when positive but because of their low sensitivity, they do not rule out obstruction. Using a combination of these techniques and transfemoral venography, it is possible to predict the presence of an iliac vein obstruction with approximately 80% accuracy. Currently, IVUS examination, a morphologic method, remains the most reliable technique in detecting iliac vein obstruction. IVUS is typically performed using a 0.035 probe (Volcano, San Diego, CA) with planimetric evaluation of the luminal areas of the common femoral vein (CFV), external iliac vein (EIV), and common iliac vein (CIV) using 125 mm 2 , 150 mm 2 , and 200 mm 2 to define normal luminal area cutoffs in the CFV, EIV, and CIV, respectively ( Table 52.1 ). Any decrease in luminal area in a symptomatic patient is considered abnormal, meriting angioplasty and stenting. Because of the high diagnostic yield with this method in symptomatic patients, IVUS remains the preferred diagnostic modality at present. With prior consent, stent correction of the detected stenosis can be performed concurrently.
| Vein | Luminal Area (mm 2 ) | Diameter (mm) |
|---|---|---|
| CFV | 125 | 12 |
| EIV | 150 | 14 |
| CIV | 200 | 16 |
| IVC | N/A | 17 |
Venous stenting differs from arterial stenting in several respects ( Box 52.2 ), and modification of the endovascular technique learned in arterial practice is essential for symptom relief.
Balloon angioplasty alone does not work because the lesion is fibrous and recoil is the rule.
Large-caliber stents approximating natural luminal size are necessary to normalize distal venous pressure; patency alone is not enough.
Stents should be extended into the inferior vena cava, otherwise the choke band at the iliocaval junction will squeeze the stent distally, causing recurrence.
Venous stents (braided) can be extended across the inguinal ligament without erosion or fracture.
Iliac veins are intolerant of residual lesions; the result is residual or recurrent symptoms.
Extensive stenting is often required; veins are very tolerant of metal load (no thrombosis).
Intravascular ultrasound is mandatory to ensure good results.
The optimal access point is at the midthigh, where the femoral vein is posterolateral to the artery. The midthigh access offers close proximity to the lesion and antegrade maneuvers while allowing adequate room above the sheath to deploy the stent below the inguinal ligament, if necessary. Access through the internal jugular vein or the popliteal vein is farther removed from the target lesion. In addition, the technique has the disadvantage of requiring prone positioning in an increasingly obese population. Large (10 or 11 French) sheaths are used. Access complications have been rare with the routine use of ultrasound guidance for access and sealant devices.
After an optional on-table venogram (omitted in patients with renal dysfunction), IVUS examination is performed and the presence of an obstructive lesion is confirmed. IVUS planimetry provides accurate measurements of even irregular stenoses, which are common in iliac veins. The luminal area cutoffs for planimetry have already been discussed. The proximal common iliac vein where the artery crosses over is the classic site for nonthrombotic obstruction. Obstructive lesions near the orifice of the hypogastric vein occur frequently but are sometimes impervious to IVUS examination. Gentle balloon dilation of this location is necessary to identify such lesions. Minor lesions are commonly present behind the inguinal ligament. In postthrombotic disease, these compressive points from arterial or ligamentous structures are particularly prone to fibrosis because thrombus resolves poorly at these locations. In severe postthrombotic disease, the entire iliofemoral segment may be involved.
Large-caliber 18-mm balloons are commonly used in normal-sized adults for predilation prior to stent placement. Iliac vein obstructions are fibrotic, and recoil is the norm after balloon dilation alone. Stent placement is therefore universally required. Large-caliber 18- to 20-mm Wallstents are deployed under fluoroscopy, with generous overlap between stents to avoid disconnection. The oversizing enables later hyperdilatation in case of stent compression or in-stent restenosis. The preferred upper landing site is the distal vena cava, just above the iliocaval confluence as determined by IVUS. A large-caliber 25- by 50-mm Z stent (Gianturco stent) is used across the iliocaval confluence for additional radial strength. All lesions in symptomatic patients should be corrected in continuity without short skip areas. Currently stents in the majority of patients are extended from the distal inferior cava to the common femoral vein ( Fig. 52.4 ). Infrainguinal deployment has not resulted in stent fractures, erosions, or stent thrombosis. Metal load from extensive stent stack length does not appear to adversely affect patency. Leaving uncorrected residual lesions has often been responsible for residual or recurrent symptoms. IVUS is used extensively during the procedure to identify lesions, determine optimal landing sites for the stent, and to ensure proper apposition between stent stack members after postdilatation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here