Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The software and hardware related to mapping technologies for cardiac arrhythmias have evolved considerably over the past decades. The history of cardiac mapping dates back to the mid-1900s when Durrer and others used the isolated heart models perfected by Langendorff and colleagues to map ventricular wall excitation via handheld and needle electrodes. Since these initial studies, multiple technologies to optimize the mapping of cardiac arrhythmias have been developed. When discussing cardiac mapping, most electrophysiologists immediately think of invasive electroanatomic mapping, involving catheters with electrodes at their tips assuming direct contact with underlying myocardium to acquire local signals and, in turn, to differentiate sites of interest-based abnormalities in the signal characteristics, voltage, and activation. The acquisition, processing, and spatial projection of these points in a three-dimensional (3D) mesh facilitate the electrophysiologist’s ability to identify the location and characteristics of substrate for complex arrhythmias and target it for ablation. The most common clinical mapping systems used are CARTO (Biosense Webster), EnSite (St. Jude Medical), and Rhythmia (Boston Scientific). However, cardiac mapping may also be thought of in the context of more basic approaches, such as using the 12-lead electrocardiogram (ECG) to localize arrhythmias, or newer approaches for noninvasive or minimally invasive mapping, such as electrocardiographic imaging (ECGI) (see Chapter 68 ) or body surface potential mapping. This chapter focuses on electroanatomic mapping in the context of the acquisition and processing of intracardiac signals to create 3D electroanatomic maps of cardiac arrhythmias.
During the formative period of invasive electrophysiology, mapping was performed using multipolar electrode catheters, fluoroscopy to localize the anatomic position within the cardiac chamber of interest, interpretation of pacing and recorded intracardiac electrograms to define the arrhythmia mechanism, and localization of the origin or critical components of a tachycardia circuit to guide ablation energy delivery. However, as the catheter was moved across the heart there was no way to store its anatomic position to “take” points; thus mapping relied on operator recall of the catheter location and approximation of lesion sites relative to one another. Each lab had its own unique way of noting the points of interest by memory. This ablation approach is convenient for arrhythmias such as atrioventricular nodal reentrant tachycardias (AVNRTs), accessory pathway–mediated tachycardias, or focal outflow tract ventricular arrhythmias, where a single ablation lesion or limited lesion sets may be capable of terminating the arrhythmia. As ablation of more complex arrhythmias such as atrial fibrillation, scar-related atrial flutters, and scar-related ventricular arrhythmias have become more common, the need for systems in which regions of abnormal signals (i.e., substrate mapping), tracking sites of ablation, and projection of the generated cardiac anatomy can be used to recognize structures close to one another has become the need of the hour. Although contemporary mapping systems have improved the efficacy and rapidity with which complex ablations may be performed, they still require an intimate understanding of how they work and their limitations to avoid potential pitfalls. A basic approach to integrating the mapping system into ablation practice is shown in Fig. 128.1 .
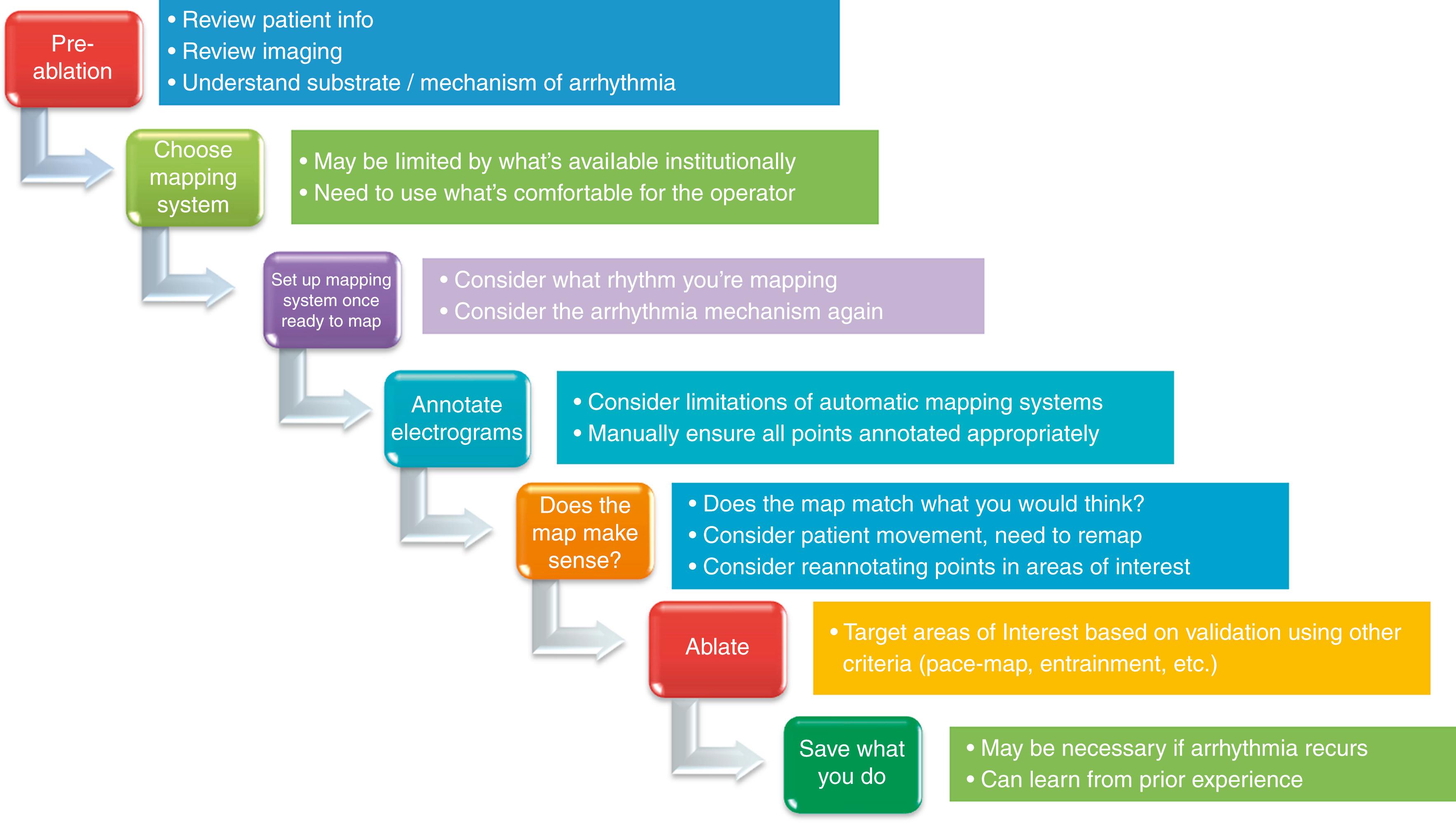
Although modern-day mapping systems may also be used for arrhythmias that have traditionally had high success rates even before the era of 3D electroanatomic mapping (e.g., accessory pathways, AVNRTs), they hold a particular utility for complex arrhythmias where the underlying cardiac substrate is complex (e.g., scar-related ventricular arrhythmias, atrial fibrillation, and complex atrial flutters). The ability of the system to “remember” what has been mapped already and reflect it back to the operator can allow the electrophysiologist to (1) recall regions where abnormal substrate exists, (2) recognize sites of prior ablation, (3) better localize subtle differences in local activation via the acquisition of a dense array of points and annotation of signals relative to one another, and (4) have an anatomic model for pure anatomically based ablation such as pulmonary vein isolation.
Three principles underlie all mapping systems: acquiring a signal, projecting that signal within a 3D framework, and accurately projecting signals from different sites relative to one another with a high degree of positional accuracy. The sum of all points, or signals, will generate an electroanatomic shell. Each point is given a position in an xyz -plane. Accurate projection of signals relative to one another typically requires that a patient not be actively moving. When acquiring a point, the local signal is determined by several factors, including the spacing of electrodes on a given catheter, postprocessing of the signal that partly depends on filter characteristics (high-pass and low-pass frequency) set by the user, contact with the tissue, positioning of the catheter relative to the underlying myocardium (i.e., whether the electrodes are parallel, oblique, or perpendicular to the underlying myocardium), and the rhythm at the time of point acquisition. This section briefly discusses how points are acquired and considerations needed when annotating such points at the time of cardiac mapping.
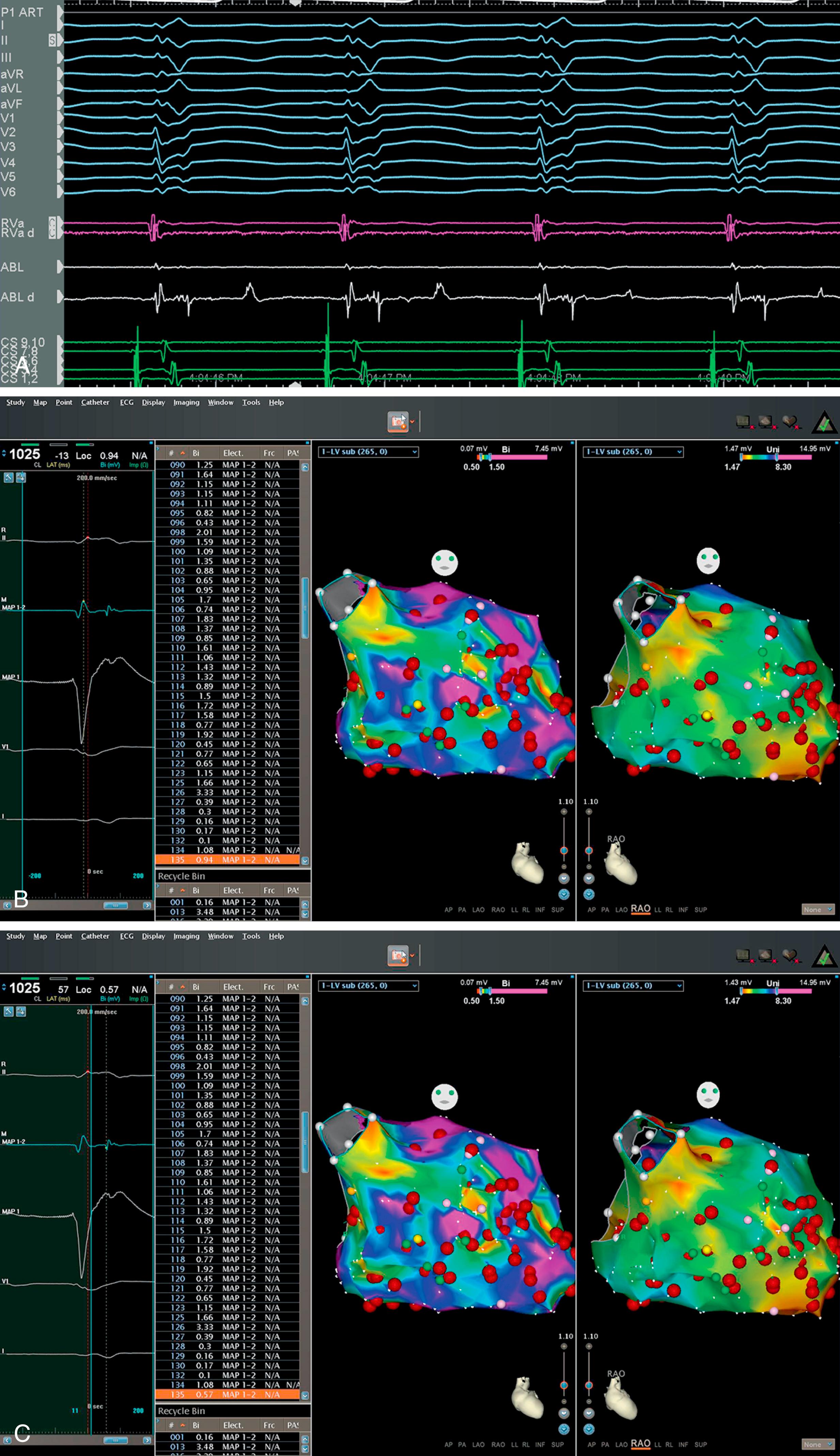
The fundamental requisite of making an interpretable map is to ensure that all points acquired either as activation points or to identify substrate are accurate and relevant to each other and the given case in general. Depending on the arrhythmia mapped, critical considerations include choosing an appropriate reference, establishing a mapping window, and deciding how an acquired point will be annotated relative to the given reference in terms of activation or voltage. This is discussed in more detail later ( Fig. 128.2 ).
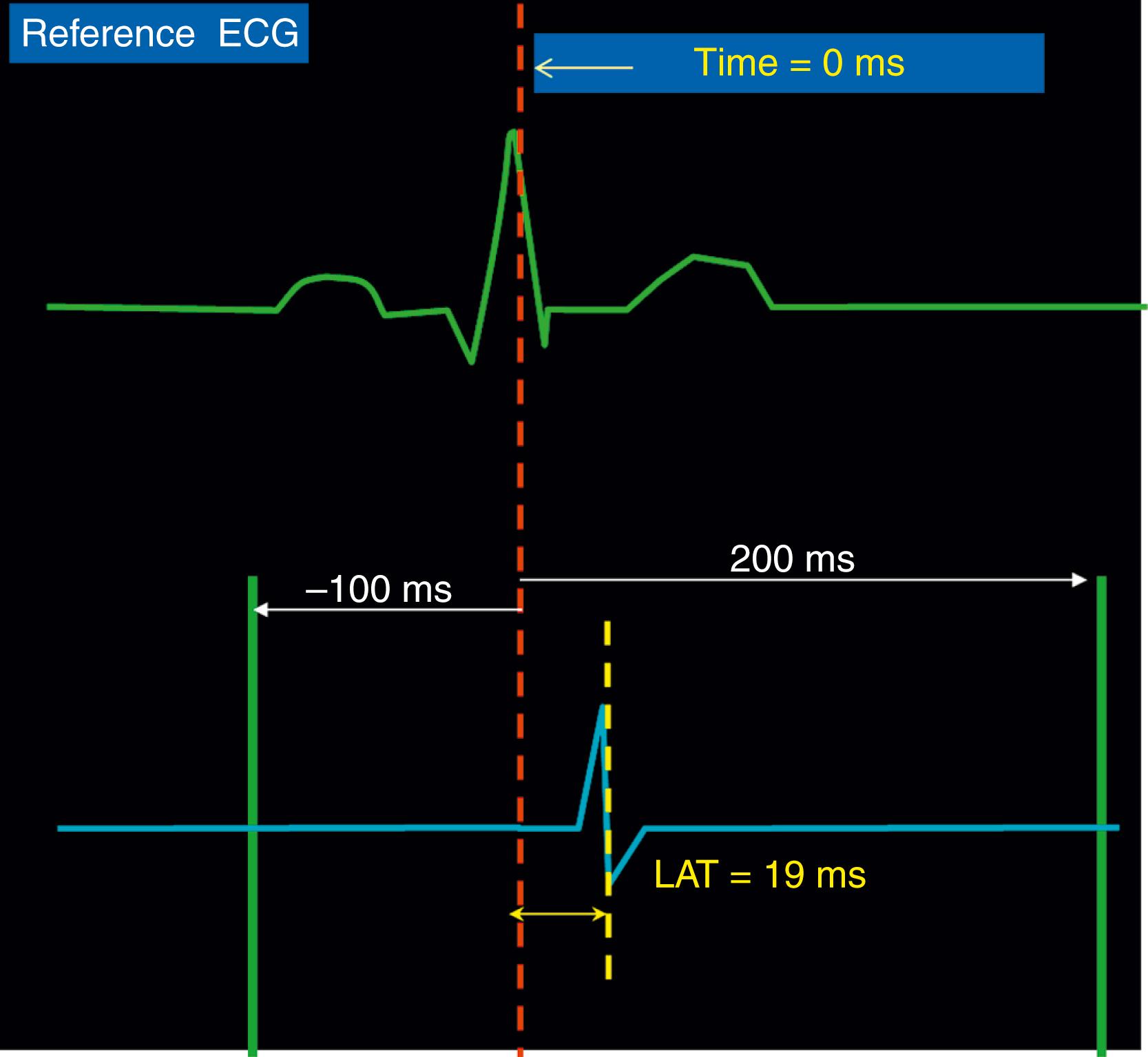
The reference is meant to be a stable reflection of cardiac electrical activation at that particular site against which an acquired local electrogram’s relative timing may be compared. The reference may either be a portion of the surface ECG (often used when mapping ventricular arrhythmias) or a local electrogram (often used when mapping atrial arrhythmias). When mapping, it is crucial to ensure that the reference electrogram has not shifted, as this may reflect either a shift in the rhythm (in which case local timing may change because of a global shift in activation pattern) or movement of the source from which the reference is being recorded (e.g., the reference catheter or the ECG patch). This can be assessed periodically, primarily when the procedure is performed under minimal or no sedation.
After a reference is chosen, the mapping window should be set up. The duration, onset, and offset of the mapping window relative to the reference signal should be decided. This is a critical step that is often overlooked. The mapping window is designed to create a period surrounding the reference within which any recorded electrograms will be acquired. Thus if an electrogram (e.g., a late potential) falls outside the window, activation of that excluded portion of the electrogram cannot be tagged without altering the window. The voltage of that excluded portion of the electrogram will also not be considered when the system reports the local voltage.
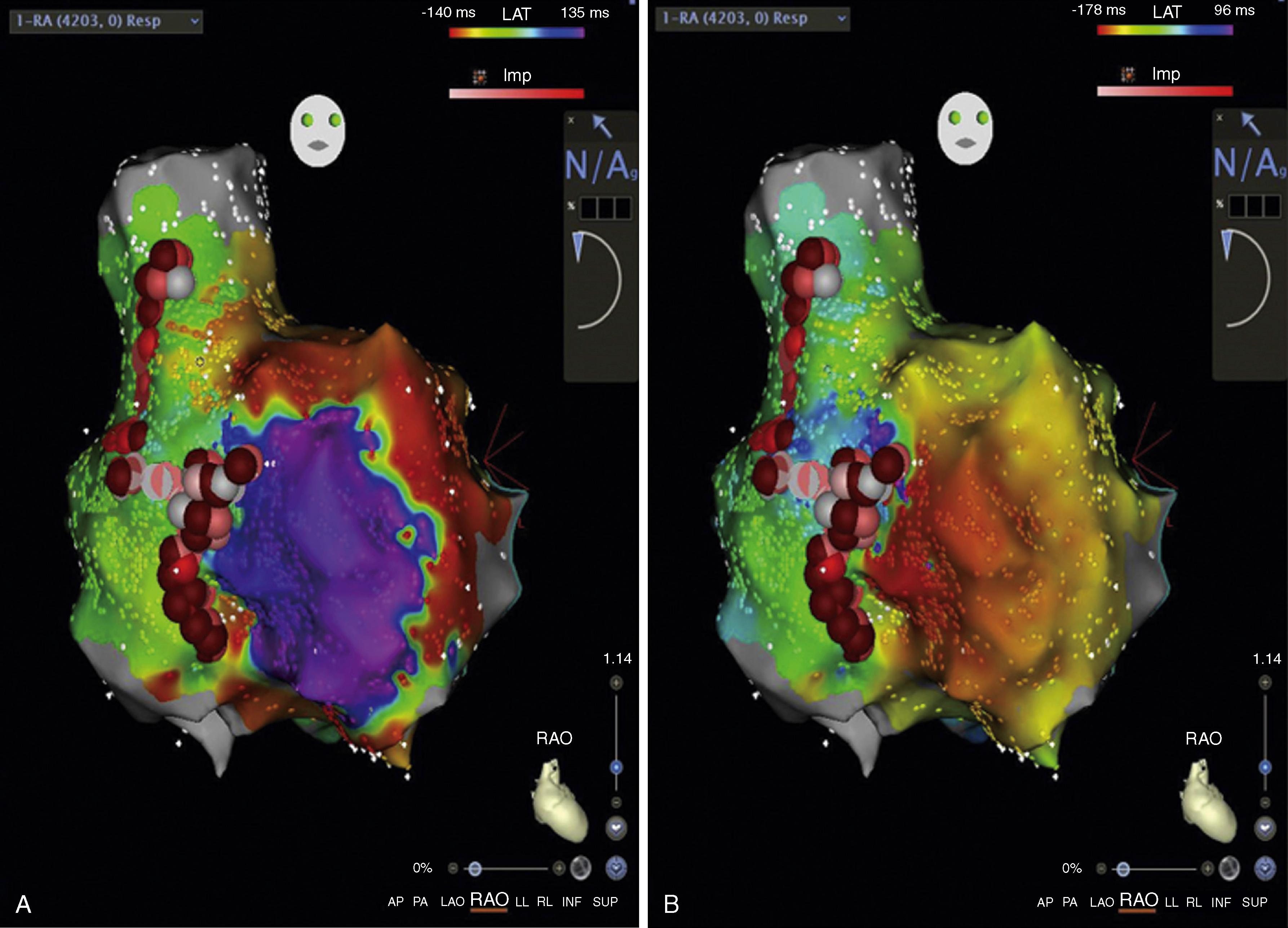
It is also essential to consider the rhythm in which one is mapping when choosing a window (i.e., whether in sinus rhythm, during a focal arrhythmia, or during a reentrant arrhythmia). When mapping a focal (microreentrant, triggered, or automatic) arrhythmia, it is crucial that the beginning of the window is set far enough before the reference to allow for the acquisition of signals from early sites responsible for arrhythmia propagation. For example, if one has a window that only extends to 10 ms pre-reference when mapping a focal arrhythmia, but the earliest signal occurs 30 to 40 ms before the reference, these early signals will not be included in the window of interest during mapping. In the case of macroreentrant arrhythmias, deciding on an appropriate window is even more critical. If the chosen window exceeds the tachycardia cycle length, more than one signal can appear in the same window. If one chooses a window less than the tachycardia cycle length, signals may be excluded, which will obviate a complete map of the entire tachycardia cycle length. Thus the window should not exceed the tachycardia cycle length ( Fig. 128.3 ). Most mapping systems allow the user to define a region where “early meets late” or where the “tail” of the arrhythmia collides with the “head.” However, where this occurs is arbitrary and depends on the offset and onset of the window relative to the reference, and defining critical sites of ablation may require the additional use of entrainment or other maneuvers. The decision to ablate at “early meets late” is fraught with several limitations, and success with such a strategy is pure serendipity. ,
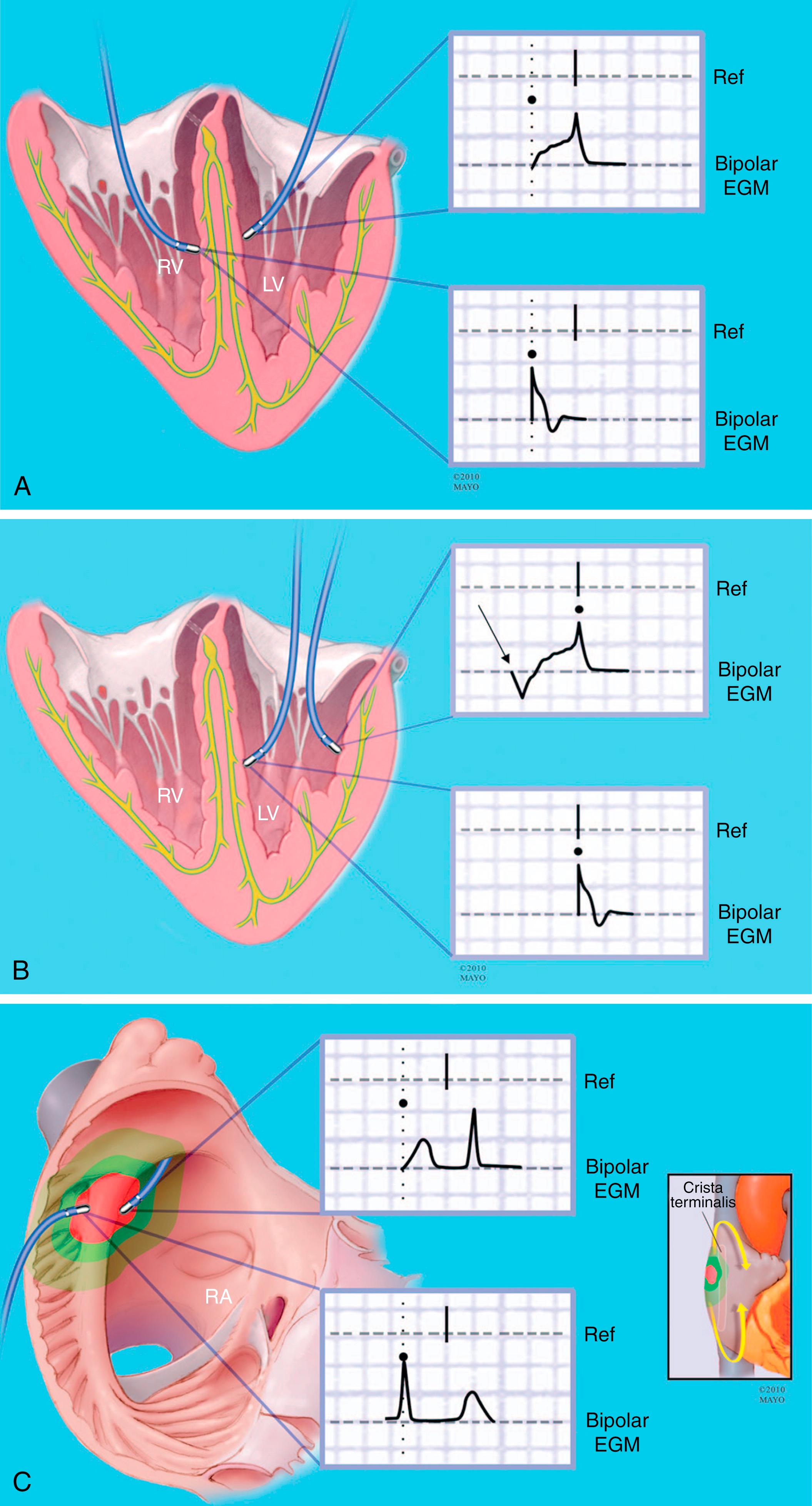
Before beginning to map, it is helpful to have a detailed plan between the operator and the person acquiring and annotating points. Once the reference and the window are set, accurate annotation of points is critical to appropriate mapping (see Chapter 127 ). Specifically, when annotating a point, one must consider whether the earliest portion of the electrogram, the peak of the electrogram, the maximum upstroke of the electrogram, or the beginning of the maximal downgoing portion of the unipolar electrogram will be used to determine local activation. Each of these approaches has its pitfalls ( Fig. 128.4 ). Annotation can become even more complex when considering electrograms at sites of diseased tissue (e.g., split potentials, far-field potentials, or fractionated electrograms). Operator considerations for annotating each electrogram are critical, including differentiating far-field from near-field potentials, recognizing when the annotation of a given point may be impossible given its complexity despite potential relevance to the underlying arrhythmia, and that there may be different considerations about where to annotate the potential when mapping points at different sites (e.g., around sites of anatomic block, within regions of scar) are critical when mapping ( Fig. 128.5 ).
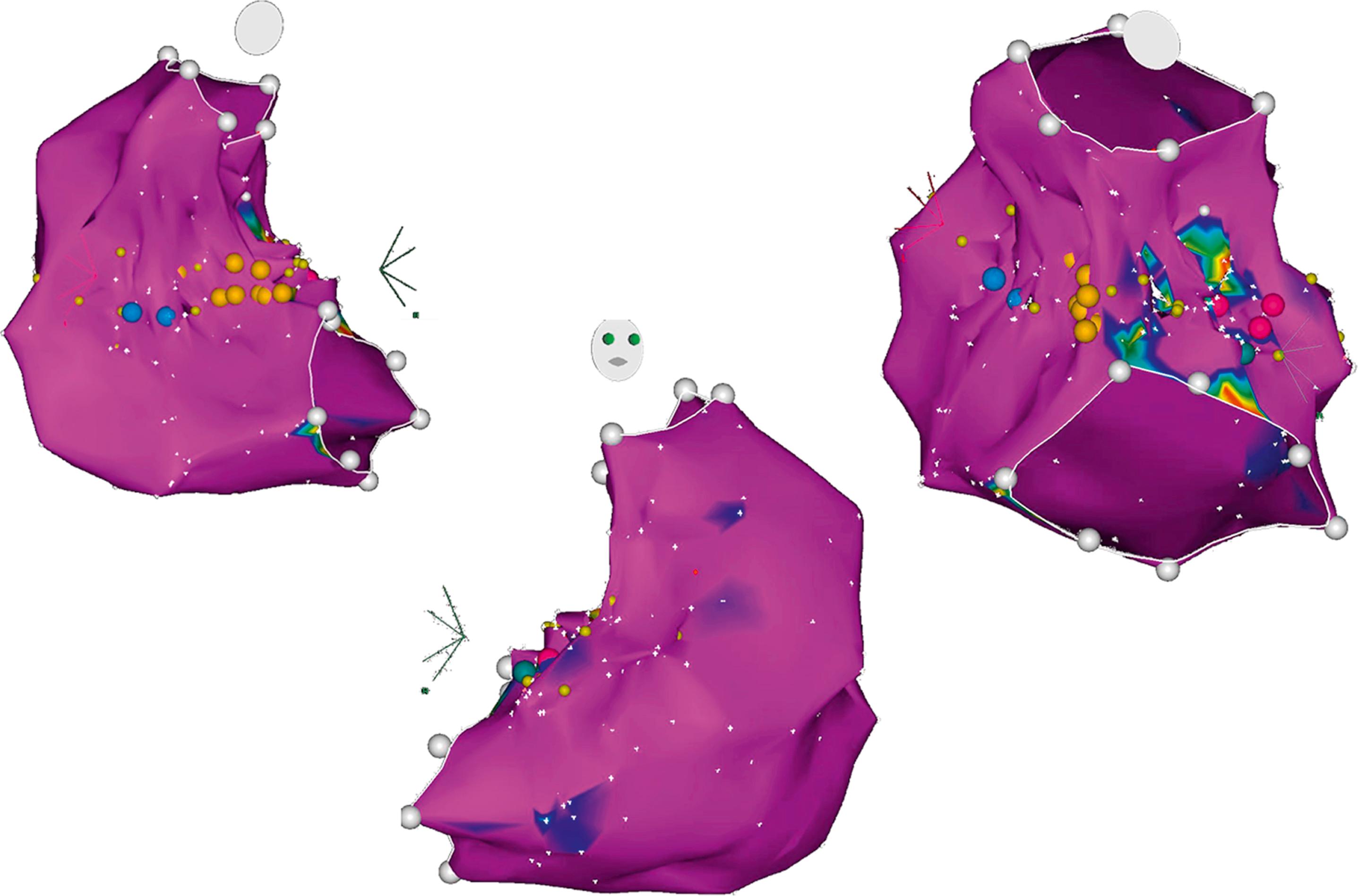
The fundamental indication for established electroanatomic mapping systems is precise nonfluoroscopic visualization of mapping catheters and reconstruction of 3D volumes of interest. The resulting 3D reconstruction is projected as a shell representing the cardiac chamber or structure of interest ( Fig. 128.6 ). Each system has proprietary features that are meant to optimize either mapping or ablation. However, the most critical difference that exists between mapping systems is how the catheters are visualized and how their 3D position is determined when acquiring points.
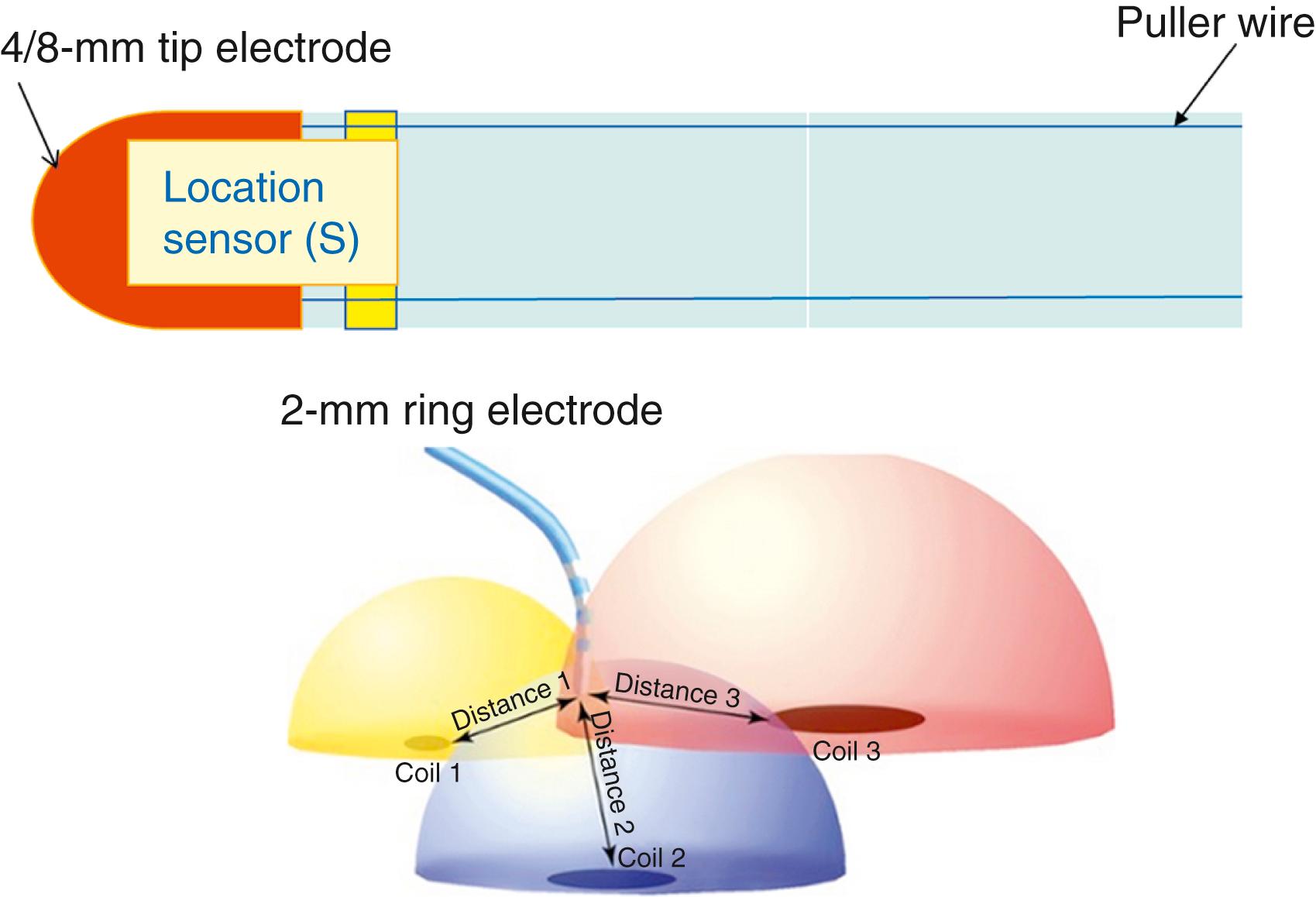
In general, mapping systems localize catheters either via a magnetic- or impedance-based system. Impedance-based systems involve emission of a low-current electrical field via three orthogonally placed skin patches along the xyz-axes. The measured voltage and impedance sensed between the catheter’s electrodes and the patches is proportional to the distance of the electrode from the patches in 3D space, allowing for localization of the catheter tip. Accuracy of the position in the space of the electrode is estimated to be in the range of 0.8 to 2 mm. Magnetic-based systems use a location sensor embedded in the catheter tip and rely on the use of proprietary mapping catheters with embedded sensors. Three miniaturized coils external to the patient generate a low-level magnetic field from which the location and orientation of the sensor within the catheter can be identified with an estimated range of error of less than 0.5 to 0.8 mm ( Fig. 128.7 ).
The CARTO mapping system uses a triangulation algorithm similar to that used by a global positioning system (GPS). The magnetic field emitter, mounted under the operating table, consists of three coils that generate a low-intensity magnetic field, approximately 0.05 to 0.2 G. A sensor attached to the patient’s skin within the working space of interest serves as a location reference. The sensor embedded proximal to the tip of the specialized mapping catheter measures the intensity of the magnetic field generated by each coil for the determination of its distance from each coil. These distances determine the area of theoretical spheres around each coil, and the intersection of these three spheres indicates the location of the tip of the catheter. The accuracy of the location determination is highest in the center of the magnetic field; therefore it is important to position the location pad under the patient’s chest. In addition to the xyz -coordinates of the catheter tip, the system can determine three orientation determinants, roll, yaw, and pitch, for the electrode at the catheter tip. The position and orientation of the catheter tip can be seen on the screen and monitored in real time as the catheter moves within the electroanatomic model of the chamber mapped. The catheter icon has four color bars (green, red, yellow, and blue), enabling the operator to view the catheter as it turns clockwise or counterclockwise.
CARTO3 and later versions integrate a hybrid of magnetic- and current-based catheter localization technology that enables the visualization of multiple catheters simultaneously without the need for fluoroscopy. This point is critical, given that earlier permutations of the system could only visualize proprietary sensor-based catheters, whereas current (impedance)-based catheter localization systems allow, in principle, localization of practically any catheter. For this system, six electrode patches are positioned at the patient’s back and chest, and the current emitted at a unique frequency from different catheter electrodes is continuously measured. Localization of the nonmagnetic electrodes can be calibrated by the detection of the magnetic sensor within the coordinate system to overcome distortions from nonuniform intrathoracic resistances. However, one limitation that persists is that visualization of catheters is limited to a 3D volume called a “matrix,” which is predefined by moving the magnetic sensor-based catheter over the region. Only catheters within this matrix will be visualized, and those beyond the matrix will not. The following sections discuss fundamental modules.
The system also allows fast anatomic mapping (FAM) for the creation of detailed anatomic end-diastolic shells using data collected simultaneously from multiple electrodes of a multipolar mapping catheter that is moved around the chamber of interest ( ). The accuracy of surface reconstructions is also improved by a unique type of respiratory gating based on measurement of the varying thoracic throughout the respiratory cycle.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here