Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Degeneration and loss of joint function are common end points for many arthropathies, the most prevalent of which is osteoarthritis (OA). Until the late twentieth century, OA was regarded as a mechanical disease, with damage to the articular surface resulting from the inevitable wear and tear of daily living. More recently, the Osteoarthritis Research Society International has defined OA as a progressive disease of the synovial joint that represents the failed repair of joint damage from stresses initiated by an abnormality in any of the synovial joint tissues, ultimately resulting in the breakdown of cartilage and bone. This definition reflects the growing trend away from viewing OA as a mechanical injury of cartilage to a disease of the entire joint. Although late-stage OA is diagnosed clinically by characteristic radiographic findings and symptoms of pain, stiffness, and disability, early-stage OA is frequently asymptomatic. When considering potential treatments for OA, it is useful to differentiate structural changes in the joint ( the disease OA ) from patient-reported symptoms ( the illness OA ). The mechanism of damage to the joint may differ widely between patients and between joints. In this respect, there is growing consensus that OA is not a single disease of joints but rather a collection of many diseases that may require different treatments.
As will be discussed in the chapter, advanced imaging techniques, such as MRI, are valuable in identifying many of the abnormalities of the joint tissues that progress to OA. Other chapters in this text edition review specific conditions and injury that may ultimately lead to secondary OA. Although OA involves the entire joint, the primary focus of the chapter is articular cartilage, with particular emphasis on MRI for identification of early cartilage injury.
Despite a high prevalence of this disease, the etiology and pathogenesis of OA are poorly understood. It is generally accepted that damage to articular cartilage occurs when local biomechanical forces exceed the material properties of cartilage, leading to structural and biochemical changes in the cartilage matrix and alteration in gene expression of the chondrocyte. These biomechanical forces are determined by many variables, including body habitus, intensity and type of physical activity, stiffness of the subchondral bone, joint trauma, as well as alignment of the joint and laxity in the stabilizing tissues. The material properties of cartilage are also influenced by many factors, including biochemical changes as a result of normal aging, genetics, diet, exercise, and coexisting systemic diseases or conditions. The complex interplay of these many variables produces an imbalance in cartilage homeostasis in which degradation of the cartilage matrix exceeds synthesis, ultimately progressing to structural loss of tissue. Recently, the concept of OA phenotypes has been proposed as a method for classifying subtypes of OA, based on patterns of these variables that may differentiate patients amenable to specific treatments directed at the root cause of progressive joint deterioration. Examples of OA phenotypes would be individuals with a more pronounced inflammatory component of OA presenting with synovitis and joint effusion or patients presenting with features of focal cartilage loss and subchondral fractures that may reflect excessive mechanical loading in the joint.
Current estimates are that symptoms and disability related to OA afflict almost 27 million Americans. With a rising mean age of the American population and growing incidence of secondary OA in younger individuals, it is projected that, by the year 2020, OA will afflict more than 60 million Americans.
Age is the primary risk factor for development of both radiographic and clinical OA. In individuals younger than 45 years, the prevalence of OA increases slowly in a linear fashion with age. After age 45 years, the rate of disease prevalence of OA increases exponentially with age, afflicting approximately 50% of people at age 65 years, increasing to 85% in those individuals 75 years or older.
Obesity has been shown to be a risk factor for knee OA; however, the relative risk for hip OA is less conclusive. Studies have shown that weight loss can reduce the risk of OA. Of note, obesity has been shown to increase the risk of OA in non–weight-bearing joints, such as the interphalangeal joints of the hand, suggesting that systemic as well as local biomechanical factors may be responsible. In the hip, anatomic variations in the femoral head and acetabulare related to developmental acetabular dysplasia and femoral acetabular impingement, as discussed in Chapter 23 , are significant risk factors for the development of hip OA.
Physical activity, particularly that related to long-term repetitive activities associated with particular occupations, can increase the risk of OA. Hip arthritis is more common in farmers. Hip and spine OA is more prevalent in miners. Unusual repetitive activities may lead to OA in atypical joints. For example, there is an increased frequency of upper extremity OA in pneumatic drill operators. Although a slightly higher incidence of OA is observed in high-performance competitive athletes, this is frequently associated with prior joint trauma, which is strongly associated with secondary OA. Moderate recreational exercise, such as running, has not been associated with an increased risk of OA. The current consensus is that moderate physical activity does not increase risk for OA and is offset by the benefits associated with reducing other comorbidities, such as obesity and metabolic syndrome.
Although once considered a noninflammatory arthropathy, there has been greater emphasis placed on the role of inflammation in OA, both in terms of its role in progressive joint damage and as a source of patient symptoms. A high prevalence of synovial inflammation is present in osteoarthritic knees, which may be detected with ultrasonography or MRI and has been shown to correlate with knee pain and structural progression.
Pain along with loss of joint mobility is a common symptom of OA. Typically, the pain is poorly localized around the joint, increases with activity, and is relieved with rest. The source of pain in OA is not entirely clear but may be associated with the presence of joint effusion, mild synovitis, and changes in subchondral bone marrow. Radiographic features of OA, such as joint space narrowing and osteophyte formation, correlate poorly with clinimetric measures of pain.
OA may be associated with transient episodes of morning stiffness. With further progression of the disease, loss of joint congruity and development of osteophytes and intraarticular osteochondral bodies can lead to loss of joint function and disability with difficulties in activities of daily living. Complaints of joint instability are common, particularly in knee OA.
Focal chondral or osteochondral lesions may have a clinical presentation that mimics meniscal pathology in the knee or labral pathology in the hip or shoulder. Symptoms consist of nonspecific pain or episodes of locking. With MRI, focal chondral abnormalities are frequently observed in subjects without joint symptoms and in otherwise healthy volunteers. Because cartilage lacks nociceptive receptors, cartilage injury alone is not responsible for pain but may contribute secondarily to pain through associated changes in subchondral bone marrow or synovium.
Under physiologic conditions, articular cartilage is exposed to high compressive and shear forces. For example, it is estimated that during a squatting maneuver, patellar cartilage undergoes compressive loads that are greater than six times body weight, with transient pressures that may be substantially higher. These forces are applied repetitively many times a day throughout the course of a lifetime. What is remarkable is not that cartilage degrades but that it is capable of functioning normally under these conditions throughout a human life span.
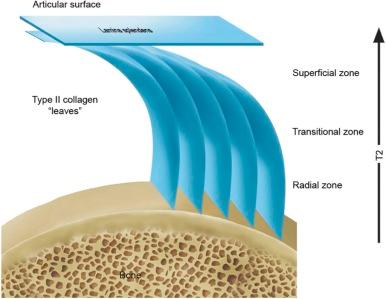
The biomaterial properties of articular cartilage are a result of the composition and organization of the extracellular matrix. Cartilage is 65% to 85% water, with water content decreasing slightly with depth from the articular surface. The major solid components of the extracellular matrix are type II collagen, which comprises 10% to 20% of the wet weight of cartilage, and large molecules of aggregating proteoglycans, termed aggrecan, which contribute 5% to 10% wet weight. The content and structure of collagen and proteoglycans in the matrix differ substantially from bone to the articular surface and strongly influence the biomaterial properties of cartilage. The components of type II collagen, tropocollagen molecules, are polymerized into larger collagen fibrils, which, in turn, are organized into a leaflike architecture. As illustrated in eFigure 47-1 and , the orientation and alignment of the collagen matrix vary with depth from the articular surface as well as regionally within the joint. Collagen fibrils cross the bone-cartilage interface at the tidemark zone and anchor cartilage to the calcified cartilage layer. In the deep layer of cartilage near bone, collagen fibrils have a preferential orientation perpendicular to the bone surface. This is frequently termed the radial zone, referring to the radial orientation of the collagen matrix as well as the columnar alignment of chondrocytes observed in this layer. Near the surface, the orientation of the collagen matrix curves tangentially in the transitional zone, becoming parallel to the articular surface in the superficial zone. The superficial layer is covered with a distinct layer of dense collagen fibers termed the lamina splendens. The tangential and superficial layers of cartilage are relatively thin in regions of the joint habitually exposed to high compressive loads, such as the central portion of the femoral condyle and tibial plateau uncovered by the meniscus. These layers are thicker in regions exposed to less load, such as articular surfaces covered by the meniscus.
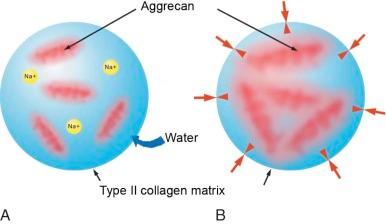
Interposed in the meshwork of type II collagen fibrils are large molecules of aggregating proteoglycans consisting of aggrecan ( eFig. 47-2 ; see ). Concentration of proteoglycans also varies within cartilage, with the highest levels found below the articular surface and decreasing in concentration near bone. The aggrecan molecules consist of a central core fiber of hyaluronic acid, which is bound to negatively charged side chains through a linking protein. The aggrecan side chain is composed of the glycosaminoglycan (GAG) molecules, chondroitin sulfate and keratin sulfate, attached to a central core binding protein. When these molecules are hydrated, the high density of negative charges on the proteoglycans binds sodium and draws water into cartilage, causing the proteoglycans to swell. Swelling of the proteoglycans is constrained by the surrounding collagen meshwork, producing an interstitial fluid pressure that contributes to the compressive stiffness of cartilage. The balance of aggrecan swelling and an intact collagen matrix is an essential feature for normal cartilage function.
The MRI relaxation properties of articular cartilage and appearance of cartilage with standard clinical MRI techniques are strongly influenced by the composition and structure of the extracellular matrix. This is most evident on proton density (PD)–weighted images. As illustrated in eFigure 47-3 , the signal intensity of cartilage varies with location in the joint as well as with depth from the articular surface. This is primarily a function of regional and zonal differences of the type II collagen matrix, which influences T2 relaxation of cartilage as well as variation in water content. Within the radial zone, the high content and anisotropic orientation of collagen fibrils provide efficient T2 relaxation, leading to low signal intensity on PD or T2-weighted images. With very high resolution images, the darker radial zone has a striated appearance with alternating fine bands of high and low signal intensity radiating from the bone cartilage interface. In lower-resolution images, individual striations are not resolved and, instead, this layer is characterized by low signal intensity. Closer to the articular surface, less fibril anisotropy and oblique orientation of the collagen matrix lead to a gradual increase in T2 relaxation time and, therefore, a relative increase in signal intensity on T2-weighted images. At the articular surface, collagen fibers are oriented parallel to the articular surface. The superficial tangential layer and the lamina splendens are approximately 20 µm thick and can be identified on MR microscopy images of excised cartilage specimens as a thin hypointense layer; however, it is too thin to resolve on routine clinical MR images.
The organization and orientation of collagen within the extracellular matrix have a strong influence on the MRI appearance of cartilage. In connective tissues, such as tendons, ligaments, and articular cartilage, the highly ordered arrangement of collagen fibers produces residual quadripolar coupling with mobile protons, an efficient mechanism for T2 relaxation. For these tissues with a highly preferred or anisotropic organization of collagen fibrils, T2 relaxation is dependent on the relative orientation of the collagen fibers with the static magnetic field (B0). Tissues containing fibers oriented parallel or perpendicular to B0 have efficient T2 relaxation and low signal intensity on T2-weighted images. However, when fibers are oriented 54 degrees relative to B0, there is averaging of the residual quadripolar coupling, which minimizes this contribution to T2 relaxation and leads to an increase in signal intensity. Because of this effect, 54 degrees is termed the magic angle, derived from the technique of magic angle spinning used to increase the T2 relaxation of crystalline samples in solid-state nuclear magnetic resonance spectroscopy.
The high concentration of collagen in the radial zone also decreases signal intensity through magnetization transfer. As suggested by the name, magnetization transfer is a process in which magnetization from a proton located on collagen is transferred to the mobile proton pool that gives rise to the MR signal. Magnetization transfer effects are most pronounced for techniques that use a large number of radiofrequency pulses, such as multislice fast spin-echo (FSE) or turbo spin-echo (TSE) techniques. The rapid application of off-resonance radiofrequency energy quickly saturates protons bound to the collagen molecules but does not saturate the mobile pool in the surrounding water. The saturated magnetization of the bound pool is then transferred either through chemical exchange or exchange of magnetization to nearby water protons in the mobile pool. Types I and II collagen demonstrate substantial magnetization transfer with FSE techniques. The effect of magnetization transfer is to decrease signal intensity of tissues rich in collagen on the MR image. For tissues such as cartilage, incidental magnetization transfer reduces signal intensity by 15% to 20% as the number of slices, and, therefore, the amount of off-resonance irradiation increases. Because gradient-echo techniques use significantly less radiofrequency energy, there is less incidental magnetization transfer with gradient-echo techniques compared with spin-echo and FSE techniques. As a result of less magnetization transfer and T2 weighting, cartilage has relatively uniform high signal intensity on T1-weighted fat-suppressed gradient-echo images.
Recognizing the normal heterogeneity of cartilage is important to avoid erroneously interpreting nonuniform signal as disease on T2-weighted images. As discussed previously, the signal intensity of cartilage normally increases toward the surface. In addition to variation in signal intensity with respect to depth from the articular surface, there are differences with respect to location in the joint and relative orientation of cartilage to the applied magnetic field. This variation in signal intensity closely follows variation in histologic and biomechanical properties of cartilage. For example, cartilage consistently exposed to high compressive load, such as tibial cartilage not covered by meniscus, has a thin superficial layer of high signal intensity, whereas the covered portion of tibial cartilage has a thicker layer of superficial hyperintensity. Goodwin and colleagues have correlated this regional variation in signal intensity with obliquity of the collagen matrix cleavage planes on freeze-fracture specimens, indicating that the relative orientation of the cartilage matrix to the applied magnetic field is a major factor.
Regional differences in cartilage T2 relaxation are most pronounced in the femoral condyle. This is a result of two processes. First, there are substantial differences in the organization of extracellular matrix and chondrocytes between the central femoral surface and posterior femoral condyles, which are not habitually exposed to high compressive load but experience shear forces during knee flexion. Whereas type II collagen in the central femoral condyle has a high degree of anisotropy, results from electron microscopy and X-ray diffraction studies indicate that the collagen matrix in the posterior femoral condyle has less anisotropy and has a fine fibrillar organization. This region of cartilage lacks the regular bands of condensed collagen seen in the central femoral condyle. Second, the oblique orientation of the collagen matrix in this region with respect to the direction of B0 is close to the magic angle of 54 degrees. As a result of differences in organization and orientation of the type II collagen matrix, cartilage in the posterior femoral condyle demonstrates uniformly high signal intensity compared with the layered pattern of signal intensity observed in the central femoral condyle. Although regional variation in the MRI appearance of cartilage has been extensively studied for the knee, similar variation is observed in other joints with MR images of sufficient resolution.
Normal cartilage function depends on a balance of aggrecan swelling and constraining forces provided by the collagen meshwork. Investigators in the early 1970s proposed that one of the earliest processes of cartilage degeneration was fragmentation of the collagen matrix. Because damaged collagen no longer constrains the proteoglycans, they swell and draw in more water. The increased water content and loss of interstitial fluid pressure allow water to move more freely through the tissue. As a result, more compressive load is carried by the solid components, leading to structural fatigue. This results in a cascade of events in which damage to the solid matrix increases mobility of water, thereby transferring more of the compressive load onto the matrix and producing further damage. Eventually, this leads to a gross loss of cartilage.
Although chondrocytes are capable of producing new proteoglycan, there is limited capacity to repair collagen damage. Most investigators believe damage to the collagen meshwork is irreversible. Several factors can increase susceptibility of cartilage collagen to damage. First, genetic mutations of the type II collagen gene have been implicated in several syndromes associated with premature onset of OA ( Fig. 47-1 ). These are collectively known as type II collagenopathies and include Stickler syndrome, Kniest dysplasia, and spondyloepiphyseal dysplasia, along with other conditions. With the relatively large degree of genetic diversity in the type II collagen gene, it is likely that inherited factors contribute to the relative risk of OA in the general population. Additional mutations have been identified in genes encoding for other cartilage macromolecules. Second, senescent accumulation of advanced glycation end products in cartilage and cross-linking of collagen fibrils make the collagen matrix more brittle and prone to fracture. Because of the slow turnover of collagen in cartilage, these byproducts accumulate with age. Age also influences chondrocyte metabolism; in particular, there appears to be a diminished capacity of older chondrocytes to synthesize proteoglycan. As a result, older cartilage may be more susceptible to damage from fatigue fractures and may have less capacity to replenish aggrecan, thereby predisposing older individuals to the development of OA. Third, two families of enzymes are observed to be unregulated in early OA and may contribute to the progression of cartilage damage. Matrix metalloproteinases break down collagen, whereas aggrecanases degrade aggrecan. In the healthy state, the activity of these degradative enzymes is balanced by that of synthetic enzymes, as well as production of specific enzyme inhibitors. Inflammatory mediators, such as interleukin-1 and tumor necrosis factor, are increased in OA and tip the balance of enzymatic pathway toward the side of matrix degradation.
As discussed previously, normal cartilage biomechanics relies on a balance of aggrecan swelling pressure and constraint by the surrounding collagen meshwork. This balance is similar to an automobile tire in which the swelling pressure from the proteoglycans is analogous to the tire pressure from the air, and the constraint of the collagen meshwork is like the walls of the tires. Just as driving on tires with low air pressure leads to rapid deterioration of the tire, low interstitial pressure in cartilage leads to deterioration of the matrix. High fluid pressure, along with the high osmolarity, viscosity, and electrostatic forces of the extracellular cartilage matrix, restricts the movement of interstitial water and provides compressive stiffness to cartilage. As illustrated in , the compression of cartilage extrudes water from the extracellular matrix near the articular surface into the synovial fluid. When the compressive load is released, the osmotic pressure in cartilage pulls water back into the tissue. The energy imparted into cartilage when it is compressed is dissipated by frictional drag forces as water moves through the extracellular matrix. For cartilage to resist compression, the movement of water through the extracellular cartilage matrix must be restricted. Under normal conditions, approximately 90% of the load is carried by water, which is the primary reason that cartilage is so resilient to repetitive compression.
In addition to compression, cartilage is also exposed to shear stress as one articular surface moves against another. The normal articular surface of cartilage has an extremely low coefficient of friction, allowing the surfaces to move smoothly. Damage to the surface, such as fibrillation or focal cartilage defects, increases friction at the surface and tensile strain in the extracellular matrix. Shear stiffness in cartilage is a function of tensile strain that develops in the type II collagen matrix. Aggrecan also contributes to shear stiffness by buttressing the collagen fibers and inflating the collagen meshwork.
Mechanical factors influence the susceptibility of cartilage to damage. It is acknowledged that high mechanical load, such as impact injuries, may exceed the material properties of even healthy tissue, producing fracture fissures through the matrix. However, a certain amount of mechanical loading is necessary for normal cartilage health. Moderate cyclical loading has been shown to increase proteoglycan production in cultured chondrocytes; however, high compressive forces, particularly static forces, have a deleterious effect on cartilage physiology. Increased levels of exercise have been shown to increase proteoglycan levels in cartilage, indicating that the tissue has the potential to adapt to changes in demands. In contrast, joint immobilization leads to rapid depletion of proteoglycan and increased susceptibility to cartilage injury.
An emerging theory for the etiology of OA and cartilage degradation is based on a growing body of evidence that indicates cartilage adapts to local biomechanical forces in the joint. In this theory, cartilage is conditioned to withstand the local forces applied to it. When local forces are substantially altered, such as after meniscal resection, with abnormal joint alignment, or with increased joint laxity, biomechanical forces are transferred to unconditioned cartilage that exceeds the stress threshold of the tissue, leading to progressive deterioration of tissue.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here