Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Immunity refers to protection against infections. The immune system is the collection of cells and molecules that are responsible for defending the body against the countless pathogens that individuals encounter. Defects in the immune system are the cause of immunodeficiency diseases, which render individuals easy prey to infections. But the immune system is itself capable of causing tissue injury and disease, called hypersensitivity disorders .
This chapter is devoted to diseases caused by too little immunity or too much immunologic reactivity. We also consider amyloidosis, a disease in which an abnormal protein, usually derived from fragments of antibodies or produced during chronic inflammatory disorders, is deposited in tissues. First, we review some important features of normal immune responses that are relevant to our understanding of immunologic diseases.
Defense against pathogens consists of two types of reactions ( Fig. 5.1 ). Innate immunity (also called natural, or native, immunity) is mediated by cells and proteins that are always present (hence the term innate ), poised to react against infectious pathogens. These mechanisms are called into action immediately in response to infection and thus provide the first line of defense. Some of these mechanisms are also involved in clearing damaged cells and tissues.
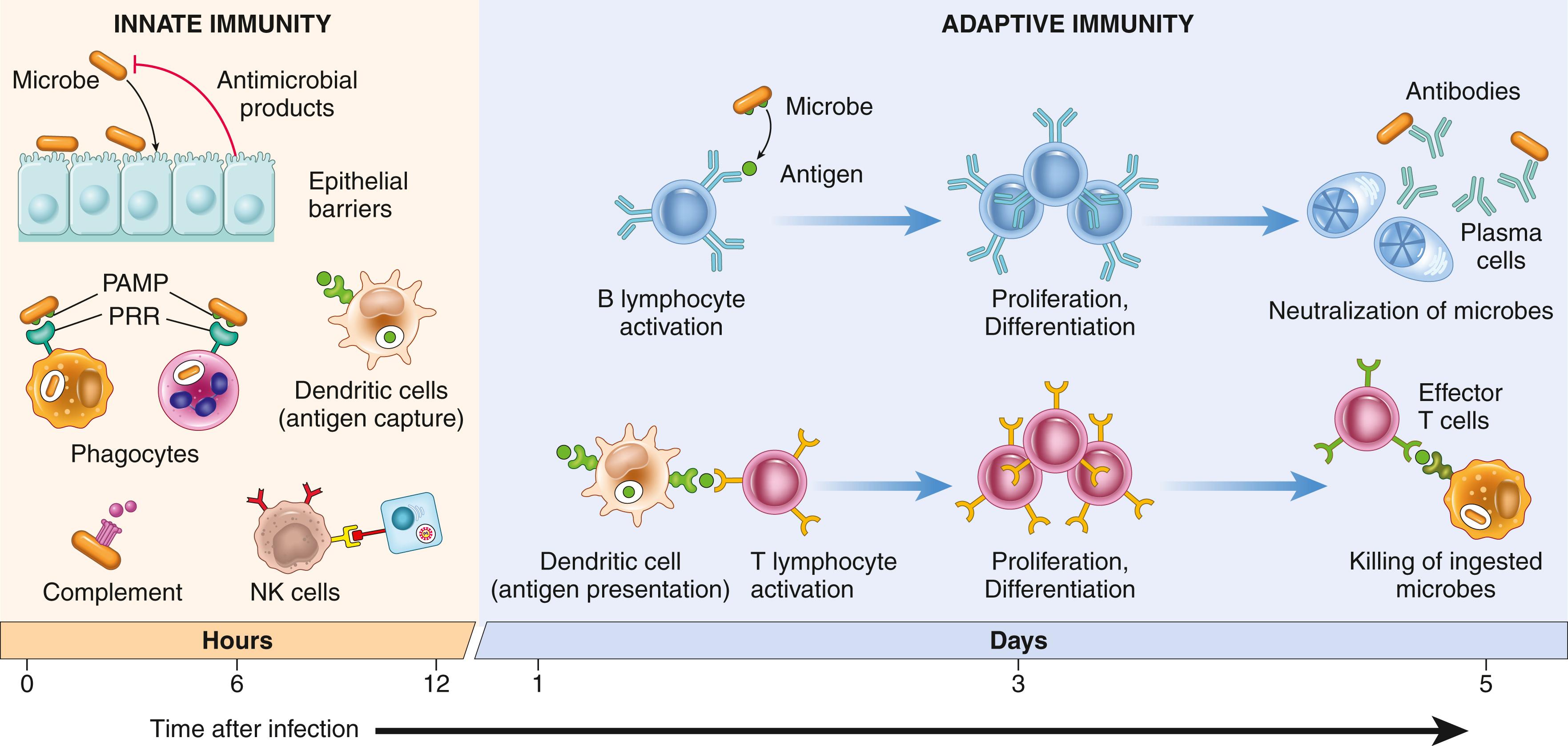
Many pathogens have evolved to resist innate immunity, and protection against these infections requires the more specialized and powerful mechanisms of adaptive immunity (also called acquired, or specific, immunity). Adaptive immunity is normally quiescent and responds (or adapts ) to the presence of infectious agents by generating potent mechanisms for neutralizing and eliminating the pathogens. By convention, the terms immune system and immune response usually refer to adaptive immunity. The adaptive immune response typically takes 3 to 7 days to become fully active; innate immune mechanisms provide host defense during this critical early window after an infection.
The major components of innate immunity are epithelial barriers that block the entry of microbes, phagocytic cells (mainly neutrophils and macrophages), dendritic cells (DCs), natural killer (NK) cells and other innate lymphoid cells, and several plasma proteins, including the proteins of the complement system ( Chapter 2 ).
Tissue-resident phagocytes, dendritic cells, and many other cells, such as epithelial cells, express receptors that detect the presence of infectious agents and substances released from dead cells. The microbial structures recognized by these receptors are called pathogen-associated molecular patterns (PAMPs) ; they are shared among microbes of the same type, and they are essential for the survival and infectivity of the microbes (so the microbes cannot evade innate immune recognition by mutating these molecules). The substances released from injured and necrotic cells are called damage-associated molecular patterns (DAMPs) . The cellular receptors that recognize these molecules are called pattern recognition receptors . It is estimated that innate immunity uses about 100 different receptors to recognize a few thousand molecular patterns.
Pattern recognition receptors are located in all the cellular compartments where pathogens may be present: plasma membrane receptors detect extracellular pathogens, endosomal receptors detect ingested microbes, and cytosolic receptors detect microbes in the cytoplasm ( Fig. 5.2 ). Several classes of these receptors have been identified.
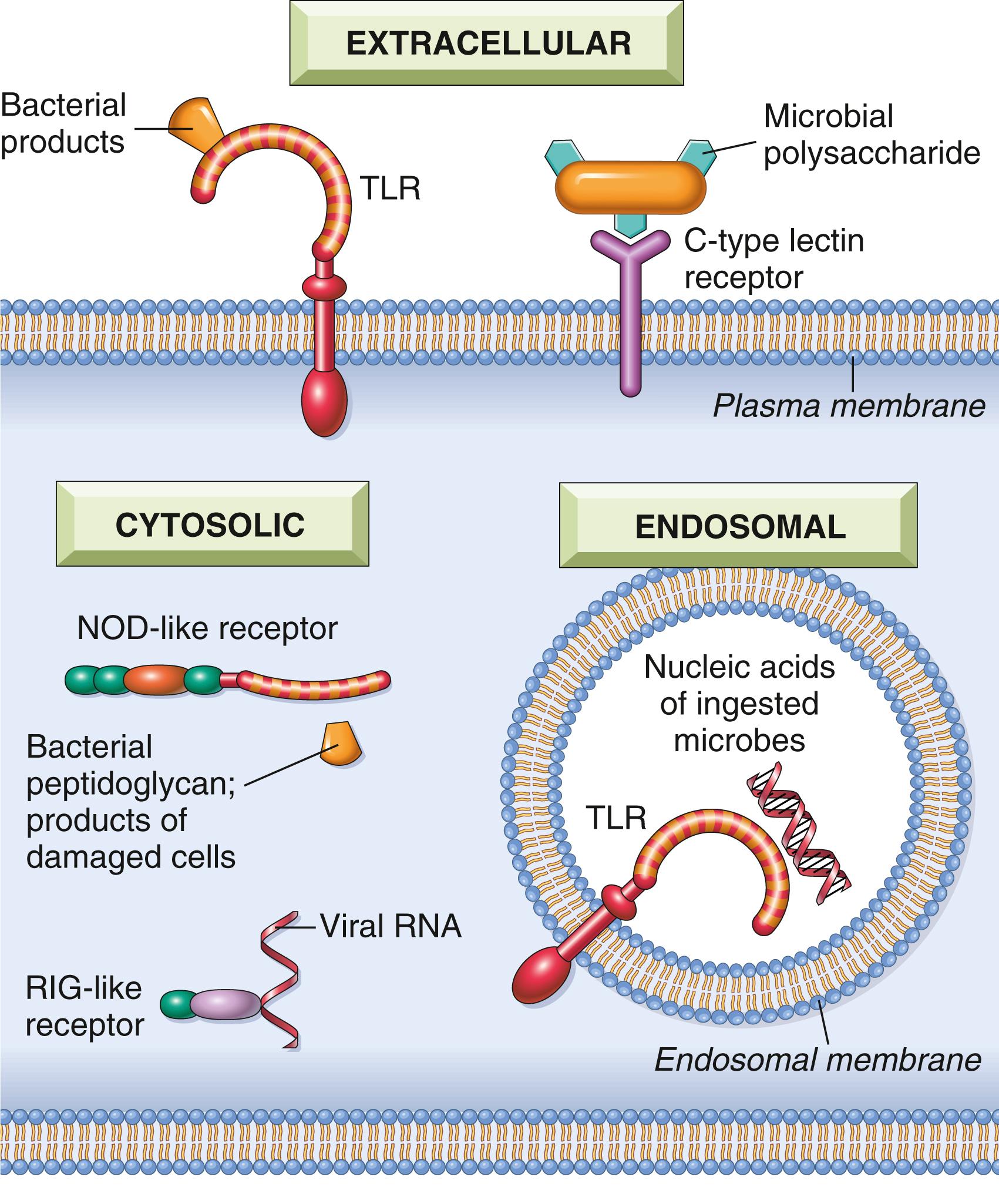
The best known of the pattern recognition receptors are the Toll-like receptors (TLRs). The plasma membrane TLRs recognize bacterial products such as lipopolysaccharide (LPS), and endosomal TLRs recognize RNA and DNA from viruses and bacteria that are phagocytosed into endosomes. Recognition of microbes by TLRs activates transcription factors that stimulate the production of mediators of inflammation (e.g., cytokines), antiviral cytokines called interferons (IFNs), and proteins such as costimulators (discussed later) that promote lymphocyte activation and the more potent adaptive immune responses.
NOD-like receptors (NLRs) are cytosolic receptors named after the founding members of this group, NOD-1 and NOD-2. They recognize a wide variety of substances, including products of necrotic cells (e.g., uric acid and released ATP); ion disturbances (e.g., loss of K + ), which indicate cell damage; and some microbial products. Several of the NLRs signal via a cytosolic multiprotein complex called the inflammasome, which activates an enzyme (caspase-1) that cleaves a precursor form of the cytokine interleukin-1 (IL-1) to generate the biologically active form. As discussed in Chapter 2 , IL-1 is a mediator of inflammation that recruits leukocytes and induces fever. Gain-of-function mutations in the NLRs result in systemic inflammatory disorders called autoinflammatory syndromes, which, as expected, respond well to treatment with IL-1 antagonists. The NLR-inflammasome pathway may also play a role in a number of chronic disorders marked by inflammation. For example, recognition of urate crystals by a class of NLRs underlies the inflammation associated with gout.
Other receptor families that play a role in innate immunity include the following.
C-type lectin receptors expressed on the plasma membrane of macrophages and DCs detect microbial (bacterial and fungal) polysaccharides and stimulate phagocytosis and inflammatory reactions.
Several types of receptors detect the nucleic acids of viruses that replicate in the cytoplasm of infected cells and stimulate the production of type I IFNs. Some of these receptors also recognize host DNA if it accumulates in the cytosol, which is often an indication of nuclear damage, leading to an inflammatory response that clears the injured cell. Excessive activation of these receptors may occur because of genetic defects in their regulation or defects in endonucleases that allow self DNA to accumulate. The resulting unregulated production of interferon causes systemic inflammatory diseases that are called interferonopathies.
G protein–coupled receptors on neutrophils, macrophages, and most other types of leukocytes recognize short bacterial peptides containing N-formylmethionyl residues, which initiate bacterial proteins, and stimulate migration of the leukocytes.
The innate immune system provides host defense by two main reactions:
Inflammation. Cytokines and products of complement activation, as well as other mediators, are produced during innate immune reactions and trigger the vascular and cellular components of inflammation ( Chapter 2 ). The recruited leukocytes destroy pathogens and ingest and eliminate damaged cells.
Antiviral defense. Type I interferons produced in response to viruses act on infected and uninfected cells and activate enzymes that degrade viral nucleic acids and inhibit viral replication.
In addition to these defensive functions, the innate immune system generates signals that stimulate the subsequent, more powerful adaptive immune response. Some of these signals are described later.
The adaptive immune system consists of lymphocytes and their products, including antibodies. In contrast to the limited number of microbial molecules recognized by the innate immune system, the adaptive immune system can recognize a vast array of foreign substances.
There are two types of adaptive immunity: humoral immunity, mediated by soluble proteins called antibodies that are produced by B lymphocytes (also called B cells ), and cell-mediated (or cellular) immunity, mediated by T lymphocytes (also called T cells ). Antibodies provide protection against extracellular pathogens in the blood, mucosal organs, and tissues. T lymphocytes are important in defense against microbes that have adapted to survive and replicate inside cells. They work by either directly killing infected cells (the function of cytotoxic T lymphocytes) or by activating phagocytes to kill ingested microbes, through the production of cytokines such as IFN-γ (made by helper T cells).
The cells of the immune system consist of lymphocytes, most of which have specific receptors for antigens and mount adaptive immune responses; specialized antigen-presenting cells (APCs), which capture and display microbial and other antigens to lymphocytes; and various other cells such as phagocytes and eosinophils. We next discuss the major cell types involved in adaptive immune responses ( Fig. 5.3 ).
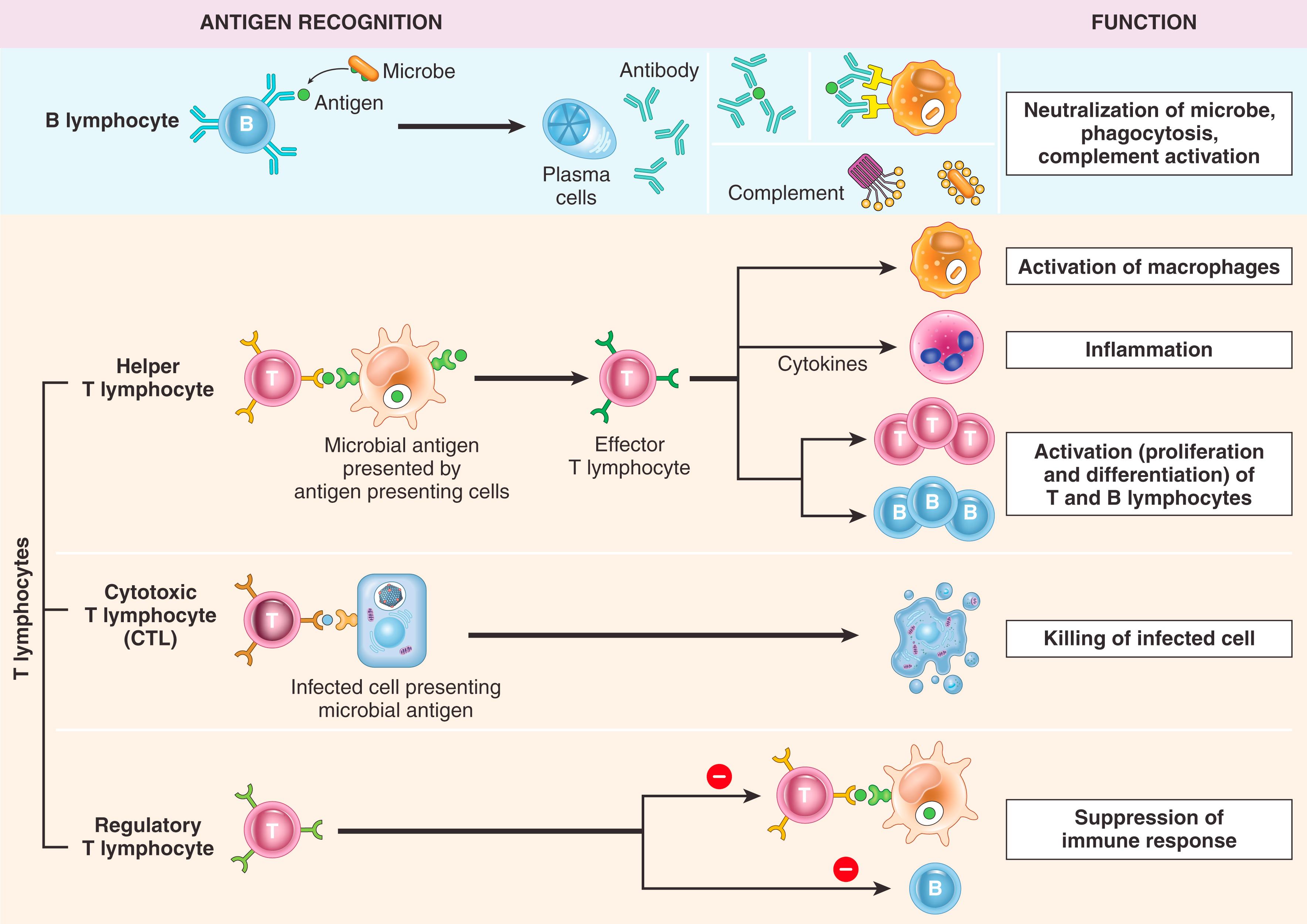
T lymphocytes, so called because they mature in the thymus, develop into the effector cells of cellular immunity following their activation and also stimulate B cells to produce antibodies against protein antigens. T cells constitute 60% to 70% of the lymphocytes in peripheral blood and are the major lymphocyte population in splenic periarteriolar sheaths and lymph node interfollicular zones. T cells cannot recognize free or circulating antigens; instead, the vast majority (>95%) of T cells see only peptide fragments of intracellular proteins displayed by molecules of the major histocompatibility complex (MHC), discussed in more detail later.
There are two major classes of T cells that are distinguished by the expression of CD4 or CD8 on their surfaces. CD4+ T cells are called helper T cells because they secrete soluble molecules (cytokines) that stimulate (help) B cells to produce antibodies and help macrophages to destroy phagocytosed microbes. The central role of CD4+ helper cells in immunity is highlighted by the severe immune defects that result from destruction of these cells by human immunodeficiency virus (HIV) infection (described later). The most important function of CD8+ T cells is to directly kill virus-infected cells and tumor cells; hence, they are called cytotoxic T lymphocytes (CTLs) .
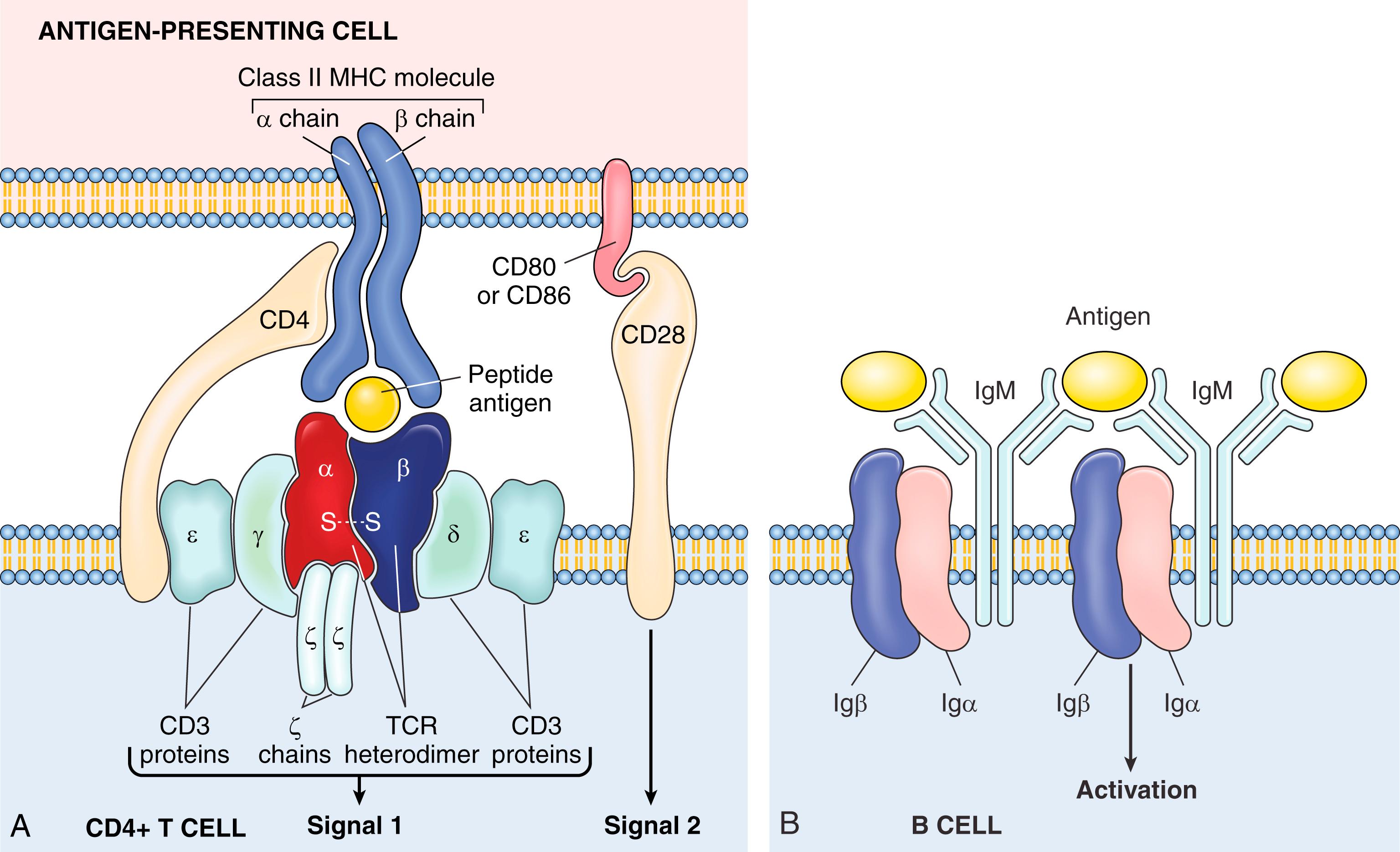
Peptide antigens presented by MHC molecules are recognized by the T-cell receptor (TCR), a heterodimer that in most T cells is composed of disulfide-linked α and β protein chains ( Fig. 5.4A ). Each chain has a variable region that participates in binding a particular peptide antigen and a constant region that interacts with associated signaling molecules. The sequence diversity of the antigen-binding portions is a consequence of the rearrangement and assembly of a multitude of TCR gene segments into functional TCR genes. T cells also express a number of other molecules that serve important functions in immune responses. The CD3 complex is noncovalently associated with the TCR and initiates activating signals following TCR recognition of antigen. During antigen recognition, CD4 molecules on T cells bind to invariant portions of class II MHC molecules (described later) on selected APCs; in an analogous fashion, CD8 binds to class I MHC molecules. CD4 is expressed on 60% to 70% of T cells, whereas CD8 is expressed on about 30% to 40% of T cells. Other important invariant proteins on T cells include CD28, which functions as the receptor for molecules called costimulators that are induced on APCs by microbes, and various adhesion molecules that strengthen the bond between the T cells and APCs and control the migration of the T cells to different tissues.
T cells that function to suppress immune responses are called regulatory T lymphocytes . This cell type is described later, when we discuss immunologic tolerance.
MHC molecules are fundamental to T-cell recognition of antigens, and genetic variations in MHC molecules are associated with graft rejection and autoimmune diseases; hence, it is important to review the structure and function of these molecules. The MHC was discovered on the basis of studies of graft rejection and acceptance (tissue, or “histo,” compatibility). The normal function of MHC molecules is to display peptides for recognition by CD4+ and CD8+ T lymphocytes. In each individual, T cells recognize only peptides displayed by that person’s MHC molecules, which, of course, are the only MHC molecules that the T cells normally encounter. This property of T-cell antigen recognition is called MHC restriction . Because T cells recognize MHC molecules, variations in these molecules among individuals elicit strong immune responses. This is the basis of graft rejection, described later.
The human MHC, known as the human leukocyte antigen (HLA) complex, consists of a cluster of genes on chromosome 6 ( Fig. 5.5 ). On the basis of their chemical structure, tissue distribution, and function, MHC gene products fall into two main categories:
Class I MHC molecules are expressed on all nucleated cells and are encoded by three closely linked loci, designated HLA-A, HLA-B, and HLA-C. Each of these molecules consists of a polymorphic α chain noncovalently associated with an invariant β 2 -microglobulin polypeptide (encoded by a separate gene on chromosome 15). The extracellular portion of the α chain contains a cleft where the polymorphic residues are located and where foreign peptides bind to MHC molecules for presentation to T cells, and a conserved region that binds CD8, ensuring that only CD8+ T cells can respond to peptides displayed by class I molecules. Class I MHC molecules bind and display peptides derived from protein antigens present in the cytosol of the cell (e.g., viral and tumor antigens).
Class II MHC molecules are encoded by genes in the HLA-D region, which contains three subregions: DP, DQ, and DR. Class II molecules are heterodimers of noncovalently linked α and β subunits. Unlike class I MHC molecules, which are expressed on all nucleated cells, expression of class II MHC molecules is restricted to a few cell types, mainly APCs (notably, dendritic cells), macrophages, and B cells. The extracellular portion of class II MHC molecules contains a cleft for the binding of antigenic peptides and a region that binds CD4. In general, class II MHC molecules bind peptides derived from extracellular proteins, for example, from microbes that are ingested and then broken down inside the cell. This property allows CD4+ T cells to recognize the presence of extracellular pathogens.
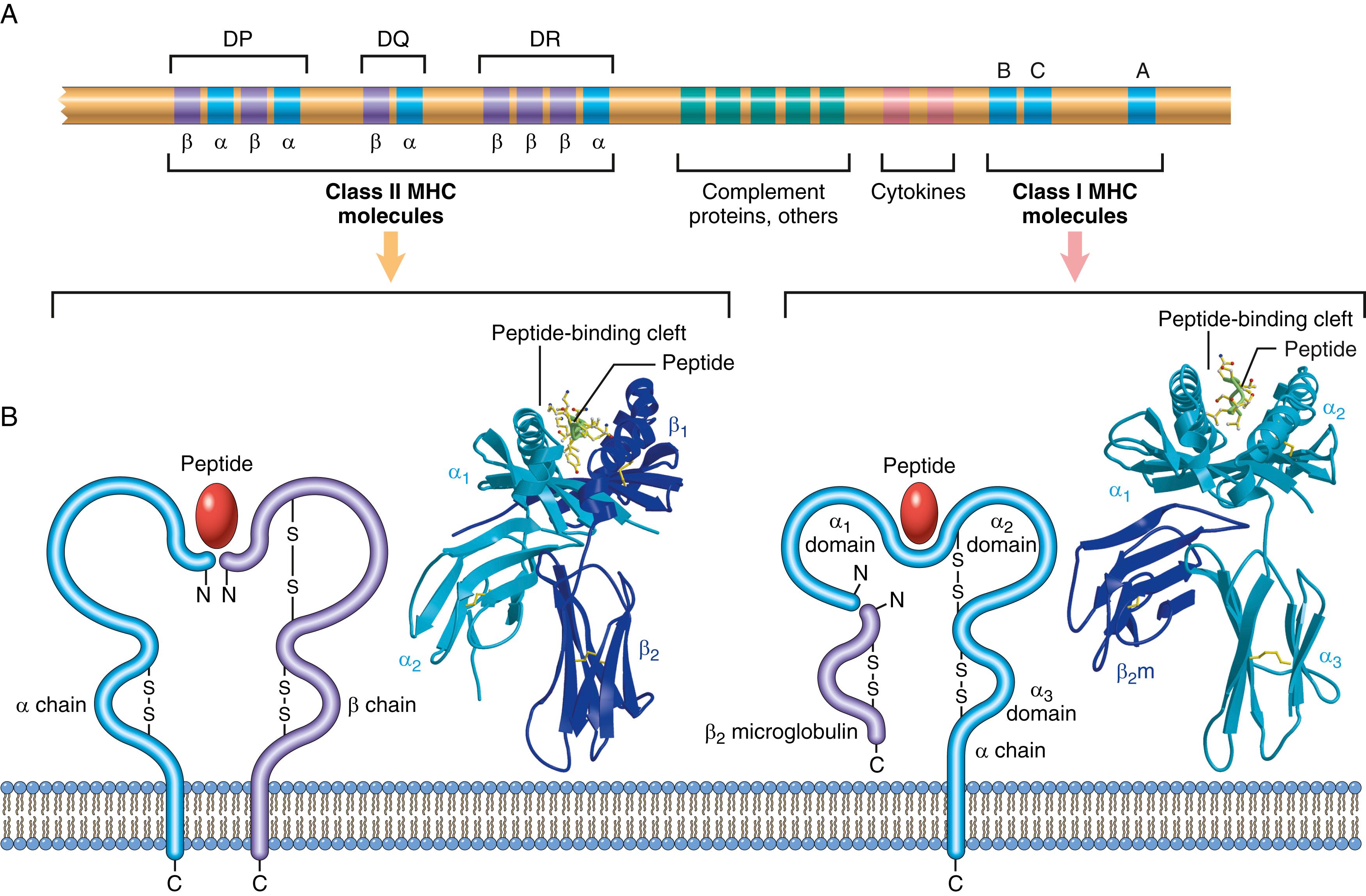
HLA genes are highly polymorphic; that is, there are alternative forms (alleles) of each gene at each locus (estimated to number over 10,000 for all HLA genes and over 3500 for HLA-B alleles alone). Each individual expresses only one set of HLA genes, and each MHC molecule can display only one peptide at a time. The many MHC variants in the population evolved to display the multitude of microbial peptides that could be encountered in the environment. As a result of this polymorphism, a vast number of combinations of HLA molecules exist in the population. HLA genes are closely linked, so they are passed from parent to offspring en bloc and behave like a single locus with respect to their inheritance patterns. Each set of maternal and paternal HLA genes is referred to as an HLA haplotype . Because of this mode of inheritance, the probability that siblings will inherit the same HLA alleles is 25%. By contrast, the probability that an unrelated donor will share the same HLA genes is very low. The implications of HLA polymorphism for transplantation are obvious; because each person has HLA alleles that differ to some extent from those of every other unrelated individual, grafts from unrelated donors will elicit immune responses in the recipient and be rejected unless the T cell response is suppressed (see discussion later). Only identical twins can accept grafts from one another without fear of rejection.
The inheritance of particular MHC alleles influences both protective and harmful immune responses. The ability of any given MHC allele to bind the peptide antigens generated from a particular pathogen will determine whether a specific individual’s T cells can recognize and mount a protective response to that pathogen. Conversely, if the antigen is an allergen and the response is an allergic reaction, inheritance of some HLA alleles may make individuals susceptible to the allergic reaction. Many autoimmune diseases are associated with particular HLA alleles. We return to a discussion of these associations when we consider autoimmunity.
B lymphocytes, so-called because they mature in the bone marrow, are the cells that produce antibodies, the mediators of humoral immunity. B cells make up 10% to 20% of lymphocytes in the blood. They are also present in bone marrow and in the follicles of secondary (peripheral) lymphoid organs.
B cells recognize antigens by means of membrane-bound antibody of the immunoglobulin M (IgM) class, expressed on the surface together with signaling molecules to form the B-cell receptor (BCR) complex (see Fig. 5.4B ). Whereas T cells recognize only MHC-associated peptides, B cells recognize and respond to many more chemical structures, including soluble or cell-associated proteins, lipids, polysaccharides, nucleic acids, and small chemicals, without requiring the MHC. As with TCRs, each antibody has a unique amino acid sequence in its antigen-binding site. B cells express several invariant molecules, such as Igα and Igβ, that are responsible for signal transduction and activation following antigen recognition by the BCR.
After stimulation, B cells differentiate into plasma cells, which secrete large amounts of antibodies. There are five classes, or isotypes, of immunoglobulins that differ in their constant regions: IgG, IgM, and IgA constitute more than 95% of circulating antibodies (IgG being at the highest concentration); IgA is the major isotype in mucosal secretions; IgE is present in the circulation at very low concentrations and is found attached to the surfaces of tissue mast cells; and IgD is expressed on the surfaces of B cells but is virtually undetectable in the blood. These isotypes differ in their ability to activate complement and recruit inflammatory cells and thus have different roles in host defense and disease states.
Natural killer (NK) cells are lymphocytes that arise from the same common lymphoid progenitor that gives rise to T lymphocytes and B lymphocytes. NK cells are innate immune cells, as they are functional without prior activation and do not express highly variable receptors for antigens. The activation of NK cells is regulated by signals from two types of receptors. Inhibitory receptors recognize self class I MHC molecules, which are expressed on all healthy cells, whereas activating receptors recognize molecules that are expressed at high levels on stressed or infected cells. Normally, signals from inhibitory receptors dominate over those of activating receptors, preventing spontaneous activation of the NK cells and killing of normal host cells. Infections (especially viral infections) and stress are associated with reduced expression of class I MHC molecules and increased expression of proteins that engage activating receptors, resulting in activation of NK cells and elimination of the infected or stressed cells. NK cells also secrete cytokines such as interferon-γ (IFN-γ), thus providing early defense against intracellular microbial infections.
Innate lymphoid cells (ILCs) are lymphocytes related to NK cells that are not cytotoxic but produce many of the same cytokines that helper T cells do. ILCs do not express antigen receptors but respond to cytokines produced as a result of cell injury and stress. Because ILCs are always present in tissues, they may be early responders to microbes that damage tissues. However, their role in host defense in humans is not established.
Numerous cell types are specialized to capture antigens and display them to lymphocytes. Of these, dendritic cells play a major role in displaying protein antigens to naïve T cells. Several other cell types present antigens to lymphocytes at various stages of immune responses.
Dendritic cells (DCs) are the most important antigen-presenting cells (APCs) for initiating T-cell responses against protein antigens. These cells have numerous fine cytoplasmic processes that resemble dendrites, from which they derive their name. Several features of DCs account for their key role in antigen capture and presentation.
DCs are located at the right place to capture antigens—under epithelia, the common site of entry of microbes and foreign antigens, and in the interstitia of all tissues, where antigens may be produced. DCs within the epidermis are called Langerhans cells.
DCs express receptors for capturing and responding to microbes (and other antigens), including TLRs and C-type lectin receptors.
In response to microbes, DCs migrate to the T-cell zones of lymphoid organs, where they are ideally positioned to present antigens to T cells.
DCs express high levels of MHC and other molecules needed for antigen presentation and activation of T cells.
Macrophages present antigens of phagocytosed microbes to T cells, which then activate the phagocytes to destroy the microbes. This is a central reaction of cell-mediated immunity. B lymphocytes present endocytosed antigens to helper T cells and receive activating signals from the T cells in humoral immune responses. All nucleated cells can present antigens of cytosolic viruses or tumor antigens to CD8+ T cells and are killed by these T cells. A specialized fibroblast with dendritic morphology, called the follicular dendritic cell (FDC), is present in the germinal centers of lymphoid follicles in the spleen and lymph nodes. These cells bear Fc receptors for IgG and receptors for C3b and can trap antigen bound to antibodies or complement proteins. These cells display antigens to B lymphocytes in lymphoid follicles and promote antibody responses but are not involved in capturing antigens for display to T cells.
The tissues of the immune system consist of the generative (also called primary, or central ) lymphoid organs, in which T lymphocytes and B lymphocytes mature and become competent to respond to antigens, and the secondary (or peripheral ) lymphoid organs, in which adaptive immune responses to microbes are initiated. The principal generative lymphoid organs are the thymus, where T cells develop, and the bone marrow, the site of production of all blood cells and where B lymphocytes mature (described in Chapter 10 ). The major peripheral organs are briefly described next.
The secondary lymphoid organs are organized to concentrate antigens, APCs, and lymphocytes in a way that optimizes interactions among these cells and the development of adaptive immune responses. Most of the body’s lymphocytes are located in these organs ( Table 5.1 ).
Lymph nodes are encapsulated, organized collections of lymphocytes, dendritic cells, and macrophages that are located along lymphatic channels throughout the body ( Fig. 5.6A ). As lymph passes through lymph nodes, resident APCs are able to sample antigens that are carried to the node in lymph from the interstitial fluids of tissues. In addition, DCs transport antigens from nearby epithelial surfaces and tissues by migrating through lymphatic vessels to the lymph nodes. Thus, antigens (e.g., of microbes that enter through epithelia or colonize tissues) become concentrated in draining lymph nodes.
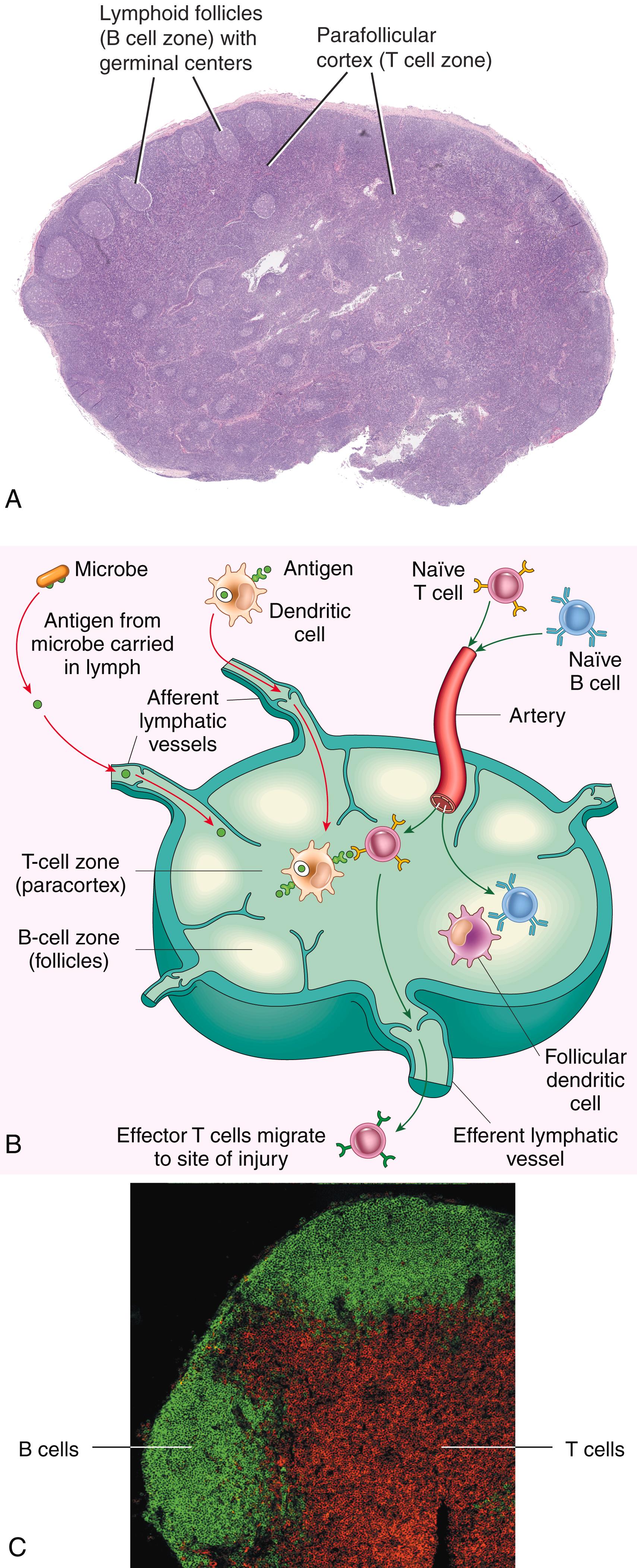
The spleen is organized into white pulp, which is where lymphocytes reside, and red pulp, which contains a network of sinusoids (through which blood flows). It plays an important role in immune responses to bloodborne antigens. Blood entering the spleen flows through the sinusoids where bloodborne antigens are trapped by resident DCs and macrophages.
The cutaneous and mucosal lymphoid systems are located under the epithelia of the skin and the gastrointestinal and respiratory tracts, respectively. They respond to antigens that enter through breaches in the epithelium. Pharyngeal tonsils and Peyer’s patches of the intestine are two anatomically defined mucosal lymphoid tissues. The large number of lymphocytes in mucosal organs (second only to lymph nodes) reflects the huge surface area of these organs.
| Tissue | Number of Lymphocytes × 10 9 |
|---|---|
| Lymph nodes | 190 |
| Spleen | 70 |
| Bone marrow | 50 |
| Blood | 10 |
| Skin | 20 |
| Intestines | 50 |
| Liver | 10 |
| Lungs | 30 |
a Approximate numbers of lymphocytes in different tissues in a healthy adult.
In the secondary lymphoid organs, T lymphocytes and B lymphocytes are segregated into different regions ( Fig. 5.6B, C ). In lymph nodes, the B cells are concentrated in discrete structures, called follicles, located at the periphery, or cortex, of each node. If the B cells in a follicle have recently responded to an antigen, the follicle develops a central pale-staining region called a germinal center . T lymphocytes are concentrated in the parafollicular cortex. The follicles contain the FDCs that are involved in the activation of B cells, and the paracortex contains the DCs that present antigens to T lymphocytes. In the spleen, T lymphocytes are concentrated in periarteriolar lymphoid sheaths surrounding small arterioles, and B cells reside in the follicles.
Cytokines are secreted proteins that mediate immune and inflammatory reactions. Molecularly defined cytokines are called interleukins, a name implying a role in communication between leukocytes. Most cytokines have a wide spectrum of actions, and some are produced by several different cell types. The majority of cytokines act on the cells that produce them or on neighboring cells, but some (like IL-1) also have systemic effects.
Different cytokines contribute to specific types of immune responses.
In innate immune responses, cytokines are produced rapidly after microbes and other stimuli are encountered, and function to induce inflammation and inhibit virus replication. These cytokines include TNF, IL-1, IL-12, type I IFNs, IFN-γ, and chemokines ( Chapter 2 ). They are produced primarily by macrophages, DCs, ILCs, and NK cells but can also be secreted by endothelial and epithelial cells.
In adaptive immune responses, cytokines are produced principally by CD4+ T lymphocytes activated by antigen and other signals. They function to promote lymphocyte proliferation and differentiation and to activate effector cells. The main cytokines in this group are IL-2, IL-4, IL-5, IL-17, and IFN-γ; their roles in immune responses are described later. Some cytokines serve mainly to limit and terminate immune responses; these include TGF-β and IL-10.
Other cytokines stimulate hematopoiesis and are called colony-stimulating factors because they stimulate formation of blood cell colonies from bone marrow progenitors ( Chapter 10 ), which increases leukocyte numbers during immune and inflammatory responses and replaces leukocytes that are consumed during such responses. They are produced by marrow stromal cells, T lymphocytes, macrophages, and other cells. Examples include IL-3, IL-7, and granulocyte-colony-stimulating factor.
The knowledge gained about cytokines has numerous practical therapeutic applications. Inhibiting cytokine production or actions can control the harmful effects of inflammation. For example, patients with rheumatoid arthritis often show dramatic responses to TNF antagonists. Other cytokine antagonists are now used to treat various inflammatory disorders. Conversely, administration of cytokines is used to boost reactions that are normally dependent on these proteins, such as hematopoiesis (e.g., following stem cell transplantation).
Adaptive immune responses proceed in steps, consisting of antigen recognition; activation, proliferation and differentiation of specific lymphocytes into effector and memory cells; elimination of the antigen; and decline of the response, with memory cells being the long-lived survivors. The major events in each step are summarized next; these general principles apply to protective responses against microbes as well as pathologic responses that injure the host.
Microbes and other foreign antigens can enter the body virtually anywhere, and it is obviously impossible for lymphocytes of every specificity to patrol every possible portal of antigen entry. To overcome this problem, microbes and their protein antigens in epithelia and other tissues are captured by dendritic cells, which then carry their antigenic cargo to draining lymph nodes through which T cells constantly recirculate ( Fig. 5.7 ). Here, the antigens are processed and complexed with MHC molecules for display on the cell surface, where the antigens are recognized by T cells. Similarly, soluble antigens are captured and concentrated in follicles in lymph nodes and the spleen, where they may be recognized by B cells via their antigen receptors.
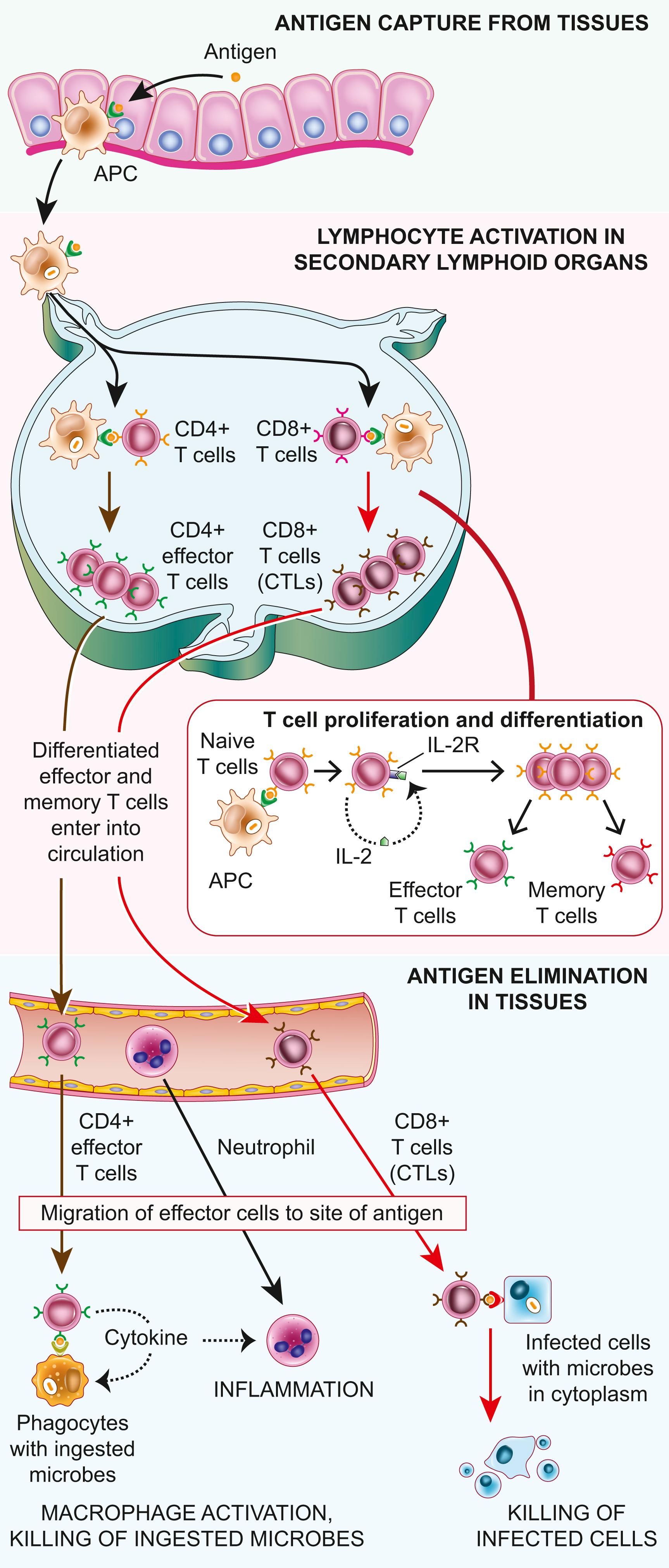
Even before microbial antigens are recognized by T lymphocytes and B lymphocytes, the microbe activates innate immune cells expressing pattern recognition receptors. In the case of immunization with a protein antigen, as in a vaccine, a microbial mimic called an adjuvant that stimulates innate immune responses is given with the antigen. As part of the innate response, the microbe or adjuvant activates APCs to express molecules called costimulators and to secrete cytokines that stimulate the proliferation and differentiation of T lymphocytes. The principal costimulators for T cells are the B7 proteins (CD80 and CD86), which are expressed on APCs and are recognized by the CD28 receptor on naïve T cells.
The reactions and functions of T lymphocytes and B lymphocytes differ in important ways and are best considered separately.
Naïve T lymphocytes are activated by antigen and costimulators in secondary lymphoid organs and proliferate and differentiate into effector cells that migrate to the site where the antigen (microbe) is present (see Fig. 5.7 ). One of the earliest responses of CD4+ helper T cells is secretion of the cytokine IL-2 and expression of high-affinity receptors for IL-2. IL-2 is a growth factor that acts on these T lymphocytes and stimulates their proliferation, leading to an increase in the number of antigen-specific lymphocytes. The functions of helper T cells are mediated by the combined actions of CD40-ligand (CD40L) and cytokines. CD40 is a member of the TNF-receptor family, and CD40L is a membrane protein homologous to TNF. When CD4+ helper T cells recognize antigens being displayed by macrophages or B lymphocytes, the T cells express CD40L, which engages CD40 on the macrophages or B cells and activates these cells. Mutations in the CD40L gene are the cause of X-linked hyper-IgM syndrome, in which both humoral and cell-mediated immunity are compromised (discussed later).
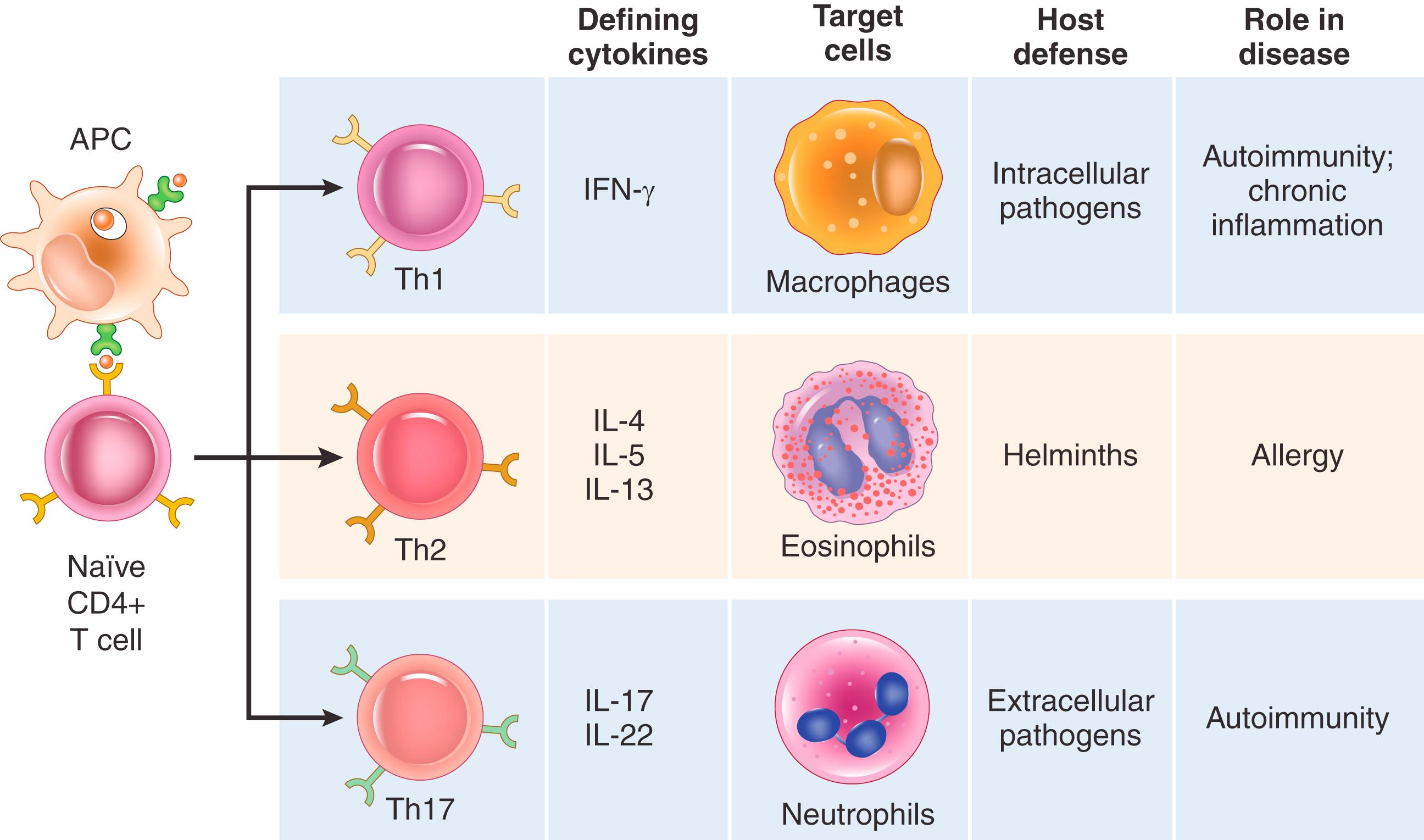
Some of the activated CD4+ T cells differentiate into effector cells that secrete different sets of cytokines and perform different functions ( Fig. 5.8 ). The three best-defined subsets are the following:
Th1 cells secrete the cytokine IFN-γ, which is a potent macrophage activator. The combination of CD40- and IFN-γ–mediated activation results in “classical” macrophage activation ( Chapter 2 ), leading to the induction of microbicidal substances in macrophages and the destruction of ingested microbes.
Th2 cells produce IL-4, which stimulates B cells to differentiate into IgE-secreting plasma cells; IL-5, which activates eosinophils; and IL-13, which activates mucosal epithelial cells to secrete mucus, and induces the “alternative” pathway of macrophage activation, which is associated with tissue repair and fibrosis ( Chapter 2 ). Eosinophils destroy pathogens such as helminthic parasites.
Th17 cells, so called because the signature cytokine of these cells is IL-17, recruit neutrophils and monocytes, which destroy some extracellular bacteria and fungi and are involved in certain inflammatory diseases.
Activated CD8+ T lymphocytes differentiate into CTLs that kill cells harboring cytoplasmic microbes, thereby eliminating otherwise hidden reservoirs of infection. The principal mechanism of killing by CTLs depends on the perforin–granzyme system. Perforin and granzymes are stored in the granules of CTLs and are rapidly released when CTLs engage their targets (cells bearing the appropriate class I MHC–bound peptides). Perforin binds to the plasma membrane of the target cells and promotes the entry of granzymes, proteases that specifically cleave and thereby activate cellular caspases ( Chapter 1 ), which induce the apoptosis of target cells.
T-cell responses are regulated by a balance between costimulatory and inhibitory receptors. The major costimulatory receptor is CD28, mentioned earlier. Other proteins of the CD28 family include two “coinhibitory” receptors, CTLA-4, which blocks and removes B7 molecules and thus reduces CD28 engagement, and PD-1, which inhibits signals from the TCR and from CD28 and thus terminates T-cell responses. Blocking these coinhibitors has proved to be a powerful approach for enhancing antitumor immune responses ( Chapter 6 ).
Upon activation, B lymphocytes proliferate and then differentiate into plasma cells that secrete different classes of antibodies with distinct functions. There are two major pathways of B-cell activation.
T cell–dependent. The response of B cells to protein antigens requires help from CD4+ T cells. B cells also act as APCs—they ingest protein antigens, degrade them, and display peptides bound to class II MHC molecules for recognition by helper T cells ( Fig. 5.9 ). The helper T cells express CD40L, which engages CD40 expressed on B cells, and secrete cytokines, which work together to activate the B cells.
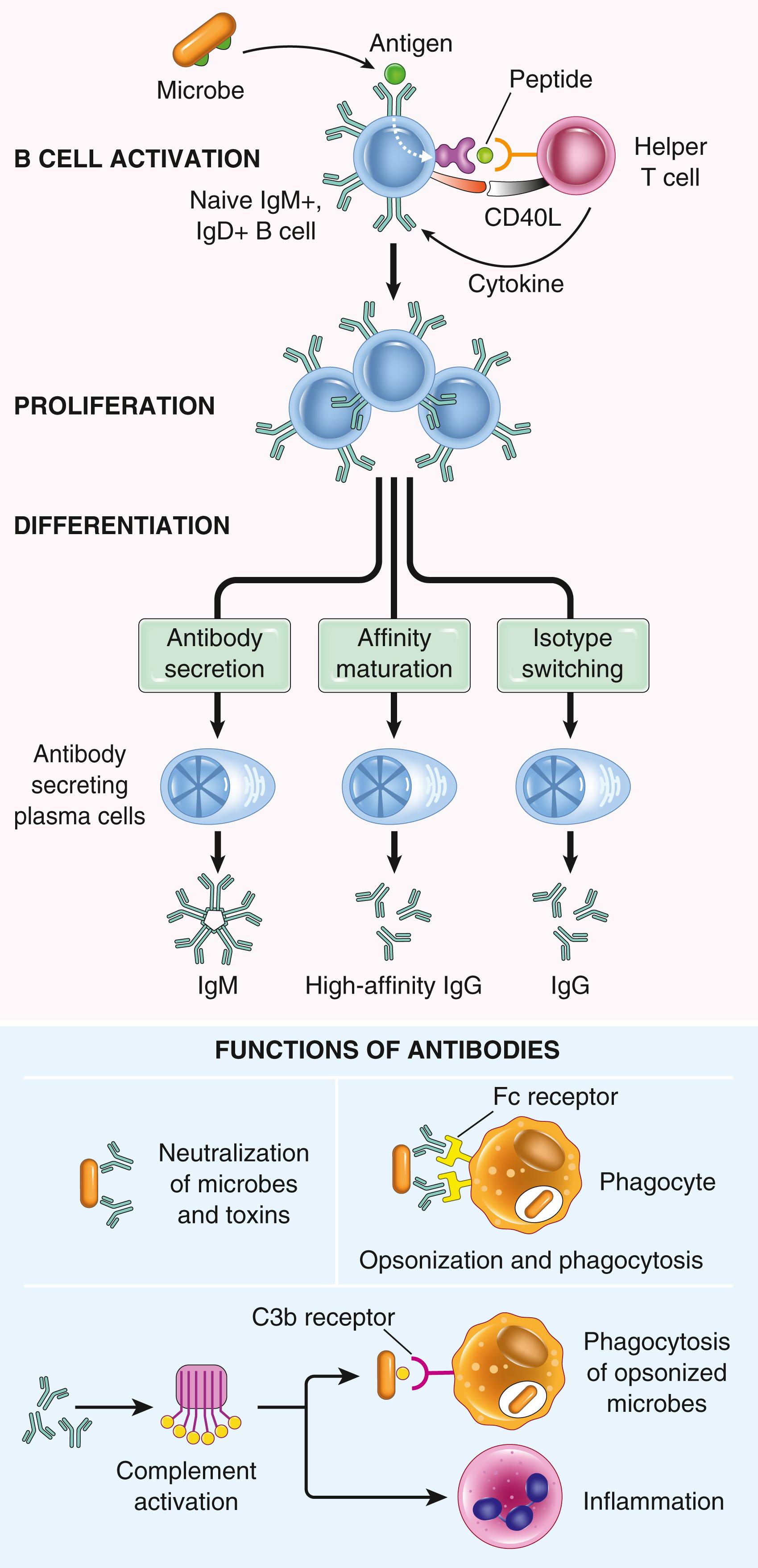
T cell–independent. Many polysaccharide and lipid antigens have multiple identical antigenic determinants (epitopes) that can cross-link several antibody molecules on each B cell and initiate the process of B-cell activation without a requirement for T-cell help.
Some of the progeny of the expanded B-cell clones differentiate into antibody-secreting plasma cells. Each plasma cell secretes antibodies with the same specificity as the cell surface antibodies (B-cell antigen receptors) that first recognized the antigen. Polysaccharides and lipids stimulate secretion mainly of IgM antibody. Protein antigens, by virtue of CD40L- and cytokine-mediated helper T-cell actions, induce the production of antibodies of different classes (IgG, IgA, IgE). Production of functionally different antibodies relies on heavy-chain class (isotype) switching, which results from DNA breaks adjacent to constant region genes followed by joining to downstream constant regions. This process changes the Fc regions of antibodies, thus increasing the range of functions that antibodies serve. The isotype-specific functions of antibodies are described later. Helper T cells also stimulate the production of antibodies with higher affinity for the antigen. This process, called affinity maturation, results from somatic mutations in the antigen-binding regions of Ig molecules followed by selection of cells that express high-affinity receptors; thus, the process improves the quality of the humoral immune response. Some activated B cells migrate into follicles and form germinal centers, which are the sites of isotype switching and affinity maturation. The helper T cells that stimulate these processes in B lymphocytes also migrate to and reside in the germinal centers and are called T follicular helper (Tfh) cells .
The humoral immune response combats microbes in numerous ways (see Fig. 5.9 ).
High-affinity antibodies of all classes bind to microbes and prevent them from infecting cells, thereby neutralizing the microbes.
IgG antibodies coat (opsonize) microbes and target them for phagocytosis by neutrophils and macrophages, which express receptors for the Fc tails of IgG molecules.
IgG and IgM activate the complement system by the classical pathway, and complement products promote phagocytosis and destruction of microbes.
IgA is secreted in mucosal tissues and neutralizes microbes in the lumens of the respiratory and gastrointestinal tracts (and other mucosal tissues).
IgG is actively transported across the placenta and protects the newborn until the immune system becomes mature. This is a form of passive immunity.
IgE activates mast cells and is involved in defense against helminths.
Circulating IgG antibodies have half-lives of about 3 weeks, which is much longer than the half-lives of most blood proteins, as a consequence of special mechanisms for recycling IgG and reducing its catabolism. Some antibody-secreting plasma cells migrate to the bone marrow and live for years, continuing to produce low levels of antibodies.
The majority of effector lymphocytes induced by an infectious pathogen die by apoptosis after the microbe is eliminated, thus returning the immune system to its basal resting state. The initial activation of lymphocytes generates long-lived memory cells, which may survive for years after the infection. Memory cells are an expanded pool of antigen-specific lymphocytes (more numerous than the naïve cells specific for any antigen that are present before encounter with that antigen) that respond faster and more effectively when reexposed to the antigen than do naïve cells. Generation of memory cells is therefore an important goal of vaccination.
This brief introduction to normal immune responses provides a background for our discussion of disorders of the immune system.
Immune responses that normally are protective are also capable of causing tissue injury. Injurious immune reactions are grouped under hypersensitivity, and the resulting diseases are called hypersensitivity diseases . This term originated from the idea that persons who mount immune responses against an antigen are sensitized to that antigen, so pathologic or excessive reactions are manifestations of a hypersensitive state. A system of checks and balances has evolved to optimize the eradication of infecting organisms without serious injury to host tissues. However, immune responses may be inadequately controlled or directed against normally harmless antigens or inappropriately targeted to host tissues, and in such situations, the normally beneficial response is the cause of disease. In this section, we describe the causes and general mechanisms of hypersensitivity diseases and then discuss specific situations in which the immune response is responsible for the disease.
Pathologic immune responses may be directed against different types of antigens.
Autoimmunity: reactions against self antigens. Normally, the immune system does not react against one’s own antigens. This phenomenon is called self-tolerance, implying that the body “tolerates” its own antigens. On occasion, self-tolerance fails, resulting in reactions against one’s own cells and tissues called autoimmunity ; diseases caused by autoimmunity are referred to as autoimmune diseases. We will return to the mechanisms of self-tolerance and autoimmunity later in this chapter.
Reactions against microbes. There are many types of reactions against microbial antigens that may cause disease. In some cases, the reaction is excessive or the microbial antigen is unusually persistent. T-cell responses against persistent microbes, such as Mycobacterium tuberculosis, may give rise to severe inflammation, sometimes with the formation of granulomas ( Chapter 2 ); this is the cause of tissue injury in tuberculosis and some other infections. If antibodies are produced against microbial antigens, the antibodies may bind to the antigens to produce immune complexes, which deposit in tissues and trigger inflammation; this is the underlying mechanism of postinfectious glomerulonephritis ( Chapter 12 ). Rarely, antibodies or T cells reactive with a microbe cross-react with a host tissue; such cross-reactivity is believed to be the basis for rheumatic heart disease ( Chapter 9 ). The SARS-CoV-2 coronavirus can induce a systemic inflammatory reaction that is an important cause of morbidity in COVID-19.
Reactions against environmental antigens. In higher-income countries, 20% or more of the population is allergic to common environmental substances (e.g., pollens, animal dander, and dust mites), as well as some metal ions and therapeutic drugs. Such individuals are predisposed to make unusual immune responses to noninfectious, typically harmless, antigens to which all persons are exposed but against which only some react.
In all these conditions, tissue injury is caused by the same mechanisms that normally function to eliminate infectious pathogens—namely, antibodies, effector T lymphocytes, and various other cells such as macrophages and eosinophils. The fundamental problem in these diseases is that the immune response is triggered and maintained inappropriately. Because the stimuli for these abnormal immune responses are difficult or impossible to eliminate (e.g., self antigens, persistent microbes, or environmental antigens), and the immune system has many intrinsic positive feedback loops (which normally promote protective immunity), once a hypersensitivity reaction starts, it is difficult to control or terminate it. Therefore, these diseases tend to be chronic and debilitating and are therapeutic challenges.
Hypersensitivity reactions can be subdivided into four types based on the principal immune mechanism responsible for injury; three are variations on antibody-mediated injury, and the fourth is T cell mediated ( Table 5.2 ). The rationale for this classification is that the mechanism of immune injury is often a good predictor of the clinical manifestations and may help to guide therapy.
| Type | Immune Mechanisms | Histopathologic Lesions | Prototypical Disorders |
|---|---|---|---|
| Immediate (type I) hypersensitivity | Production of IgE antibody → immediate release of vasoactive amines and other mediators from mast cells; later recruitment of inflammatory cells | Vascular dilation, edema, smooth muscle contraction, mucus production, tissue injury, inflammation | Anaphylaxis; allergies; bronchial asthma (atopic forms) |
| Antibody-mediated (type II) hypersensitivity | Production of IgG, IgM → binds to antigen on target cell or tissue → phagocytosis or lysis of target cell by activated complement or Fc receptors; recruitment of leukocytes | Phagocytosis and lysis of cells; inflammation; in some diseases, functional derangements without cell or tissue injury | Autoimmune hemolytic anemia; Goodpasture syndrome |
| Immune complex–mediated (type III) hypersensitivity | Deposition of antigen-antibody complexes → complement activation → recruitment of leukocytes by complement products and Fc receptors → release of enzymes and other toxic molecules | Inflammation, necrotizing vasculitis (fibrinoid necrosis) | Systemic lupus erythematosus; some forms of glomerulonephritis; serum sickness; Arthus reaction |
| Cell-mediated (type IV) hypersensitivity | Activated T lymphocytes → (1) release of cytokines, inflammation and macrophage activation; (2) T cell–mediated cytotoxicity | Perivascular cellular infiltrates; edema; granuloma formation; cell destruction | Contact dermatitis; multiple sclerosis; type 1 diabetes; tuberculosis |
The main types of hypersensitivity reactions are as follows:
In immediate (type I) hypersensitivity , also called allergy, the injury is caused by Th2 cells, IgE antibodies, and mast cells and other leukocytes. Mast cells release mediators that act on blood vessels and smooth muscle as well as cytokines that recruit and activate inflammatory cells.
Antibody-mediated disorders (type II hypersensitivity) are caused by secreted IgG and IgM antibodies that bind to antigens in tissue or on a cell surface. Antibodies injure cells by promoting their phagocytosis or lysis and injure tissues by inducing inflammation. Antibodies may also interfere with cellular functions and cause disease without cell or tissue injury.
In immune complex–mediated disorders (type III hypersensitivity), IgG and IgM antibodies bind antigens, usually in the circulation, and form antigen-antibody complexes that deposit in vascular beds and induce inflammation. The leukocytes that are recruited (neutrophils and monocytes) damage tissues by releasing lysosomal enzymes and generating toxic free radicals.
T cell–mediated (type IV) hypersensitivity disorders are caused mainly by immune responses in which T lymphocytes of the Th1 and Th17 subsets produce cytokines that induce inflammation and activate neutrophils and macrophages, which are responsible for tissue injury. CD8+ CTLs may also contribute to injury by directly killing host cells.
Immediate hypersensitivity is a tissue reaction that occurs rapidly (typically within minutes) after the interaction of antigen with IgE antibody bound to the surface of mast cells. The reaction is initiated by entry of an antigen, which is called an allergen because it triggers allergy. Many allergens are environmental substances against which some individuals are predisposed to mounting Th2 and IgE responses, which are responsible for the clinical and pathologic manifestations of the reaction. Immediate hypersensitivity may occur as a mild reaction (e.g., seasonal rhinitis, hay fever), or it can be severely debilitating (e.g., asthma) or even fatal (e.g., anaphylaxis).
Most immediate hypersensitivity reactions follow a stereotypic sequence of cellular responses ( Fig. 5.10 ):
Activation of Th2 cells and production of IgE antibody. Only a subset of individuals exposed to common environmental antigens make strong Th2 and IgE responses. The factors that contribute to this propensity are discussed later. Th2 cells secrete several cytokines, including IL-4, IL-5, and IL-13, which are responsible for the reactions of immediate hypersensitivity. IL-4 and IL-13 stimulate allergen-specific B cells to undergo heavy-chain class switching to IgE. IL-5 activates eosinophils that are recruited to the reaction, and IL-13 stimulates mucus secretion from epithelial cells. Th2 cells are often recruited to the site of allergic reactions in response to locally produced chemokines; one of these chemokines, eotaxin, also recruits eosinophils to the same site.
Sensitization of mast cells by IgE antibody. Mast cells are derived from precursors in the bone marrow and widely distributed in tissues, often residing near blood vessels and nerves and in subepithelial locations. Mast cells express a high-affinity receptor for the Fc portion of the ε heavy chain of IgE, called FcεRI. Although the serum concentration of IgE is very low (in the range of 0.1 to 10 μg/mL), the affinity of the mast cell FcεRI receptor is so high that the receptors are always occupied by IgE. These antibody-bearing mast cells are sensitized to react if the specific antigen (the allergen) binds to the antibody molecules. Basophils, circulating cells that resemble mast cells, also express FcεRI, may be recruited into tissues, and may contribute to immediate hypersensitivity reactions.
Activation of mast cells and release of mediators. When a person who has been sensitized by exposure to an allergen is reexposed to that allergen, the allergen binds to antigen-specific IgE molecules on mast cells, usually at or near the site of allergen entry. Cross-linking of these IgE molecules triggers a series of biochemical signals from the associated FcεRI receptor that culminate in the secretion of various mediators from the mast cells.
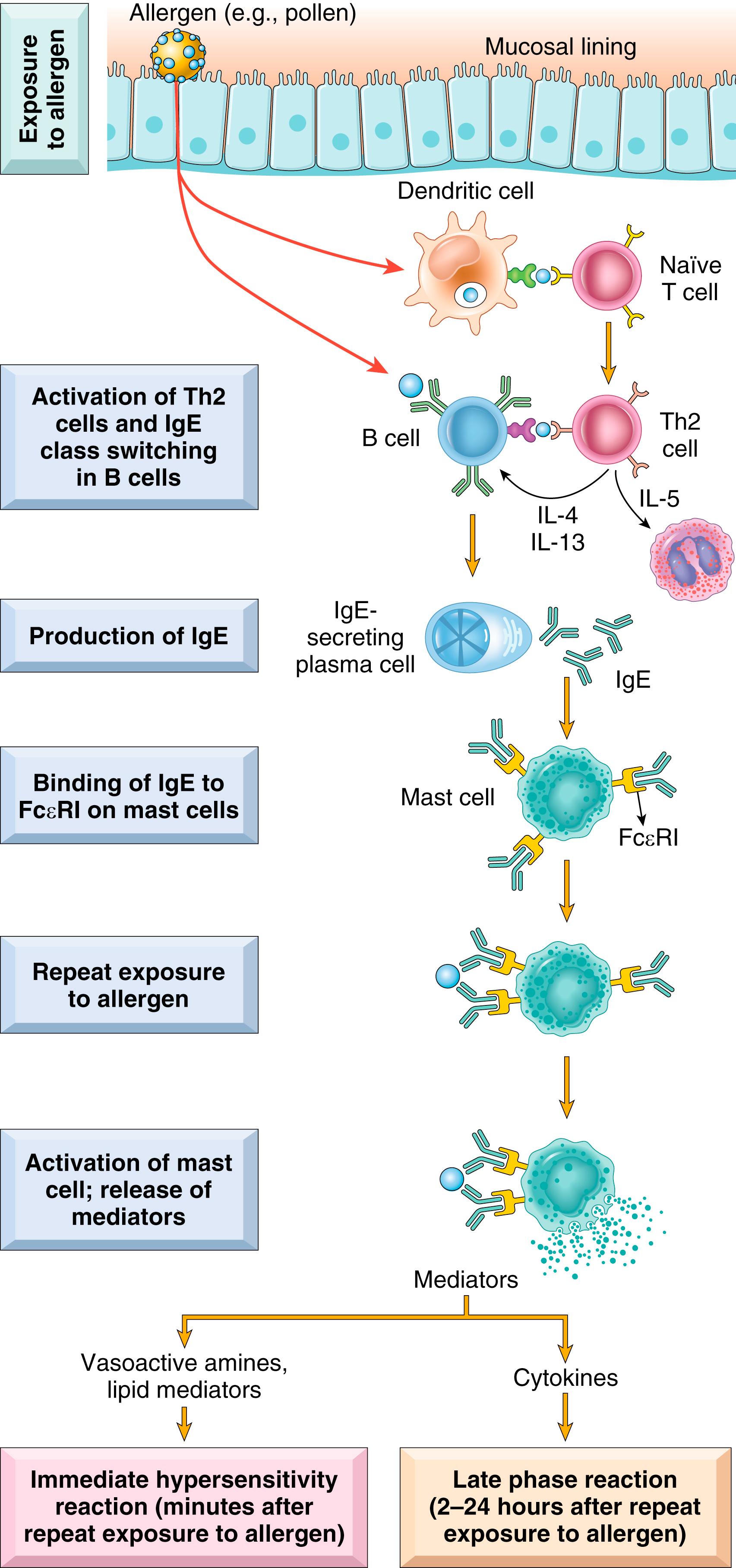
Three groups of mediators are important in different immediate hypersensitivity reactions:
Vasoactive amines released from granule stores. The granules of mast cells contain histamine , which is released within seconds or minutes of activation. Histamine causes vasodilation, increased vascular permeability, smooth muscle contraction, and increased mucus secretion. Other rapidly released mediators include neutral proteases (e.g., tryptase), which may damage tissues and also generate kinins and cleave complement components to produce additional chemotactic and inflammatory factors (e.g., C5a) ( Chapter 2 ). The granules also contain acidic proteoglycans (e.g., heparin, chondroitin sulfate), which seem to serve as a storage matrix for the amines.
Newly synthesized lipid mediators. Mast cells synthesize and secrete prostaglandins and leukotrienes by the same pathways as do other leukocytes ( Chapter 2 ). These lipid mediators have several actions that are important in immediate hypersensitivity reactions. Prostaglandin D2 (PGD 2 ) is the most abundant mediator generated by the cyclooxygenase pathway in mast cells. It causes intense bronchospasm as well as increased mucus secretion. The leukotrienes LTC 4 and LTD 4 are the most potent vasoactive and spasmogenic agents known; on a molar basis, they are several thousand times more active than histamine in increasing vascular permeability and in causing bronchial smooth muscle contraction. LTB 4 is highly chemotactic for neutrophils, eosinophils, and monocytes.
Cytokines. Activated mast cells secrete several cytokines that are important for the late-phase reaction. These include TNF and chemokines, which recruit and activate leukocytes ( Chapter 2 ).
The reactions of immediate hypersensitivity did not evolve to cause human discomfort and disease. The Th2 response plays an important protective role in combating parasitic infections, mainly by destruction of helminths by eosinophil granule proteins. Mast cells are also involved in defense against bacterial infections and animal venoms.
Susceptibility to immediate hypersensitivity reactions is genetically determined. An increased propensity to develop immediate hypersensitivity reactions is called atopy . Atopic individuals tend to have higher serum IgE levels and more IL-4–producing Th2 cells than does the general population. A positive family history of allergy is found in 50% of atopic individuals. Genes that are implicated in susceptibility to asthma and other atopic disorders include those encoding HLA molecules (which may confer immune responsiveness to particular allergens), cytokines (which may control Th2 responses), a component of the FcεRI receptor, and ADAM33, a metalloproteinase that may be involved in tissue remodeling in the airways.
Environmental factors are also important in the development of allergic diseases. Exposure to environmental pollutants, all too common in industrialized societies, is an important predisposing factor for allergy. Dogs and cats living in the same environment as humans can develop allergies, whereas chimps living in the wild do not despite their much closer genetic similarity to humans. This simple observation suggests that environmental factors may be more important in the development of allergic disease than genetics. Viral infections of the airways are important triggers for bronchial asthma, an allergic disease affecting the lungs ( Chapter 11 ). Bacterial skin infections are strongly associated with atopic dermatitis.
It is estimated that 20% to 30% of immediate hypersensitivity reactions are triggered by nonantigenic stimuli such as temperature extremes and exercise and do not involve Th2 cells or IgE. It is believed that in these cases of nonatopic allergy, mast cells are abnormally sensitive to activation by various nonimmune stimuli.
The incidence of many allergic diseases is increasing in higher-income countries, perhaps related to a decrease in infections during early life. This observation has led to an idea, called the hygiene hypothesis, that early childhood and even prenatal exposure to microbial antigens educates the immune system such that subsequent pathologic responses against common environmental allergens are prevented. Thus, too little exposure to potential allergens and, perhaps, microbes in childhood may predispose individuals to allergies later in life. This idea has received support from clinical studies demonstrating that exposing infants to peanuts reduces the incidence of peanut allergy later in life.
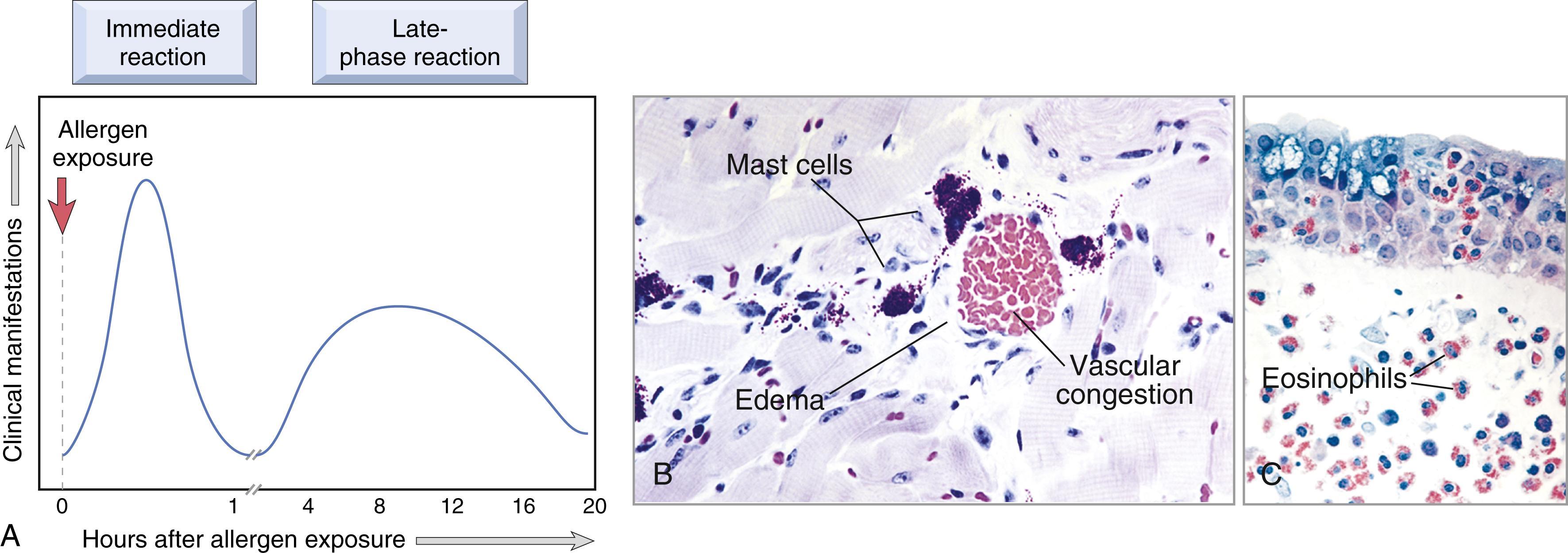
Often, the IgE-triggered reaction has two well-defined phases ( Fig. 5.11 ).
The immediate response, usually evident within 5 to 30 minutes after exposure to an allergen and subsiding by 60 minutes, is stimulated by mast cell granule contents and lipid mediators. It is characterized by vasodilation, vascular leakage, and smooth muscle spasm.
A second, late-phase reaction usually begins 2 to 8 hours later and may last for several days. It is stimulated mainly by cytokines and is characterized by inflammation as well as tissue injury, such as mucosal epithelial cell damage. The dominant inflammatory cells in the late-phase reaction are neutrophils, eosinophils, and lymphocytes, especially Th2 cells. Neutrophils are recruited by various chemokines; their roles in inflammation were described in Chapter 2 . Eosinophils, which are recruited by eotaxin and other chemokines released from epithelium, produce granule proteins that are toxic to epithelial cells as well as leukotrienes and other factors that promote inflammation. Th2 cells produce cytokines that have multiple actions, as described earlier. These recruited leukocytes can amplify and sustain the injurious inflammatory response, even in the absence of continuous allergen exposure. Because inflammation is a major component of many allergic diseases, notably asthma and atopic dermatitis, therapy includes antiinflammatory drugs such as corticosteroids.
Immediate hypersensitivity reactions may occur as systemic disorders or as local reactions ( Table 5.3 ). The route of antigen exposure often determines the nature of the reaction. Exposure to protein antigens (e.g., in bee venom) or drugs (e.g., penicillin) that enter the circulation may result in systemic anaphylaxis . Within minutes of the exposure in a sensitized host, itching, urticaria (hives), and skin erythema appear, followed rapidly by profound respiratory difficulty caused by pulmonary bronchoconstriction and hypersecretion of mucus. Laryngeal edema may exacerbate matters by causing upper airway obstruction. In addition, the musculature of the entire gastrointestinal tract may be affected, with resultant vomiting, abdominal cramps, and diarrhea. Without immediate intervention, there may be systemic vasodilation with a fall in blood pressure (anaphylactic shock), and the patient may progress to circulatory collapse and death within minutes.
| Clinical Syndrome | Clinical and Pathologic Manifestations |
|---|---|
| Anaphylaxis (may be caused by drugs, bee sting, food) | Fall in blood pressure (shock) caused by vascular dilation; airway obstruction due to laryngeal edema |
| Bronchial asthma | Airway obstruction caused by bronchial smooth muscle hyperactivity; inflammation and tissue injury caused by late-phase reaction |
| Allergic rhinitis, sinusitis (hay fever) | Increased mucus secretion; inflammation of upper airways and sinuses |
| Food allergies | Increased peristalsis due to contraction of intestinal muscles, resulting in vomiting and diarrhea |
Local reactions generally occur when the antigen is confined to a particular site, such as the skin (following contact), the gastrointestinal tract (following ingestion), or the lung (following inhalation). Atopic dermatitis (eczema), food allergies, allergic rhinitis (hay fever), and certain forms of asthma are examples of localized allergic reactions. However, ingestion or inhalation of allergens also can trigger systemic reactions if the allergen is absorbed into the circulation, as happens in cases of peanut allergy. Sometimes, infants develop atopic dermatitis, followed, later in life, by allergic rhinitis and asthma. These three disorders are grouped under the atopic triad , and their sequential development has been called the atopic march .
Therapy for allergies relies on corticosteroids (to reduce inflammation) and agents to counteract the effects of mediators (such as antihistamines, leukotriene antagonists, bronchodilators for asthma, and epinephrine to correct the drop in blood pressure in anaphylaxis). Antibodies that block Th2 cytokines or their receptors or neutralize IgE are now used for the treatment of asthma, atopic dermatitis, and peanut allergy.
Antibody-mediated (type II) hypersensitivity disorders are caused by antibodies directed against target antigens on the surface of cells or other tissue components. The antigens may be normal molecules intrinsic to cell membranes or in the extracellular matrix, or they may be adsorbed exogenous antigens (e.g., a drug metabolite). These reactions are the cause of several important diseases ( Table 5.4 ).
| Disease | Target Antigen | Mechanisms of Disease | Clinicopathologic Manifestations |
|---|---|---|---|
| Autoimmune hemolytic anemia | Red cell membrane proteins | Opsonization and phagocytosis of red cells | Hemolysis, anemia |
| Autoimmune thrombocytopenic purpura | Platelet membrane proteins (Gpllb : Illa integrin) | Opsonization and phagocytosis of platelets | Bleeding |
| Pemphigus vulgaris | Proteins in intercellular junctions of epidermal cells (desmogleins) | Antibody-mediated activation of proteases, disruption of intercellular adhesions | Skin vesicles (bullae) |
| Vasculitis caused by ANCA | Neutrophil granule proteins, presumably released from activated neutrophils | Neutrophil degranulation and inflammation | Vasculitis |
| Goodpasture syndrome | Protein in basement membranes of kidney glomeruli and lung alveoli | Complement- and Fc receptor–mediated inflammation | Nephritis, lung hemorrhage |
| Acute rheumatic fever | Streptococcal cell wall antigen; antibody cross-reacts with myocardial antigen | Inflammation, macrophage activation | Myocarditis, arthritis |
| Myasthenia gravis | Acetylcholine receptor | Antibody inhibits acetylcholine binding; complement-mediated injury | Muscle weakness, paralysis |
| Graves disease (hyperthyroidism) | TSH receptor | Antibody-mediated stimulation of TSH receptors | Hyperthyroidism |
| Pernicious anemia | Intrinsic factor of gastric parietal cells | Neutralization of intrinsic factor, decreased absorption of vitamin B 12 | Abnormal erythropoiesis, anemia |
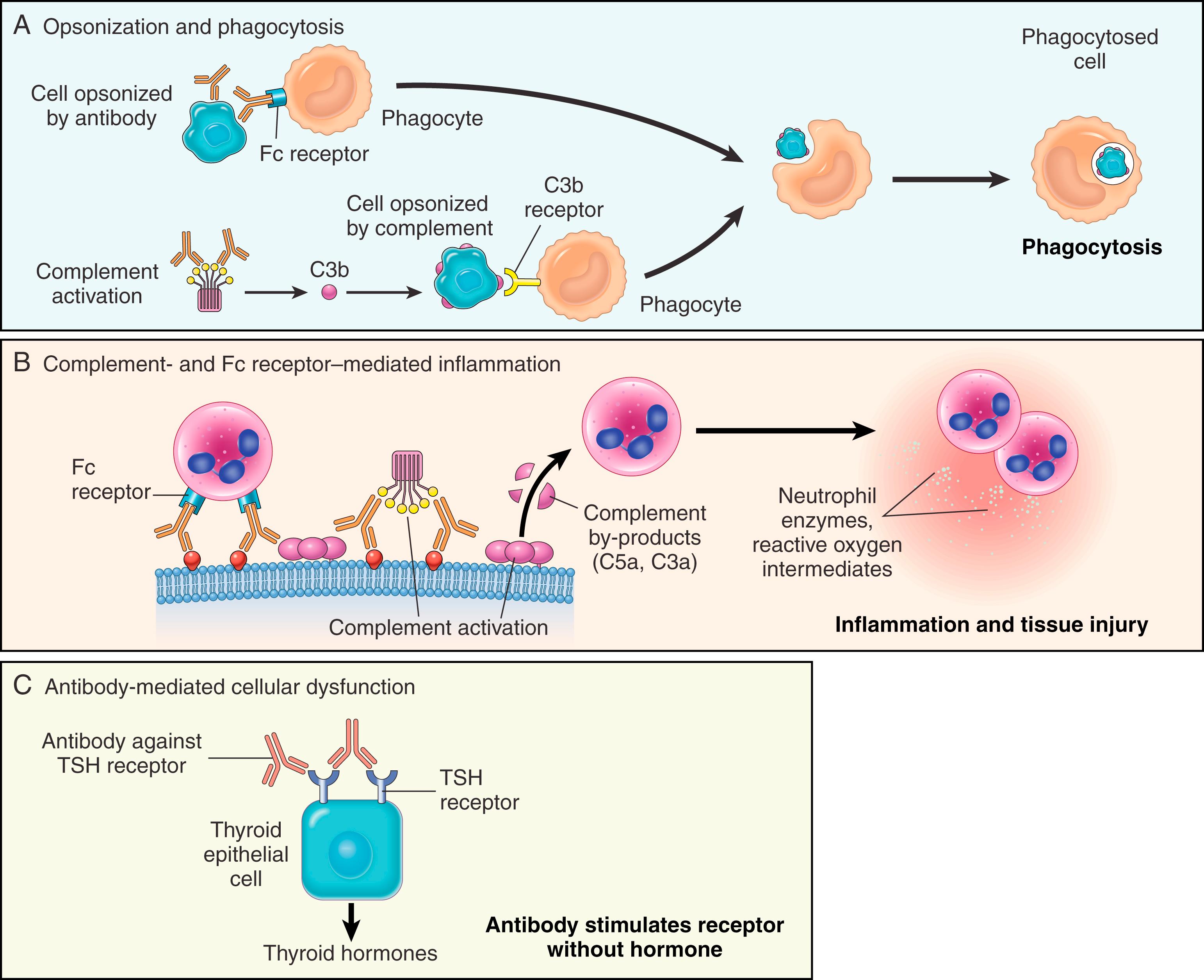
In type II hypersensitivity, antibodies cause disease by targeting cells for phagocytosis, activating the complement system, or interfering with normal cellular functions ( Fig. 5.12 ). The antibodies that are responsible are typically high-affinity IgG and IgM antibodies capable of activating complement and, for IgG, binding to phagocyte Fc receptors.
Opsonization and phagocytosis. When circulating cells, such as red cells or platelets, are coated (opsonized) with autoantibodies, with or without complement proteins, the cells become targets for phagocytosis by neutrophils and macrophages (see Fig. 5.12A ). These phagocytes express receptors for the Fc tails of IgG antibodies and for breakdown products of the C3 complement protein and use these receptors to bind and ingest opsonized particles. Opsonized blood cells are usually eliminated by macrophages in the spleen, which is why splenectomy is of clinical benefit in some antibody-mediated diseases.
Antibody-mediated cell destruction occurs in the following clinical situations: (1) transfusion reactions, in which cells from an incompatible donor react with preformed antibody in the host ( Chapter 10 ); (2) hemolytic anemia of the fetus and newborn (erythroblastosis fetalis), in which IgG anti–red cell antibodies from the mother cross the placenta and cause destruction of fetal red cells ( Chapter 4 ); (3) autoimmune hemolytic anemia, neutropenia, and thrombocytopenia, in which individuals produce antibodies to their own blood cells ( Chapter 10 ); and (4) certain drug reactions, in which a drug attaches to plasma membrane proteins of red cells and antibodies are produced against the drug-protein complex.
Inflammation. Antibodies bound to tissue antigens activate the complement system by the classical pathway (see Fig. 5.12B ). Products of complement activation serve several functions ( Chapter 2 ), one of which is to recruit neutrophils and monocytes, triggering inflammation in tissues. Leukocytes may also be activated by engagement of Fc receptors, which recognize the bound antibodies. Antibody-mediated inflammation is responsible for tissue injury in some forms of glomerulonephritis, vascular rejection in organ grafts, and other disorders.
Antibody-mediated cellular dysfunction. In some cases, antibodies directed against an essential protein impair or dysregulate important functions without directly causing cell injury or inflammation. In pernicious anemia, antibodies against intrinsic factor, which is required for absorption of vitamin B 12 in the stomach, lead to a deficiency of this vitamin and abnormal hematopoiesis; antibody-mediated damage to gastric epithelial cells may also contribute. Previously, it was thought that in myasthenia gravis, antibodies specific for the acetylcholine receptor in neuromuscular junctions of motor end plates of skeletal muscles inhibited neuromuscular transmission, resulting in muscle weakness, but without injury. However, recent clinical studies have shown benefit with complement inhibition therapies, suggesting that antibody- and complement-mediated damage to motor end plates may be involved in disease pathogenesis. Antibodies can also stimulate excessive cellular responses; e.g., in Graves disease, antibodies against the thyroid-stimulating hormone receptor stimulate thyroid epithelial cells to secrete thyroid hormones, resulting in hyperthyroidism (see Fig. 5.12C ).
Antigen–antibody (immune) complexes that are formed in the circulation may deposit in blood vessels, leading to complement activation and acute inflammation. In some cases, the complexes may be formed at sites where antigen has been “planted” previously (in situ immune complexes) . The antigens that form immune complexes may be exogenous, such as a foreign protein that is injected or produced by an infectious microbe, or endogenous, if the individual produces antibody against self antigens (autoimmunity) ( Table 5.5 ). Immune complex–mediated diseases tend be systemic because the complexes can deposit in blood vessels anywhere in the body, but often preferentially involve the kidney (glomerulonephritis), joints (arthritis), and small blood vessels (vasculitis), all of which are common sites of immune complex deposition.
| Disease | Antigen Involved | Clinicopathologic Manifestations |
|---|---|---|
| Systemic lupus erythematosus | Nuclear antigens (circulating or “planted” in kidney) | Nephritis, skin lesions, arthritis, others |
| Poststreptococcal glomerulonephritis | Streptococcal cell wall antigen(s); may be “planted” in glomerular basement membrane | Nephritis |
| Polyarteritis nodosa | Hepatitis B virus antigens in some cases | Systemic vasculitis |
| Reactive arthritis | Bacterial antigens (e.g., Yersinia ) | Acute arthritis |
| Serum sickness | Various proteins (e.g., foreign serum protein such as horse antithymocyte globulin) | Arthritis, vasculitis, nephritis |
| Arthus reaction (experimental) | Various foreign proteins | Cutaneous vasculitis |
The pathogenesis of immune complex diseases can be divided into three phases ( Fig. 5.13 ).
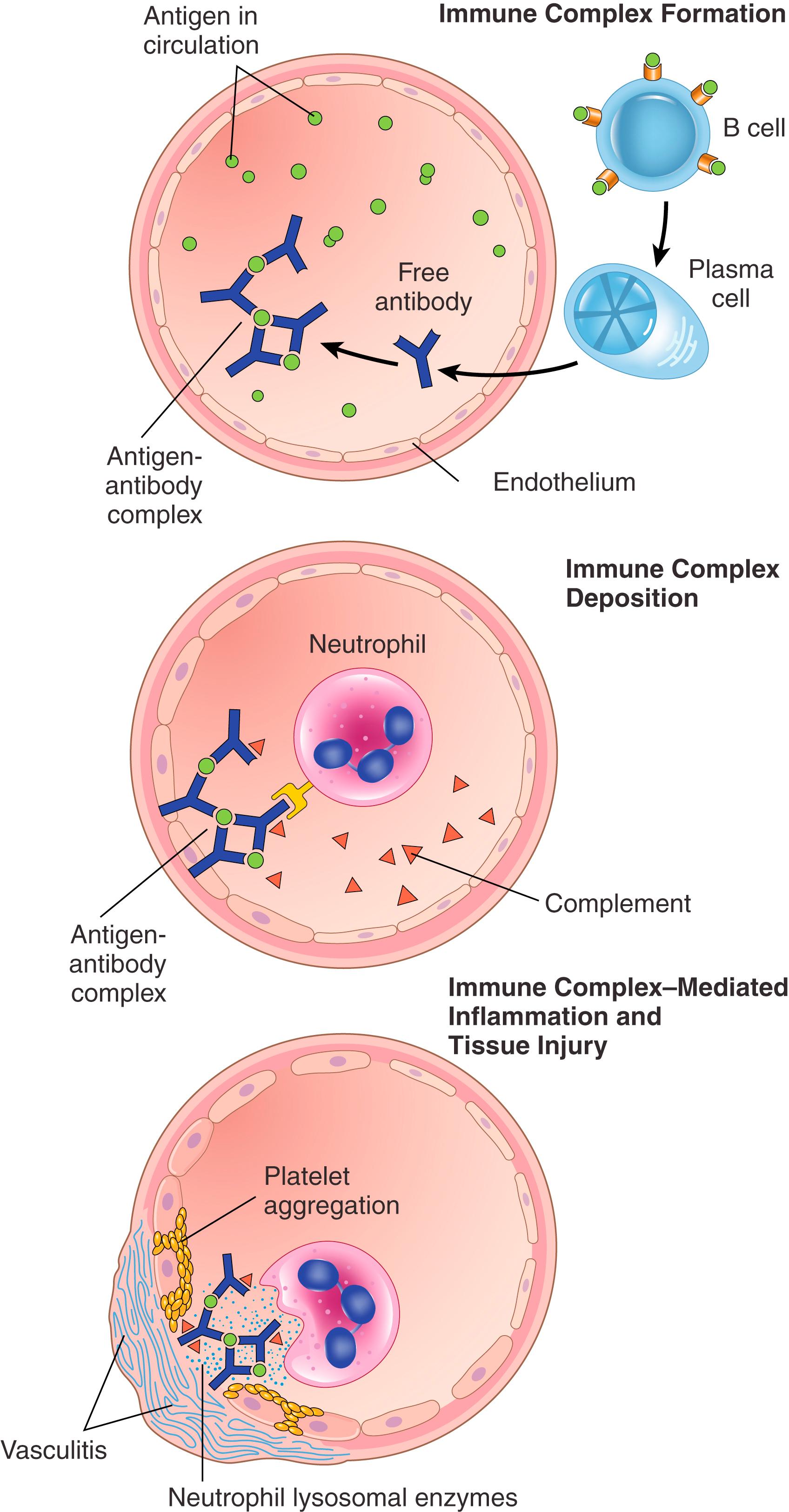
Protein antigens trigger immune responses that result in the formation of antibodies, typically about 1 week after the introduction of the antigen. These antibodies are secreted into the blood, where they react with the antigen still present in the circulation and form immune complexes.
In the next phase, the circulating antigen-antibody complexes are deposited in various tissues. The factors that determine whether immune complex formation will lead to tissue deposition and disease are not fully understood, but the major influences seem to be the characteristics of the complexes and the features of the vasculature. In general, the most pathogenic immune complexes are those that are produced in amounts and size that are not efficiently cleared by phagocytes in the spleen and liver. Organs where blood is filtered at high pressure to form other fluids, like urine and synovial fluid, are sites where immune complexes become concentrated and deposit; hence, immune complex disease often affects glomeruli and joints. The endothelium in these tissues is also often fenestrated, promoting passage of immune complexes between endothelial cells.
Once deposited in tissues, immune complexes initiate an acute inflammatory reaction via complement activation and engagement of leukocyte Fc receptors. Typically, the antibodies are IgG or IgM, both of which activate complement by the classical pathway. Deposition of complement proteins can be detected at the site of injury. Consumption of complement during the active phase of the disease decreases serum levels of C3, which can be used as a marker for disease activity, e.g., in systemic lupus erythematosus (SLE). During this phase (approximately 10 days after antigen administration), clinical manifestations such as fever, urticaria, joint pain (arthralgia), lymph node enlargement, and proteinuria appear. Wherever complexes deposit, the tissue damage is similar. The resultant inflammatory lesion is termed vasculitis if it occurs in blood vessels, glomerulonephritis if it occurs in renal glomeruli, arthritis if it occurs in the joints, and so on.
The principal morphologic manifestation of immune complex deposition in blood vessels is acute vasculitis, associated with fibrinoid necrosis of the vessel wall and variable neutrophilic infiltration ( Chapter 2 ). When deposited in kidney glomeruli, the complexes cause glomerulonephritis and can be seen on immunofluorescence microscopy as granular deposits of immunoglobulin and complement and on electron microscopy as electron-dense deposits along the glomerular basement membrane ( Chapter 12 ).
Much of what we know about systemic immune complex disease is derived from studies of acute serum sickness, which was once a complication of the administration of large amounts of foreign protein (e.g., in serum from immunized horses used for protection against diphtheria). In modern times, the disease is infrequent and is usually seen in individuals who receive antibodies from other individuals or species, e.g., horse or rabbit antithymocyte globulin administered to deplete T cells in recipients of organ grafts.
Acute serum sickness induced by administration of a single large dose of antigen is characterized by fever, rash, and arthritis; the lesions tend to resolve as the complexes are cleared by phagocytes. A form of chronic serum sickness results from repeated or prolonged exposure to an antigen. This occurs in several diseases, such as systemic lupus erythematosus (SLE), which is associated with persistent antibody responses to autoantigens. In many diseases, the morphologic changes and other findings suggest immune complex deposition, but the inciting antigens are unknown. Included in this category are several vasculitides, such as polyarteritis nodosa.
A model of local immune complex diseases is the Arthus reaction, in which an area of tissue necrosis appears as a result of acute immune complex vasculitis. The reaction is produced experimentally by injecting an antigen into the skin of a previously immunized animal. Immune complexes form as the antigen diffuses into the vascular wall at the site of injection and binds to preformed antibody, triggering the same inflammatory reaction and histologic appearance as in systemic immune complex disease. Within a few hours, the injection site develops edema and hemorrhage, occasionally followed by ulceration.
Several autoimmune disorders, as well as pathologic reactions to environmental chemicals and persistent microbes, are known to be caused by T cells ( Table 5.6 ). Two types of T-cell reactions are capable of causing tissue injury and disease ( Fig. 5.14 ). The most frequent is cytokine-mediated inflammation, in which the cytokines are produced mainly by CD4+ T cells. Direct cell cytotoxicity mediated by CD8+ T cells may also contribute to tissue injury. This group of diseases is of great clinical interest because T-cell reactions are increasingly recognized as the basis of chronic inflammatory diseases, and many of the new rationally designed therapies for these diseases have been developed to target T cells.
| Disease | Specificity of Pathogenic T Cells | Principal Mechanisms of Tissue Injury | Clinicopathologic Manifestations |
|---|---|---|---|
| Rheumatoid arthritis | Collagen? Citrullinated self proteins? |
Inflammation mediated by Th17 (and Th1?) cytokines; role of antibodies and immune complexes? | Chronic arthritis with inflammation, destruction of articular cartilage |
| Multiple sclerosis | Protein antigens in myelin (e.g., myelin basic protein) | Inflammation mediated by Th1 and Th17 cytokines; myelin destruction by activated macrophages | Demyelination in CNS with perivascular inflammation; paralysis |
| Type 1 diabetes | Antigens of pancreatic islet β cells (insulin, glutamic acid decarboxylase, others) | T cell–mediated inflammation, destruction of islet cells by CTLs | Insulitis (chronic inflammation in islets), destruction of β cells; diabetes |
| Inflammatory bowel disease | Enteric bacteria; self antigens? | Inflammation mediated by Th1 and Th17 cytokines | Chronic intestinal inflammation, obstruction |
| Psoriasis | Unknown | Inflammation mediated mainly by Th17 cytokines | Plaques in the skin |
| Contact sensitivity | Various environmental chemicals (e.g., urushiol from poison ivy or poison oak, therapeutic drugs) | Inflammation mediated by Th1 (and Th17?) cytokines | Epidermal necrosis, dermal inflammation, causing skin rash and blisters |
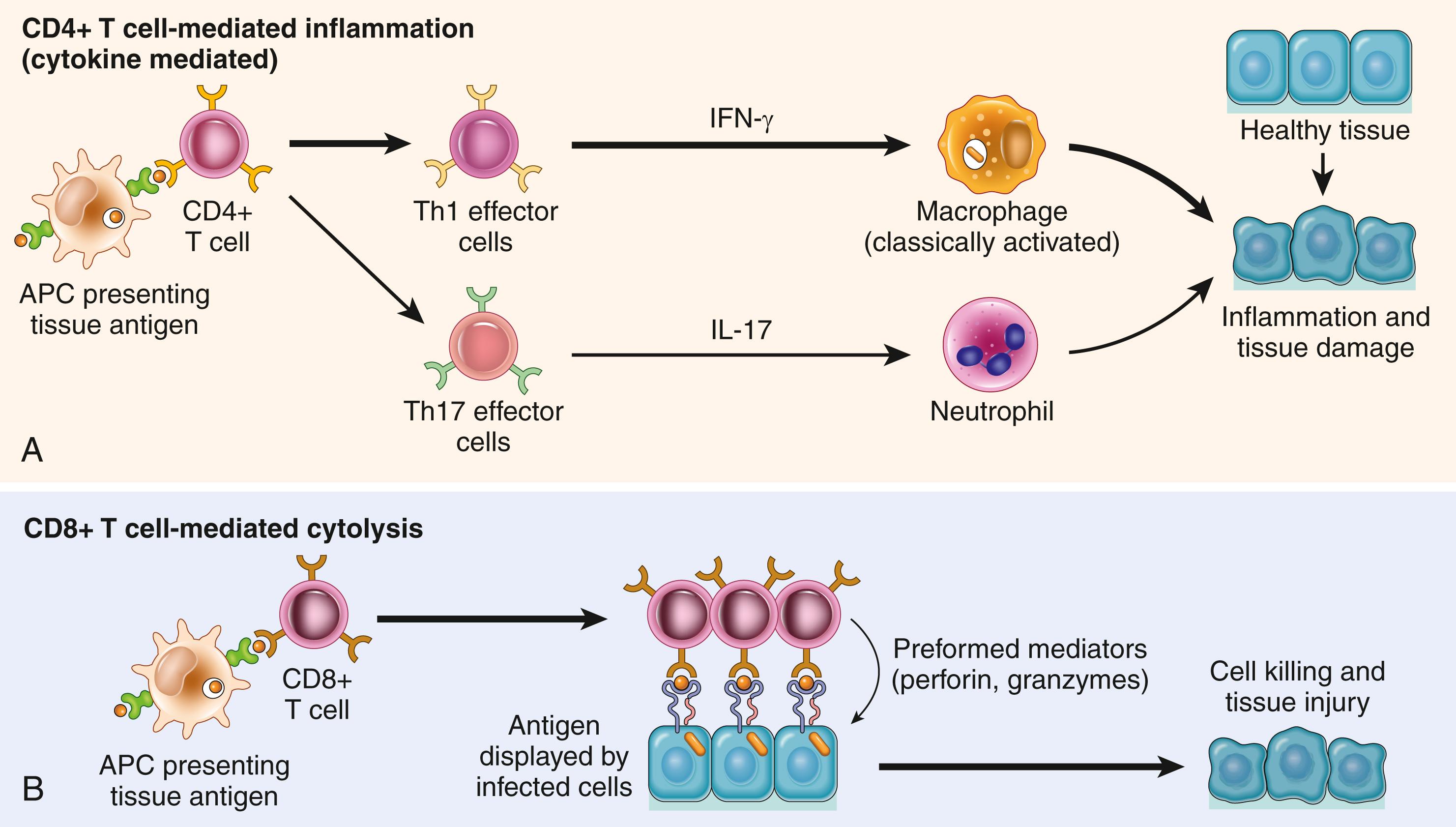
In CD4+ T cell–mediated hypersensitivity reactions, cytokines produced by the T cells induce inflammation that may be chronic and destructive. The prototype of T cell–mediated inflammation is delayed-type hypersensitivity (DTH), a tissue reaction to antigens given to individuals who are already sensitized. In this setting, an antigen administered into the skin results in a detectable cutaneous reaction within 24 to 48 hours (hence the term delayed, in contrast to immediate hypersensitivity).
As described earlier, naïve T cells are activated in secondary lymphoid organs by recognition of peptide antigens displayed by dendritic cells and the T cells differentiate into effector cells. Classical T cell–mediated hypersensitivity is a reaction of Th1 effector cells, but Th17 cells also may contribute, especially when neutrophils are prominent in the inflammatory infiltrate. Th1 cells secrete cytokines, mainly IFN-γ, which are responsible for many of the manifestations of delayed-type hypersensitivity. IFN-γ–activated (so-called classically activated, or M1) macrophages produce substances that destroy microbes and damage tissues and mediators that promote inflammation ( Chapter 2 ). Activated Th17 cells secrete cytokines that recruit neutrophils and monocytes.
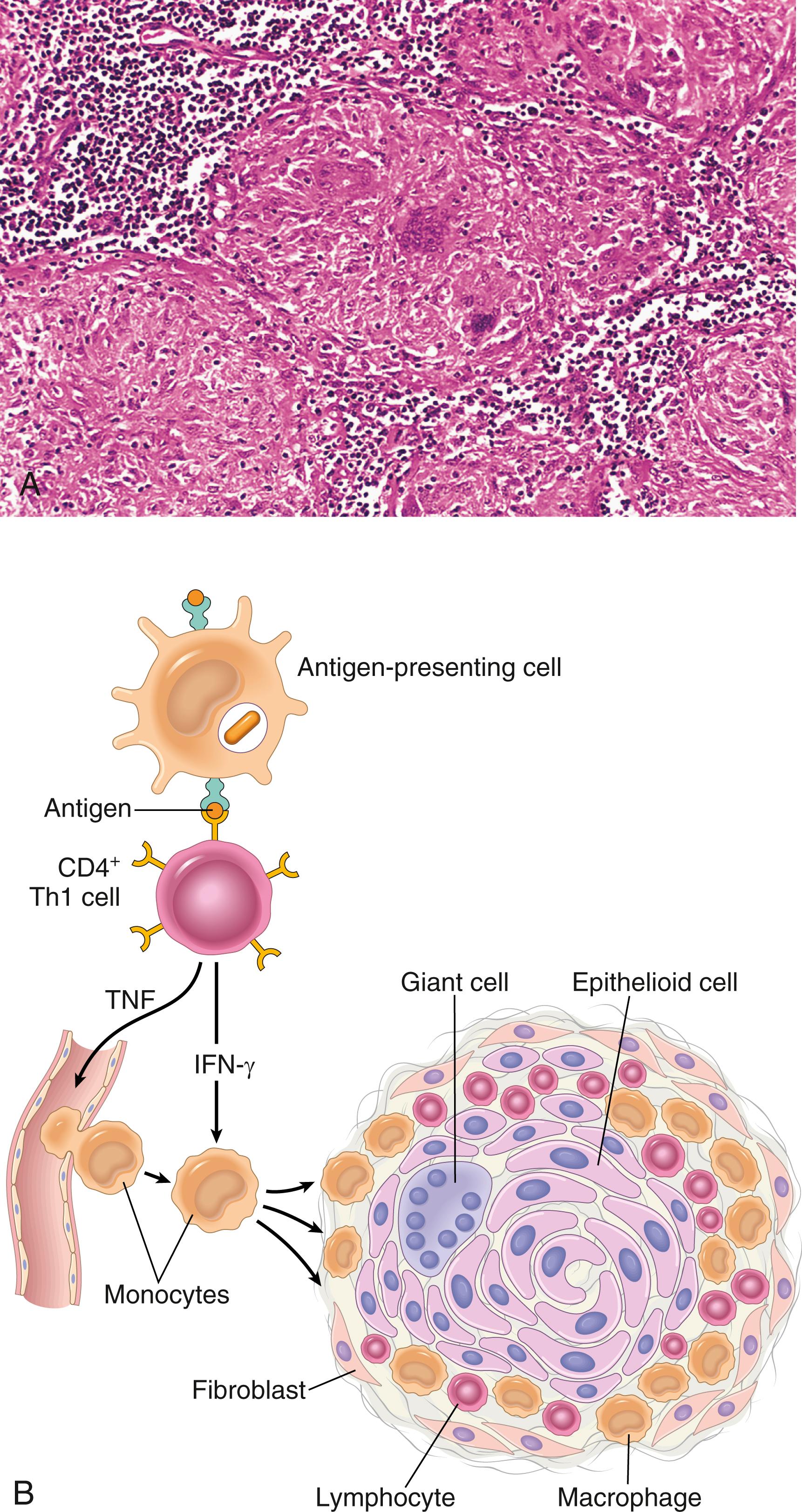
The classic example of DTH is the tuberculin reaction (known in clinical medicine as the PPD skin test ), which is produced by the intracutaneous injection of purified protein derivative (PPD, also called tuberculin ), containing protein antigens of the Mycobacterium tuberculosis bacillus. In a previously exposed individual, reddening and induration of the site appear in 8 to 12 hours, reach a peak in 24 to 72 hours, and then slowly subside. Morphologically, delayed-type hypersensitivity is characterized by the accumulation of mononuclear cells, mainly CD4+ T cells and macrophages, around venules, producing perivascular “cuffing” ( Fig. 5.15 ). Prolonged DTH reactions against persistent microbes or other stimuli may result in a special pattern of reaction called granulomatous inflammation ( eFig. 5.1 ), described in Chapter 2 . The release of IFN-γ from blood cells stimulated with mycobacterial antigens in vitro is another widely used test for tuberculosis.
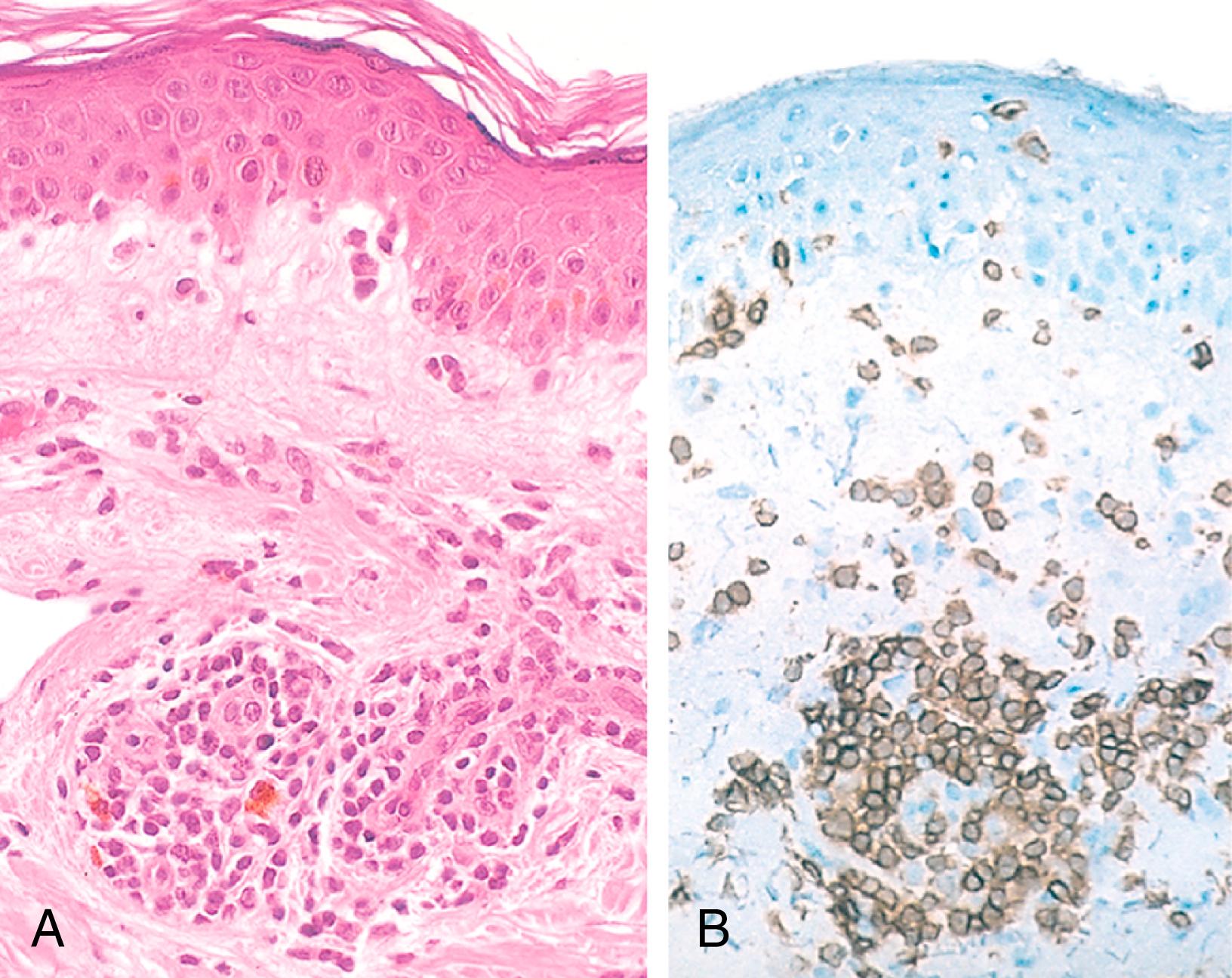
Contact dermatitis is a common example of tissue injury resulting from DTH reactions. It may be evoked by contact with urushiol, the antigenic component of poison ivy and poison oak, and presents as a vesicular dermatitis. It is thought that in these reactions, the environmental chemical binds to and structurally modifies self proteins, and peptides derived from these modified proteins are recognized by T cells that elicit the reaction. The same mechanism is responsible for many drug reactions, among the most common hypersensitivity reactions of humans. The responsible drug (often a reactive chemical) alters self proteins, including MHC molecules, and these neoantigens are recognized as foreign by T cells, leading to cytokine production and inflammation. Drug reactions often manifest as skin rashes.
CD4+ T cell–mediated inflammation is the basis of tissue injury in many organ-specific and systemic autoimmune diseases, such as rheumatoid arthritis and multiple sclerosis, as well as diseases probably caused by uncontrolled reactions to bacterial commensals, such as inflammatory bowel disease (see Table 5.6 ).
In this type of T cell–mediated reaction, CD8+ CTLs kill antigen-expressing target cells. Tissue destruction by CTLs may be a component of some T cell–mediated diseases, such as type 1 diabetes. CTLs directed against cell surface histocompatibility antigens play an important role in organ transplant rejection, which is discussed later. They also play a role in reactions against viruses. In a virus-infected cell, viral peptides are displayed by class I MHC molecules and the complex is recognized by the TCR of CD8+ T lymphocytes. The killing of infected cells leads to elimination of the infection but, in some cases, causes cell damage (e.g., in viral hepatitis). CD8+ T cells also produce cytokines, notably IFN-γ, and are involved in inflammatory reactions resembling DTH, especially following virus infections and exposure to some contact sensitizing agents.
Now that we have described how the immune system can cause tissue damage, we turn to autoimmune disorders, which are the result of failure of tolerance to self antigens and in which disease is caused by hypersensitivity reactions.
Autoimmunity refers to immune reactions against self (“auto”) antigens. Autoimmune diseases are fairly common, estimated to affect 5% to 8% of the U.S. population. Autoimmune diseases may be organ-specific, in which the immune responses are directed against one particular organ or cell type and result in localized tissue damage, or systemic, characterized by lesions in many organs ( Table 5.7 ). In systemic diseases that are caused by immune complexes and autoantibodies, the lesions principally affect the connective tissues and blood vessels of involved organs. Therefore, these diseases are often referred to as collagen vascular diseases or connective tissue diseases, even though the immunologic reactions are not specifically directed against constituents of connective tissue or blood vessels.
| Organ-Specific | Systemic |
|---|---|
| Diseases Mediated by Antibodies | |
| Autoimmune hemolytic anemia Autoimmune thrombocytopenia Autoimmune atrophic gastritis of pernicious anemia |
Systemic lupus erythematosus ANCA-associated vasculitis |
| Myasthenia gravis | |
| Graves disease | |
| Goodpasture syndrome | |
| Diseases Mediated by T Cells a | |
| Type 1 diabetes mellitus | Rheumatoid arthritis |
| Multiple sclerosis | Systemic sclerosis (scleroderma) b |
| Sjögren syndrome b | |
| Diseases Postulated to Be Autoimmune | |
| Inflammatory bowel diseases (Crohn disease, ulcerative colitis) c | Polyarteritis nodosa b |
| Primary biliary cholangitis b | |
| Autoimmune (chronic active) hepatitis | |
a A role for T cells has been demonstrated in these disorders, but antibodies may also be involved in tissue injury.
b An autoimmune basis of these disorders is suspected, but not proven.
c These disorders may result from excessive immune responses to commensal enteric microbes, autoimmunity, or a combination of the two.
Normally, individuals are unresponsive (tolerant) to their own (self) antigens, and autoimmunity results from a failure of self-tolerance. Therefore, understanding the pathogenesis of autoimmunity requires familiarity with the mechanisms of normal immunologic tolerance.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here