Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The disorders included in this chapter are grouped under the heading “obstructive lung diseases.” These diseases share the characteristic of increased resistance to airflow, which can be caused by either an increase in the resistance of the conducting airways or an increase in lung compliance due to emphysematous lung destruction, or both. The airflow limitation is usually progressive and is associated with an abnormal inflammatory response of the lungs. Obstructive lung diseases include emphysema, chronic bronchitis, asthma, and bronchiectasis. Although these diseases have distinctive clinical and pathologic features, patients can show features of more than one of these disorders. The combination of emphysema and chronic bronchitis is particularly frequent, due to the strong linkages of these diseases to a common etiologic factor: tobacco smoking. The term chronic obstructive pulmonary disease (COPD) is commonly used to refer to both processes and is defined physiologically by airflow limitation measured during forced expiration. A component of asthma may also contribute to COPD in some patients. Small-airway abnormalities also form part of the constellation of changes contributing to airflow limitation in smokers and are discussed in more depth in Chapter 21 . COPD is now the third leading cause of death in the United States and is projected to be the third leading cause of death worldwide by 2020. The current clinical consensus definition of COPD emphasizes that it is a preventable and treatable disease state characterized by airflow limitation that is not fully reversible. The risk factor of exposure to cigarette smoke and other noxious particles or gases is well known in COPD. More recent research has focused on the modulating role of individual genetic variations and its implications for disease classification, disease severity, and treatment.
Disorder characterized by abnormal permanent enlargement of airspaces distal to the terminal bronchiole, with destruction of their walls and without obvious fibrosis
Estimated to affect 5% to 8% of the U.S. population
Primarily affects smokers and individuals with A1AT deficiency
A component of chronic obstructive pulmonary disease, which is the third leading cause of death in the United States
Can lead to pulmonary hypertension and cor pulmonale, especially if concurrent chronic bronchitis exists
Can cause death from respiratory failure or rupture of bullae with tension pneumothorax or hemorrhage
Male predominance (male-to-female ratio = 2:1)
Symptoms in smokers usually develop after age 50 but can occur earlier in A1AT deficiency, especially with concurrent smoking
Dyspnea is characteristic
If coexisting chronic bronchitis is present, individuals will have a productive cough
Physical examination: “barrel chest,” decreased breath sounds, prolonged inspiration
Spirometry: decreased FEV 1
With advancing disease, hypoxemia develops
Hyperinflated lungs, tapering vascular shadows with hyperlucency, “pushed down” diaphragms, bullae
Damage is irreversible
Smoking cessation is central to treatment plan to avoid progression
A1AT deficiency is treated with A1AT replacement therapy
Surgical therapies include bullectomy, lung volume reduction therapy, and lung transplantation
Enlarged and hyperinflated lungs
Bullae may be present, especially in the apical regions in smokers
Several types of emphysema, which can occur in combinations and with varying severities:
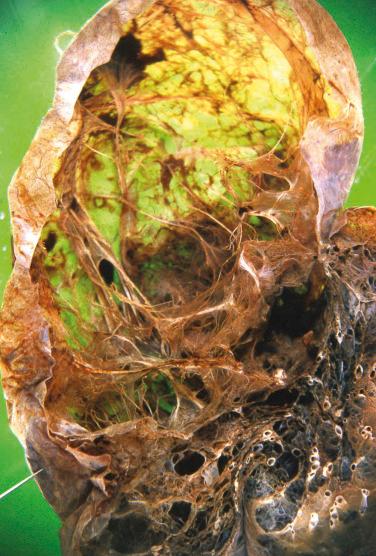
Centriacinar (centrilobular): enlarged airspaces in centers of lobules (primarily at the level of the respiratory bronchiole), often with blackened walls, surrounded by unaffected parenchyma; upper lobes affected more than lower lobes
Panacinar (panlobular): airspace enlargement throughout the lobule; in A1AT deficiency, lower lobes are affected more than upper lobes
Paraseptal: enlarged airspaces along interlobular septae and pleura
Irregular: Localized airspace enlargement adjacent to a focal lesion, usually a scar
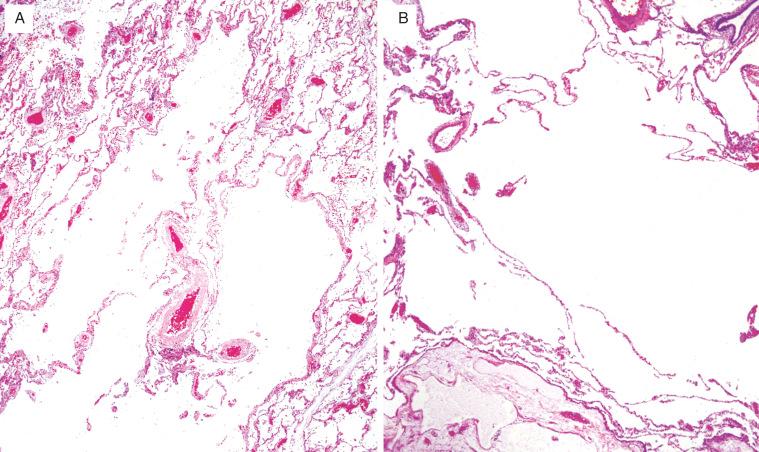
Alveolar wall destruction with “free-floating” portions of septae that appear disconnected from other parenchymal structures, leading to airspace enlargement
Overinflation secondary to partial airway obstruction or due to artifactual overinflation with formalin
Emphysema is a disorder characterized by abnormal permanent enlargement of airspaces distal to the terminal bronchiole, with destruction of their walls and without obvious fibrosis. It is often associated with chronic bronchitis and produces physiologic airflow obstruction. There are four described variants that affect different regions of the pulmonary lobule: (1) centriacinar or centrilobular, (2) panacinar or panlobular, (3) paraseptal, and (4) irregular. Centriacinar emphysema is the most common form of emphysema and is most closely associated with long-standing tobacco use. Panacinar emphysema is characteristic of α-1-antitrypsin (A1AT) deficiency. Paraseptal emphysema may coexist with other types of emphysema, but paraseptal blebs/bullae are also considered to be a cause of pneumothorax in young, otherwise healthy, and predominantly male adults. Irregular emphysema occurs at the periphery of large scars or other focal lesions. Patients may have more than one type; mixtures of centriacinar and paraseptal emphysema are common in smokers, and centriacinar emphysema can progress in smokers to involve whole acini. Large bullae can also develop, compress adjacent lung tissue, and further compromise ventilation, or these structures may spontaneously rupture, leading to a pneumothorax.
The pathogenesis of emphysema has received extensive study and remains a subject of continued investigation. The protease-antiprotease theory suggests that emphysema results from an imbalance between activities of proteases (especially elastase) and antiproteases. Supporting this theory is the association between human A1AT deficiency and emphysema, and the observation that exposure of the lungs of experimental animals to certain proteolytic enzymes can reproduce lesions of emphysema. The inflammatory response triggered by smoking may play an important role in pathogenesis; smokers with severe emphysema appear to accumulate substantially increased numbers of neutrophils, macrophages, T-lymphocytes, and eosinophils compared with smokers with normal lung function. Neutrophil and macrophage activation is believed to occur with release of proteases, especially elastase. Inactivation of antiproteases by reactive oxygen species introduced via tobacco smoke and activated neutrophils has also been described, further promoting tissue injury.
A1AT is a serine protease inhibitor synthesized by the liver and secreted into the blood. Its major function is the inhibition of neutrophil proteases, and it represents the most important inhibitor of protease activity in the lung. The A1AT protein is encoded by the protease inhibitor (PI) locus gene on chromosome 14q32.1 and manifests an autosomal codominant pattern of allelic expression. The PI locus is highly polymorphic, and about 100 variants have been identified to date. The PiMM phenotype is the most common phenotype, occurring in 90% of the general population. Individuals homozygous for PiZ (PiZZ) have markedly reduced A1AT activity and are the most commonly affected by symptomatic emphysema, with 80% to 90% eventually developing emphysema. Heterozygous PiSZ individuals have 30% to 35% of normal activity and are at a higher risk as well. Tobacco smoking accelerates the progression of emphysema in patients with A1AT deficiency.
In the United States 4% to 5% of males and 1% to 3% of females are estimated to have emphysema. The most important environmental cause is tobacco smoking, and A1AT deficiency accounts for another significant subset of patients with emphysema. Patients typically develop symptoms after age 50. Dyspnea is characteristic, whereas cough is not usually conspicuous unless there is coexisting chronic bronchitis. Respiratory rate increases with use of accessory respiratory muscles. Thoracic examination may reveal a “barrel chest” due to hyperinflation, diffusely decreased breath sounds, hyperresonance on percussion, and prolonged inspiration. Clinical laboratory testing may include measurement of A1AT level, genotyping of A1AT in patients with A1AT levels greater than 7 mmol/L, hemoglobin/hematocrit measurement to detect polycythemia, and arterial blood gas testing. Pulmonary function testing shows reduction in forced expiratory volume in 1 second (FEV 1 ), as a reflection of airflow obstruction. A1AT deficiency is underrecognized and underdiagnosed. The diagnosis should be given particular consideration in nonsmokers with fixed airflow obstruction and in individuals with a family history of emphysema or liver disease.
Chest radiographs reveal hyperinflation with hyperlucency, rapidly tapering vascular shadows, “pushed down” diaphragms, and a long, narrow heart shadow. High-resolution computed tomography (HRCT) is a more sensitive and specific test for emphysema ( Fig. 20.1 ) and will reveal bullae that may be less apparent on routine x-rays. However, HRCT is not part of routine evaluation and is usually done in instances when lung volume reduction surgery is planned or if another abnormality (eg, a lung cancer) is suspected.
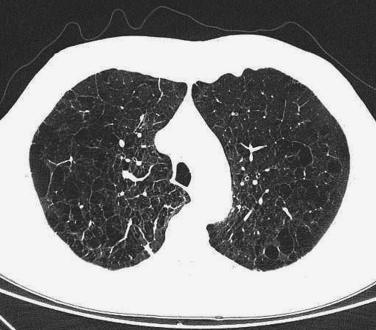
The lungs usually appear enlarged and hyperinflated and have less than normal weight. Blebs or large, air-filled bullae ( Fig. 20.2 ) may be present, particularly in the apices. In centriacinar emphysema, enlarged airspaces are visible in the centers of the lobules and are surrounded by normal-appearing parenchyma ( Fig. 20.3 ). This type of emphysema is usually most pronounced in the central regions of the upper lobes. The walls of the enlarged airspaces often have a blackened appearance. In A1AT deficiency, panacinar emphysema is usually most severe in the lung bases and involves the entire lobule. Paraseptal emphysema appears as enlarged airspaces along interlobular septa and pleura. In irregular emphysema, the abnormal airspaces lie adjacent to focal lesions such as scars. Combinations of more than one type of emphysema are common, and bullae (emphysematous airspaces measuring more than 1.0 cm in diameter) may develop in any type.

Histologically, there is alveolar wall destruction with isolated, or “free-floating,” portions of septae that appear to be disconnected from other parenchymal structures ( Fig. 20.4 , [CR] ). Enlargement of alveolar sacs without airway destruction can occur secondary to subtotal airway obstruction or as an artifact caused by overinflation with formalin and should not be overdiagnosed as emphysema. In centriacinar emphysema, airspace enlargement occurs in the proximal portion of the acinus/lobule (primarily at the level of the respiratory bronchiole), and distal alveoli are relatively spared. A small artery is often seen adjacent to the enlarged airspace and represents a clue to the centriacinar location of the emphysema. Groups of pigment-containing macrophages are also frequently seen in peribronchiolar regions. Panacinar emphysema results in diffuse acinar destruction and airspace enlargement, involving all portions of the acinus/lobule ( [CR] ). In paraseptal emphysema, the centriacinar structures are preserved, and the site of emphysematous airspace enlargement lies adjacent to an interlobular septum or the pleura. In irregular emphysema, the emphysematous airspaces lie adjacent to a focal lesion, usually a scar.
Emphysematous destruction of airspace structures is irreversible and can lead to death, either directly or as a consequence of a complication such as bullous rupture with tension pneumothorax or hemorrhage. Goals of therapy include improving quality of life and preventing further damage. For smokers, smoking cessation is the most essential part of a comprehensive treatment plan.
Clinically defined by a chronic productive or mucus-producing cough on most days of the month for at least 3 months in at least 2 consecutive years, with no other identifiable cause
Affects 4%–5% of the U.S. population
A component of chronic obstructive pulmonary disease, which is the third leading cause of death in the United States and has a mortality rate of 50% at 10 years after diagnosis
Can lead to pulmonary hypertension and cor pulmonale
Can also cause death from respiratory failure due to severe disease, often with superimposed respiratory tract infection
Female dominant (male-to-female ratio = 1:2)
Most affected individuals are more than 45 years of age
Associated with smoking (more than 90%); exposure to dusts, fumes, or toxins; airway infections; allergic airway injury
Symptoms include cough with excessive sputum production, dyspnea
Superimposed respiratory tract infections are common and can be associated with worsening respiratory symptoms, fever, and chills
Spirometry: decreased FEV 1
With advancing disease, hypoxemia develops
Nonspecific but may show bronchial wall thickening
High long-term mortality with death related to respiratory failure from exacerbations and infections
Treatment strategies are geared toward reducing bronchial irritation and treating infections: smoking cessation, antibiotics for infections, bronchodilators, supplemental oxygen
Airways are filled with abundant mucus or mucopurulent secretions
Bronchial walls often appear thickened and the mucosal surfaces hyperemic
Abundant mucus in bronchial and bronchiolar lumens, with variable acute inflammation depending on the presence or absence of infection
Enlargement of the mucous glands in the bronchial wall
Increased bronchial goblet cells, goblet cell metaplasia of bronchioles
Chronic inflammatory infiltrates in the mucosa and submucosa of bronchi and bronchioles
Increased BALT in small airways
Submucosal and adventitial fibrosis in bronchi and bronchioles
Squamous metaplasia and reserve cell hyperplasia
Thickening of the bronchial basement membrane
Chronic bronchitis is defined by clinical manifestations of a chronic productive or mucus-producing cough on most days of the month for at least 3 months in at least 2 consecutive years, with no other identifiable cause. In the United States this disorder affects 4% to 5% of the population, most over the age of 45. Chronic bronchitis is associated with airflow obstruction, which is demonstrable by pulmonary function testing. It can occur as an isolated condition but is often associated with emphysema or asthma. Direct bronchial damage related to tobacco use, bacterial and viral infections, allergic injury, and environmental pollution have been implicated in its pathogenesis. Periodic symptomatic exacerbations are common and are usually precipitated by infections. Household air pollution (HAP) as a consequence of indoor combustion of solid biomass fuels for heat and cooking is associated with COPD and is a major global health problem. By comparison to tobacco smoke, HAP results in greater bronchial involvement and disproportionately affects women.
Radiologic studies often show bronchial wall thickening, but this finding is nonspecific.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here