Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Duplex sonography is a valuable, functional addition to anatomic sonography of the pediatric brain.
Various quantitative metrics can be extracted from the flow curves, including peak systolic, end-diastolic flow velocity, time average maximum mean blood flow velocity, and resistive index.
The quantitative markers may be influenced or altered by mechanical ventilation, patent ductus arteriosus, extracorporeal membrane oxygenation (ECMO), arteriovenous shunting, and pathologies that are affected by cerebral hemodynamic autoregulation.
In hypoxic ischemic injury and general brain edema, the resistive index values typically drop to low values.
Duplex sonography may help to identify and explore arterial and venous malformations such as the vein of Galen aneurysmal malformation.
Continuous and pulsed echo technique, or duplex sonography in which gray-scale tissue imaging is combined with blood flow sampling, has been in use for the past decades in the evaluation of normal and abnormal neonatal intracranial hemodynamics. In addition, color Doppler sonography renders valuable information about the direction of the blood flow in relation to the transducer. This technique allows better understanding of altered intracranial flow or exploring the complex angioarchitecture of vascular malformations. Continuing improvements in transducer design and sensitivity allow routine imaging of the intracranial vasculature in newborns and young infants, including the identification of flow in submillimeter arteries (e.g., lenticulostriate arteries) and patency of dural venous sinuses (e.g., superior and inferior sagittal sinus) of the brain. The wide dynamic range and high sensitivity of power Doppler sonography, in which blood flow is represented as various shades of a single color (amplitude-coded color or color flow Doppler energy), is especially helpful to depict low-velocity and low-amplitude blood flow. Power Doppler sonography, however, lacks information about flow direction. Furthermore, analysis of the sampled flow spectra/curves and postprocessing software programs offer extraction of objective semiquantitative data (e.g., peak systolic velocity [PSV], end-diastolic flow velocity [EDV], resistive index [RI], and time-averaged mean blood flow velocity [TAV]). These semiquantitative data can be collected serially, giving valuable real-time information about progression of disease or recovery. Analysis of the shape of the spectral pattern with delayed systolic flow acceleration, flattened systolic upstroke, slow diastolic deceleration, or reversal of flow during diastole may provide important information about the cerebral hemodynamics. This chapter describes how brain ultrasonography combining multiplanar, multiapproach anatomic and functional/hemodynamic data has become an invaluable noninvasive bedside imaging modality supporting diagnosis, monitoring, guidance of treatment options, and estimation of prognosis for neonates with a wide variety of neurologic diseases.
Three different scanning approaches to the neonatal brain have worked well, each with its own advantages and limitations. The anterior fontanelle approach is the most common and easiest to use. The basilar, internal carotid, and anterior cerebral arteries, as well as the internal cerebral veins, vein of Galen, and superior sagittal, inferior, and straight sinus, can be routinely sampled on midsagittal scans ( Fig. 46.1A-B ). The smaller thalamostriate arteries and opercular branches of the middle cerebral artery (MCA) may be seen on angled parasagittal images.
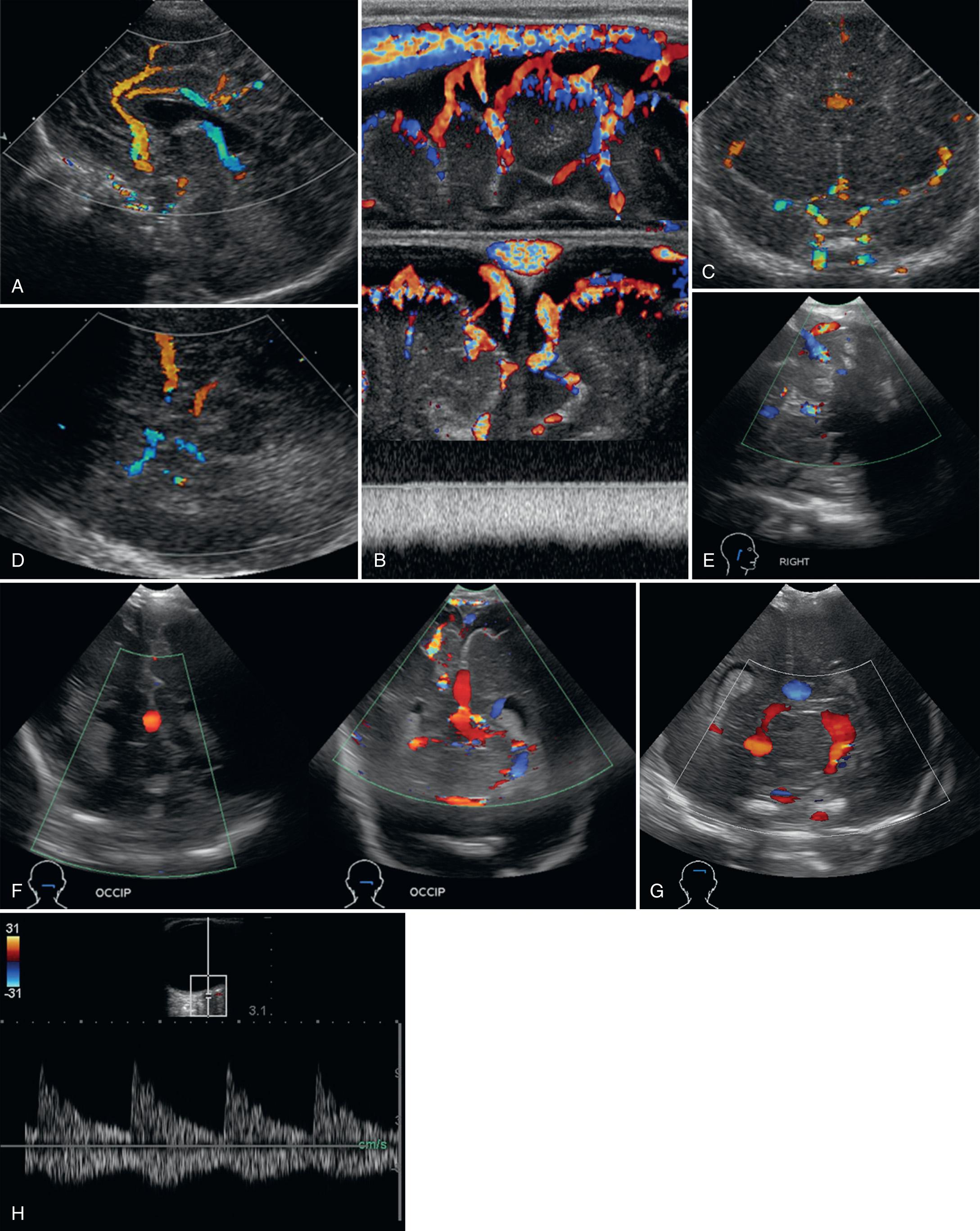
On coronal scans through the anterior fontanelle, the supraclinoid internal carotid arteries, M1 segments of the MCAs, thalamostriate arteries, A1 segments of the anterior cerebral arteries, and the cavernous sinus can almost always be visualized on anteriorly angled scans (see Fig. 46.1C ). Paired terminal, caudothalamic/thalamostriate, and internal cerebral veins, the basilar artery, straight sinus, and transverse sinuses can be seen on more posteriorly angled scans. A major drawback of the coronal plane is the near perpendicular angle between the MCA and the ultrasound beam, such that measured frequency shifts from flowing red blood cells approach zero, making the MCA appear to have no flow.
The temporal bone approach is best for the MCA. The transducer is placed in axial orientation approximately 1 cm anterior and superior to the helix of the ear. Using the thin temporal bone as an acoustic window, adequate penetration for imaging and Doppler studies can be achieved in most full-term neonates. This approach allows visualization of the major branches of the circle of Willis (see Fig. 46.1D ). In most premature infants, both MCAs can be easily insonated from one side of the head.
The mastoid fontanelle approach is an angled axial scan. This acoustic window is located approximately 1 cm behind and superior to the helix of the ear at the junction of the posterior parietal, temporal, and occipital bones. It is the preferred approach for imaging flow in the transverse dural sinuses, and the torcular Herophili in selected patients (see Fig. 46.1E ).
Dedicated images of the posterior fossa circulation may be obtained using the foramen magnum approach or suboccipital approach (see Fig. 46.1F ). The neonate is placed in lateral recumbent position with the head gently flexed. The transducer is placed in the midline, just inferior to the occipital protuberance, and angled cephalad in either sagittal or axial orientation. Alternatively, the posterior fontanelle (see Fig. 46.1G ) at the junction between the lambdoid and sagittal suture can be used as an acoustic window. The posterior fossa arterial and venous vasculature can be depicted in most children.
Finally, a transorbital approach (see Fig. 46.1H ) may be selected to evaluate the ophthalmic artery and the internal carotid siphon. Typically, the transducer is positioned over the closed eyelid and angled slightly medially and upward toward the region of the orbital apex/optic nerve canal.
For the best visualization of the intracranial vascular system, the image should be electronically magnified and the color region of interest restricted to enhance color sensitivity and frame rate. Color gain should be carefully adjusted and balanced to maximize vascular signal, limit flow aliasing, and minimize tissue motion artifacts, and the lowest bandpass filter should be used to maximize low-flow sensitivity necessary for evaluating venous structures. A 7- to 15-MHz linear transducer is recommended for examining the superficially located superior sagittal sinus. Visualization of the smaller arterial branches of the middle and anterior cerebral arteries can also be accomplished in the majority of normal premature and full-term infants but often requires a higher frequency (7 or 10 MHz) vector or sector transducers capable of detecting lower velocity and amplitude signal. The use of power Doppler sonography is recommended when directional information is of secondary importance and detection of flow is primary.
Acoustic window should match sampled vessel.
Electronically magnify image.
Restrict color region of interest to enhance color sensitivity and frame rate.
Adjust color gain to maximize vascular signal and minimize aliasing and tissue artifact.
Use lowest bandpass filter to maximize low-flow sensitivity and evaluate venous structures.
Use 7- to 15-MHz linear transducer for superior sagittal sinus.
Use power-mode Doppler for smallest vessels when flow direction is not needed.
Insonication angle for spectral data collection should be less than 60 degrees.
Sample volume box should be centered to vessel lumen, avoiding sharp curves of vessel.
For specific vessel evaluation, spectral Doppler imaging can be performed using 3.5- to 15-MHz probes, depending on the depth and location of the target vessel. To ensure reliable and reproducible quantitative hemodynamic metrics, care should be given to maintain an angle of insonication of less than 60 degrees while sampling the intravascular flow. Furthermore, comparison of serial data requires a standardized, uniform angle of insonication of the same vessel during subsequent studies. In addition, factors that may alter the diameter of the sampled vessels should be taken into account, such as acute changes in systemic blood pressure, Pa co 2 , Pa o 2 , and hematocrit.
During pulsed wave and color flow Doppler examinations, signal is obtained by imparting energy into tissues. Although intracranial Doppler ultrasound is safe, biologic effects may be identified in the future. Thus Doppler exposure should be time limited, and the signal intensity should be maximized by increasing gain and not transducer power output settings. The ALARA principle (as low as reasonably achievable) should be followed to limit energy exposure to tissues.
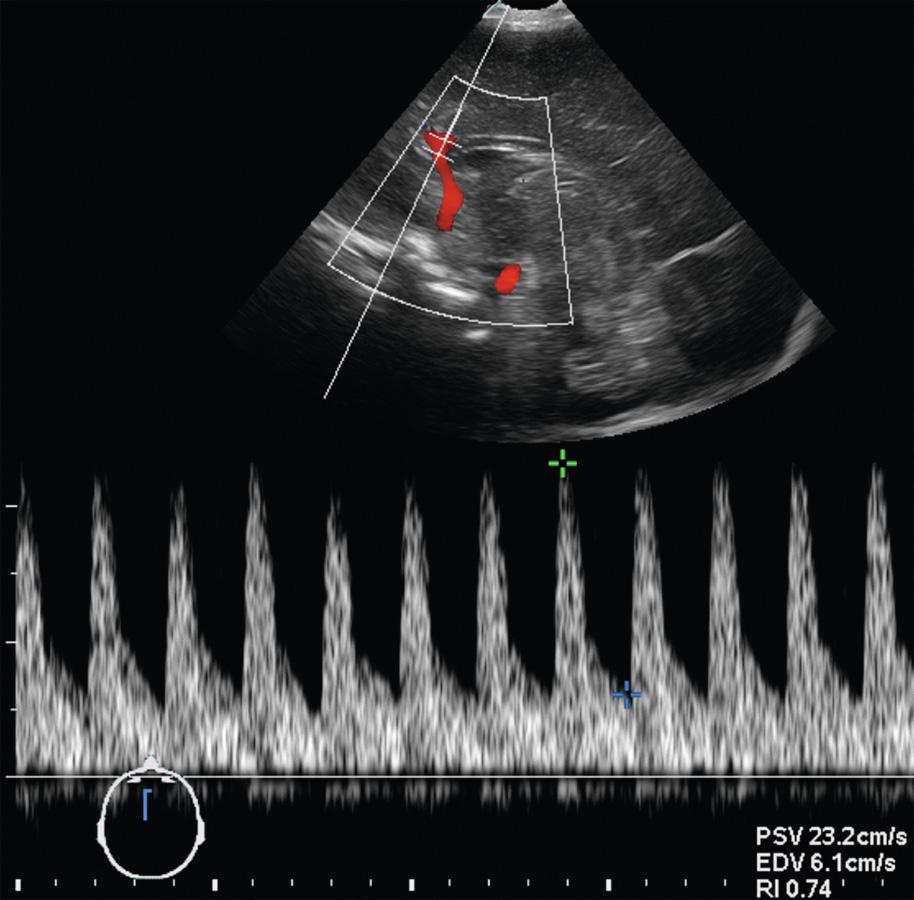
The peak systolic velocity, end-diastolic flow velocity, resistive index, and time-averaged mean flow velocity (TAV) are the most commonly used semiquantitative spectral Doppler measures for monitoring intracranial hemodynamics ( Fig. 46.2 ).
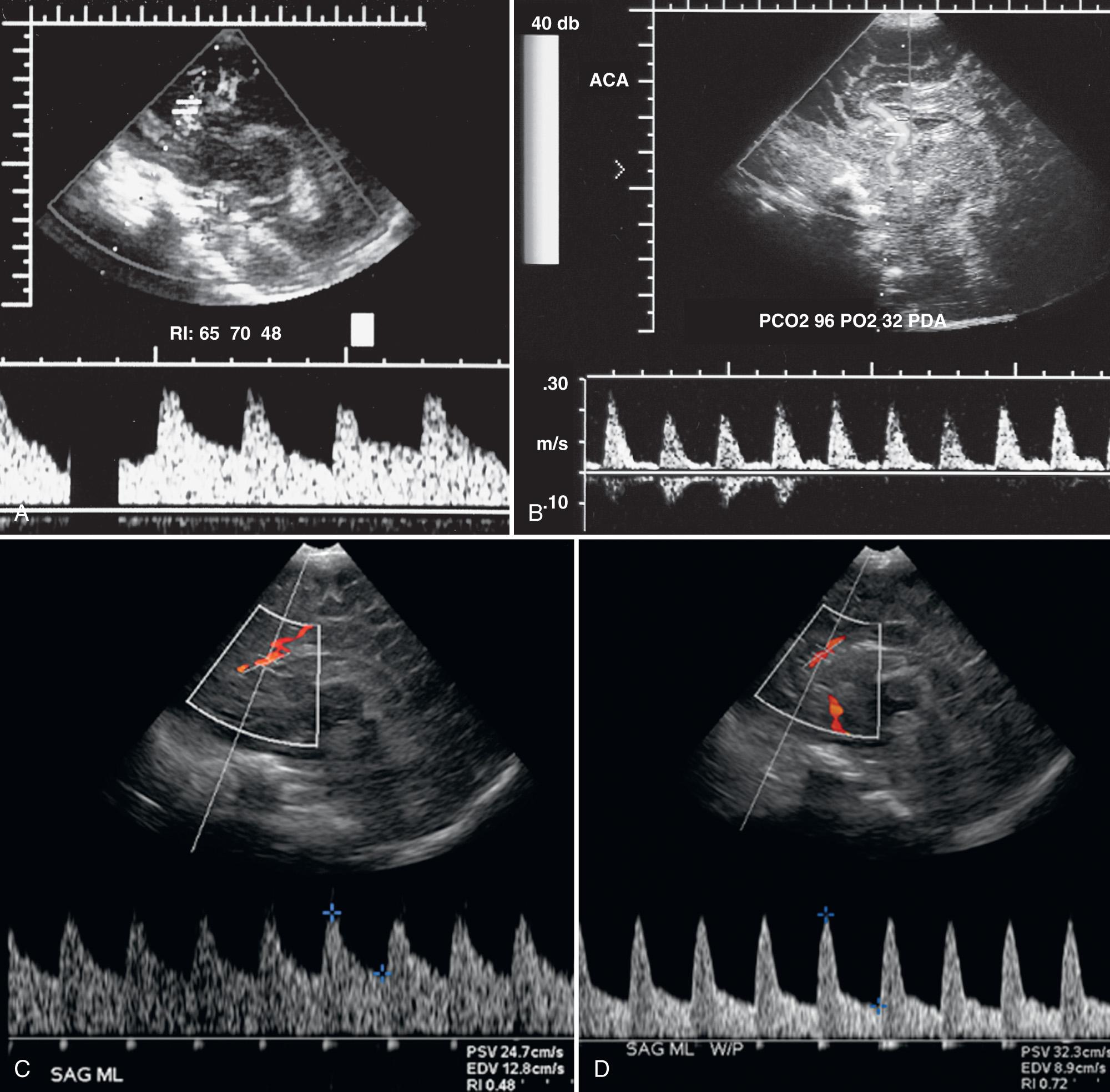
One should, however, be aware of the various factors that may modify the measured or calculated RI values, which include both technical causes and patient comorbidities outside of the brain ( Fig. 46.3 , Table 46.1 ).
| Factor | Effect on RI |
|---|---|
| High-pass filter settings | Increased |
| Transducer scanning pressure | Increased |
| Patent ductus arteriosus | Increased |
| Elevated heart rate | Decreased |
| Decreased cardiac output | Decreased |
With increasing filter settings, lower velocities are not depicted, resulting in a falsely elevated RI. Transducer pressure on the anterior fontanelle may transiently increase intracranial pressure (ICP), which in turn preferentially reduces flow during diastole and increases RI, especially in neonates with impaired cerebral autoregulation (e.g., secondary to hypoxic ischemic injury [HII]). In infants with symptomatic patent ductus arteriosus, resistance to flow in the cerebrovascular bed is higher than pulmonary vascular resistance. This results in shunting of blood away from the brain during diastole and an elevated intracranial RI. During tachycardia, the arterial pressure wave has less time to dissipate before another systolic ejection occurs. Intracranial RI is artificially lower because diastolic velocities are measured at middiastole, when velocities are higher, rather than during end diastole. Left ventricular dysfunction (decreased cardiac output) results in a decreased systolic pressure wave, lowered systolic velocities, and a reduced RI.
The RI is only a weak predictor of cerebrovascular resistance under most physiologic conditions. Mean blood flow velocity measures are the most informative indices of cerebral blood flow (CBF). Although accurate placement of the sample volume and angle of insonation are required, a strong correlation has been demonstrated between mean blood flow velocity and changes in global CBF under a variety of clinical and experimental conditions.
Arterial hemodynamics in the cerebral circulation are affected by normal maturational events in the healthy newborn. The RI in the anterior cerebral artery decreases from a mean of 0.78 (range, 0.5-1.0) in preterm infants to a mean of 0.71 (range, 0.6-0.8) in full-term newborns. This trend is associated with increasing diastolic flow velocities and may be related to peripheral changes in cerebrovascular resistance or to changes proximal to the site of recording, such as a closing ductus arteriosus and a diminishing left-to-right shunt. In full-term infants, RI may also change over the first few days of life. In a study of 476 normal newborns weighing over 2500 g at birth, anterior cerebral artery RI decreased from a mean of 70.6 ± 7 (range, 51-87) to 68.3 ± 6 (range, 51-83) within the first 24 hours.
Table 46.2 provides the range of published peak systolic and end diastolic velocities and RI values in several intracranial arteries. Although the range of normal values is broad, no great variability should be seen in the individual patient. Changes of more than 50% from baseline values should be considered abnormal. There are no consistent differences in instantaneous blood flow velocity among the major branches of the circle of Willis or between right-sided and left-sided structures. The measured blood flow velocities may, however, vary with pressure applied to the anterior fontanel. In healthy preterm and term neonates a tendency toward higher RI values can be observed with anterior fontanel compression, and a statistically significant increase in RI values may be observed in children with presumed impaired autoregulation of brain perfusion caused by hydrocephalus or brain edema related to HII.
| Artery | Peak Systolic Velocity (cm/sec) | End-Diastolic Velocity (cm/sec) | Resistive Index |
|---|---|---|---|
| Internal carotid | 12-80 | 3-20 | 0.5-0.8 |
| Basilar | 30-80 | 5-20 | 0.6-0.8 |
| Middle cerebral | 20-70 | 8-20 | 0.6-0.8 |
| Anterior cerebral | 12-35 | 6-20 | 0.6-0.8 |
| Posterior cerebral | 20-60 | 8-25 | 0.6-0.8 |
a Values modified from Archer LN, Evans DH, Levene MI. Doppler ultrasound examination of the anterior cerebral arteries of normal newborn infants: the effect of postnatal age. Early Hum Dev. 1985;10(3-4):255-260 ; Horgan JG, Rumack CM, Hay T, et al. Absolute intracranial blood-flow velocities evaluated by duplex Doppler sonography in asymptomatic preterm and term neonates. Am J Roentgenol. 1989;152(5):1059-1064 ; Allison JW, Faddis LA, Kinder DL, et al. Intracranial resistive index (RI) values in normal term infants during the first day of life. Pediatr Radiol. 2000;30(9):618-620 ; and Raju TN, Zikos E. Regional cerebral blood velocity in infants. A real-time transcranial and fontanellar pulsed Doppler study. J Ultrasound Med. 1987;6(9):497-507.
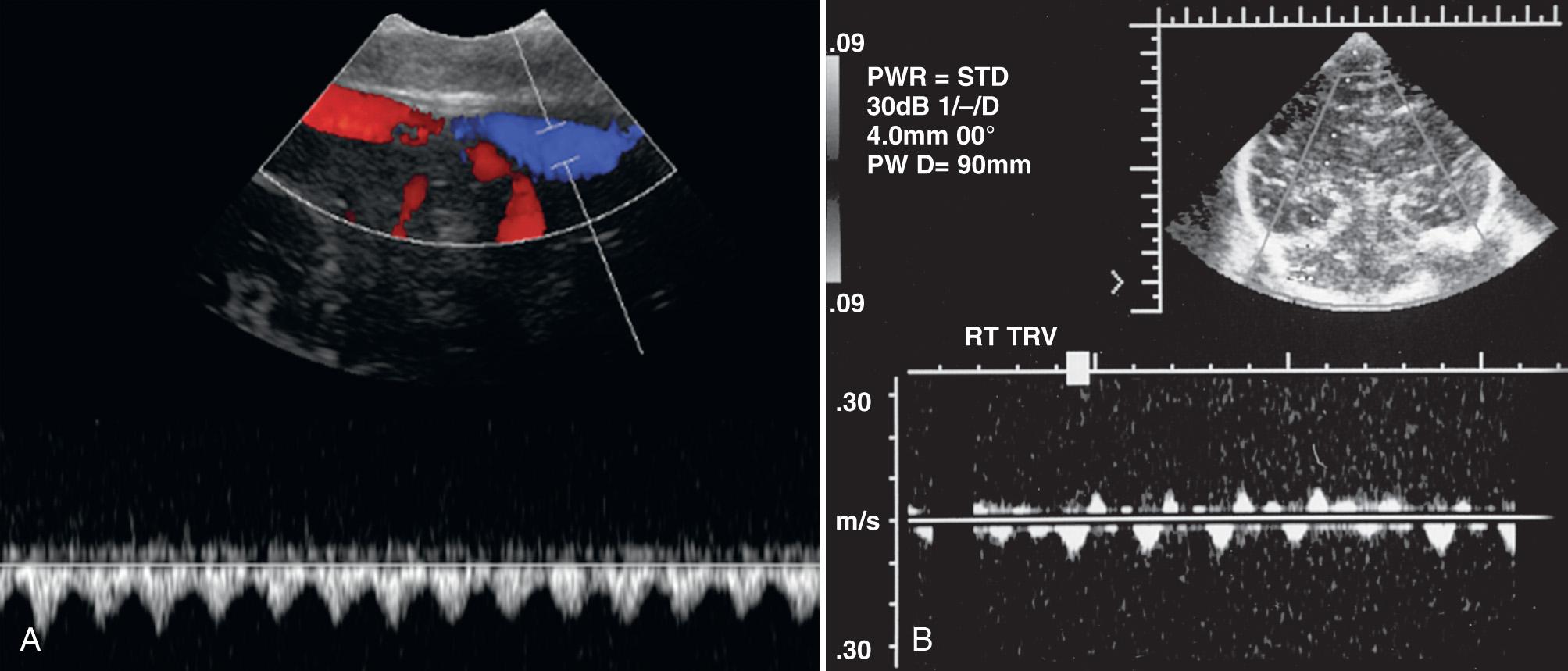
Venous blood flow is continuous in the intracerebral veins and dural sinuses. Cardiac pulsations of variable amplitude may occasionally be propagated into the larger venous structures, such as the vein of Galen and the sagittal and transverse sinuses ( Fig. 46.4 ). High-amplitude or sawtooth patterns of pulsatility are not normal and may be seen in infants with elevated right-sided heart pressures or tricuspid regurgitation. Although respiratory changes are not usually observed during normal quiet respiration, marked changes in velocity can occur during forceful crying or are seen secondary to high-frequency ventilation settings. Table 46.3 provides ranges for mean blood flow velocities in several intracranial veins and sinuses.
| Vessel | Mean TAV a (cm/sec) |
|---|---|
| Terminal veins | 3.0 ± 0.3 |
| Internal cerebral veins | 3.3 ± 0.3 |
| Vein of Galen | 4.3 ± 0.7 |
| Straight sinus | 5.9 ± 1.0 |
| Superior sagittal sinus | 9.2 ± 1.1 |
| Inferior sagittal sinus | 3.5 ± 0.3 |
a Time-averaged mean blood flow velocity (± standard error of the mean). Values modified from Taylor GA. Intracranial venous system in the newborn: evaluation of normal anatomy and flow characteristics with color Doppler US. Radiology. 1992;183(2):449-452.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here