Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pancreatic pseudocysts, abscesses, and walled-off necrosis (WON) are types of pancreatic fluid collections (PFCs) that arise as a consequence of pancreatic injury. These sequelae of pancreatic injury result from a disruption of the main pancreatic duct (PD) and/or side branches. Ductal disruption can occur secondary to acute pancreatic injury (i.e., acute pancreatitis, trauma, surgical resection, or inadvertent damage to the pancreas during abdominal surgery) or chronic injury (i.e., chronic pancreatitis, autoimmune pancreatitis). Ductal injury subsequently leads to formation of a collection of fluid with or without solid debris.
Endoscopic therapy is directed at drainage of fluid and solid components using a transmural approach, treatment of PD disruption or stricture using a transpapillary approach, or a combination of both techniques in select patients. Treatment of PD disruption is believed to decrease the long-term recurrence rate and improve the outcome after successful resolution of a collection. This chapter discusses endoscopic approaches to PFCs.
Classification systems for defining types of PFCs are useful for understanding mechanisms of formation and allowing comparisons of therapies between and among disciplines. Since the previous edition of this text, the classification and nomenclature of PFCs have been revised, and they will likely continue to evolve.
When intervention is indicated, the therapeutic approach can be simplified by addressing three questions: (1) Is the collection a result of pancreatitis or does it represent a cystic neoplasm (see Chapter 51 )? (2) Is the collection composed primarily of liquid or does it contain significant solid debris? (3) What is the PD anatomy? Using these three basic questions, the short-term and long-term approaches to the patient with a PFC can be formulated. The approach to collections that are composed primarily of fluid is different from the approach to those containing significant solid debris, because liquefied collections can be managed with placement of small-diameter stents via a transpapillary or transmural approach alone, whereas those with solid debris may require direct endoscopic necrosectomy, placement of large-bore self-expandable metal stents (SEMS), adjuvant percutaneous catheters, and direct removal of necrotic debris from the collection with or without placement of irrigation catheters.
Acute fluid collections. Acute fluid collections arise early in the course of acute pancreatitis and are usually peripancreatic in location. These collections usually resolve without sequelae but may evolve into pancreatic pseudocysts.
Pancreatic pseudocysts.
Acute pancreatic pseudocysts. Acute pseudocysts arise as sequelae of acute pancreatitis, require at least 4 weeks to encapsulate, and are devoid of significant solid debris. Acute pancreatic pseudocysts usually form as a result of limited pancreatic necrosis in conjunction with a PD leak ( Fig. 56.1 ). Alternatively, areas of pancreatic and peripancreatic fat necrosis may completely liquefy over time and become a pseudocyst. Despite the requirement of at least 4 weeks for a pseudocyst to mature, this time period does not define the collection as a pancreatic pseudocyst. Significant pancreatic necrosis (≥30%) may evolve into a collection that resembles a pseudocyst radiographically but has been present for more than 4 weeks (see the Walled-Off Necrosis section of this chapter). By definition, collections that contain significant solid debris are not pseudocysts, and endoscopic treatment of these collections by pseudocyst drainage methods alone often results in infection of the remaining unremoved solid debris.
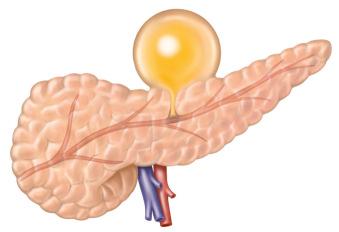
Chronic pseudocysts. A chronic pseudocyst arises as a sequela of chronic pancreatitis because of downstream pancreatic obstruction from fibrotic strictures and/or stones. This results in a pancreatic ductal disruption, blowout, or leak and accumulation of pancreatic fluid. These collections do not contain solid debris and usually do not arise as a result of acute inflammatory processes ( Fig. 56.2 ).
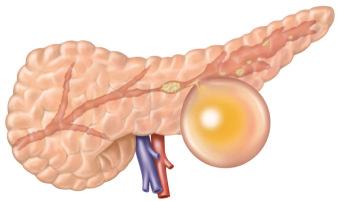
Pancreatic pseudocysts can be subdivided into sterile and infected (the latter is referred to as pancreatic abscess in some nomenclature systems).
Pancreatic abscesses. True pancreatic abscesses are rare and are not synonymous with infected pancreatic pseudocysts. However, for the purposes of this chapter and based on pending revisions of the existing nomenclature, an abscess will be considered as an infected PFC that contains little to no solid debris (as opposed to infected pancreatic necrosis, which is described later). The authors believe that when this definition is used, abscesses can be drained through modest-sized catheters without an absolute need for irrigation or debridement.
In general, the indications for drainage of a liquefied PFC are driven by symptoms and/or signs of infection and not merely by the presence of a collection on cross-sectional imaging. Symptoms related to sterile pancreatic collections include abdominal pain, often exacerbated by eating, weight loss, gastric outlet obstruction (nausea and vomiting), obstructive jaundice, and ongoing PD leaks. PD leaks can manifest as pancreatic ascites or high-amylase pleural effusion and pancreatic fistulae and are discussed in Chapter 54 . Infection is considered an absolute indication for drainage.
For many years a size cutoff of 6 cm and persistence of a collection were used as criteria for drainage. However, patients may remain asymptomatic with collections ≥6 cm with little risk of adverse events such as rupture, infection, or bleeding, whereas endoscopic intervention is associated with a finite (as well as potentially higher) risk of adverse events. Progressive enlargement of a collection is one exception that is cited as an indication for drainage, although even then such patients potentially can be followed until they become symptomatic.
Before undertaking drainage of a liquefied pancreatic collection, a predrainage evaluation should be performed that includes the following:
Establish whether or not the collection represents a PFC or a “masquerader” of a PFC such as a cystic neoplasm or other entity ( Box 56.1 ; Fig. 56.3 ). If the patient does not have a well-documented history of acute or chronic pancreatitis, the endoscopist should be concerned that the collection does not represent a pseudocyst or other inflammatory collection. Endoscopic ultrasonography (EUS) allows for diagnosis of cystic neoplasms, as discussed in Chapter 51 . Clinical presentation and cross-sectional imaging with CT and magnetic resonance imaging (MRI) can also be useful in making the differentiation between cystic neoplasms and inflammatory PFCs (see Chapter 34 ).
Cystic pancreatic neoplasm
Duplication cyst
True pancreatic cyst
Pseudoaneurysm
Solid necrotic neoplasm (e.g., retroperitoneal sarcoma)
Lymphocele
Gallbladder
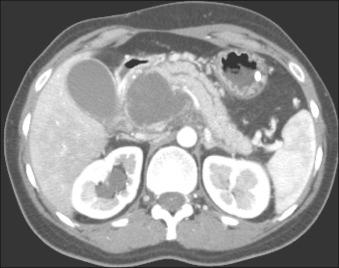
Establish whether the collection is predominantly liquid or contains a significant amount of solid debris.
Establish the relationship of the collection to surrounding luminal and vascular structures.
Consider underlying etiologies of true pancreatic pseudocyst that have implications for alternative or adjuvant therapies, such as pancreatic cancer, autoimmune pancreatitis, and intraductal pancreatic mucinous neoplasms (IPMNs).
In addition to a complete history and physical examination, the following evaluation should be undertaken before endoscopic drainage:
Coagulation profile in patients with a suspicion of coagulopathy and/or liver disease, especially when transmural drainage is considered. Management of antithrombotic agents, as appropriate for high-risk bleeding procedures (see Chapter 10 ).
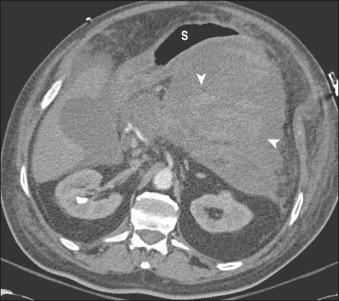
Contrast-enhanced abdominal computed tomography (CT). This allows assessment of the precise location of the collection in relation to the stomach and duodenum in anticipation of possible transmural drainage. Additionally, the relationship of the collection to potential intervening vascular structures can be assessed. Surrounding varices from splenic vein or portal vein thrombosis also may be visualized. The finding of inhomogeneity within the collection suggests the presence of underlying solid debris and/or blood ( Fig. 56.4 ).
Consideration should be given to the following additional studies:
Endoscopic ultrasonography. EUS can be used before drainage to allow assessment of the presence of significant solid debris that may alter the management strategy. In addition, if there is uncertainty as to whether the collection represents a true pseudocyst or other noninflammatory cystic lesion, EUS allows a definitive diagnosis to be achieved by using ultrasonographic features, aspiration and analysis of cyst contents, biopsy of the cyst wall, and even confocal imaging with a probe passed through a fine-needle aspiration (FNA) needle (see Chapter 28 ). Once the endoscopist is certain that the lesion in question is a PFC and the decision has been made to proceed with endoscopic drainage, EUS is used to guide transmural drainage, as discussed in the next section.
MRI with or without magnetic resonance cholangiopancreatography. MRI also allows detection of the presence of solid debris, and so plans for removal and/or alternative drainage strategies can be chosen depending on local expertise and necrosis drainage preferences. Magnetic resonance cholangiopancreatography (MRCP) can define the ductal anatomy and be augmented by secretin stimulation. Secretin-enhanced MRCP (S-MRCP) may demonstrate the presence or absence of an ongoing ductal disruption.
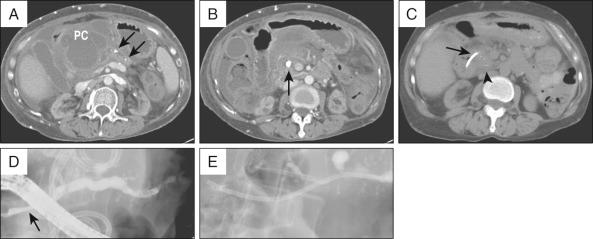
Liquefied PFCs may be drained using a transpapillary approach, transmural approach, or a combination of these. The decision to use one approach over the other depends on the size of the collection, its proximity to the stomach or duodenum, and the ability to enter the PD and/or reach the area of disruption. For example, although the intended approach to draining a pseudocyst that formed from an obstructing PD stone may be transpapillary ( Fig. 56.5, A and B ), failure to negotiate a guidewire beyond the obstructing ductal stone may require transmural drainage. Assessment and treatment of the ductal stone at a later date by other techniques, such as extracorporeal shock wave lithotripsy (ESWL), can then be performed ( Fig. 56.5, C to E ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here