Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The therapeutic basis of all electrosurgery is the use of high frequency, alternating electric current to produce heating in living cells. The heating can be manipulated to achieve a desired tissue effect such as cutting, tissue ablation, desiccation, or a combination of these. Electrical energy to produce heating and tissue effects has been a part of endoscopy since the early 1970s. Common indications include biliary sphincterotomy, polypectomy, hemostasis, and the ablation of vascular lesions of multiple origins. Incorrect use of electrosurgery may contribute to poor patient outcomes such as post-polypectomy serositis, perforation, immediate or late hemorrhage, and sphincterotomy-associated complications.
Generator manufacturers give guidance as to suggested settings and output choices for various procedures. However, because there are many patient and physician technique variables that are outside of the manufacturer's knowledge regarding the generator's eventual impact, there can be no one “magic” setting that is guaranteed to always produce a known result. This is why physician discretion and knowledge remains important.
A clinician with no electrosurgical cognitive competence may be unable to troubleshoot, make appropriate alternate output selections, or be flexible in unusual situations. Without a good understanding of electrosurgical technology, it will be more difficult for a physician to expand his or her own clinical expertise into more advanced procedures such as endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). Clinical understanding of the fundamentals of the technology is known to promote better patient outcomes.
From the clinician's point of view, this understanding and competence has been hampered by problems with nomenclature, lack of standardized training, inappropriate marketing messages, and the perpetuation of incorrect understandings and/or outdated beliefs. All these have conspired to make electrosurgery “the most commonly used, but most often misunderstood technology.”
There is a lack of uniformity in names for output selections used by different manufacturers ( Table 6.1 ). Similar names may have either similar or different characteristics from one manufacturer's generator to another. The terms themselves may be intuitively misleading. For example, a waveform may be called COAG but is very capable of cutting tissue. Some terms in common usage are manufacturer trademarks. SoftCoag refers specifically to an Erbe Elektromedizin GmbH (Tübingen, Germany) generator output, whereas TouchSoft is registered to Genii, Inc. (St. Paul, MN) for a technically equivalent, very low voltage output. The constant misuse of other terms such as electrocautery add to the confusion and seems particularly out of step with medical practice, which relies on precise terminology in everything from reporting diagnoses (diverticulosis is not diverticulitis) to recording methodologies for others to reproduce. Recently, both the Journal of Minimally Invasive Gynecology and the journal Gastrointestinal Endoscopy have addressed this issue and begun to promote standardization of terms. As far as possible, this chapter conforms to these new guidelines.
This chapter outlines the basic principles of electrosurgery pertaining to flexible endoscopy and attempts to relate the fundamentals of the technology directly to common and emerging clinical procedures. The use of proprietary or brand-specific terms is minimized, and only the most reliably referenced and up-to-date information to date is included.
Early pioneers of electrosurgical devices discovered that applying an electrical current to biologic tissue produces different effects. The first effect is electrolytic. Charged molecules in tissue flow toward opposite electrode poles if the current applied is direct or alternates slowly. Alternating the current more rapidly eliminates the electrolytic effect and produces heating at a cellular level. However, a current alternating at less than 100 kHz results in undesired neuromuscular effects. The shocking result of applying a household current of 60 Hz is well known. Alternating at a very a high frequency (300 kHz) nearly eliminates neuromuscular effects but retains the desired cellular heating. This thermal effect is the basis of all electrosurgery. Because this frequency is in the AM radio range, this energy is often referred to as radio frequency (RF), and a radio frequency electrosurgical generator is an acceptable term for an electrosurgical generator unit (ESU).
High-frequency alternating current is generated by an ESU and delivered to tissue via an assortment of suitable accessories. Water within cells heated very quickly vaporizes and causes the cell membranes to burst. These bursting cells separate tissue. We use the accessory to guide the burst cells along a cleavage plane and say the tissue has been electrosurgically “cut.” Cells that heat more slowly dry out (coagulate) without bursting. The proportion of how many cells burst and how many coagulate, as well as how much tissue is involved, is referred to as the tissue effect. Many variables combine to make this end result ( Fig. 6.1 ). Heating the tissue directly, producing both cutting and coagulation, sets electrosurgery apart from electrocautery. Because the intracellular water is heated directly by the RF energy and not by conduction from an already heated accessory, electrosurgery should never be referred to as cautery . Cautery devices produce coagulation by the passive transfer of heat from a heated accessory and can never produce electrosurgical cutting. This is why it is incorrect to use the term electrocautery for RF technology. True cautery devices found in gastroenterology practices include the Olympus Heat Probe (Olympus Corp., Tokyo, Japan).
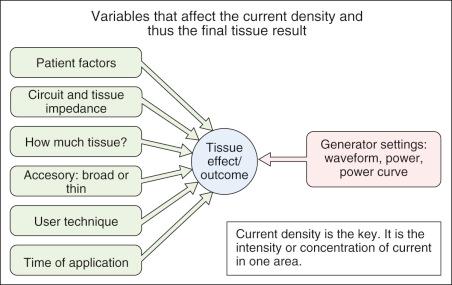
Current density is the defining variable in determining specific tissue effects in electrosurgery; yet it is the sum of the effects of all the other variables (see Fig. 6.1 ).
Current density is the measure of current concentration or, by definition, the current per unit area. The rate of heat generation, and therefore the resulting therapeutic effect, is a function of the current density. It is a measure of intensity. Mathematically, the temperature rises as a square of the current density. Current that will boil water on a square millimeter area will not even feel warm on a square-centimeter area. The dramatic difference in surface area between the active electrode and the dispersive (grounding) pad is perhaps the best-known example of this principle. Current density depends on the applied voltage, current, and type of waveform, as well as the tissue impedance, the size of the electrode, and the time that current is flowing. Understanding the different characteristics of tissue heating under different circumstances is essential to understanding electrosurgery.
The physical law that relates all the electrosurgical variables is summarized by Ohm in the equation:
where P is power, I is current, and R is resistance (for our purposes, resistance and impedance are used synonymously). The P = V x I derivation of this equation relates the type of waveform to total power ( P ). Power combines both voltage ( V ) and current ( I ). For example, if power is to remain constant as voltage increases, then current must decrease. Ohm's Law defines another principle crucial to a clinical understanding of electrosurgery: as impedance in tissue rises, power (either current or voltage or both) decreases. If “nothing is done” as the therapy produces coagulation, and therefore the tissue impedance rises, the generator power must drop (stall).
To address this problem, research turned in the late 1980s to using microprocessors to measure and respond to changing impedance. First patented in 1986, microprocessor monitoring allows an ESU to track a resistance baseline and subsequent changes in the resistance during the electrosurgical activation. Using millions of data points, the generator's software programming (algorithm) tells the generator how to regulate the current and/or voltage (which together form power) being delivered during the activation. A graphic representation of how a particular output is designed to react to changes in impedance is called a Power to Impedance Curve or Power Curve . Sometimes marketers will use the informal term power dosing to explain this. Power curves associated with each output selection are so important to understanding the operation of the ESU that regulatory bodies require these graphs to be included in every user manual.
Power to impedance curves tend to be either “narrow” or “broad.” By convention, power is shown on the vertical axis with increasing resistance progressing to the right on the horizontal axis ( Fig. 6.2 ). The horizontal axis is also likely to correlate with time as tissue resistance increases when tissue becomes coagulated with increasing time of application.
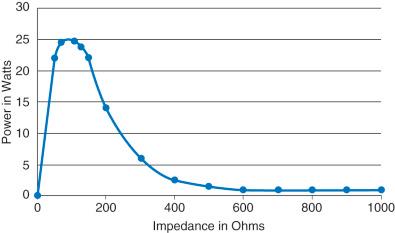
The power curve dictates that the power setting displayed on an ESU is not the power that will be delivered over the entire activation. Depending on the algorithm defining the curve, the power setting displayed may be a more or less close approximation of the actual or average power delivered, or simply a target maximum power. In all cases, the average power delivered will be less than the maximum or peak power delivered.
Outputs with narrow type power curves have been long utilized in flexible endoscopy for contact coagulation using very familiar bipolar endostasis probes. Matched with low-voltage continuous outputs and a narrow power curve, this bipolar method is a frequent and effective choice for hemostasis and control of bleeding.
In Fig. 6.2 , 25 watts have been selected by the user and will be displayed on the ESU. To the ESU's algorithm, this selected power represents only the maximum (or target) power to be delivered. The ESU is programmed to ramp up quickly in the low-resistance situation characterized by frank bleeding. The target maximum is reached, but the power drops quickly as soon as the resistance reaches approximately 100 to 300 Ohms, which correlates well with the appearance of the characteristic whitish eschar, indicating adequate coagulation. This power curve delivers exactly what is desired: rapid onset of hemostasis with self-limiting depth of injury. With the low voltage of the output and this power curve, the tissue tends not to overly dry out, which would cause the bipolar accessory to stick. This well-matched output mode, power curve, and appropriate accessory provides the desired clinical effect of reliable and quick hemostasis.
A nearly identical narrow power curve and voltage cap, matched with monopolar accessories, is usually named SoftCoag (Erbe) or TouchSoft (Genii). These soft coagulation outputs are suggested for use with monopolar hot biopsy forceps (HBF) or newer monopolar contact coagulation accessories such as the Olympus Coagrasper or the Genii TouchSoft Coagulator. One study described successful use of a snare tip with a soft coagulation output for touch-ups during the resection of large colonic lesions. These monopolar counterparts do require the use of a return dispersive electrode (grounding pad) and require higher initial power settings to overcome the higher resistance in the monopolar circuit. TouchSoft or soft coagulation power maximums are usually set at 40, 50, or 60 watts versus the 15 to 25 watts that are very common for bipolar contact applications.
For snare polypectomy, sphincterotomy, or other procedures that depend on a smooth advance of an electrode, the narrow power curve would not be suitable, as stalling would result. Instead, a broad power curve ( Fig. 6.3 ) where cutting with various levels of concurrent coagulation (depending upon the waveform and accessory selected) is the choice. These outputs usually ramp more slowly toward an effective power and maintain the effectiveness over a broad range of impedances. The result is a smooth and reliable transection. Again, in all cases the average power delivered over the activation will be less than the maximum or peak power. A marketing term called constant power is sometimes applied to broad curves but is mostly inaccurate. The power delivered is dynamically driven by the algorithm of the ESU as it responds to impedance/resistance measures. Rigid terms such as constant , always , and never are to be avoided in scientific and medical use for good reason. It is similarly not correct to refer to one entire ESU as a constant power generator . Every ESU in current use today has some outputs of both curve types included in its available selections. It is the physician's good understanding of the available outputs and their associated power curves that allow matching to suitable accessories to produce desired and optimal clinical results.
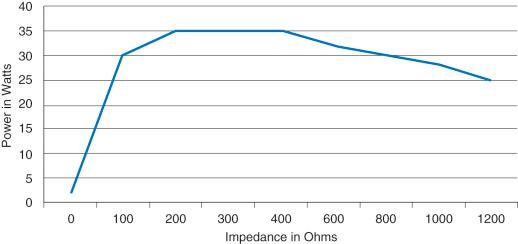
Broad power curves are ideal for most applications that require at least some cutting because the transection is done smoothly without stalling. The generator overcomes the Ohm's law directed drop in power by automatically adjusting the voltage and/or current to keep the cut progressing smoothly over a wide (broad) range of impedances. For ESUs intended for flexible endoscopy, it is now very common to also provide an additional microprocessor or software control layer over some of the broad curve output choices in order to interrupt or fractionate the cut. Named EndoCut (Erbe), Pulsed Cut (Genii), Pulsed Blend Cut (Genii), etc., these types of outputs have multiple options for adding more or less coagulation to the cutting advance. Multiple studies have shown a sole but important benefit of the pulsing or interruption of the advance: a significant reduction in uncontrolled or “zipper” cuts in sphincterotomy.
Microprocessor control on modern generators is very precise in reading dynamic impedance and holding current and/or voltage (power) changes tightly to the algorithm design demanded by the power curve. For this reason, often the “watts” shown on the generator display may be absent or only an indication of a maximum power target. In any case, and with every power curve, the average power delivered over the total activation will be less than the peak power delivered. Often the generator requires a certain lag time to reach the programmed maximum power, may on occasion even slightly overshoot it, and will ramp down from the peak depending on the impedance. It is customary for record keepers to chart only the maximum selected power shown on the generator display.
As noted, the controlling concept of the final tissue effect is the current density at the treatment site. But many variables affect the current density. Some are under the physician's control, and some are not. Only a few are functions of the ESU (see Fig. 6.1 ).
A defining variable that is a selection on the electrosurgery generator is the high-frequency waveform, or output mode. In solid-state generators (the only type now produced), continuous sinusoidal waveforms with fairly low voltage increasingly give way to a higher-voltage waveform that is more and more interrupted (modulated) to take the tissue effect from one of mostly cutting to one of mostly coagulation ( Fig. 6.4 ).
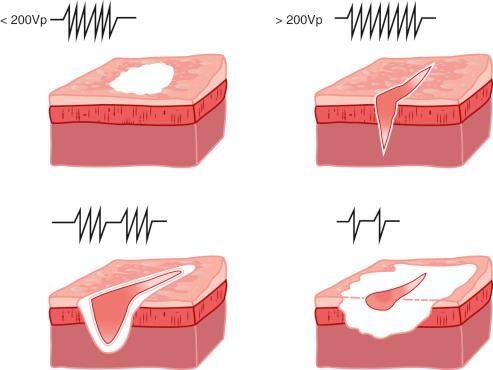
A continuous high-frequency waveform with a peak voltage of at least 200Vp will produce an intensity of current sufficient to create micro electric sparks between the active electrode and the target tissue. Such high-current density along the leading edge of the electrode causes cells to literally explode, separating the tissue as if it were cut. As this cell vaporization continues, a micro steam layer is formed, which helps to propagate the cutting effect.
Along the edges of the cut, there will always be a margin of cells whose distance from the active electrode allows them to heat more slowly. These cells simply coagulate. This is why it is often said, “Only a cold cut is a pure cut.” It is better to use the term cut rather than pure cut , but the literature does not yet reflect this new standard. The depth of the coagulated margin along the cut is related to the height of the peak voltage in the cutting waveform, with higher voltages leaving a thicker margin of coagulation. The coagulation margin is also influenced by the thickness of the electrode. A thin wire leaves less coagulation than a flat blade or a thicker endoscopic dissection wire or knife.
Even with a continuous waveform, if the voltage never goes above 200Vp, no cutting can occur. Such outputs lack the intensity to initiate the initial sparking necessary to produce the cutting effect. A superficial coagulation results instead. These waveforms have been described earlier in the discussion about bipolar and monopolar soft coagulation output choices with narrow power curves.
To produce deeper thermal injury with less electrosurgical cutting and an increasingly greater proportion of coagulated cells, the continuous cut waveform is interrupted or modulated. Modulating the waveform delivers the energy, even at equivalent power settings, more slowly. To increase the depth of the coagulation, the voltage spikes are increased. This is necessary because along the margins of the electrode path, the impedance is rising as the tissue is coagulated. The higher voltage spikes force the current through the desiccated layer along the cut margin, increasing the depth of the coagulation.
The modulated output that for a particular generator provides the deepest coagulation (hemostasis) with only some cutting may be named either coag or forced coag . Users often erroneously call these outputs pure coag . The term is erroneous because as long as the voltage spikes (at least sometimes) over 200Vp, some electrosurgical cutting is expected. This common but imprecise use of terminology can promote confusion. The most common coag waveform type (which often has approximately a 6% duty cycle and crest factor of approximately six) has been a first choice for polypectomy for decades; this is because the cutting action along the snare wire allows a clean transection, whereas the coagulation remaining promotes reliable hemostasis. With the advance of ESU technology offering a greater number of output choices on many ESUs, waveforms with a bit more cutting action and comparatively less thermal spread have gained nearly equal use. These waveforms may have names such as blend coag or swift coag . Robust, evidence-based data as to what waveform should be preferred is currently absent. Therefore, an optimal guideline for waveform choice is not available, leaving this to the physician's discretion, preference, and experience.
Many ESUs are equipped with a varying number of modulated waveforms that fall between the extremes of nearly all cut or nearly all coag. These are typically named the blended currents . This is perhaps the most descriptive name, as it correctly implies that some of the cells will be vaporized, or cut, whereas some will be coagulated. Increasingly modulated waveforms with increasingly high voltages will produce tissue effects with increasing hemostasis and decreasing cutting.
The qualitative descriptions of waveforms can be quantified by assigning them a duty cycle or a crest factor. The duty cycle is the numerical percentage of the time the waveform is on (spiking) and the time it is off (interrupted). A waveform with a 6% duty cycle is on 6% of the time and off 94% of the total time. The common coag or forced coag types discussed previously are those with approximately a 6% duty cycle. Any continuous waveform has a 100% duty cycle. It will be the peak voltage that will determine if a 100% duty cycle waveform produces pure (intense) electrosurgical cutting or only soft coagulation. Crest factor is similarly used to quantify the degree to which a waveform is modulated, but is more informative for modulated waves in that it includes information about the peak voltage. Modulated waveforms with low duty cycles and high crest factors produce more and deeper coagulation than waveforms with low crest factors and high duty cycles ( Table 6.2 ). Either duty cycle or crest factor information can be found in every generator's user manual.
A physician with a good knowledge of the principles of current density, the waveform choices on the ESU at hand, and its microprocessor controlled power curves is in a very good position to control, avoid, or compensate for all the other non-ESU variables that enter into the final tissue effect (see Fig. 6.1 ).
One of these non-ESU variables is the operator's technique, which includes the tension applied to the accessory. Some degree of tissue tension is necessary for progression of an incision (e.g., polypectomy or sphincterotomy). However, overzealous tension may result in poor control of cutting, leading to a rapid incision with inadequate vessel coagulation and increased risk of hemorrhage. A slow advance can promote more coagulation. Highly skilled endoscopists performing ESD procedures are able to control small bleeding episodes simply by slowing the cut advance markedly. This holding staunches the bleeding with no change whatsoever in the accessory, the waveform output, or the power selection.
Resistance in an entire monopolar circuit or in the tissue itself varies before and during the incision. Highly fibrotic or scarred tissue is more resistant (and harder to cut) than tissue with a high water content. Tissue resistance is a dynamic variable that alters as tissue desiccates during the incision. The entire resistance between an argon probe tip and the dispersive electrode plays a role in the length of the plasma arc that is generated during argon coagulation procedures. Blood flow is a variable that may help dissipate heat at the site of the tissue. Similarly, submucosal saline injection may act as a “heat sink” and help dissipate energy and prevent transmural injury during polypectomy and other procedures.
The most common method for completing the electrical circuit in gastroenterology procedures is to use a monopolar accessory and a remotely placed dispersive (grounding) pad. The current flows from the ESU and is concentrated at the treatment site by the accessory (e.g., a snare, sphincterotome, HBF, or needle-knife). The concentrated energy at the site has high current density which promotes tissue heating. The energy then flows through the patient's tissue following a path of least resistance to the dispersive electrode on the skin and hence back to the generator, completing an electrical circuit ( Fig. 6.5 ). Because the surface area of the pad is so much larger than the active accessory, the current density is very low at the pad site. Proper pad placement is important to evenly disperse the energy and avoid any increase in skin temperature under the pad. Most modern ESUs have dispersive electrode safety alarms available when used in conjunction with “split” or “dual” pads. Patented in 1983, this technology advance is inexpensive and highly recommended, as it markedly reduces the risk of a patient skin burn under the pad.
Bipolar (multipolar) accessories are different from monopolar electrodes in that both active and return functions are included in the tip of the instrument. Current flows through a relatively small area of tissue, and there is no need for a dispersive electrode on the patient's skin ( Fig. 6.6 ). The most common flexible endoscopic use for this technology is for contact coagulation using a flexible bipolar hemostasis probe that is supplied by many manufacturers ( Fig. 6.7 ). Bipolar hemostasis probes have been shown to be effective devices for hemostasis for bleeding peptic ulcers, arteriovenous malformations, and other hemorrhagic lesions. Optimal results are obtained by using moderate to firm tamponade in the case of active bleeding. Power settings of 15 to 25 watts are most common. These probes are best used with generator outputs that have continuous low-voltage outputs paired with a narrow power curve in which the microprocessor control design allows the energy to ramp up quickly in the low-resistance case of frank blood, but to limit the power delivery automatically as the tissue becomes coagulated. Flexible endoscopic bipolar snares, biopsy forceps, and sphincterotomes have also been developed but have not been successfully or widely adopted.
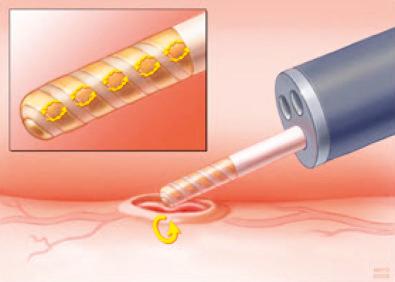
A newer, important use of bipolar current for thermal therapy is radiofrequency ablation employed to ablate Barrett's esophagus. In this situation, the current moves from one adjacent electrode on a balloon catheter to another. This energy passes via the mucosa and is sufficient to ablate the mucosa, while being superficial enough (< 1 mm depth of ablation) to prevent significant submucosal damage, and minimizing stricture formation.
High-frequency electrosurgical energy can be applied to the tissue in either of two ways. The first and far most common method in flexible endoscopy is to have the accessory in direct contact with the surface of the tissue. A well-recognized list of common flexible endoscopic therapies and accessories employ this method: polypectomy and EMR/snares; sphincterotomy/sphincterotomes and needle-knives; contact coagulation/bipolar endostasis probes; coagraspers; and contact coagulators.
In the second way, by using especially high-voltage waveforms with or without the addition of argon (or other) gas to the treatment field, the energy can travel to the tissue surface without the accessory being in contact with the surface.
Argon-assisted coagulation and ablation (also called argon plasma coagulation [APC], argon beam coagulation [ABC], or simply argon coagulation [ArC]) is by far the more prominent noncontact technique in flexible endoscopy. It has many useful applications including tumor debulking, ablation of vascular malformations, and other hemostasis and ablation therapies. Argon coagulation also has a role in “tidying up” residual adenoma after piecemeal polypectomy or EMR, although overuse in this setting may suggest suboptimal prior resection technique.
In argon-assisted coagulation, argon gas flows from a special catheter tip and becomes electrically charged (ionized) by high voltage, which is concurrently delivered to an electrode in the catheter tip by a specially equipped ESU. The resulting conductive gas plasma arc provides a medium for current flow from the catheter tip to an adjacent mucosal surface. This is a monopolar application and requires the use of a dispersive pad to complete the electrical circuit. It can be used effectively by positioning the catheter tip either in a tangential or perpendicular direction to the mucosal surface. Tangential or perpendicular applications are possible because the arc will always follow the current direction to the nearest tissue without regard to the probe tip design. Although this is a noncontact method, the arc length (and therefore the distance from the tissue surface) achieved is important and variable.
This method can be controlled to promote a very superficial pliant eschar or provide deeper effects and tumor debulking by using longer activations often at higher powers. Although accepted as a generally safe tool, it has been shown that argon coagulation can cause transmural injury at high power outputs, and there have been reports of perforation using this device. In addition, pulsed argon modes have been shown to produce a high rate of pain and patient neuromuscular responses. Nonetheless, the relative ease of use, noncontact speed, ability to arc around folds, and relatively shallow depth of injury make argon coagulation a versatile and indispensable therapy.
The character of the argon beam varies with the several types of systems being marketed. In general, various manufacturers offer standard, amplified, or linear beams. Amplified beams have been termed high power due to their higher intensity and correspondingly greater thermal injury. These require that power settings be set lower than either standard or linear beams to compensate.
The nature of the high-voltage waveform used to ionize the argon imparts different characteristics to each beam design. It is therefore very important not to assume that power settings or application times will be constant as a user moves from one device to another. It is important to learn and understand the power and time settings suggested by individual manufacturers. Please note that often time of application, either by a first pass of energy or repeated passes, can greatly affect the depth of thermal injury. It is possible to achieve only a few cell layers deep superficial effect or a deeper mucosal injury by varying only the time of application. See Box 6.1 : Argon Clinical Pearls to aid in successful outcomes. These usage tips are appropriate no matter which ESU/argon system is in use.
Physicians should be sure to use power settings recommended for the system in use—they are NOT all the same!
Purging the connector and argon probe with nonionized argon at least once before inserting into the endoscope is universally recommended. All systems are equipped with buttons for the automatic activation of this function.
In addition to the initial purge, it may also be helpful to add another short purge after the probe has been inserted into the scope and is nearing the target site (be sure the tip of the probe is not touching any tissue). This helps bring the gas fully over the ignition electrode which is located at the end of the probe and makes sure an adequate non-ionized argon cloud is surrounding the target site.
Sometimes approaching the tissue with the probe in a tangential position will help get close to the target treatment site without touching.
It is OK to gently “lay down” the probe tip and then pull back without delivering energy to gauge distance from the tissue.
Don't extend the probe too far out of the scope; working closely gives better control. Be sure you see the tip of the probe extending beyond the tip of the endoscope.
Keep your foot on the energy delivery pedal long enough to get ignition started.
There are three popular application techniques: “spot welding,” “painting,” and “ignite and drag.” Each has different uses and slightly different techniques. It helps to practice on ex vivo or animal models.
For the painting technique, move the scope not the probe.
Increasing the time increases the penetration depth.
Putting the dispersive (grounding pad) on the flank can shorten the impedance path and help make the beam longer.
Don't turn up the gas flow trying to make the beam longer. This will only dilute the ions already activated.
1 liter per minute flow rate is sufficient for most applications. Increasing the gas flow will lead to more distension of the patient's gut.
Monitor the patient for distension during the procedure, and use suction frequently, especially for lengthy or pediatric applications. Recall that argon gas is flowing when the foot pedal is depressed even if the beam has not ignited.
It is not true that having a side or circumferential tip on the argon probe will make it safe to touch the tissue.
Do not use argon coagulation to treat varices, hemorrhoids, or other lesions requiring tamponade or coaption.
It is possible to induce electrosurgical current to arc in a noncontact fashion without the aid of argon gas. To ionize mixed air in the gap between the accessory and tissue requires high voltages. (Argon gas forms a more stable plasma even in the lower range of these voltages, which is one reason argon is chosen.) The waveforms used to ionize mixed air have crest factors of at least seven or eight and may be named either spray coag or fulgurate . The ionization of mixed air is less consistent and tends to produce a variable, often charlike and less pliable eschar than when argon gas is present. Smoke may also be produced. These drawbacks, along with historic concerns about capacitive coupling when high voltages are used with endoscopes, have made the use of these nonargon-assisted methods rare in flexible endoscopy, although the method is fairly common in open surgery.
Recently, however, the use of spray coag/fulgurate waveforms have been introduced by some Japanese physicians as an optional output choice for the dissection stage of ESD and peroral endoscopic myotomy (POEM) procedures. No studies of tissue effect and safety of this waveform in these settings are known to have been published to date.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here