Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors would like to acknowledge the contributions of Drs. Marybeth Ezaki and Peter Carter.
Very few congenital anomalies of the upper limb can be restored to normal function and appearance. The goals of treatment are therefore to maximize functional potential, preserve sensation, and minimize scarring. The surgeon should offer support, first to the family and later to the child, to help them cope with the impact of knowing that even with today’s medical technology, the limb cannot be restored to normal. Surgical intervention should be undertaken only when the intended procedure is to be done for the child, not merely to the child, and when the goals are clearly understood by the family and, when possible, the child.
Some surgical indications for common congenital conditions are straightforward, and the literature detailing the techniques is abundant. Surgery for other, less common conditions requires careful, sometimes creative preoperative planning and the ability to change these plans intraoperatively in an acceptable fashion if aberrant anatomy precludes a beneficial outcome.
In general, the goal of surgical treatment of congenital upper limb anomalies is to position the hands so that bimanual activity within the field of vision is possible. Preservation of joint motion, when possible, is desirable to allow the greatest reach of the limbs, especially with regard to reaching the face and perineum. Because hand function requires intact sensation, scars should be placed carefully and peripheral nerves protected throughout the limb. Position, motion, and strength for grasp and release are the goals of surgery on the nondominant hand, which will stabilize objects for manipulation by the dominant hand. Position, motion, and strength for pinch and fine motor function are the goals of surgery on the dominant hand.
The upper limb bud appears along the crest of Wolf ( Fig. 12.1 ) at approximately 23 days of gestation. Mesodermal proliferation within the limb bud depends on a critical vascular structure that grows with the limb and supplies nutrients and oxygenation. The limb bud is innervated from its inception by nerves derived from the neuroectoderm. The leading edge of the limb bud ectoderm is a thickened ridge called the apical ectodermal ridge (AER) ( Fig. 12.2 ). It functions as an advancing mobile command center and contains the “architectural blueprint” for the limb’s structure. The AER is programmed to allow sequential transcription of crucial segments of DNA. The time-linked sequence of genetic transcription directed by the AER controls the proximodistal differentiation of limb structures. A number of fibroblast growth factors are involved in maintaining outward growth. Genetically encoded limb anomalies are “blueprint” variations and are built into the limb construction process.


Differentiation of the underlying mesodermal substrate occurs through the interplay of concentration gradients of growth factors and cellular mediators. On the caudal edge of the limb bud, a zone of polarizing activity ( Fig. 12.3 ) has been identified as being crucial to the craniocaudal orientation of the limb. The Hox gene family plays a role in this radioulnar (or tibiofibular) orientation of the limb. The WNT7 group of genes plays a role in dorsoventral patterning. The increasing number of clinically recognized conditions that can be mapped to specific gene mutations is contributing to a better understanding of the control of the three axes of limb development.

Vascular ingrowth supplying the advancing progress zone ( Fig. 12.4 ) of undifferentiated mesodermal cells is critical to the development of limbs of normal length and size. Disruption of this vascular support limits the amount of mesodermal substrate and results in the spectrum of transverse limb deficiencies known as symbrachydactyly ( Fig. 12.5 ).


The final act of the AER is programmed self-destruction through a gene that codes for an endonuclease. This gene triggers a process known as apoptosis ( Fig. 12.6 ), or programmed cell death. Dissolution of the interdigital webbing occurs at the end of the embryonic period, at approximately 56 days of gestation. The limbs continue to grow during the fetal period.

A requirement for normal prenatal development is a protected uterine environment. Congenital limb abnormalities may also be due to teratologic, deforming, or disruptive influences on development. Teratogenic agents may affect development at the genetic transcription stage or may interfere with posttranscription growth factors. Physical forces may cause deformation of the growing embryo or fetus or disruption of normal development; an example of the latter is the amnion disruption sequence (amniotic band syndrome; Fig. 12.7 ).

Associated anomalies correlating with the timing of development of other organ systems may occur in a child with an upper limb abnormality. Recognized associations include anomalies of the heart, thoracic contents, spine, and kidneys, all of which develop concomitantly with the upper limb. Known hereditary associations also include hematopoietic and gastrointestinal conditions and lower limb anomalies ( Fig. 12.8 ).

The history taking begins with asking the parents to describe the child’s problem. This description may bring into focus a more specific inquiry. The expanded description of the problem should include any functional limitations in age-appropriate activities and—elicited by gentle inquiry—the emotional status of the family and child in dealing with the deformity. An offer of occupational therapy to assist in activities of daily living (ADLs) and referral to support networks of families with similar children or to psychological counseling can be made at this juncture.
Questions about the pregnancy should elicit information about previous pregnancies and miscarriages (a clue to possible genetic conditions); illnesses and exposure to disease, chemicals, radiation, or drugs during the pregnancy; difficulties with the pregnancy, such as leakage of amniotic fluid or premature labor; and any antenatal testing such as amniocentesis or chorionic villus sampling and the reason for the testing. Information elicited about the delivery includes the infant’s gestational age, birth weight, Apgar scores, condition at delivery, and anything abnormal about the placenta or umbilical cord. (A single umbilical artery is abnormal and is one of the abnormalities in the VACTERL association [vertebral abnormalities, anal atresia, cardiac abnormalities, tracheoesophageal fistula and/or esophageal atresia, renal agenesis and dysplasia, and limb defects; see Box 12.4 ].)
V ertebral
A nal
C ardiopulmonary
T racheo E sophageal
R enal
L imb
Information to be obtained about the newborn period includes any extended hospital stay, diagnostic tests ortreatment required, bleeding problems or the need for blood transfusions, nutritional status, and attainment of appropriate developmental milestones.
The pertinent family history for a child with a limb abnormality includes questions about similar anomalies in the families of both parents.
The physical examination is focused by the complaint but includes inspection of the entire child. The examiner should pay special attention to overall muscle tone while handling the child. A floppy infant may be morphologically normal but developmentally delayed. An appreciation of the appropriate reflexes according to the age of the child helps the examiner detect central nervous system dysfunction. Craniofacial features such as premature closure of sutures, abnormal head shape, facial asymmetry, crumpled or abnormal ears, and micrognathia suggest syndromes associated with limb abnormalities. As the observer becomes more familiar with the dysmorphic features, other facial features characteristic of syndromes will be recognized ( Fig. 12.9 ).

The neck and chest should be palpated for symmetry and integrity of the clavicle and ribs, the presence and bulk of the pectoralis and latissimus musculature, and symmetry of the breast tissue. The lower limbs are checked for stability of the hips, length, and symmetry, and any deformity is noted.
The spinal examination includes a check of alignment and range of motion (ROM) of the neck and palpation of the dorsal spinal elements. Any cutaneous manifestation of spinal dysraphism, such as a hairy patch or dimple, should be investigated ( Fig. 12.10 ). In the infant this is easily done utilizing a spinal ultrasound.

Examination of the limbs includes observation and documentation of all pertinent findings. The limbs can be inspected before the examiner evaluates the active and passive ROM of all joints. Any asymmetry in size should be noted. Motor examination includes observation of active ROM, palpation of muscle bulk, assessment of any musculotendinous contractures or spasticity, and in older children, evaluation of strength and measurement of strength grades. Any anomalies should be noted in anatomic descriptive terms and by classified or named conditions if the diagnosis is clear. Pejorative terms such as lobster claw or clubhand should be avoided in favor of simpler descriptive terms such as cleft, deviated, bowed, or angled .
Assessment of sensation may be inferred from the texture, temperature, presence of sweat and papillary ridges, and integration of the limb or digit into function. Discrete sensory testing is impossible in a young child but usually possible in older children. Asymmetry in the size of limbs or parts of limbs may be a clue to asymmetric neural innervation of the limb, vascular or neural malformation, or a possible underlying tumor.
In the case of anomalous formation of limb structures, the examination should focus on both the appearance and function of the limb. Observation of the child while using the limb in developmentally appropriate activities is important. A description of the functional limitations, such as an inability to touch the mouth with the hands or to bring the feet into a plantigrade position, should be included in the record to provide a clearer picture of the abnormality.
The examination of the child may need to be repeated to obtain a clear picture of the problem and the potential treatment. Establishing rapport with an older child is important in formulating a plan for treatment and may take more than one visit. Having several small, washable, nonlatex toys available for the child to play with is helpful for observing active ROM of the upper limbs and fine motor dexterity ( Fig. 12.11 ). White coats tend to provoke conditioned negative behavior and thus make examination of the child more difficult.

Examining a young child is very different from examining an older child or adult. Tricks of the trade include using toys, decoys, diversions, and patience.
Rational timing of hand procedures in children depends on the parent, the child, the procedure, the surgeon, the hand itself, and the anesthesiologist. All must be ready for the procedure to achieve the best result. A logical approach makes decision making easier for the surgeon and parent and leads to a better result for the patient.
Especially in children with a congenital deformity, the attitude of the parents must be one of cooperation. Parents need to work through a bereavement process by mourning loss of the “perfect baby” that they had anticipated before birth. The time required for family members to experience this bereavement is variable. The grief process may lead to unrealistic expectations of the surgical procedure and the surgeon. The parent and the surgeon must be patient and allow grieving to take its course. This is one reason why all but the most trivial reconstructive surgical procedures on a child’s hand are best deferred until after the first 6 months of life. Bradbury lists three stages of the grieving process.
In the denial phase, the parent tends to minimize the impact of the deficit: “There’s nothing my child can’t do!”
This phase is important for the surgeon to recognize in the parent because the anger may be directed toward the surgeon or may focus on the obstetrician’s failure to make a prenatal diagnosis. With a diagnosis such as Erb palsy, the parent may be unable to get past the assignment of blame. Before reconstructive surgery the parent must understand that the only real solution is not “what if” but “what now.” Before this the parent is not capable of understanding informed consent.
In the distress phase parents experience feelings of guilt, anxiety about future pregnancies, and loss of control. It may be helpful to discuss with the parents the time of fetal development when the deformity occurred. Genetic counseling for the parents may be useful.
At times, parents may seek to abdicate their responsibility for decisions until the child is an adult and can decide for himself or herself. This is inappropriate and should be strongly discouraged.
Two considerations related to the child’s maturity level are important: cooperation and self-awareness.
Children’s capacity to participate in a helpful way in their care varies with age. We have found Bradbury’s “cooperation milestones” to be clinically useful ( Table 12.1 ). They help the surgeon make reasonable decisions regarding the timing of treatment.
| Age | Milestones |
|---|---|
| Infants (<6 mo) |
Accept strange adults Accept splinting with encouragement Less accepting of passive stretching Best comforted by parents |
| Babies (6–18 mo) |
Anxious with strangers Easily distracted and reassured Tolerate splinting less well than infants do Passive stretching more effective |
| Toddlers (18 mo to 4 yr) |
Very resistant to control and restrictions Short attention span |
| Children (4–7 yr) |
Understand the rules Flexible in thinking Conjure up strange ideas about surgery (e.g., fear of waking up) |
| Children (7–12 yr) |
Logical in their thinking Compliant Can make meaningful decisions Still dependent on parents |
| Adolescents | Fragile body image Very aware of their appearance Socially dependent on peers Fearful of death (anesthesia) Distrustful of adults |
Another consideration is the child’s self-awareness, or when the child with a deformity learns “I’m different.” Although the child may notice a difference in the deformed hand by 3 years of age or earlier, self-consciousness rarely develops before approximately 5 years of age, when the child begins to interact with people outside the family. The deformity poses great emotional stress for the child at two important times. The first is between 5 and 7 years and the second is in early adolescence. Adolescent boys, more so than girls, seem to be particularly prone to acute self-consciousness, perhaps because of their comparative inability to talk about their deformity with their friends.
It is important for surgeons treating these children to have appropriate interest, training, and experience. Dealing with the child, the family, and the tediousness of the operations and dressings demands a special commitment of time and patience to achieve optimal results. The first surgeon who operates on the child’s hand has the best chance of achieving a good-quality result. Even small gains in function may be important for use of the hand. The hand has many emotional and physical differences from the lower extremity. Although the hand does not bear weight, it needs stability, power, and at the same time, precision, flexible movement, and high-quality sensation. The hand also has unique importance cosmetically and is never covered by clothing. Because of these considerations, pediatric hand surgery is usually best done by orthopaedic or plastic surgeons with a special interest in children’s hand deformities. Recently, special training in pediatric hand surgery has become available at some centers. Performance of hand operations is never an emergency (with the rare exception of neonatal compartment syndrome or a congenital band causing vascular compromise). As a rule, within reason, later tends to be better.
Two unique characteristics of a child’s hand direct the appropriate timing of surgical intervention: growth of the hand and the risk for a disproportionately thick scar created in infants’ skin at the site of hand incisions.
The hand increases in size 10-fold between the time that it is fully formed, at 8 weeks of gestation, and birth. After birth, the hand doubles in size nearly twice before reaching adult size. The first doubling occurs early in childhood, at approximately 2 to 3 years, and delaying surgical correction until 2 to 3 years of age allows precise execution of a surgical plan ( Table 12.2 ).
| Age | Length |
|---|---|
| Fetal age to 8 wk | 5 mm |
| Birth (fetal age to 40 wk) | 60 mm |
| 30 yr | 120 mm |
| Adult (full size) | 200 mm |
Fetal skin has the remarkable characteristic of healing without scarring, a property that only bone maintains into adult life. Soon after birth, however, the infant skin may make an unexpectedly robust, hypertrophic scar at the site of surgical incisions. This is in contrast to the more favorable nature of deep scarring around the tendons and joints of older children. This superficial hypertrophy of the scar in the skin incision is particularly a problem for the hand surgeon because the hand’s great mobility demands absence of scar in areas of the skin that change length with hand motion. Areas that are especially important are the palmar surface of the digits and the webs ( Fig. 12.12 ). Inaccurate positioning of incisions, especially in these areas, is a common cause of “web creep” after syndactyly. The poor results in children from “tiny Z-plasties” or any small flap are usually related to inappropriate scar placement in the skin of a young child.
![FIG. 12.12, Precision design of digital skin incisions is even more critical in a child’s hand than in an adult’s because of scarring in the hands of small children. The figure illustrates the fundamental principles for the correct design of these incisions. Note that the incisions are based on the axis of rotation of the interphalangeal joints, as shown in the lateral-view illustration (A). The oblique view (B) shows the three basic interaxial lines (longitudinal [L] , transverse [T] , oblique [O] ) from which all correct incisions are derived. The palmar view (C) shows the three digital diamonds containing skin that changes length with finger motion. Any longitudinal scar located within these diamonds will hypertrophy and contract. DIP , Distal interphalangeal; PIP , proximal interphalangeal. (D) In full digital flexion, points 1, 2, 3, and 4 touch, as do the side limbs of the diamonds, to maintain length. FIG. 12.12, Precision design of digital skin incisions is even more critical in a child’s hand than in an adult’s because of scarring in the hands of small children. The figure illustrates the fundamental principles for the correct design of these incisions. Note that the incisions are based on the axis of rotation of the interphalangeal joints, as shown in the lateral-view illustration (A). The oblique view (B) shows the three basic interaxial lines (longitudinal [L] , transverse [T] , oblique [O] ) from which all correct incisions are derived. The palmar view (C) shows the three digital diamonds containing skin that changes length with finger motion. Any longitudinal scar located within these diamonds will hypertrophy and contract. DIP , Distal interphalangeal; PIP , proximal interphalangeal. (D) In full digital flexion, points 1, 2, 3, and 4 touch, as do the side limbs of the diamonds, to maintain length.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/DisordersoftheUpperExtremity/11_3s20B9780323567695000120.jpg)
Finally, because of the elective nature of the surgery and the nonfatal aspect of these conditions, an unexpected anesthetic catastrophe is especially shocking when it occurs during reconstruction of a child’s hand. An anesthesiologist trained in the care of children and whose practice is mostly pediatrics is optimal for hand reconstruction because the surgical procedure itself carries almost no risk for death. The operating room in which the procedure is done should be equipped with appropriate instruments for children. Most anesthesiologists agree that non–life- or limb-threatening conditions requiring operations under general anesthesia are best delayed until after 6 months of age—and even later in children born prematurely, whose lungs may be less mature than would be expected by chronologic age. This is especially true as recently there has been controversy regarding the potential effects of early anesthesia on the developing brain.
We have developed certain guidelines for scheduling surgery on the hand in children. These are “soft” guidelines, and it must be remembered that timing also varies with the experience of the surgeon and anesthesiologist and the emotional readiness of the parent. In addition, children with hand anomalies often have serious visceral and systemic problems that take precedence over treatment of the hand anomalies (e.g., cardiac, renal, hematologic, spinal, and tracheoesophageal problems). Once these problems are attended to, accurate execution of the surgical procedure on the hand must be balanced against giving the child the best opportunity to begin using the surgically altered hand. Finally, to some degree, pressure from parents may speed up or delay the time of surgery. Acknowledging all of this, Box 12.1 shows a few guidelines that we have found useful.
Floating polydactyly: The floating digit is usually ulnar (postaxial) and dangling from a narrow skin and vascular tether. Ligation is well accepted by the mother, who has recently seen the umbilical cord desiccate, just as the polydactylous digit will. If ligation–amputation is not done, as the child gets older, the family or patient may develop a morbid attachment to the useless part, which may complicate the best plans for reconstruction.
Strangulating amniotic bands: Bands causing obvious vascular compromise that need to be released to maintain viability (or more often amputation of a nonviable part) are rare. Z-plasty correction of the clefts formed by the band in these small hands should wait until the child is larger.
Border syndactyly: Especially the thumb–index web and the ring–small finger web, which cause serious loss of function of the thumb and tethering of the ring or index finger.
Complex osseous syndactylies: Especially those with transversely positioned bones, which progressively deform the hand as it grows. Some cases of central polydactyly may be optimally treated now.
Apert polysyndactyly: Because of the number of operative procedures required in these children, we like to start early and often perform them bilaterally with two teams of surgeons.
Macrodactyly needing amputation: Though rare, massive macrodactyly may be present at such an early age that amputation is the only reasonable course of action.
Broad-based polydactyly: Cases that could not be treated safely before 6 months of age with ligation under local anesthesia.
Thumb duplications: Here, the skill of the surgeon may alter the timing of reconstruction of a duplicated thumb. Preaxial thumb polydactyly is unique in that reconstruction is almost never a simple amputation, and careful consideration of ligament reconstruction is critical for long-term joint stability and alignment.
Simple or cutaneous syndactyly involving the long and ring fingers or the long and index fingers: By 12–18 months the hand is much larger than an infant’s hand, and precise construction of flaps and skin grafts leads to a more predictable result. Even greater delay may be appropriate in a child with an understanding parent.
Pollicization: We prefer the age of 18–24 months to allow technical precision in the execution of this multistep, exacting procedure.
Angular deformities requiring osteotomy and fixation: The larger size of the bone allows more accurate and effective use of Kirschner wires.
Thumb–index web reconstructions: Thumb–index web reconstruction affords the most important opportunity to improve hand function. We do these operations earlier if bony connections are present in the web and do them later when only cutaneous restraint is present.
Hypoplastic thumbs, hands with ulnar dysplasia, and complex polysyndactyly are frequently treated at this time.
The dressings applied after an operation are of special importance in hand surgery, for which uncomplicated wound healing usually makes the difference between a good result and a disaster. This is particularly important in operating on the hands of children. The combination of a child’s wiggly hand and the small flaps and skin grafts that cannot tolerate motion is a recipe for disaster during the postoperative period. Small children seem to do their best, even if unconsciously, to thwart a successful outcome. Therefore, the objective of the hand dressing is primarily to protect children from themselves. Preventing problems is by far the favored strategy in hand surgery. The only reasonable means of preventing problems is with a carefully applied, rigid dressing, previously made of plaster but now usually made of fiberglass or semirigid casting tape.
What follows is a method that we have developed over the years for treating younger children. In children younger than 8 or 9 years or who are not likely to understand the importance of leaving the dressing on, the following method has worked well for us.
The anesthesiologist must keep the child completely still during application of the entire dressing because movement by the child almost always prevents application of a well-fitted dressing without pressure points.
Short-arm casts are rarely used in young children. In children younger than 8 or 9 years, we rarely use anything but a long-arm cast.
After operations on the fingers or palm of a small child, we also use a safety cast or mitten cast. This cast covers the fingers (which have been loosely wrapped with gauze and cotton padding) entirely in a loose-fitting shell to prevent the child from getting to the fingers and to keep unwanted food, toys, and other items out. The surgeon must be certain that vascular compromise is not present before final application of the “finger cage” because continuous direct observation is sacrificed for protected wound healing.
Of course, when the surgeon is concerned for any reason (if the child has persistent or unusual hand pain, an unexplained fever, or a foul odor emanating from the hand), the dressing should be removed and the wound inspected. In a small or uncooperative child, this may best be done under general anesthesia so that another dressing can be applied carefully.
Changing the dressing before primary healing in 3 to 4 weeks is rarely necessary when each step of the dressing application is done with meticulous care. Leaving the responsibility of dressing application to the most junior or inexperienced member of the operating team can destroy the best efforts of the operating surgeon. Similarly, changing the cast for no reason before healing may severely complicate such operations as tendon repairs and skin grafts. Unless applied under general anesthesia, a subsequent new dressing on a small, uncooperative child rarely fits as well as the one applied initially.
The Texas Scottish Rite Hospital hand dressing application protocol has several steps, which are illustrated in Figs. 12.13 to 12.22 :
Wound closure
Primary dressing
Gentle compression components
Padding and skin protection
Rigid outer shell

We prefer to use fine absorbable sutures (6-0 plain gut) dyed blue with the marking pencil for easy visualization (see Fig. 12.13 ). This obviates later suture removal, saves time, and reduces anxiety. These fine absorbable sutures are weak and may be supplemented with wound closure strips cut short. The wound must be protected with the rigid dressing described later.
The primary dressing goes directly on the wound. We prefer a single layer of fine mesh gauze treated with petroleum jelly and antibiotic (see Fig. 12.14 ) . Use of petroleum jelly-treated gauze minimizes sticking of the dressing to the wound, and if a single layer is used, no maceration of skin will occur because blood from the wound can easily pass into the dressing. This important drainage of blood into the dressing is facilitated by the application of a saline-soaked dressing sponge of appropriate size (see Fig. 12.15 ) . The well-moistened gauze also contours well to the complex shape of the hand and digits.


This primary dressing is altered when a skin graft is present by applying the Xeroform in a single sheet combined with dry gauze just covering the wound. This dressing is then secured with paper tape (Steri-Strips) and, once secured, is moistened with saline. We call this the “Steri-Strip stent” and have found it most useful for securing the skin graft, as is required, for example, in reconstruction of syndactyly (see Fig. 12.16 ).

This layer holds the primary dressing in place and is carefully applied with just enough compression to accomplish this aim, but not so much that the circulation is restricted. Applying this layer requires some practice and is critical to success. Illustrating the technique of applying just the correct amount of compression is difficult.
The first layer of the compression components consists of fluffed gauze formed from single-dressing gauze opened up and shaped into cones. These cones are gently placed between the fingers to prevent maceration, but they are not forced into the webs, which would restrict the circulation (see Fig. 12.17 ) . The fluffs are secured with the appropriate-size roller gauze (e.g., Conform, Kling) in 2- or 3-inch widths (see Fig. 12.18 ). Although gentle compression is required to make this first compression layer fit the hand well, all tightness around the forearm or wrist and circumferential dressings around a finger must be avoided. After proper application of this layer, the hand should be secured in the proper position of slight wrist extension and thumb abduction. (The exception is after flexor tendon reconstruction, in which case some wrist flexion is required to protect the tendon repair.)


Next, a layer of cotton cast padding (Webril) is applied to protect the skin and bony prominence and make removal of the rigid shell easier (see Fig. 12.19 ). Plaster or fiberglass sticks to gauze dressing and is more difficult and time-consuming to remove later. Orthopaedic felt applied to the upper portions helps protect the skin from the very abrasive fiberglass (see Fig. 12.20 ) .


The tourniquet must now be deflated, before the next stage, to verify unobstructed and unequivocal digital circulation. If the circulation is in doubt, it must be restored, which may occasionally require removal of the dressing. The hand should now be kept elevated because during the next few minutes, during posttourniquet hyperemia, arm volume will be equilibrating before application of the rigid outer shell.
This important layer has two functions. The first is to hold the primary dressing in the position applied, and the second is to function as armor plating to protect the wound. Plaster-of-Paris casts have worked well in the past and have the advantage that they can be molded accurately. However, fiberglass is lighter and more durable and simplifies cleanup. We have found that a new, softer, semirigid fiberglass (Softcast) has the advantage that it can be removed weeks later by simply unwrapping it. Softcast obviates use of a noisy cast saw, which is especially frightening to children and parents when used around the hand (see Fig. 12.20 ).
Next, in small children the digits are first well padded and covered with cast padding completely enclosed by a fiberglass mitten. Some active digital movement by the child under the fiberglass is possible (see Fig. 12.21 ). A 2-inch sling of stockinette can be fashioned and put around the child’s chest or neck as indicated (see Fig. 12.22 ). It should be removed for sleep. Alternatively, a larger sock can be used to cover the cast and can be safety-pinned to the shirt for support.


Hand therapy in pediatrics is both similar to and at the same time very different from hand therapy in adults. In children, the therapist treats not only the patient but the immediate family and often the extended family as well. This patient–family unit varies tremendously in its ability to comprehend and comply with treatment protocols. The members of this complex unit often carry a special psychological burden associated with a congenital anomaly, which can ultimately affect the child’s self-esteem and body image ( Fig. 12.23 ). The hands of children are also different in several ways from those of adults. Although tendons, nerves, and bones all repair more rapidly in children, well-worn aphorisms such as “get them moving” are replaced in pediatric patients with “slow them down.” Early-motion protocols are difficult or unreasonable in most pediatric treatment situations. In addition, growth of the hand and limb, a consideration totally lacking in adults, is of paramount importance in children.

The goal of therapy in children is to guide the patient toward maximum functional independence. However, in severely disabled children, this may still include lifelong dependence on assistive devices or alternative methods of accomplishing even basic ADLs.
This section focuses on the aspects of hand therapy unique to pediatric patients, with special attention directed to congenital anomalies. Such aspects include ADLs, splinting after injury or surgery, and support and guidance for long-term functional independence.
Children with limb deficiencies develop remarkable ways to substitute for missing parts. ADLs can include bathing, dressing, eating, and perineal care, as well as school activities such as writing, cutting, computer use, or even carrying books or a lunch tray ( Fig. 12.24 ). ADLs can also include extracurricular activities such as sports, music, art, and dance. These extracurricular activities can be a very important part of promoting self-esteem and self-confidence. Guiding each child toward independence—or allowing participation in an activity through the use of assistive devices or alternative methods and techniques—is of utmost importance ( Fig. 12.25 ).


When determining which piece of equipment or alternative technique is most appropriate for each child, it is imperative that the therapist first look at available function by assessing ROM, strength, sensation, and dexterity. An example of this therapeutic approach can be seen in Fig. 12.26A and B with a small child who has limited grip strength and is unable to manipulate heavy eating utensils and may be better off using a plastic spoon/fork or a wraparound utensil (see Fig. 12.26 ) that requires no grip strength at all. In addition, to promote participation in a specific activity, it is necessary for the child to be interested in the task and feel a sense of accomplishment once it is attempted or completed. Coaching the parents to teach and encourage their child will reinforce the child’s functional independence, which in turn will enhance self-esteem and confidence. It is also important to remind parents and other caregivers that a child with a congenital hand difference may complete activities very differently from another child or adult with a five-digit hand, but how it looks matters little if the task can be attempted or accomplished ( Fig. 12.27A ). Kids can also learn from other peers or older teens/adults who have the same hand difference (see Fig. 12.27B ). This opportunity can be provided at hand camps and/or hand support groups in their local area.


Proper splinting of the pediatric hand and upper extremity is an acquired skill that requires a working knowledge of anatomy, an understanding of the condition being treated, attention to detail, considerable practice, and an unusual degree of patience. Splinting in pediatrics also requires the ability to work quickly, as well as to develop an effective method for holding a desired hand position as the splint material cools. Distraction is an important tool in pediatric splint making ( Fig. 12.28 ). Parents can be coached on how to help distract the child during the fabrication process to prevent unwanted movement and crying.

Therapists splint for a variety of reasons, including: to increase function, to protect or rest a joint, to increase ROM, to decrease pain, and to stabilize. It is important for the therapist to understand why a splint is needed and what function it will serve before the fabrication process begins. A comprehensive physician’s note or, at times, a verbal communication with the surgeon, coupled with an in-depth conversation with the child and parent, will help determine the expected function of the splint. For splints used in the postoperative period, an operative note should be available to the therapist, and it is preferable that the surgeon discuss in detail his or her objectives and concerns directly with the therapist who will fabricate the splint. Several manuals of splinting detail the fabrication of standard splints, to which the reader is referred.
Early splints were fabricated from plaster of Paris or metal. The first thermoplastic hand-splinting material to be introduced was Prenyl (Johnson & Johnson Medical New Brunswick, NJ) in 1964. It was soon followed by Aquaplast, Polyform, and Orthoplast (polymeric hydrocarbon). Since then, a number of other products have become available, some with properties that make them particularly useful to hand therapists caring for children. The therapist should take the time to practice carefully with any new material and learning the often subtle and unique characteristics and properties of the particular polymer. Such characteristics include drapability, softness, setup time, color, thickness, and memory (capacity for remolding). The best splints are ones that fit comfortably, accomplish their intended purpose, and are worn faithfully. Achieving these goals is more the result of the therapist’s skill and understanding of the chosen material than of which polymer is actually used. Complete proficiency in application of the material, close follow-up with each patient, and making adjustments as needed are the tenets of effective splinting.
When continuous immobilization is required, the splint may be secured with strapping material or Coban (3M, St. Paul, MN) over the straps at the palm, wrist, and elbow levels to secure the correct splint position. If the child removes the splint when unsupervised, additional measures may be required to ensure consistent wearing patterns. Such measures may include placing a sock over the top of the splint and securing it with a safety pin at the shoulder ( Fig. 12.29 ) or using a lace-up method. The wrist strap may also be split along most of its length so that one tail can go around the wrist and the other across the hand and through the thumb web space to secure the hand in the splint ( Fig. 12.30A and B ) .


Children 4 months to 2 years of age are historically harder to splint. At this age, children have a short attention span and lack the ability to reason, but they have developed significant function in their hands. Therefore, use of splinting at this age is often difficult or impossible. If, however, a splint is required and a circumferential cast is not preferable, special precautions should be taken. Small splints at this age can become choking hazards, so detailed explanations and precautions must be given to caregivers. A splint that is fabricated larger than actually needed and that crosses several joints may be more reliably kept in place and is less likely to become a choking hazard ( Fig. 12.31 ). Also, being able to secure the splint as indicated previously can help prevent unwanted removal. Stretching can often maintain or increase ROM until a child is old enough to understand why a splint is necessary. This may not occur until school age (5 to 6 years).

Static splints are molded directly on the hand or limb to maintain one or more joints in a desired position. They are used to support healing joints and tissues after injury or surgery. These splints can also be used to maintain or improve ROM. Static splints are generally comfortable, well tolerated, and relatively easy to fabricate. Because a static splint does not have any moving parts, frequent remolding is often required because ROM and the degree of edema change. In small children, simple static splints are especially useful and often tolerated better by the child, as well as the caregiver ( Fig. 12.32A and B ).

This form of splinting is especially effective in adult patients but is rarely used in children. Dynamic splints consist of a static splint as the base with the addition of self-adjusting resilient components such as rubber bands, springs, or an elastic line to create a mobilizing force, which provides passive ROM. This feature can be used to increase passive ROM of a stiff joint, replace absent muscle function, or protect injured or repaired structures.
It is important to set the tension of the components carefully to achieve the desired ROM while maintaining proper healing. To be most effective, frequent readjustments are required because the resistance of tissue can fluctuate. Tension pulled too tightly can overstretch joints and tendons and lead to inflammation of tissues. Tension that is too loose is ineffective.
Splint forces can be difficult for some patients to control, which makes tolerance problematic and in some cases impossible. It is also imperative to take special precautions in patients with vascular insufficiency, thin tissue, an insensate hand, or edema.
Elastic components tend to become fatigued over time, thus decreasing their effectiveness on joints. This makes routine splint adjustments a priority to ensure correct fit and continued function of the splint. It is also important to assess whether the patient has adequate strength in the opposing direction to overcome the resistance and move through the desired ROM to obtain proper tissue healing, maintain ROM, and prevent adhesions. Dynamic splints are most useful in resolving contractures with a “soft” end-feel. Careful patient selection is important for effective use of dynamic splints, especially with pediatric patients. Extensive education and explanation are required for successful outcomes and compliance, and small children are rarely suitable candidates for wearing these complicated splints ( Fig. 12.33 ).

Serial casting is used most often to resolve contractures with a “hard” end-feel by statically positioning a joint near the end of its elastic range and maintaining that position for a time. Because constant stretch is achieved without any additional stress or force added until the cast is changed, this type of splinting is well tolerated. Casting and serial static splinting provide low-load, end-range positioning, which facilitates relaxation and lengthening of tissues. Pressure is distributed over a large surface area, which aids in comfort and vascular flow. Because it is difficult for the patient to remove the cast, compliance is typically very good. The splint or cast must be remolded or recasted frequently to accommodate increases in ROM. Clamshell splinting can be a good alternative if the child is not a good candidate for serial casting or lives far away and is unable to make weekly appointments for cast changes ( Fig. 12.34A and B ).

Static-progressive splinting involves a static splint base with inelastic components such as hook-and-loop tabs, progressive hinges, turnbuckles, or screws to position joints statically. Static-progressive splinting can be useful in resolving contractures with a hard or soft end-feel. These splints are usually well tolerated over long periods because they hold stretched tissue near the end of its elastic limit and do not stress beyond it. They provide low-load, end-range positioning, which has been shown to facilitate relaxation and lengthening of tissues.
Patients have the capability with a static-progressive splint to make their own incremental adjustments in tension to accommodate changes in ROM. This splinting technique is reserved for older and, in particular, very compliant patients.
Custom splints are fabricated by hand and are used for patients who require a special fit. These splints are frequently required for the small hands of children with congenital deformity. Custom splints can provide more support than prefabricated splints do and allow therapists to customize the fit to the individual patient. Custom splints provide exact joint alignment for improved healing and are used frequently following surgical repair ( Fig. 12.35A and B ) . Custom splints require skill, time, and a working knowledge of the materials used. Custom splints are more expensive than prefabricated splints because the cost of materials and labor needs to be factored into the cost of the splint. With each fabricated splint, it is important that the child and caregiver have a good understanding of the wear and care of the splint and the purpose behind its use. Allowing color choices for material and straps adds ownership, creates participation, and makes the process fun! Kids leave with a splint they helped design, which increases adherence to wear schedules and proper care of the splint ( Fig. 12.36A to C ) .


Prefabricated splints are made by a manufacturer, purchased through a catalog, and sit on the shelf ready for use. This allows quick fitting time, a material that can easily be washed, and in some cases, increased comfort. The ability to place the limb in a precise position is compromised with a prefabricated splint; however, most prefabricated splints have some room for adjustment and limited customization ( Fig. 12.37 ).

Occasionally it is preferable to fabricate a combination splint that allows the benefits of both splints. A combination splint can use a custom splint to hold the desired position (usually hand based), with a prefabricated splint placed over it for comfort and added protection ( Fig. 12.38 ). Or a prefabricated splint can be used to hold joints in place while fabricating a custom splint over the prefab splint ( Fig. 12.39A to C ) . This can be used with children who have increased tone and are difficult to splint across several joints at the same time. It is often quicker to fabricate a portion of a splint rather than an entire splint, and a combination splint can also be more comfortable, which increases compliance.


Splinting the fingers of children can be challenging. An injured finger can sometimes be splinted effectively by attaching it to an adjacent finger with a buddy strap ( Fig. 12.40A and B ) . A buddy strap also encourages ROM because the injured finger is attached to a stronger finger. A variety of types are available, although softer ones tend to be tolerated better by children. Tape or Coban can also be used for this purpose. Care must be taken to ensure that application of the strap or tape does not affect the circulation.

Silipos is a unique material that is very effective for scar management and available in a variety of sizes. It is a stretchy tube lined with a gel pad that is impregnated with mineral oil. The finger tubes come with a closed tip that provides a smooth contour for fingertip amputations. These tubes provide compression and allow full ROM ( Fig. 12.41 ).

The Finger-Hugger is used for proximal interphalangeal (PIP) and distal interphalangeal (DIP) joint extension ( Fig. 12.42A and B ). It comes with two inserts that slip into a pocket. One is more flexible to encourage extension but allows some motion. The second is a static aluminum insert with adjustable spring tension that provides gentle compression but is comfortable to wear because it better distributes the force across the dorsal aspect of the finger.

profile and has a light spring force, which allows longer wearing times and greater tolerance ( Fig. 12.43A ). The LMB flexion splint (see Fig. 12.43B and C ) may be used for swan-neck deformities and extension tightness.

Oval-8 ring splints may be used for swan-neck deformities. They are low profile, which makes them ideal if multiple splints are required on adjacent fingers ( Fig. 12.44A and B ). They can also allow the patient to see the potential benefit of surgical tenodesis or capsulodesis for more permanent correction of the deformity.

A Joe Cool splint ( Fig. 12.45A and B ) is used to position the thumb and is often used on children with cerebral palsy. It applies pressure on the index–thumb web space and places the thumb in a more functional position. Care must be taken to avoid hyperextension of the metacarpophalangeal (MCP) joint during wear.

A prefabricated short basic opponens (SBO) splint ( Fig. 12.46A and B ) can be used for moderate tone in the thumb or after surgery when rigid splinting is not required. Support of the thumb after surgery or trauma can decrease pain and increase function by providing support to soft tissue during periods of stress on the thumb in the performance of ADLs.

The most common wrist splints used in pediatrics are the wrist cock-up splint, the dorsal-blocking splint, and the long basic opponens splint.
The wrist cock-up splint ( Fig. 12.47 ) is used to block motion at the wrist. Care needs to be taken to ensure that the wrist is not in an ulnar-deviated position, especially in children with abnormal tone.

The dorsal-blocking splint ( Fig. 12.48 ) is used for flexor tendon injury or other types of injury to the flexor side of the forearm. Because the splint is placed on the dorsal side of the hand, a drapable material is most desirable.

The long basic opponens splint or thumb spica splint ( Fig. 12.49A and B ) is used after surgery on the thumb or after trauma. The thumb can sometimes be supported with a hand-based opponens splint if the wrist is uninjured and movement at the wrist level would not affect the healing tissues in the thumb.

The tone and positioning (TAP) splint is a pronation/supination splint that is effective for lack of range caused by weakness or deficient tone. The forearm strap can be changed if a deficit in both directions is present, thus avoiding the need for two separate splints. When pronation is needed for functional tasks, such as using a keyboard or holding/carrying objects that require two hands, the forearm wrap is started over the ulnar border of the forearm ( Fig. 12.50A and B ).

When a child is carrying objects that require a palm up position, such as a lunch tray at school, the hand needs to be in supination. The strap then needs only to be rewrapped over the radial border of the forearm. Because the neoprene is stretchable, the child can move against the splint. When the child relaxes, the splint returns the forearm to the desired position.
Splinting the elbow is a challenge in pediatrics because all elbow splints restrict motion. When moderate support is needed for soft tissue, an elbow sleeve ( Fig. 12.51 ) is well tolerated by children. However, when gains need to be made in flexion, a static elbow flexionsplint ( Fig. 12.52 ) may result in better compliance than a heavier hinged splint. Elbow extension is usually best addressed with a static extension splint worn at night ( Fig. 12.53A and B). When more aggressive elbow extension is needed serial casting works nicely over time to extend the elbow. The clinician must pick compliant patients and families, provide good cast instruction, and follow up weekly to change cast and monitor improvements ( Fig. 12.54A to C ).




It is vital for a therapist to receive a good, clear prescription from the referring physician. This may be the only method of communication available between physician and therapist, so the physician’s intentions and recommendations for each patient must be clearly stated on paper. A good prescription includes the full diagnosis (not a vague complaint such as “wrist pain”). It should also include any surgeries performed by the physician and which, if any, structures were repaired. The opportunity for the therapist to read an operative note before the initial evaluation with a patient can be very helpful.
Next, it is important for the surgeon to state specifically the exact requirements. If a splint is recommended, it is important to include the desired position, the wearing schedule, and approximately how long the patient should wear the splint. If exercises are recommended, the surgeon should indicate whether passive ROM, active ROM, active assisted ROM, strengthening, or some combination of these is required or need to be avoided.
It is also important for therapists to know from the start whether any precautions or limitations in ROM and in initiating strengthening exercises need to be taken into account. ADLs can also be addressed by the occupational therapist, so if this is a need, it can be added to the prescription as well. Since both OTs and PTs by profession can be hand therapists it is also important to indicate which discipline the physician would prefer or is even available to the patient.
Last, it is important to specify when the patient is to return to the physician, so a detailed progress note can be sent with the patient for the physician to read at the time of the visit. Economic pressure on health care has led to a trend to decrease therapy visits and encourage therapists to teach home exercise programs to patients. We need to be conscious of our patients’ funding and ability to pay and provide the best care with the given resources in the shortest amount of time. Patients need to be empowered to become, in a sense, their own therapist, not only to save resources but also to encourage participation in care. This direct participation gives the patient autonomy and, more important, pride in accomplishment and therapeutic gains—a great motivational tool. Ideally, the therapist is the teacher and the patient becomes responsible for the exercise program.
Whenever an incision is made in the skin, a scar forms as a normal part of wound healing. A scar may require up to 1 to 2 years to mature. Frequently, while the scar is maturing, tendons can become adherent to the scar. Such adhesions can prevent normal tendon gliding and result in decreased ROM. Through proper scar management, adhesions can be minimized. Once sutures are removed and the eschar has separated, scar management can begin safely. This can include scar massage and the use of a scar pad.
Scar massage is performed 4 to 6 times per day with a lubricating substance such as petroleum jelly or cocoa butter. The massage technique consists of rotating two fingers or the thumb over the scar in a clockwise and counterclockwise direction and exerting as much pressure as the patient can comfortably tolerate.
A scar pad is often used to help soften, flatten, and decrease redness in the scar, as well as decrease hypersensitivity. It performs best when held in place with a compressive wrap such as Coban to apply pressure on the scar for continued remodeling ( Fig. 12.55A to C ).

Elastomer inserts may also be used during splint application for compression of scar tissue through the application of firm pressure on the maturing scar. Many products are available, and the choice depends on the condition of the healing tissue.
Rolyan Elastomer and Otoform (hydroxyl-polydimethyl siloxane with fillers, auxiliaries; not vulcanized) are in a putty form that can be spread thin or formed to fit areas requiring bulk, such as the web areas ( Fig. 12.56A to F ) . Silicone or gel sheets are best used when a smooth, flat surface of compression is needed. These sheets are available in a variety of weights (thicknesses) and densities to achieve the desired result as appropriate for the healing tissue. Scar pads that are not used under a splint require some method of attachment to ensure uniform compression.
Coban, Tubigrip, and Medigrip are some effective materials for holding scar conformers in the desired position. The therapist needs to determine the best material to use for each individual patient to ensure the best possible outcome. Attaching a scar conformer to the finger is a challenge to therapists who treat children because their fingers are small and it is difficult to keep wraps in place. For this reason, the conformer may have to be attached with the use of a splint ( Fig. 12.57 ).

It has been said that the first surgeon who sees the injured hand most affects the final result. This is especially true in children. Accurate early diagnosis is more difficult in a child than in an adult because patient cooperation may be minimal or absent. The chance of missing significant injury is reduced when the surgeon and parent realize that a complete and accurate diagnosis may often require seeing the child more often than just in the emergency department. It is perfectly acceptable and in fact often crucial to insist that a follow-up examination be done a day or so later in the clinic or physician’s office, when less blood, less hysteria, and less distraction make a more complete and reliable examination possible. A second examination is particularly useful in cases of possible nerve injury, for a sensory examination can be done more reliably in the quiet of the physician’s office than in a hectic emergency department. Occasionally, even this is not sufficient, and when the surgeon cannot rule out a nerve or tendon injury, the wound may need to be explored under general anesthesia.
It is important to realize that almost no hand injury is a life-threatening emergency and that treatment should always be delayed until the anesthetic risk is minimized by a period of fasting and until a well-rested operating team with appropriate light, instruments, and training is available. Simple closure of the skin with delay is the treatment of choice until all these elements are in place. This is especially important in children with hand injuries because children eat constantly and are frequently difficult to anesthetize. Patience and a deliberate course of action will lead to the best outcomes.
As in other areas, the diagnosis is based on the history of the injury, observation, findings on physical examination, and radiographs. Only very rarely are more sophisticated examinations necessary. Careful and thoughtful observation of the injured hand by the surgeon is by far the most important factor in evaluating an uncooperative child. This observation must be made in the context of what has been called “topographic anticipation.”
The surgeon’s knowledge of the topographic anatomy of the injured site is used to anticipate the physical findings associated with probable injuries. By first asking the question “What structures are at risk?” the surgeon is much more likely to notice the critical findings associated with the injury. In most cases this assessment is best done by critically observing the rest position and use of the hand by the child. More information is gained by simply observing the child’s hand use from across the room than by wrestling the child from the mother’s arms. A lacerated flexor tendon is the prototypic example of the usefulness of topographic anticipation ( Fig. 12.58 ).

Alteration of the hand’s resting position in a child with a tendon laceration may be obvious or subtle (see Fig. 12.58 ). Furthermore, when wound exploration reveals laceration of a tendon sheath, it is very likely that the tendon itself has sustained injury. This is especially true if the finger was flexed at injury. If the wound is inspected with the finger in extension, the site of a partial tendon laceration will move distally in the tendon sheath and be hidden from view ( Fig. 12.59 ). During the first 5 to 7 days after injury, a partially injured tendon is especially vulnerable to failure. If a partial injury is suspected, the hand is splinted in a posture to protect the tendon from rupture. Some hand surgeons prefer to explore such injuries in the operating room under general anesthesia so that accurate assessment of the extent of the partial laceration and appropriate repair can be accomplished.

Surgical exploration and the technique of tendon repair in children are identical to exploration and repair in adults, except for the size of the structures involved. This topic has been covered thoroughly in other hand surgery texts and will not be discussed here. The complex six-strand tendon repair techniques described in the modern hand surgery literature have little place in these small-caliber structures. Magnification, appropriate instrument size, and careful technique can usually resolve most other differences in the technical aspects of tendon repair, and good outcomes can be expected after four-strand flexor tendon repair in children, , although extension lag and loss of flexion are common after extensor tendon repair in this population. The more critical challenges in children are immediate postoperative care to prevent rupture and, later, rehabilitation to develop gliding of the repaired tendon.
Postoperative immobilization should be complete and uninterrupted in a small child. Although successful results have been reported with age-adapted early mobilization, , our treatment regimen consists of an extra week or two of immobilization in an attempt to prevent rupture in young children and infants. Excessive immobilization of more than 4 weeks for flexor tendons or 6 weeks for extensor tendons is not warranted and in small children can lead to stiffness. On the other hand, changing the casts early just to “have a look” may reward the surgeon with rupture and should not be done unless infection or swelling leading to compromise is strongly suspected.
The type of rehabilitation possible in very young children is limited but becomes more like that in adults as the child’s cooperation level improves with age. In adolescence, compliance may sometimes be poor, but this is less of a problem if the teenager can be made to feel responsible and involved in the treatment program.
When a primary repair fails, we rarely repeat the attempt in a young, uncooperative child. If tendon repair is unsuccessful in a preschool child, surgery should wait to be repeated until the child’s hand is larger and the child is better able to understand and cooperate with the treatment program.
The diagnosis of a nerve injury is usually subtle and depends heavily on topographic anticipation. The surgeon is well served by expecting the worst instead of just hoping for the best. We have seen a toddler with a minor wound over the posteromedial aspect of the elbow weeks later with the first recognized sign of nerve injury: an anesthetic fingertip that was bitten off by the child. In addition to being very superficial in the hand, wrist, and elbow, the peripheral nerves virtually always run beside an artery. With a good history of arterial bleeding after a wound, one should carefully examine the patient for a nerve injury. Children with suspected nerve injuries, more than almost any patient, deserve a follow-up assessment in the relative quiet of the clinic or office setting because the diagnosis is easily missed the day of injury.
Absence of a sweat pattern of the anesthetic finger is a useful finding, and recognition of this finding is more often possible during a second examination. Absence of a sweat pattern is best identified by the dry or slick quality of the affected finger when the surgeon gently strokes the injured digit and uses a normal digit for comparison. Loss of intrinsic function may also be a subtle finding (especially in the median nerve) and is best noticed by comparing the resting position of the normal hand and thumb with that of the injured one. Again, topographic anticipation allows the subtle findings of nerve injury to be noticed. Finally, it is completely appropriate for a competent hand surgeon to explore the wound under anesthesia when the diagnosis is sufficiently suspected.
Fingertip injuries in children are very common. As a general rule, the less done for these injuries, the better. Even wounds with some exposed bone can heal with dressing changes alone if no tendon has been exposed. More extensive wounds may require full- or split-thickness grafts or local rotation flaps. Pedicle grafting in children is rarely indicated in the acute treatment of all but the most catastrophic hand wounds.
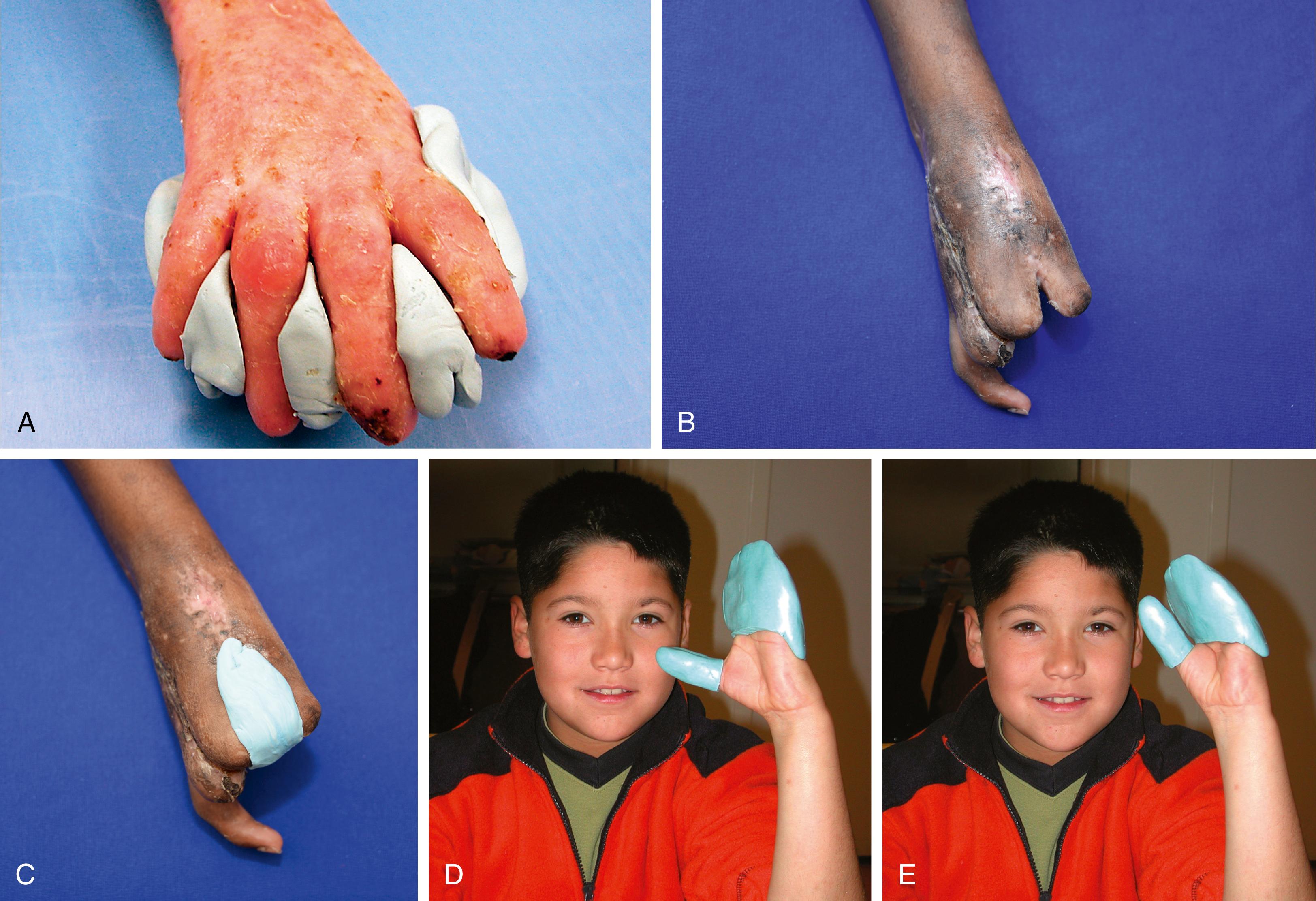
Burns are treated as in adults, with the surgeon remembering that the initial assessment frequently underestimates the severity of children’s burns. Child abuse may be manifested as burns; especially suspect are cigarette burns and bilateral hand and foot burns in a stocking-glove distribution. Treadmill burn injuries usually occur on the palmar surface of a young child’s hand and are treated by early Silvadene dressing changes, splinting, and antibiotics, with later aggressive scar management and mobilization.
Characteristically, children tend to burn their hands more often on the palms because they lack understanding of what is too hot to hold. Severe flexion contractures are frequent after these injuries. Fortunately, the palmar skin is thicker, and the deeper and important structures such as the nerves, tendons, and tendon sheath are usually unharmed. Even when the finger seems hopelessly contracted after palmar burns, reconstruction after the scar matures frequently yields a marked improvement in function. Careful release of the anterior cicatrix, while protecting the tendon sheath, tendons, and nerves, is followed by precision full-thickness skin grafting. First, a transverse incision that accurately joins the axis of rotation of the joints is made. This incision must carefully avoid the tendon sheath and nerves. With gentle dissection, the contracted cicatrix is removed, and as the finger extends, the linear incision is converted into diamond-shaped defects. The resulting defects are covered with carefully tailored, full-thickness skin grafts whose margins conform to the rules of hand surgery incisions ( Fig. 12.60 ). Frequently this approach can restore nearly complete movement of the finger and may greatly improve the function of the hand.

The great advantage of radiographs in diagnosing adult fractures is limited in children by the cartilaginous nature of much of a young child’s bone. The value of simultaneous comparison views of the injured side and the normal side cannot be overestimated, especially when the structures are placed side by side on the same radiographic plate. This simple and inexpensive detail can often provide more information than expensive computed tomography (CT) or magnetic resonance imaging (MRI) studies can.
A true lateral radiograph of the injured digit is important and may reveal a subtle but often significant injury. The technician should use the fingernail as a topographic landmark to obtain a true lateral view ( Fig. 12.61 ).

In general, most fractures in a child’s hand are treated nonoperatively because children’s hands have a remarkable ability to recover useful movement by remodeling fractures that would clearly need open reduction in an adult. Minimal displacements and malalignments heal better with closed treatment. Simply protecting the hand from use for 3 or 4 weeks is usually adequate. However, reduction of rotational malalignment is critical before immobilization.
Serious rotational malalignment, markedly displaced intraarticular fractures, and some displaced epiphyseal fractures may require early open reduction and internal fixation. Open reduction of a nascent malunion is especially difficult in the small bones of the hand when substantial fracture healing has taken place. Vigorous dissection around these small bones can occasionally result in devascularization and should be avoided. A more in-depth discussion of hand and finger fractures can be found in Chapter 29 .
Once healing has begun and the fracture site is nontender or callus is seen on the radiograph, a wise surgeon often delays operative treatment to avoid excessive cicatrix, loss of tendon gliding, or avascular fragments near joints. However, delayed surgical treatment of fractures of the small bones in the hand can lead to some of the most disastrous complications in hand surgery. Care of these complications may go on for months, only to yield a final result that is unsatisfactory to the surgeon, patient, and parent ( Fig. 12.62 ). It is often best to let fracture remodeling complete itself and then address the finger from a clinical rather than just a radiographic aspect. Later, after remodeling has taken place, an osteotomy can be done more safely in an area of the bone that is more likely to heal quickly and cause the least amount of tendon scarring. Even then, if the finger looks straight, has functional ROM, and is painless, aggressive treatment of the radiographic finding is not justified. The surgeon must remember that precise control of the small fragments of phalangeal osteotomies in young hands is not usually possible and that small malalignments are rarely improved, even by the most experienced surgeon.

Vascular trauma in the upper limb can result from direct vascular injury and lead to a nonviable distal extremity or can result from a compartment syndrome caused by pressure in an unyielding fascial compartment. Complete loss of distal circulation should be addressed by a vascular surgeon and is not discussed here. Repair of smaller distal vessels in a nonviable hand or digits is discussed at the end of this chapter in the section “Microsurgery.”
Forearm compartment syndrome has been the dread of pediatric orthopaedic surgeons since Volkmann first described the condition in 1881. The clinical setting for compartment syndrome rarely involves massive open trauma because in these cases the fascial covering is disrupted enough to prevent secondary ischemic muscle death. Instead, the condition is all too often unexpected and, despite aggressive early fasciotomy, may not be preventable.
The classic clinical signs of pain, pallor, paralysis, pulselessness, and paresthesia are not reliable in young children. Increasing need for pain medication is the most sensitive indicator of compartment syndrome. Although a supracondylar fracture of the humerus is the classic association, both-bone forearm fractures, blunt trauma, and even extravasation of blood or fluids may be the inciting cause. Pain on passive digital extension that is referred to the proximal part of the forearm may be especially suggestive.
Although compartment pressure measurements have been popular in the literature, they are time-consuming and occasionally falsely negative. They are rarely used at our institution with the exception of obtunded patients.
The fasciotomy incision used should not only allow access to the entire compartment but also anticipate the possible need for tendon transfer during later reconstruction ( Fig. 12.63 ). Undermining of skin flaps should be kept to a minimum in the swollen extremity, but complete release of forearm fascia is required from above the lacertus fibrosus to and, if necessary, including the carpal tunnel.

Rare cases of newborns with compartment syndrome have been reported ( Fig. 12.64 ). , The appearance is characteristic and warrants emergent fasciotomy. The cause is controversial, but if not recognized it will lead to late muscle contracture similar to what is seen in more typical cases later in life.

Compartment syndrome in the hand is less common but occurs most often in conditions with soft tissue trauma associated with intact skin. Recognition depends on suspicion on the part of the orthopaedic surgeon and evidence of swelling and pain. Release of the fascia of the interosseous muscles is best accomplished with three small longitudinal incisions placed over the dorsum of the hand.
Successful reconstruction of a child’s hand relies on the well-established principles of hand surgery developed for adults, with some special considerations for children. Effective reconstruction is based on understanding the architecture and physiology of the hand as an organ of motion, strength, and sensibility. A short discussion of mechanics and function of this unique element of the musculoskeletal system is appropriate because any surgeon’s reconstructive plan must, as much as possible, restore sensibility , motion , and strength .
The primary consideration in any hand reconstructive procedure is the ability of the hand to feel. Wilder Penfield’s homunculus illustrates the great importance given to the hand by the sensory cortex of the brain ( Fig. 12.65 ). Although operations designed to improve the appearance of a crippled upper limb are an important part of pediatric upper limb reconstruction, the child’s ability to feel with and protect the hand is essential for improving hand use. The primary place of sensibility in all treatment of children with a hand deformity or injury cannot be overemphasized. It is the fundamental reason for failure of long-term use of upper limb prostheses ( Fig. 12.66 ).


Fortunately for the pediatric hand surgeon, the results of all peripheral nerve reconstructions are considerably better in children than in adults. The better results are due more to central reintegration of the defective signal from the periphery and less to faster or better growth of axons or the shorter distances of axonal growth needed in a child’s shorter limb. However, the nerve injury must be recognized, repaired, and held in contact long enough for the child to realize these advantages. In addition, the aftermath of nerve injury is not always more favorable in children than in adults. Particularly in very young and rapidly growing children, nerve injuries can affect the length of growing bones, as well as the shape of joints. The shortened extremity in a patient with polio or the shortened, denervated digit illustrates the first, and the deformed shoulder joint of an incompletely recovered patient with Erb palsy illustrates the second.
Reconstruction of the complex function of the hand can be facilitated by dividing its mechanics into two components, a fixed portion surrounded by a collection of mobile units.
The fixed component is made up of the index and long finger metacarpals and the distal row of carpal bones. These six rigid bones form two arches at right angles to one another. The transverse arch formed by the distal carpal row is a Roman or semicircular arch. At a right angle to this arch in the sagittal plane, the metacarpals and carpus form another arch whose shape is a common cycloid. The fixed unit of the hand is the foundation of all hand motion and power. For practical purposes, no movement occurs between these bony elements. The stability and alignment of this combined “foundation” are controlled in space by the strong flexor and extensor muscles of the wrist ( Fig. 12.67 ).

The mobile components include three groups of movable parts. The first part is the highly mobile thumb ray radiating off the radial volar side of the fixed unit. Its complex conical motion and great strength are the result of a collection of the three joints empowered by the intrinsic and extrinsic muscles of the thumb ( Fig. 12.68 ). The second mobile component consists of the two minimally mobile ulnar metacarpals, which in conjunction with the thumb, allow flattening and cupping of the palm. The third and highly mobile component is made up of the 12 bones of the four fingers. The interaxial lengths of these bones make it possible for the sensate fingertip to move in a motion commonly found in nature—the equiangular spiral ( Fig. 12.69 ). This spiral motion of the fingers accounts for the adaptability of the hand to objects of any size and is a consequence of the fact that the distance between the axes of the joints of the bones of the finger precisely follows the Fibonacci sequence.


Reconstruction of any complex hand injury or anomaly must first address restoration of the fixed unit’s stability. Here, fusions and osteotomies are used to restore the two arches and their stability. When adequate power in the wrist motors is absent and transfers are not available, wrist fusion is required to allow the fingers and digits to experience the motion provided by the muscles originating above the wrist. In general, operations to restore the fixed unit or other elements of the skeleton require prolonged immobilization. As soon as skin coverage is achieved, bone reconstruction takes priority. Bone reconstruction must not be combined with operations that require early movement such as tenolysis, tendon repair, or tendon transfer. It is a simple but too often ignored surgical principle that one cannot hold something still and move it at the same time.
Reconstruction of the mobile portion of the hand requires wrist stability, flexible digital joints, gliding tendons, and the combined power of the extrinsic and intrinsic muscles. Without the balanced function of these four components, the long cantilever of the wrist and multiarticulated finger falls into the familiar claw deformity ( Fig. 12.70 ). Although children maintain joint mobility and tendon gliding better than adults do, the surgeon must be certain that passive motion of joints has developed before adding active muscle power. If the surgeon cannot move the child’s joints easily with his or her own hands, it is not possible that the child’s diminutive muscle that the surgeon transfers to the area can move them.

Another important principle in reconstructing the mobile unit is the synergy of wrist and finger motion ( Fig. 12.71 ). Active finger extension cannot be restored without wrist flexion power that is at least equal to the power of the extrinsic finger extensor muscles. Conversely, finger flexion requires active, strong, simultaneous contraction of the wrist extensor. The inability of a patient with radial nerve palsy to make a fist is convincing evidence of this critical synergism. When the synergistic wrist motor is not available, wrist arthrodesis is required.

The decision regarding which children can be helped by surgical reconstruction, at what age it should be carried out, and which operative procedure would be appropriate is the critical challenge for pediatric hand surgeons. Although in some cases this decision is straightforward, in others it can be very complicated. Guidelines from adult surgery are important, but some additional factors need to be considered before embarking on reconstruction in children. Such factors include the child’s variable and often limited ability to cooperate in the postoperative period, the risk associated with anesthesia in early life, the size of the structures, absence of critical parts, the need for growth, and occasionally the reduced life span of some patients with particular congenital syndromes. The timing of reconstruction in children is discussed in detail earlier in this chapter, and the reader is encouraged to review this material now.
Surgical decisions by those treating children can sometimes be difficult to make. The child’s disabilities and deformities may seem overwhelming to the surgeon and parent. Furthermore, making a selection from a long list of possible treatments is one of the most important and sophisticated tasks in hand surgery. The goal is to get the best result with the least insult to the limb. This is important because the healing capacity of the tissues is compromised by repeated operations. We have found the following guidelines useful for decision making in complex reconstruction problems.
What is the patient ’ s problem? This question must be answered after the diagnosis has been made. Sometimes it can be difficult to get children or parents to verbalize how their problem really affects their daily lives. The surgeon must observe and listen carefully to both the parent and child to discover the child’s exact needs.
Goals of treatment, short and long term. Treatment of the patient’s short-term goal requires the surgeon to remain focused on the patient’s problem, as noted previously, and to detect losses that are critical to the patient’s daily life. Reduced to its basic elements, the hand needs the power of pinch and grasp with durable, sensate coverage. The long-term goal of treatment is to make the patient as independent as possible. Care must be taken to avoid making the patient dependent on the medical team any more than necessary.
Reasonable methods to achieve these goals. The key here is reasonableness. Surgeons must carefully consider the probable responses of the child, the parent, and the tissues of the child’s hand to the planned operation. In addition, surgeons must realistically evaluate what they can actually deliver in the operating room and postoperative period.
Reasonable time schedule. Both the surgeon and the family find comfort when a realistic time schedule is agreed on at the beginning. This schedule may need revision later, but the child’s exit from the medical environment should not be delayed more than necessary. Although follow-up to the treatment is important, the final goal of all surgery is maximal independent life for the patient. When a treatment plan is open ended, the child and parent tend to delay taking responsibility for this ultimate goal.
Outcome evaluation method. All too often, failure to take preoperative photographs, to measure and record joint angles and grip strength, and to perform functional tests before treatment renders an honest, accurate assessment of the result at the end of treatment impossible. This is as important as establishing the type of treatment.
Classification of congenital limb anomalies is made difficult by the spectrum of manifestations of developmentally related conditions, the myriad terms derived from Greek and Latin for the same condition, and the overlap in clinical appearance of conditions with different etiologies. In particular, etiologic information may erode a neat anatomic classification.
A good classification system should allow a reproducible “fit” by any user, should group the spectrum of findings of the same anomaly consistently into the same category, and should be specific and useful. It should allow retrieval of information and facilitate communication among geneticists, surgeons, pediatricians, and embryologists. Moreover, a good classification system should be flexible enough to accommodate new information about developmental and genetic etiologies of the conditions classified.
The classification system adopted by the International Federation of Societies for Surgery of the Hand (IFSSH) is currently the most commonly used system for classifying limb abnormalities. It is based on a mixture of anatomic sites and presumed developmental errors. A single anomaly or group of anomalies may fit into more than one category, or it may not be easily classified into any group. In its simplest form, the classification system adopted by the IFSSH in 1976 is presented in Box 12.2 . However, this system does not accommodate our increased understanding of the developmental biology and molecular pathways responsible for upper limb malformation.
Failure of formation
Transverse arrest
Longitudinal arrest
Failure of differentiation (separation) of parts
Soft tissue involvement
Skeletal involvement
Duplication
Overgrowth
Undergrowth
Constriction ring syndrome
Generalized abnormalities and syndromes
To better classify hand differences Oberg, Manske, and Tonkin (OMT) developed a classification which placed congenital hand differences into Malformations, Deformations, or Dysplasias groups. This OMT classification system has now been accepted by the IFSSH as an appropriate replacement for the previously used Swanson Classification ( Box 12.3 ).
MALFORMATIONS
Abnormal axis formation/differentiation—entire upper limb
Proximal-distal axis
Brachymelia with brachydactyly
Symbrachydactyly
Poland syndrome
Whole limb, excluding Poland syndrome
Transverse deficiency
Amelia
Clavicular/scapular
Humeral (above elbow)
Forearm (below elbow)
Wrist (carpals absent/at level of proximal carpals/at level of distal carpals) (with forearm/arm involvement)
Metacarpal (with forearm/arm involvement)
Phalangeal (proximal/middle/distal) (with forearm/arm involvement)
Intersegmental deficiency
Proximal (humeral – rhizomelic)
Distal (forearm – mesomelic)
Total (phocomelia)
Whole limb duplication/triplication
Radial-ulnar (anterior-posterior) axis
Radial longitudinal deficiency—thumb hypoplasia (with proximal limb involvement)
Ulnar longitudinal deficiency
Ulnar dimelia
Radioulnar synostosis
Congenital dislocation of the radial head
Humeroradial synostosis—elbow ankyloses
Madelung deformity
Dorsal-ventral axis
Ventral dimelia
Furhmann/Al-Awadi/Raas-Rothschild syndromes
Nail patella syndrome
Absent/hypoplastic extensor/flexor muscles
Unspecified axis
Shoulder
Undescended (Sprengel)
Abnormal shoulder muscles
Not otherwise specified
Arthrogryposis
Abnormal axis formation/differentiation—hand plate
Proximal-distal axis
Brachydactyly (no forearm/arm involvement)
Symbrachydactyly (no forearm/arm involvement)
Transverse deficiency (no forearm/arm involvement)
Wrist (carpals absent/at level of proximal carpals/at level of distal carpals)
Metacarpal
Phalangeal (proximal/middle/distal)
Radial-ulnar (anterior-posterior) axis
Radial deficiency (thumb—no forearm/arm involvement)
Ulnar deficiency (no forearm/arm involvement)
Radial polydactyly
Triphalangeal thumb
Ulnar dimelia (mirror hand—no forearm/arm involvement)
Ulnar polydactyly
Dorsal-ventral axis
Dorsal dimelia (palmar nail)
Ventral (palmar) dimelia (including hypoplastic/aplastic nail)
Unspecified axis
Soft tissue
Syndactyly
Camptodactyly
Thumb in palm deformity
Distal arthrogryposis
Skeletal deficiency
Clinodactyly
Kirner deformity
Synostosis/symphalangism (carpal/metacarpal/phalangeal)
Complex
Complex syndactyly
Synpolydactyly—central
Cleft hand
Apert hand
Not otherwise specified
DEFORMATIONS
Constriction ring sequence
Trigger digits
Not otherwise specified
DYSPLASIAS
Hypertrophy
Whole limb
Hemihypertrophy
Aberrant flexor/extensor/intrinsic muscle
Partial limb
Macrodactyly
Aberrant intrinsic muscles of hand
Tumorous conditions
Vascular
Hemangioma
Malformation
Others
Neurologic
Neurofibromatosis
Others
Connective tissue
Juvenile aponeurotic fibroma
Infantile digital fibroma
Others
Skeletal
Osteochondromatosis
Enchondromatosis
Fibrous dysplasia
Epiphyseal abnormalities
Others
SYNDROMES ∗
∗ The specified syndromes are those considered most relevant; however, many other syndromes have a limb component categorized under “B. Others”.
Specified
Acrofacial dysostosis 1 (Nager type)
Apert
Al-Awadi/Raas-Rothschild/Schinzel phocomelia
Baller-Gerold
Bardet-Biedl Carpenter
Beales
Catel-Manzke
Constriction band (amniotic band sequence)
Cornelia de Lange (types 1-5)
Crouzon
Down
Ectrodactyly-ectodermal dysplasia-clefting
Fanconi pancytopenia
Fuhrmann
Goltz
Gorlin
Greig cephalopolysyndactyly
Hajdu-Cheney
Hemifacial microsomia (Goldenhar syndrome)
Holt-Oram
Lacrimoauriculodentodigital (Levy-Hollister)
Larsen
Leri-Weill dyschondrosteosis
Moebius sequence
Multiple synostoses
Nail-patella
Noonan
Oculodentodigital dysplasia
Orofacialdigital
Otopalataldigital
Pallister-Hall
Pfeiffer
Pierre Robin
Poland
Proteus
Roberts-SC phocomelia
Rothmund-Thomson
Rubinstein-Taybi
Saethre-Chotzen
Thrombocytopenia absent radius
Townes-Brock
Trichorhinophalangeal (types 1-3)
Ulnar-mammary
VACTERLS association
Others
It is important to recognize coexisting anomalies associated with upper limb anomalies to plan treatment, counsel the family, and work with other providers of health care. The conditions may affect treatment decisions, anesthesia techniques, the timing of surgery, and the coordination of multiple disciplines in treatment of the child.
Anomalies may be characterized as syndromes (patterns whose causation is understood), associations (anomalies that occur in combination but whose underlying causation is not known), and sequences (anomalies whose coexistence results from a cascade of events during development). Anomalies may be associated on the basis of genetic coding, timing of development of other organ systems, or recognized patterns of disruption. Known syndromes of heritable anomalies are now finding explanation in localization of the genetic error and an understanding of how the incorrect protein affects morphogenesis. Multiple anomalies may be associated as a result of a generalized insult to the developing embryo, with the particular association reflecting the timing of the insult. The classic diagnosis in this category is the VACTERL association.
It has long been known that radial longitudinal deficiency (RLD) (radial dysplasia) is one of the components in a number of conditions, including the potentially life-threatening Holt-Oram syndrome, Fanconi anemia (FA), and thrombocytopenia–absent radii (TAR) syndrome. This list was at one point limited enough to be included in a text such as this. General information has traditionally been found in books such as Smith ’ s Recognizable Patterns of Malformation (fourth edition) and Clinical Syndromes (third edition) , but given the rate of new information and reports, the Online Mendelian Inheritance in Man (OMIM) website, maintained by the National Institutes of Health (NIH; available at http://www.ncbi.nlm.nih.gov/Omim/ ), is an extremely useful source of continuously updated information. Links to PubMed, a reliable source of medical literature maintained by the NIH, and additional informative websites are provided. A web search by diagnosis will usually produce information posted and maintained by families and support groups for children with a particular diagnosis. These sites are kept current by dedicated volunteers; most information, however, is not peer reviewed.
In general, anomalies that affect the upper and lower limbs simultaneously are often the result of genetic alterations either with known heredity or as new mutations.
Mutations in regions that code for fibroblast growth factors or receptors have been shown to be responsible for a number of limb malformations in association with craniofacial abnormalities. Different base pair substitutions cause different alterations in the three-dimensional configuration of the protein. This change in shape prevents normal binding of the factor with the receptor site. Some of the resulting syndromes are listed in Table 12.3 . Other examples are the different phenotypes of cleft hands and feet that map to different chromosomes. Three different mutations have been localized.
| FGFR | Syndrome | Comments |
|---|---|---|
| FGFR 1 | Pfeiffer syndrome | Overlap in syndromes and protein mutation |
| FGFR 2 | Noack syndrome Carpenter syndrome |
|
| FGFR 2 | Apert syndrome Crouzon syndrome Jackson-Weiss syndrome |
Different mutations of the same protein producing phenotypic differences |
| FGFR 3 | Achondroplasia Hypochondroplasia Thanatophoric dwarfism |
Autosomal dominant mutations, chromosome 4p16.3 |
Abnormalities associated with radial-sided deficiency (dysplasia), including hypoplastic thumbs, may be associated with other conditions, such as cardiac, renal, vertebral, and hematopoietic disorders. These kinds of known associations must be investigated further as part of the treatment plan. Consultation with other pediatric disciplines is important in planning reconstructive care for the child.
RLD includes the radius, radial carpus, and radial digits. The defect may be proximal, distal, intercalary, or total. It may involve proximal parts of the limb.
RLD has been estimated in the literature to occur in 1 in 50,000 to 1 in 100,000 live births. The incidence of all radial ray–deficient limbs, including patients with hypoplastic thumbs alone, is approximately 1 in 30,000. The prevalence has been reported to be slightly higher in boys at 3:2. At our institution we have treated more than 400 patients with radial dysplasia deficiencies and have noted an equal sex ratio.
Radial deficiencies can be either isolated or associated with other anomalies, often as part of a recognized malformation syndrome. The molecular basis of isolated radial ray deficiency is still unknown. In some of the syndromic associations the genetic lesion has been identified. Patients with Holt-Oram syndrome have a mutation on chromosome 12 at the location of the TXB5 gene. The etiology of FA, which may be associated with radial ray deficiency, is also known. It is an autosomal recessive disease. Eleven FANC genes are known. An individual must have two abnormal FANC genes to have FA. They may be homozygous for one FANC mutation or heterozygous for two FANC gene mutations. The cause of TAR syndrome has not yet been identified but can be passed in an autosomal recessive fashion. Both environmental factors and drugs have been associated with RLD. Maternal exposure to antiepileptic drugs, particularly valproic acid, has been associated with radial deficiency. Radial aplasia has been observed with increased frequency in fetal alcohol syndrome. Other drugs associated with radial deficiency include thalidomide, phenobarbital, and aminopterin.
There seems to be a critical time in embryogenesis in which the risk for radial ray defects is increased. In humans, the upper limb forms between the fourth and eighth postovulatory weeks. The insult, whether environmental or genetic, seems likely to occur in the critical time between weeks 4 and 5 of embryonic development.
RLD has a wide range of phenotypes. In some cases, it may be a subtle finding noticed incidentally on radiography as a mildly hypoplastic thumb and missing or fused carpal bones. The most severe manifestations are characterized by total absence of the radius and deficiency of the proximal part of the upper limb, with a shortened humeral segment and hypoplastic glenoid giving the limb a phocomelic appearance ( Fig. 12.72 ). The defect may be unilateral or bilateral. Bilateral defects are more likely than unilateral defects to have a syndromic association.

The signs and symptoms of RLD are distinctly different from those of ulnar longitudinal deficiency (ULD), as discussed in the following sections ( Table 12.4 ).
| ULD | RLD | |
|---|---|---|
| Inheritance | Sporadic | Genetic |
| Associated anomalies | Musculoskeletal | Visceral |
| Syndromes | Rare | Common |
| Involved digits | Any | Radial |
| Joint stability | Wrist stable Elbow unstable |
Wrist unstable Elbow stable |
| Clinical features | Partial absence | Total absence |
| Bilateral | 25% | 100% |
RLD is more common than ULD. The relative prevalence is approximately 2:1 at our institution.
RLD is more likely to be bilateral. The defect of the radius is more often total in radial deficiency than in ulnar deficiency.
Because of the contribution of the distal end of the radius to the wrist joint, the wrist is very abnormal in the more severe types of radial dysplasia. In contrast, patients with ULD usually have a stable wrist joint. The elbow joint in RLD is usually present and stable. With the exception of the thumb, the hand is relatively normal.
RLD is associated with a high frequency of anomalies in other organ systems, including most commonly the heart, the hematopoietic system, and the VACTERL association. In contrast, ULD is associated with other musculoskeletal deformities, such as fibular hemimelia, but not usually with visceral abnormalities. It is important for the clinician to note the high frequency of problems in other organ systems that occur in children with radial ray deficiency. All cases of radial deficiency, including thumb hypoplasia with a normal radius, merit evaluation for associated conditions. At our institution the rate of systemic anomalies was approximately 40% in patients with RLD and 12% in those with radial ray deficiency restricted to the thumb. It has been reported to be even higher in some institutions, which further emphasizes the need for further investigation in these patients.
The most common syndromic associations are described in the following sections.
The association of radial deficiency and cardiac anomalies (hand–heart syndrome) was described by Holt and Oram in 1960. By definition, upper limb and cardiac anomalies are always present. However, in either case they may be subtle.
The most common cardiac anomalies are ventricular septal defect, atrial septal defect, and conduction abnormalities. In particular, the conduction abnormalities may not be obvious during the initial evaluation and may not be clinically manifested until adulthood.
The cause of Holt-Oram syndrome is a mutation on chromosome 12 in the TXB5 gene. It is autosomal dominant with complete penetrance. The phenotype is variable, and 85% of cases are new mutations.
New cases of Holt-Oram syndrome can be confirmed with genetic testing. The heritable nature of this condition also needs to be discussed. Patients require cardiology follow-up. In addition to septal defects, they are at higher risk for such complications as congestive heart failure, arrhythmia, heart block, atrial fibrillation, endocarditis, and sudden death.
FA is an autosomal recessive disorder. The carrier rate is approximately 1 in 300. Patients are either homozygous or doubly heterozygous for 1 of the 11 different FANC genes. The protein products of these genes combine to form a complex involved in DNA repair. Bone marrow failure (pancytopenia) occurs in up to 90% and is often fatal. Onset is at approximately 6 years of age. Patients are also at risk for acute myeloid leukemia (10%) and myelodysplastic syndrome. In adult survivors, the cumulative incidence of solid tumors (e.g., hepatic, esophageal) is 30% by 45 years of age.
FA can be diagnosed before the onset of clinical disease. Cultured peripheral lymphocytes exhibit fragility when exposed to DNA cross-linkers such as diepoxybutane. FA can also be tested for by flow cytometry. At present, these tests are too costly for use as routine screening tests.
Approximately 40% of patients with FA have radial abnormalities, but it is not known how many patients with radial deficiency have FA. At our institution the prevalence is between 1% and 5%.
We currently do not screen every patient with radial deficiency for FA. Patients at high risk, such as those with an affected sibling, who have a 25% chance of having FA, should be screened. Patients with FA may have other features that raise clinical suspicion, the most common of which are skin pigmentation (55%), short stature (51%), and abnormal gonads (33% of boys). Early diagnosis of FA may be lifesaving. Not only does it allow adequate monitoring of hematologic status, but it also allows search for a potential bone marrow donor to begin before bone marrow failure develops.
Though somewhat ethically controversial, the family may wish to conceive another child who may be suitable for cord blood transfer. This involves embryonic selection of offspring who do not have FA and are human leukocyte antigen (HLA) compatible with the affected sibling. The first cord blood transfer was done in France in 1988 and used a naturally conceived, HLA-identical sibling who was tested for compatibility in utero. The recipient was still alive and well 15 years later. ,
The TAR syndrome is the association of radial defect and hypomegakaryocytic thrombocytopenia. No genetic lesion clearly associated with the TAR syndrome has yet been identified. In a few cases a familial pattern suggestive of autosomal recessive inheritance has been seen, but this is not the case in the majority. ,
The radial deficiency in TAR syndrome is almost always characteristic. In affected individuals the radius is totally absent. Even though the thumbs are present they are usually hypoplastic with impaired function because of lack of thumb interphalangeal (IP) motion and an MCP flexion contracture. The proximal part of the limb may also be very foreshortened and give a phocomelic appearance.
The thrombocytopenia is manifested as episodic bleeding from early in infancy. Thrombocytopenia is present in the first week of life in 50% and by 4 months of age in 90%. The severity of the thrombocytopenia is significant. In one review of 100 cases, 20 patients died of hemorrhage. Platelet counts were lower than 10,000/mm 3 in these cases. The incidence of hemorrhage is limited to the first 18 months of life.
Pediatric orthopaedists should be familiar with the manifestations of TAR syndrome. The diagnosis is confirmed by laboratory tests showing thrombocytopenia. Cardiac anomalies occur in approximately one third of patients with TAR syndrome. Other anomalies include musculoskeletal defects in the lower limb.
Patients need hematologic care, and cardiac anomalies need to be excluded. Cows’ milk must be avoided in the first year of life because it can cause gastrointestinal bleeding. Surgery on the upper limb should also be avoided until the child reaches approximately 18 months to 2 years of age and is cleared by hematology.
The differential diagnosis for TAR syndrome includes IVIC syndrome, named for the Instituto Venezolano de Investigaciones Cientìficas, where it was first described. It is also known as oculootoradial dysplasia, and patients may have thrombocytopenia and radial deformities, as well as other anomalies.
The VACTERL association is the association of congenital anomalies in a number of systems ( Box 12.4 ). Three anomalies must be present to make the diagnosis. Cases usually occur as sporadic events, although some with more than one family member affected have been reported. ,
The frequency of the VACTERL association is increased in the offspring of diabetic mothers. An increased frequency has also been reported with statin use during pregnancy. Statins downregulate cholesterol, which may have a secondary effect on the sterol-dependent sonic hedgehog pathway.
The cause is thought to be a defect in mesodermal development and may be related to defects in the sonic hedgehog signaling pathway. An association between VACTERL with hydrocephalus and VACTERL without hydrocephalus and FA is recognized. Some authors have recommended chromosome breakage studies for patients with the VACTERL association who have radial ray anomalies.
It is essential that any child with a radial deficiency of any degree be evaluated with a complete history and physical examination. At our institution, one fourth of all patients with a hypoplastic thumb or radial clubhand had a syndromic association ( Table 12.5 ).
| System | Condition |
|---|---|
| Cardiac | Holt-Oram syndrome VACTERL association Ventriculoradial dysplasia |
| Chromosomal | Trisomies 13, 17, and 18 Deletions 4q and 14q |
| Craniofacial | Acrorenalial–ocular syndrome Craniosynostosis–radial aplasia (Baller-Gerold syndrome) Cleft lip/palate Oculoauriculovertebral dysplasia (Goldenhar syndrome) Orofacial–digital syndrome (Juberg-Hayward syndrome) Roberts SC phocomelia syndrome Treacher Collins syndrome Eye–radial dysplasia (Duane syndrome) Möbius syndrome Hemifacial microsomia |
| Cutaneous | Rothmund-Thompson syndrome (cutaneous poikiloderma) |
| Developmental delay | de Lange syndrome |
| Hematologic | Thrombocytopenia–absent radius syndrome Fanconi anemia Anemia–triphalangeal thumb (Aase-Smith syndrome) |
| Renal | Renal–radial ray aplasia (Sofer syndrome) |
| Skeletal | Cervical rib–radial ray defect (Funston syndrome) Costovertebral dysplasia–humeroradial synostosis (Keutel syndrome) Klippel-Feil syndrome VACTERL association |
| Teratogenic | |
| Other | IVIC (Instituto Venezolano de Investigaciones Cientìfcas) syndrome |
Earlier classifications of thumb hypoplasia (Blauth) and radial clubhand (Bayne and Klug) have been modified by Manske into the current classification of radial deficiency ( Figs. 12.73 and 12.74 ; Table 12.6 ).


| Type | Thumb | Carpus | Distal Radius | Proximal Radius |
|---|---|---|---|---|
| N | Hypoplastic or absent | Normal | Normal | Normal |
| 0 | Hypoplastic or absent | Absence, hypoplasia, or coalition | Normal | Normal, radioulnar synostosis, or congenital dislocation of the radial head |
| 1 | Hypoplastic or absent | Absence, hypoplasia, or coalition | >2 mm shorter than the ulna | Normal, radioulnar synostosis, or congenital dislocation of the radial head |
| 2 | Hypoplastic or absent | Absence, hypoplasia, or coalition | Hypoplasia | Hypoplasia |
| 3 | Hypoplastic or absent | Absence, hypoplasia, or coalition | Physis absent | Variable hypoplasia |
| 4 | Hypoplastic or absent | Absence, hypoplasia, or coalition | Absent | Absent |
From a surgical point of view, the most important anatomic differences occur at the level of the wrist.
The radial ray is defined as the radius, scaphoid, trapezium, thumb, and associated soft tissue structures. All or part of these structures can be missing, depending on severity, and the defect can be longitudinal or intercalary.
A fibrous anlage is frequently present in cases in which all or part of the radius is missing (types II to IV). This is an important deforming force and needs to be excised as part of corrective surgery.
Because of the deficiency of radial structures, the median nerve is often one of the most radial structures and is very superficial at the level of the wrist. Care must be exercised by the surgeon to not damage it in the approach or mistake it for the anlage.
Other muscles in the upper limb may be absent, abnormal, or accessory ( Fig. 12.75 ). The radial artery is absent in 86.5% of cases. The ulnar artery is usually present and normal. A significant number of patients have persistence of the embryologic median artery of the upper limb. In some cases the anterior interosseous artery is larger. A brachiocarpalis muscle, acting as a deforming force at both the elbow and wrist joints, is consistently found in patients with TAR syndrome.

From a functional point of view, it must be remembered that 80% of the load-bearing part of the wrist joint is missing when the distal end of the radius is absent. For this reason, radial clubhand does not resemble clubfoot in the lower limb. Corrective surgery can realign the limb, but it cannot replace this anatomic and biomechanical defect. Recurrence of the deformity after corrective surgery is common. This contributes to the already deficient growth potential of the ulna. The ulna tends to curve in association with the missing radius. In untreated cases the ulna grows to approximately 60% of the length expected.
In cases of complete radial aplasia, the hand is fixed in pronation, and the patient substitutes wrist flexion for supination.
The elbow joint may be normal or abnormal. An ulnohumeral synostosis may be present, which is important to note because procedures that stiffen the wrist joint or cause the hand to deviate away from the face have no place in this situation.
It is also important to note the condition of the fingers. The index and sometimes middle finger may be stiff and lacking in function because of aberrant flexor and extensor structures. This is particularly important to note in cases in which pollicization may be considered.
Plain radiographs are all that is required to diagnose and classify deficiency involving the radius itself. The forearm deformity can be classified in infancy. In cases of subtle radial dysplasia involving just the carpal bones or the thumb, radiographs obtained once the carpal bones have ossified will be more accurate. If microvascular surgical treatment is planned, specialized studies of the local vasculature may be necessary, especially in patients who previously have undergone surgery.
Plain radiographs can be very useful as tools for monitoring the clinical progress of radial dysplasia, particularly after surgery. We recommend that a standardized view (posteroanterior [PA], neutral rotation) be used to allow adequate comparison ( Fig. 12.76 ).

Before embarking on any operative treatment, it is important to remember that it is easy to make these children worse in trying to make them better.
Even results that look good in the postoperative period may not be satisfactory in the longer term because of either recurrence of the deformity or arrest of distal ulnar growth brought on by the original procedure. All reports detailing various surgical approaches are plagued by small numbers, absence of long-term follow-up, and lack of standardized assessments of functional impairment and activity performance. ,
It has been said that “The story of the surgical treatment of radial ray defect … consists of descriptions of many operations and reports of successful short term results. … The graveyard of discarded procedures—documented by book after book and article after article …”
The general principles of treatment can be listed as follows:
Stretching and splinting should be started early in infancy to maintain soft tissue length.
Surgical correction needs to address the tight structures, most notably the fibrous anlage on the radial side of the wrist.
The limb, especially the forearm, is destined to be short. At best, the affected ulna will grow to only 60% of the length expected. Eighty percent of the growth of the forearm comes from the distal end of the ulna. Aggressive surgery in early childhood that prompts distal ulnar growth arrest will result in a very short forearm. Care must be taken to protect the distal ulnar physis and its blood supply.
Before surgery attempting to improve the appearance of the forearm is undertaken, the potential to disturb the function of the limb must be considered carefully, especially with bilateral cases. In some cases, the limb is so tight and so deficient that even if an elbow is present, operative intervention offers no improvement in function or appearance.
Recommendations for treatment, based on age, for infants and young children are as follows:
0 to 6 Months : Splinting is effective and well tolerated at this age. Splints should ideally be above the elbow for increased control because the limb is so small. Serial casting can also be used. Both must be changed frequently.
6 to 18 Months : After 6 months, splinting is less well tolerated. A stretching program for the parents to follow is then instituted, combined with night splinting if possible.
2 to 3 Years : Splinting and stretching are not as effective by this age. In addition, the limb has now almost doubled in size since birth, which makes any subsequent operative treatment easier.
In cases requiring operative intervention, our current treatment of the forearm involves the use of a bilobed flap ( Fig. 12.77 ), soft tissue release, ulnar osteotomy, and temporary longitudinal wiring of the carpus to the distal end of the ulna in the corrected position. The soft tissue release must include resection of the anlage, if present. The longitudinal wire does not seem to cause arrest of distal ulnar growth. Some recurrence of deformity over time in all but the mildest cases is to be expected.

Thumb hypoplasia is managed according to the severity of the level of involvement. Blauth type I thumbs may require first web space lengthening or even no surgical treatment. Addition of opponensplasty and ulnar collateral ligament reconstruction to the web space lengthening may be considered for type II thumbs, and type IIIA thumbs may require tendon transfers in addition to the aforementioned procedures.
After 2 years of age, pollicization may also be considered for Blauth type IIIB, IV, and V thumb hypoplasia, and in fact it can be one of the most helpful operative treatments of radial ray deficiency in which the principal defect is an inadequate thumb.
When deciding whether the patient is a suitable candidate for pollicization, the following needs to be considered:
Flexibility of the index finger
Orientation of the wrist on the forearm (position of the pollicized digit)
Whether the index finger is functional
Pollicization of a stiff index finger that the child does not use for prehensile activities rarely results in a favorable outcome because the child tends to continue to ignore the pollicized digit. This is especially important for a child who uses the little finger for pinch. In this case the ulnar side of the hand is often better positioned for prehensile activities.
In the presence of recurrent deformity, another partial correction and wrist fusion can be considered. By this time the distal end of the ulna will have grown almost as much as possible. It must be remembered that wrist fusion in these patients eliminates any rotation of the hand as well because they have a one-bone forearm. Fusion is contraindicated if wrist flexion is required for function.
Centralization aims to improve the position of the carpus on the forearm and may increase the overall length of the limb. The first centralization procedure was performed by Sayre in 1894. Since then, many variations have been developed to achieve the best long-term result with the fewest complications. Problems with centralization have included recurrence, growth arrest, and loss of motion at the wrist joint ( Fig. 12.78 ).

Centralization improves the appearance at least temporarily but has not been shown to improve function. In longer-term follow-up studies, patients tend to have either a recurrent deformity with a more flexible wrist or reasonable maintenance of alignment with a stiff wrist. In patients with good elbow function, postcentralization recurrent deformity may be addressed by ulnocarpal epiphyseal arthrodesis to stabilize the carpus.
Radialization was developed by Buck-Gramcko in the 1980s as another modification of centralization in an attempt to reduce the risk for recurrence. Today, it is usually combined with preoperative soft tissue distraction. The carpus is then fixed on top of the distal end of the ulna with a longitudinal wire. Radial-sided tendons are transferred ulnarly. An Ilizarov device may be added to lengthen the ulna. Complications have included overcorrection and recurrence.
Before undertaking any centralization or radialization procedure it must be remembered that when these children become adults limitations do not seem to be related to wrist position.
Ilizarov correction can be used to lengthen the ulna through osteotomies in conjunction with realignment procedures. It can also be performed at the time of wrist arthrodesis. Radial lengthening for type II and III radial dysplasia has also been described, as has gradual soft tissue distraction in preparation for centralization. , The patient’s and family’s enthusiasm for a longer arm must be tempered by the surgeon. The need for possible multiple lengthenings should be discussed. Problems associated with this procedure include progressive contracture and nerve injury. Neurologic changes during lengthening are very difficult to monitor in young children. In general, lengthening procedures in these patients are associated with high risk and low benefit.
This technique, developed by Vilkki in the 1990s, aims to replace the missing radial strut with tissue that has some growth potential. In this operation, a metatarsophalangeal (MTP) joint unit is transferred from the foot to the radial side of the wrist via microsurgical techniques.
Although some good short-term results have been reported, complications have included arrest of the growing epiphysis and recurrence of deformity.
Transfer of the vascularized proximal end of the fibula to the radius has been reported in the tumor literature and has been described as treatment of radial dysplasia, but long-term results are not yet available in congenital cases.
Life-threatening problems in other organ systems must be dealt with before surgery on the upper limb.
In patients with an ulnohumeral synostosis, surgery on the wrist is contraindicated because mobility of the wrist needs to be preserved so that the patients can get their hand to the mouth.
Bilateral centralizations often worsen function and are rarely advised.
Though not the first to report it, Sprengel’s description of congenital elevation of the scapula in 1891 was the first to draw attention to the deformity that bears his name. Sprengel deformity is due to arrest of caudal migration of the scapula during the 9th through 12th weeks of gestation; it also leads to arrest in the development of accompanying bone, muscle, and cartilage. Scapular stabilizers may be absent, hypoplastic, or completely replaced by fibrous tissue. In addition, associations with rib cage and cervicothoracic abnormalities, scoliosis, chest wall asymmetry, Klippel-Feil syndrome, torticollis, and pulmonary and renal disorders are common. An aberrant omovertebral bone, well visualized on CT, is present 16% to 55% of the time and connects the scapula with the cervical spine. ,
Patients often have an asymmetric, painless, bony mass in the web of the neck, normal glenohumeral motion, and severely restricted scapulothoracic motion. Shoulder abduction is limited by three factors: (1) scapular fixation from both abnormal anatomy and an omovertebral bone restricts the normal glenohumeral and scapulothoracic synchronous abduction of 2:1, (2) the inferior pole of the hypoplastic scapula is medially rotated with the glenoid facing caudally, and (3) weak or absent scapular muscles provide insufficient power for shoulder abduction. Multidirectional shoulder instability has also been reported.
Surgical intervention is indicated for functional deficiencies and severe cosmesis and is generally thought to be optimally timed when the child is younger than 8 years, , although improvement has been reported in the older age group. , The Cavendish score was developed to grade the severity of the deformity, as well as improvement after surgery ( Table 12.7 ).
| Grade 1 (very mild) | Shoulder joints level Deformity not visible with the patient dressed |
| Grade 2 (mild) | Shoulder joint level or nearly level Deformity visible with the patient dressed |
| Grade 3 (moderate) | Shoulder joint elevated 2–5 cm |
| Grade 4 (severe) | Shoulder elevated >5 cm Superior angle of the scapula near the occiput |
Surgery has generally been advocated for patients who are Cavendish grade 3 or 4. , Those with minimal limitation and deformity may be treated by resection of the superomedial angle of the scapula and omovertebral bone.
Many variations in surgical technique for addressing Sprengel deformity exist and have been reported as case series in the literature. Direct comparison of results is nearly impossible because of authors’ modifications of technique, differences in assessment of outcomes, and inconsistent classification of the severity of the preoperative deformity. Nevertheless, the two main surgical objectives when treating Sprengel deformity are lowering the position of the scapula and releasing the abnormal connections between the scapula and vertebra/chest wall.
Green initially described an extraperiosteal release of the muscles attached to the medial aspect of the scapula, reattachment after the scapula was brought distally, resection of the supraspinous portion of the scapula, and then holding the scapula in its new distal position with spring wire traction and spica casting for 3 weeks (see ePlate 12.1 on expertconsult.com ). In an attempt to simplify surgery and decrease complications, Woodward introduced a technique consisting of release of the origins of the trapezius, levator scapulae, and rhomboids from the spinous processes; reattachment of them distally; and resection of the omovertebral bone, supraspinous fossa, or prominent superomedial angle of the scapula (see ePlate 12.2 on expertconsult.com website). The Mears procedure adds an oblique osteotomy through the body of the scapula and release of the long head of the triceps to subperiosteal resection of the muscles and omovertebral bone attaching to the medial part of the scapula. Addition of a clavicular osteotomy to the surgical procedure is advocated for severe deformity and older patients to prevent brachial plexus injury. Maintenance of favorable functional and cosmetic long-term results has been reported for almost all variations of the Green and Woodward procedures, , , and good midterm results have been demonstrated with the Mears procedure. ,

First, an osteotomy of the clavicle is performed. The patient is placed in the lateral decubitus position, and the upper half of the chest, the entire neck, the entire upper limb, and the posterior aspect of the neck are fully prepared and draped. It is vital that the level of the contralateral normal scapula be visible during surgery. An alternative method is to place the patient in the supine position and prepare the neck and upper half of the chest, perform the osteotomy of the clavicle, and then turn the patient to the prone position and reprepare and redrape. This author finds it expedient to use the former method.
(A) A supraclavicular curvilinear incision is made 2 cm above the clavicle in line with the skin creases of the neck and centered over the midportion of the clavicle. It is best to make the skin incision with the neck in slight flexion (not hyperextension). The subcutaneous tissue is divided in line with the skin incision, and the wound is pulled down directly over the clavicle.
(B) The deep fascia is incised; any superficial veins are clamped and coagulated. The periosteum of the clavicle is divided longitudinally on its anterior aspect and, with a periosteal elevator, is gently elevated circumferentially around the clavicle. Two smaller Chandler elevators are placed deep to the clavicle to protect the subclavicular vessels and the brachial plexus.
(C) With a bone cutter or an oscillating electric saw, the clavicle is sectioned at one or two sites with its posteroinferior cortex left intact (if two sites are used, they should be 3 cm apart). Then by gentle force, a greenstick fracture of the clavicle is produced. The periosteum is closed. The skin is closed with subcuticular running suture. Morcellation of the clavicle is not recommended.
In an older patient the incision may be extended laterally so that the tip of the coracoid process and the origins of the short head of the biceps brachii and the coracobrachialis muscles are exposed. The cartilaginous tip of the coracoid process is sectioned, and then the wound is closed as already described. The purpose of this step in a child older than 10 years is to prevent compression of the neurovascular bundle against the rib.
(D) The patient is turned to the prone position with the head and neck extending beyond the operating table and supported on a headrest. The chin piece of the headrest should be well padded, and during the procedure the anesthesiologist should frequently check the chin for pressure areas. Anchoring the buttocks to the operating table with 2- or 3-inch-wide adhesive tape will prevent the patient from slipping caudally. Care should be taken to guard the sterility of the operating field. First, the vertebral border, the level of the inferior angle and the spine of the elevated scapula, and those of the opposite normal scapula are palpated and marked with indelible ink. A midline skin incision is made that begins at the spinous process of the fourth cervical vertebra and extends distally to terminate at the spinous process of the tenth dorsal vertebra (C4–T10).

(E) The skin and subcutaneous tissue are divided in line with the skin incision, and a place between the subcutaneous tissue and fascia underlying the trapezius muscle is developed. Dissection is extended laterally to expose the spine of the scapula. Next, the inferior margin of the trapezius muscle, which runs obliquely upward and laterally to the scapular spine, is isolated. Its free lateral border is mobilized and retracted proximally and medially. The insertion of the entire trapezius muscle (superior, middle, and inferior parts) on the scapular spine is sectioned, elevated extraperiosteally, and marked with 2-0 Mersilene suture. Inferiorly, the lower fibers of the trapezius muscle are separated from the subjacent latissimus dorsi muscle with Metzenbaum scissors.
(F) The detached trapezius muscle is reflected medially to expose the underlying muscles and scapula. The spinal accessory nerve, which is the motor nerve of the trapezius, should not be injured.


(G and H) The supraspinatus muscle is then detached from the scapula extraperiosteally to the greater scapular notch. The transverse scapular artery and suprascapular vessels and nerve must be identified and protected in the lateral portion of the wound as they enter the infraspinatus fossa and pass through the greater scapular notch.

(I) The omovertebral bar (bony, cartilaginous, or fibrous) is excised by first sectioning it at the scapular end with a bone cutter and then gently detaching its attachment to the cervical vertebra. At the cervical level it may be attached to the spinous process, lamina, or transverse process of one of the lower cervical vertebrae (fourth to seventh).
(J) The insertion of the levator scapulae muscle on the superior angle of the scapula and the insertions of the rhomboideus muscles, major and minor, on the medial border of the scapula are extraperiosteally dissected, divided, and retracted, and their free ends are marked with 2-0 Mersilene suture.

(K) The superior margin of the scapula is then retracted posteriorly, and starting medially, the supraspinous portion of the subscapularis muscle is elevated extraperiosteally from the anterior surface of the scapula.
(L) Next, a staphylorrhaphy probe is placed in the scapular notch to protect the suprascapular nerves and vessels, and with bone-cutting forceps or an osteotome, the supraspinous part of the scapula along with its periosteum is excised. (Currently, this author preserves the normal anatomy of the scapula because its supraspinous portion is often tilted anteriorly toward the rib cage, in which case a greenstick fracture is produced and the tilted portion elevated.)

(M) The attachments of the latissimus dorsi muscle to the scapula are then divided extraperiosteally, and by blunt dissection a large pocket is created deep to the superior part of the latissimus dorsi muscle.
(N) The medial border of the scapula is everted by retracting it posteriorly and laterally, and the insertions of the serratus anterior muscle to the vertebral margin and to the angle of the scapula are freed extraperiosteally and marked with 2-0 Mersilene suture.

(O) Thick fibrous bands may connect the scapula to the chest wall. They should be divided to mobilize the scapula so that it can be displaced distally enough.

(P) Then, by direct pressure and without traction on the arm, the scapula is gently displaced distally to the desired position. The possibility of stretching and damaging the brachial plexus must always be kept in mind, and vigorous manipulations should be avoided. The inferior angle and distal quarter of the scapula should be in the large pocket deep to the superior part of the latissimus dorsi muscle.
(Q) If winging of the scapula is present, the inferior pole of the scapula is attached to the adjacent rib with two or three absorbable sutures. If the rhomboid muscles and other scapulocostal muscles are hypoplastic or fibrotic and marked winging of the scapula is noted, this author recommends fixing the scapula on the rib cage in a lowered and more laterally rotated position. The winging will be corrected, and the laterally rotated fixed position of the scapula will enable the patient to abduct the shoulder fully at the glenohumeral joint.
(R) Next, while the assistant holds the scapula in its lowered position, the divided and marked muscles are reattached in the following order: (1) the supraspinatus to the base of the scapular spine, (2) the subscapularis to the vertebral border, and (3) the serratus anterior to the vertebral border at a level more proximal than its original position.
(S) (4) The levator scapulae muscle, lengthened if necessary, is attached to the superior border of the scapula. (5) The rhomboids are attached to the medial border of the scapula at a more proximal site than the original position.


(T) (6) The superior part of the trapezius is reattached to the scapular spine approximately inches medial to its original position. (7) The inferior part of the trapezius is attached to the spine of the scapula more laterally and proximally than before. (8) The superior edge of the latissimus dorsi is attached to the inferolateral edge of the laterally advanced lower part of the trapezius. In the distal part of the incision, the origin of the lower part of the trapezius is followed, excess tissue is excised, and the free muscle edges are overlapped and sutured. The increased tension in this part of the muscle will serve as an added measure to hold the scapula in its lowered position. The wound is closed in layers. Closure of the skin should be subcuticular. If an associated pterygium colli is present, a Z-plasty repair may be performed.
The shoulder is immobilized in a Velpeau cast. Make sure that the elbow is not elevated. The patient is discharged from the hospital in 3 or 4 days. Approximately 4–6 weeks postoperatively, the cast is removed and active shoulder abduction and scapular depression exercises are performed to increase muscle strength. Passive exercises of the glenohumeral and scapulocostal joints are carried out to increase range of joint motion.

The operation is performed with the patient in the prone position, the head supported on a craniotomy headrest, and the neck in slight flexion. The sides and back of the neck, both shoulders, the trunk down to the iliac crests, and the upper limb on the involved side are prepared and draped. One should be able to manipulate the shoulder girdle and arms during the operation without contaminating the surgical field.
(A) A midline longitudinal incision is made that extends from the spinous process of the first cervical vertebra to that of the ninth thoracic vertebra.
(B) The subcutaneous tissue is divided in line with the skin incision. The wound margins are undermined laterally to the medial border of the scapula. The muscle arrangement should be clearly visualized.

(C) Next, the lateral border of the trapezius muscle is identified at the distal part of the wound. By blunt dissection, the lower portion of the trapezius is separated from the subjacent latissimus dorsi muscle.
(D) With a sharp scalpel the tough and tendinous origin of the trapezius muscle is detached from the spinous process. Numerous sutures are passed at the entire origin of the muscle to mark it and for use at later reattachment.

(E) In the upper part of the incision the origins of the rhomboideus major and minor muscles are sharply divided and tagged with sutures. A well-defined deep layer of fascia separates the rhomboids and the upper part of the trapezius from the serratus posterior superior and erector spinae muscles. It is vital to maintain a proper tissue plane. Preserve the aponeurosis and muscle sheet intact for secure fixation of the scapula at its lowered level.
Next, the entire muscle sheet is retracted laterally to expose the omovertebral bone or fibrous band if present. The omovertebral bar is excised extraperiosteally; it usually extends from the superior angle of the scapula to the lower cervical vertebrae. It is best to use a bone cutter for resection. Avoid injury to the spinal accessory nerve, the nerves to the rhomboids, and the descending scapular artery. The contracted levator scapulae muscle is sectioned at its attachment to the scapula. Fibrous bands attached to the anterior surface of the scapula usually restrict its downward displacement; if present, they are sectioned. Next, the scapula is everted, and the serratus anterior muscle is detached from its insertion on the vertebral border of the scapula. A periosteal elevator is used to elevate the supraspinatus muscle extraperiosteally from the supraspinous portion of the scapula and the subscapularis muscle from the deep surface of the scapula midway between the superior and inferior angles. The supraspinous portion of the scapula is resected with its periosteum. The suprascapular vessels and nerves and the transverse scapular artery should be protected from injury. These steps are illustrated on Plate 12.1 , steps K and L , of the modified Green scapuloplasty.

(F) Next, the scapula is lowered to its normal level and held in the corrected position by an assistant. The subscapularis muscle is reattached to the vertebral border of the scapula, and the supraspinatus muscle is resutured to the scapular spine. The serratus anterior muscle is reattached to the vertebral border of the scapula at a more proximal level. The latissimus dorsi muscle is reattached to the scapula. Proceeding cephalocaudally, the thick aponeurosis of the trapezius and rhomboid muscles is sutured to the spinous processes at a more distal level. It is essential that an assistant maintain the corrected level of the scapula.
(G) Because the origin of the trapezius muscle distal to the ninth thoracic vertebra is not disturbed, a redundant fold of aponeurotic tissue is created in the distal end of the trapezius muscle. This fold of soft tissue is excised and resutured.
The wound is closed in usual fashion. The skin closure is subcuticular.
A Velpeau bandage is applied and worn for 3–4 weeks. The patient is allowed to be up and around the day after the operation. After removal of the Velpeau bandage, postoperative exercises similar to those described for the modified Green scapuloplasty are carried out.
Congenital pseudarthrosis of the clavicle is a rare condition defined as a discontinuity in the midshaft of the clavicle that is present at birth, and it is a distinct clinical entity from cleidocranial dysostosis, neurofibromatosis, posttraumatic nonunion, and neonatal fracture. This anomaly typically occurs in females on the right side and can be associated with cervical ribs. Bilateral involvement is seen in 10%, and multiple families with cases of familial congenital pseudarthrosis of the clavicle have been described, although no direct genetic causal link has been found. , It may not be diagnosed until later in childhood when the cosmetic deformity becomes more prominent or the pseudarthrosis becomes symptomatic with strenuous activity.
The etiology of congenital pseudarthrosis is unknown, but it is hypothesized that pressure from the underlying subclavian artery in utero may cause pressure on the fetal clavicle and subsequent pseudarthrosis. This hypothesis is supported by the more proximal position of the right subclavian artery (in comparison to the left side) as it passes through a narrow space between the middle third of the clavicle and the first rib. Left-sided pseudarthrosis has been reported in conjunction with dextrocardia, thus further supporting this theory. The presence of cervical ribs may also be a contributing factor in this pressure phenomenon. Other theories include in utero positioning in the left occiput anterior position leading to pressure of the maternal pubic bones on the right fetal clavicle and failure of coalescence of the medial and lateral primary ossification centers of the clavicle. Histologic evaluation of resected pseudarthroses reveals hyaline cartilage caps covering both ends of the pseudarthrosis. , In one reported case, preoperative tetracycline labeling confirmed that the cartilage caps were undergoing enchondral ossification, similar to developing physes, which lends credence to the hypothesis that failure of the ossification centers to fuse leads to the pseudarthrosis.
Patients initially seen in infancy most often have a firm, painless prominence in the middle third of the clavicle. By childhood, shoulder girdle asymmetry with a mobile midclavicular prominence is present. Older patients may complain of pain with activities, but cosmetic concerns because of the drooping shoulder and a prominent midclavicular bulge are usually paramount. Development of the rest of the extremity is usually normal, with normal findings on neurologic and vascular examination, although thoracic outlet syndrome and brachial plexus compression have been reported in conjunction with this diagnosis. , Radiographic findings include smooth, well-defined bone margins surrounding the pseudarthrosis site that may be tapered, beak shaped, or enlarged.
Although descriptions of surgical treatment of pseudarthrosis of the clavicle predominate in the medical literature, no absolute indications for surgical intervention outside of thoracic outlet syndrome are recognized, , , , and certainly no studies have compared outcomes or the satisfaction of patients treated surgically versus conservatively. Since most patients are asymptomatic with few functional limitations , ; surgery is generally reserved for those with unacceptable cosmetic deformity and symptoms such as pain and functional deficits. Multiple reports describe techniques of open reduction, resection of the pseudarthrosis, and rigid plate or K-wire fixation with or without bone grafting. Younger patients may require only early resection without grafting and fixation. Union rates are high, although one case of failure of plating combined with bone marrow aspirate and synthetic bone matrix has been reported that was successfully salvaged with a free vascularized fibula graft, as well as two cases of failure of internal fixation with bovine cancellous xenograft. Patient satisfaction and cosmetic outcome are generally good following surgical treatment, although shoulder girdle symmetry is not always achieved. However, one case of massive brachial plexopathy after surgical treatment has been reported.
Goller is said to have first described this deformity in 1693. However, it was Priestly in 1856 who presented a case of a newborn with an absent ulna and a hand with only a thumb and index finger. He recognized a longitudinal deficiency state different from deformities with transverse amputations.
Ulnar deficiency is thought to be due to disruption in expression or signaling of the sonic hedgehog gene, an ulnarizing influence in the forelimb. , From studies in human embryos, the deficiency probably occurs during weeks 4 and 5 of fetal development, in the earliest stages of upper limb formation. Ogino and Kato induced longitudinal deficiency states in rats with busulfan. Ulnar deficiency probably develops earlier than radial deficiency in embryologic development.
ULD occurs as part of several syndromes whose genetic loci have been identified, and these syndromes are discussed elsewhere (see Chapter 21 ). However, the genetic details of the isolated spontaneous and most common form of ULD are unknown.
Although the old terms ulnar clubhand and radial clubhand suggest that these two conditions are similar, patients with these conditions differ in almost every respect:
Anomalies of the heart and the hematopoietic and gastrointestinal systems are common in RLD but, even though reported, are rare in ULD.
Other musculoskeletal anomalies are absent in radial deficiency but are often present in ulnar deficiency. These anomalies vary widely and include proximal femoral focal deficiency, fibular and tibial ray deficiency, phocomelia, scoliosis, clubfeet, absent patellae, congenital dislocation of the hip, coxa vara, and spina bifida.
The wrist is usually unstable and a significant clinical problem in RLD. The wrist of a patient with ULD generally needs little treatment of the deformity, which is usually mild and rarely unstable.
The elbow in radial deficiency, if present, is stable. In patients with ulnar deficiency, the elbow may be stable, unstable, or fused.
In radial deficiency, either the hand is normal or only the radial components are affected. In ulnar deficiency, the deficit in the hands may occur on either the radial or the ulnar border of the hand, or on both.
Total absence of the radius is the most common manifestation of radial deficiency. In ulnar deficiency, partial absence is far more common.
The prevalence of radial deficiency is greater than the prevalence of ulnar deficiency, but it may not be as high as previously thought. At the Texas Scottish Rite Hospital for Children in Dallas, 147 ulnar-deficient limbs and 430 radial-deficient limbs have been treated. The reported incidence of ulnar deficiency is 1 in 100,000 live births.
To assist in surgical decision making for patients with this rare deformity and after consideration of the efforts of many previous investigators, we have settled on two clinically useful classification systems: the Bayne classification for forearm and elbow deformities and the Manske classification for hand deformities ( Fig. 12.79 ).

The Bayne classification is widely used and focuses on the elbow and forearm. The stability of the wrist is usually good in forearm variants unless the ulnar anlage is present.
The Manske classification of the hand defect associated with ulnar dysplasia is more important from a functional standpoint because the substantial functional gains are derived more often from surgical operations on the hands of these patients and less often from operations on their forearms or elbows. The surgeon’s main focus should be the hand, especially the thumb–index web space.
Type 0 ulnar longitudinal dysplasia has recently been proposed to describe those with isolated ulnar-sided hand deficiency with normal forearms.
In some of the ulnar deficiency states, a curious fibrocartilaginous mass thought to represent the anlage of the absent portion of the ulna may be present. It is most often seen in Bayne types II and IV (see Fig. 12.79 ) and originates proximally in the distal end of either the ulna or the humerus. In the proximal portion, the ulnar anlage is formed of hyaline cartilage. Distally, the mass continues as fibrocartilage and may insert into the distal end of the radius, the carpal mass, or both. The structure has been described by Riordan as being similar to a fiberglass fishing rod, which allows bending but no increase in length.
Controversy exists over the significance of this fibrocartilage anlage and its effect on progressive bowing of the radius, deviation of the wrist, and dislocation of the radial head at the elbow. Some authors have suggested that the anlage is unimportant and requires no surgical treatment because follow-up in nonsurgically treated patients has not shown convincing evidence of progressive deformity. Others have suggested that early resection of the anlage at 6 months of age is indicated in healthy children.
No unusual imaging techniques are required. As in cases of RLD, clinical measurement of deformity should augment the radiographic measurements of bowing and radial deviation, which are vulnerable to inconsistency in positioning for the radiographic study.
Reasonable treatments of ULD are founded on the fact that the most important functional gains for these patients usually come from operations on their hands, not on their wrists or forearms. In particular, crucial gains are derived from improving the thumb–index web space.
Careful clinical assessment of the ulnar deviation of the wrist and passive correction should be recorded initially and at subsequent follow-up examinations because wrist deformity, elbow radial head dislocation, and radial bowing may occasionally be progressive. Relying on the radiograph alone is not adequate. Progression verified by careful clinical measurements of the ulnar deviation deformity is the strongest indication for anlage resection in Bayne types II and IV. Improving the aesthetic appearance of the malaligned hand and forearm unit is a reasonable goal of this treatment.
Early splinting and stretching of the ulnar-deviated wrist are reasonable when the wrist is deviated more than 30 degrees. Infants younger than 6 months usually tolerate a splint better than stretching. After 6 months, if the wrist still shows 30 degrees or more of fixed ulnar deviation, surgical correction should be considered.
With evidence of greater than 30 degrees of ulnar deviation or evidence of progression, resection of the anlage is appropriate for a Bayne type II or IV forearm. Most of these children are usually otherwise healthy (unlike their radial dysplastic counterparts). Early treatment at 6 months of age is appropriate because as the child grows, the forearm will almost double in length twice, and the first doubling occurs in the first 3 years of life. Early resection affords the greatest possibility of reducing the tether of growth by the anlage.
The procedure is carried out with a lazy-S incision over the ulnar aspect of the forearm and wrist. Because the flexor carpi ulnaris is absent, the ulnar nerve and artery, when present, may lie immediately under the incision in the subcutaneous tissue. Once the neurovascular structures are identified and retracted, the anlage is dissected. It is a firm, fibrous structure that originates from the proximal residual ulna in type II or from the humerus in type IV (see Fig. 12.79 ). It is critical that distal dissection carefully and completely expose the attachment of the fibrous anlage to the carpus and, when present, to the radius. The structure is completely resected from the carpus and distal end of the radius. It should be easy to passively deviate the wrist at least to neutral after resection. Complete proximal excision of the anlage is less important. Osteotomy of the radius is appropriate if excessive bowing is present. After surgery, a reasonable period of follow-up stretching and splinting is instituted, usually for approximately 6 months.
Although forearm and wrist surgeries are best done during the first year of life, hand operations should be done later. Syndactyly and first web reconstructions are important procedures to improve use of the hands in these children. Better results come from more precisely done operations on slightly larger hands, and we prefer to delay hand surgery in these children until the second year of life.
Rotational osteotomies of the metacarpals are indicated for hands with digits that are aligned in the same plane. These flat hands make prehension with the pulp of the digits impossible, and osteotomies to rotate the metacarpals or phalanges into opposition can improve prehension. The rotation achieved at surgery has a tendency to return slowly to the preoperative state, and concomitant realignment of muscle power with tendon transfers may help prevent derotation.
Creation of a one-bone forearm should be reserved for older children with type II dysplasia and is indicated only when the instability of the forearm is truly disabling, which is rarely the case. The price paid in loss of forearm rotation by a child with a severely disabled hand is rarely worth the additional stability or questionable cosmetic improvement afforded by a one-bone forearm. In our experience and that of others, any function gained from increased stability is greatly offset by the loss of forearm rotation needed to position the hand for use.
In selected type IV cases, osteotomy of the elbow synostosis may be useful, especially when the elbow deformity positions the hand behind the child and away from the opposite, uninvolved hand ( Fig. 12.80 ). Although the benefit of this surgery is potentially great, vascular compromise secondary to tethering of vessels at the osteotomy is a serious risk. Because of the potential for catastrophic complications and loss of the limb, this is not an operation for an inexperienced pediatric upper limb surgeon.

Radial head resection for types II and III has been considered when the head is dislocated. However, we try to avoid radial head resection because pain is not usually a problem for these children. Radial head excision may increase elbow instability and should generally be avoided.
Careful reexamination of 60 limbs in which upper extremity “phocomelia” was diagnosed revealed that nearly all limbs had defects consistent with a proximal continuum of either radial or ulnar longitudinal deficiencies.
Figs. 12.81 and 12.82 show the proposed extension of the Bayne classification of radial and ulnar longitudinal dysplasia for children with a proximal limb anomaly. Physicians who treat these children, whether pediatrician or orthopaedic surgeon, should recognize the association of potentially life-threatening medical conditions with any form of radial dysplasia.


Synostoses represent a failure of differentiation of parts. Although the precise cause of synostosis of the forearm is unknown, the time of occurrence is almost certainly during the earliest portion of embryonic limb development. No teratogenic trigger is known, but in a study by Jaffer and colleagues, 2 of 15 infants with fetal alcohol syndrome had the anomaly. At approximately 5 weeks after conception, the elbow forms from the three cartilaginous condensations representing the humerus, radius, and ulna. For a short period these cartilage analogues share a common perichondrium. A cavitation process ensues in which the three distinct bones are formed. If this process fails, enchondral ossification results in the bony synostosis. Because the forearm bones separate at a time when the fetal forearm is in pronation, essentially all forearm synostoses are fixed in this position.
The condition occasionally affects other members of the family, usually in an autosomal dominant inheritance pattern.
Because the event that causes radioulnar synostosis occurs so early in fetal development, when all organ systems are forming, it may be seen in conjunction with other syndromes, including Apert syndrome (acrocephalosyndactyly), Carpenter syndrome (acropolysyndactyly), arthrogryposis, mandibulofacial dysostosis, Klinefelter syndrome, and Poland anomaly. , Approximately one third of these patients have other anomalies, but no common pattern is seen. , Cardiovascular (tetralogy of Fallot and ventricular septal defects), thoracic (absence of the first rib or pectoral muscles), genitourinary, gastrointestinal, and central nervous system (microcephaly, hydrocephalus, encephalocele, mental retardation, delayed milestone attainment, hemiplegia) anomalies, as well as other musculoskeletal anomalies, are seen in association with radioulnar synostosis, but it is an isolated anomaly in one third of cases.
Boys are slightly more often affected with radioulnar synostosis than girls are (3:2 ratio). The lesion is bilateral in 80% of cases. The diagnosis is often delayed, and patients frequently lack functional complaints when the position of fixed pronation is moderate and carpal compensatory hypermobility is adequate. Treatment of affected children is usually sought between 3 and 6 years of age. The amount of functional limitation caused by radioulnar synostosis and the need for operative treatment have been controversial, with some noting that operative correction is rarely indicated and that when surgical treatment is selected, it should be based more on limitations in function than on physical or radiographic findings.
Others have reported that most patients do have functional limitations related to the synostosis, including difficulty using a spoon or pencil, buckling belts, fastening buttons, and grasping small objects. Some patients have also reported difficulty with sporting activities.
It is important that a careful clinical measurement of the patient’s exact forearm fixed position be made as accurately as possible because intraoperative decisions are based in part on this assessment. All degrees of fixed pronation are seen, but the most common are less than 30 degrees of pronation (40%) and more than 60 degrees of pronation (40%). By comparing the angle of pronation with the plane of the palm, one may assess the compensatory rotation through the carpus, which is usually increased in these patients and enhances use of the hand, especially when the shoulder is normal.
Radiographs typically show proximal radioulnar coalescence, but extensive synostosis extending distally into the forearm is occasionally seen. In our experience this is associated with Holt-Oram syndrome. Several classification systems based on radiographic appearance have been devised. Tachdjian noted three types :
Type I: True congenital radioulnar synostosis, or the headless type. Here the radial head is absent and a bony fusion of the radius to the ulna is present. The distal ends are fused and the radius is bowed, is thicker than the ulna, and is not attached to it distally.
Type II: Dislocated radial head type. The malformed radial head is posteriorly dislocated and the proximal end of the radius is fused with the ulna just below.
Type III: No bony synostosis is present, but a thick fibrous interosseous ligament forms and attaches to each bone just distal to their proximal ends and prevents rotation. This is the rarest type in the Tachdjian classification.
In the classification of Cleary and Omer, four radiographic types represent a continuum from fibrous to complex bony synostosis. Cleary and Omer did not believe that this classification was helpful in assessing function or making decisions about treatment:
Type I: Fibrous synostosis, with no bone changes but a stiff and smaller forearm (6/35, or 17%)
Type II: Osseous synostosis, radial head present and reduced (3/35, or 9%)
Type III: Osseous synostosis, radial head present and posteriorly dislocated (20/35, or 57%)
Type IV: Osseous synostosis, radial head present and anteriorly dislocated (6/35, or 17%)
Sachar and colleagues reported progressive dislocation of the radial head in some patients.
In our experience, most patients and parents envision restoration of forearm motion and are often not satisfied with adjustment of the position of the fixed forearm. This has led a few surgeons to attempt correction of the synostosis by inserting various material, both inert and biologic. Poor follow-up of these patients has precluded acceptance of any of these techniques by most hand and pediatric surgeons.
Even a simple positional change of the forearm may be associated with complications. Surgical intervention has been recommended by most surgeons only when a significant amount of pronation (usually > 60 degrees) is associated with functional limitations and complaints. Recommendations for the optimal postoperative position of the forearm vary ( Table 12.8 ).
| Authors (Reference) | Recommendation | Comments |
|---|---|---|
| Green and Mital ( ) | 20 degrees of supination | 20–35 degrees for the dominant hand in bilateral cases |
| Simmons and Waters ( ) | 20 degrees of pronation | Neutral for the nondominant hand in bilateral cases |
| Ogino and Hikino ( ) | Neutral to 20 degrees of supination for unilateral or the nondominant hand in bilateral cases | Allows the use of chopsticks |
| Hung ( ) | 70%–100% pronation | |
| Tsujino and Hooper ( ) | Neutral to 30 degrees of pronation for the dominant hand, neutral for the nondominant hand | |
| Ramachandran et al. ( ) | 10 degrees of supination for all | Compensatory movements of the shoulder and wrist allow most activities of daily living |
Although correction by the Ilizarov method has been reported, we have no experience with it.
The reported results of surgical treatment by rotational osteotomy and the complications have varied, with rates of good to excellent results ranging from 82% to 100% and complication rates from 15% to 33%. However, no study has demonstrated objective improvement after surgery with validated outcomes scores. Reported complications include wound infection, loss of correction, vascular compromise, compartment syndrome, and nerve injury.
We frequently advise against any surgical treatment. Although many patients definitely have limitations in function, most want full forearm rotation, not just an adjustment of the fixed position. The risk associated with surgical intervention in these cases should be weighed against the benefit of surgically changing the fixed position. When indicated, we favor osteotomies of the radius and ulna as reported previously.
In earlier practice it was thought that children with amyoplasia or “classic” arthrogryposis should not undergo surgery on the upper limbs. The children often adapted to the internally rotated shoulders, the stiff extended elbows, and the flexed wrists by developing a crossed-limb bimanual position that was functionally limited. Today, much greater function is achieved with early repositioning of the upper limbs, passive range of the elbows, adding active elbow flexion when possible, and bringing the wrists into extension to allow access to desktops and computers, as well as for feeding and personal care. The following principles must be respected:
Joint motion should be preserved or enhanced and not intentionally sacrificed.
Passive motion of a joint must be gained before restoring active motion.
Bimanual functional patterns should be facilitated; both hands are usually needed for all activities because multiple joints are involved and motion and strength are limited.
The entire upper limb should be considered a functional unit, and adjustments in position of the shoulder, elbow, wrist, and hand may be necessary. The hands should be positioned to access a working tabletop, especially important in this computer age.
The ultimate goal for most patients is independent living and employment, and proper upper limb management may help make this goal easier. Our preference is to complete the major changes in limb position by 4 years of age. We believe that by 8 years of age, use patterns are so well established that the child will have a much harder time adapting well to a new limb position, and decisions for older children must be made especially carefully.
Our first goal is to achieve passive elbow flexion. The second priority is to correct humeral rotation and then reposition the wrist and correct the thumb-in-palm deformity. Surgical procedures can be combined to minimize the number of anesthetic inductions needed, and they can often be done at the time of lower limb surgery if the patient and parents can tolerate the additional postoperative immobility.
When these patients are seen early in life stretching and splinting is indicated as there is a “catch-up” period during the first year of life and improvements in both active and passive motion of the upper extremities can frequently be seen. In general, physical findings in the upper extremities are symmetric. If significant asymmetry is noted then further investigation of the C-spine is warranted.
Importantly, every child is different and treatment plans must be individualized.
The shoulder is often contracted into adduction and internal rotation by joint incongruity and soft tissue contractures. Scapulothoracic motion may also be limited, in addition to the lack of glenohumeral motion. Typically, little active flexion or abduction of the shoulder is available. The major obstacle to function of the elbow and hand is the internal rotation contracture of the shoulder. Consequently, one of the most useful procedures in the upper limb is an external rotation osteotomy of the humerus to allow the forearm to clear the body as the elbow flexes. This allows the hands to function at the anterior midline. The osteotomy may be done at the midshaft level and fixed with a plate or be done at the supracondylar level with crossed-pin fixation. After surgery the arm should be immobilized only long enough to achieve early bony union. An osteotomy cannot be done at the same time that the elbow release is done because rehabilitation differs for the two procedures.
The elbow deformity is usually full extension without active flexion but with retention of some active triceps function. Many children have some variable passive flexion, but many others have a fixed contracture in full extension, and it may be difficult to determine the axis of joint motion. The goal of surgical care of the upper limb is elbow flexion greater than 90 degrees. Tricepsplasty followed by assiduous exercising will predictably increase the passive ROM of the elbow and allow hand-to-mouth activities without requiring subsequent tendon transfer surgery. Elbow stability in extension can usually be preserved, and if an elbow flexion contracture develops, modified crutches can be used. Most children who require extensive ambulatory aids for their lower limb involvement do not continue to be community ambulators into adulthood, and deferral of upper limb treatment to preserve crutch gait will compromise their potential use of the upper limbs in a computer-based livelihood later in life.
Posterior elbow release with tricepsplasty is done through a posterior curvilinear incision. The ulnar nerve should be released from its tunnel and transferred anteriorly into a subcutaneous or submuscular tunnel to protect it. A soft tissue sling is secured to the medial epicondyle to prevent posterior slipping of the nerve after surgery.
The triceps is lengthened through a long W incision, with the lateral and medial limbs of the W extending into the triceps expansion on both sides of the olecranon. The central fibers of the triceps tendon are released from the tip of the olecranon. The posterior capsule of the elbow is opened and the elbow gently flexed to serially release the most posterior fibers of the collateral ligaments as necessary and allow easy passive flexion greater than 90 degrees. Care must be taken to make sure that the motion is flexion and not “hinging,” and release of the proximal anterior capsule may be necessary to make room for the coronoid process and prevent this hinge phenomenon. Care must also be taken to avoid fracture or epiphyseal separation during manipulation into flexion. It should be possible both to flex the elbow and to maintain medial–lateral stability. The triceps is closed in a long V-to-Y design with the medial and lateral limbs closed over the central tongue of the triceps tendon and the distal defect at the olecranon closed primarily.
After surgery, the elbow is splinted in at least 90 degrees of flexion for the first 3 weeks. Passive ROM exercises are begun and a resting splint is used for another 3 weeks. The parents should be taught to work with the child over the next several months to maintain both flexion and extension. Parents must know that this is arduous and time-consuming and that children often do not enjoy the therapy sessions.
The ideal tissue to gain active elbow flexion would be an expendable muscle, synergistic with elbow flexion, that can be appropriately aligned and has good strength. It cannot be unopposed or a flexion contracture will develop. Unfortunately, this combination is not available for most children with this disorder. Preoperative selection of a muscle–tendon unit for transfer is difficult and not aided by imaging studies. It may be necessary to make an incision over the proposed muscle to evaluate its bulk, color, and contractility (with electrical stimulation) before using that muscle for transfer.
The entire pectoralis major muscle can be transferred by mobilizing it on its neurovascular pedicle. The muscle is routed so that the tendon of insertion is transferred to the coracoid or acromion, which becomes the new origin of the muscle ( Fig. 12.83 ). The broad fascia of the origin is mobilized and transferred to the ulna, either directly or with a graft if necessary. This may be a good option if the pectoralis major is strong. The disadvantage of this transfer is that the scars may be disfiguring, especially in girls, with resulting breast asymmetry if done unilaterally. In addition, the result achieved deteriorates over the long term with the development of an intractable flexion contracture.

For this transfer the entire latissimus dorsi is mobilized on its pedicle and moved anteriorly through the axilla ( Figs. 12.84 to 12.86 ). The original tendon of insertion is moved to the coracoid or acromion, where it becomes the origin of the new muscle arrangement. The remainder of the muscle and its fascial prolongation are attached to the ulna distal to the coronoid process. This transfer is used if the latissimus is strong, but in most arthrogrypotic children the muscle is fibrotic and not of satisfactory quality for transfer.



This transfer is feasible because the long head of the triceps has a separate neurovascular pedicle and is sufficiently independent from the rest of the triceps to be easily separated. The size of the triceps muscle is variable in these children and directed examination may be very difficult. We use MRI to assess the size of the lateral, medial, and long head of triceps muscle. Besides having an adequate long head, sufficient lateral and/or medial head must be present to allow active elbow extension otherwise a severe elbow flexion contracture can result after transfer. A fascia lata graft is used to prolong the tendon and allow insertion into the proximal end of the ulna. Although the muscle is not large, satisfactory active elbow flexion can be gained without loss of active elbow extension in selected cases. A description of this procedure can be found in a recent publication.
Complete triceps transfer is mentioned only to be condemned. It had been mentioned often in the literature and is still used by some surgeons; however, the resulting unopposed elbow flexion is functionally worse than the lack of elbow flexion. Fixed, resistant contractures develop and are difficult, if not impossible, to salvage.
Transfer of the flexor pronator muscle origin to the anterior aspect of the humerus to flex the elbow works only if the patient is able to isolate the muscle before surgery and can also stabilize the wrist against excess flexion with the radial wrist extensors. In children with arthrogryposis, this operation usually results only in unacceptable greater wrist flexion because the child is unable to extend the wrist in a cocontraction at the same time that the transferred flexor muscle origin flexes the elbow. This procedure should not be done.
Theoretically, the gracilis can serve as a free muscle donor to the anterior aspect of the arm to flex the elbow. Innervation would have to be by nerve transfer from the intercostals or other donor tissue, which sacrifices function of a muscle in or near the upper limb. In addition, the gracilis is often abnormal, absent, or fibrotic in children with arthrogryposis. However, this has been reported to be a successful option in select patients. ,
The wrist in arthrogryposis is usually flexed and deviated ulnarward, and a small ROM is available. All the structures on the volar side are contracted, including the joint capsule, the tendons, the skin, and subcutaneous tissue. The radial wrist extensors are fibrotic and nonfunctional, but the extensor carpi ulnaris is often spared. Carpal coalitions are common.
Early stretching and splinting have been recommended, but their efficacy is uncertain. Overzealous, painful stretching is clearly inappropriate. Surgical treatment is indicated to reorient the wrist into a neutral position while maintaining available motion. We have found midcarpal biplanar wedge resection to be the most useful procedure for correcting flexion and ulnar deviation. Simultaneous release of tight volar structures and transfer of an appropriate tendon to provide radial extension can be done if a donor tendon is available. Frequently, the extensor carpi ulnaris is available and works well for this transfer. , , ,
Release of the tight volar structures is accomplished through a transverse or longitudinal incision on the flexor surface of the distal third of the forearm. The fascia over the wrist flexors is opened. If the flexor carpi ulnaris has muscle bulk and excursion, it may be lengthened either at the intramuscular level or by Z-lengthening. In the usual case the flexor carpi ulnaris, flexor carpi radialis, and palmaris longus are fibrotic and should be divided at the wrist to increase passive extension. The superficial fascia should also be divided transversely.
A second dorsal incision is made at the level of the proximal carpal row. The digital and thumb extensors are isolated and protected. The two radial wrist extensors are isolated from the dorsal capsule and divided as far proximally as possible in the distal end of the forearm. These tendons usually become confluent with the dorsal capsule and are difficult to dissect. No muscle will be functioning to extend the wrist through these tendons. The extensor carpi ulnaris is mobilized from the insertion, across the wrist, and into the midforearm, where it is exposed in another incision. It is divided at its insertion and pulled into the proximal part of the wound, with care taken to keep all healthy musculature attached to the tendon. The extensor carpi ulnaris is then transferred across the dorsal aspect of the wrist, under subcutaneous fat, into the wound at the dorsum of the wrist.
The radiocarpal capsule is left intact. Just distal to the radiocarpal joint, a distally based capsular flap can be developed and reflected toward the metacarpals to expose the bony or cartilaginous dorsal carpus. A biplanar wedge of bone is scored, incised, and then removed from the midcarpus, distal to the wrist joint. The two cuts are made with the wrist supported in its maximally extended position, with the proximal cut perpendicular in both planes to the long axis of the forearm and the distal cut perpendicular in both planes to the long axis of the metacarpus. This creates an asymmetric wedge, wider dorsally and radially, that when removed from the wrist allows the defect to be closed and the wrist to be brought to neutral position ( Figs. 12.87 and 12.88 ).


The cuts are adjusted so that the surfaces can be apposed. Great care must be taken to preserve the radiocarpal joint. Several nonabsorbable sutures are placed before closing the defect and transfixing the wrist with a heavy Kirschner wire. The sutures are tied to secure the carpus in a coapted position, and then the dorsal capsule is trimmed and closed over the osteotomy. The tendon of the extensor carpi ulnaris is sutured to the stump of the radial wrist extensor tendons with nonabsorbable suture ( Fig. 12.89 ).

The wrist is splinted and casted for 6 weeks and then protected in a removable splint for at least another 6 to 12 months.
If the child is still young, the unossified carpus can be cut with a scalpel. In a child with more bone present, small osteotomies will be needed.
Proximal row carpectomy was used in the past, but because the capitate and radial articulations are grossly abnormal and because carpal coalitions are usually present, albeit not obvious in a young child, this operation results in increased stiffness and destruction of the only mobile joint in the wrist.
Wrist fusion should be avoided because all motion is lost at the wrist of an arthrogrypotic child. It can be reserved for a salvage operation to correct a stiff, unacceptable position in an older child who has no other option to preserve motion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here