Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hepatic ablative techniques remain an important tool in the armamentarium against both primary and metastatic liver tumors. Liver resection continues to be the gold standard in management; however, ablative therapies are useful for patients with limited disease and compromised liver function, and in some cases they can be curative. Because the majority of malignant liver tumors present as unresectable disease, there has been an increased interest in minimally invasive ablative techniques to prolong survival.
Liver failure continues to be a major cause of death in patients with unresected liver tumors, especially in the setting of preexisting liver dysfunction. , Ablation can be used in combination with parenchymal-sparing resection to control disease progression within the liver. Intrahepatic recurrences are common after liver resection for primary and metastatic malignancies. Hepatic ablation is a useful tool to manage recurrences in liver remnants that are inadequate for re-resection.
The most common modalities of thermal ablation include radiofrequency ablation (RFA; see Chapter 96B ), microwave ablation (MWA; see Chapter 96C ), and cryoablation (or cryotherapy). One of the original ablative techniques, cryotherapy has largely been replaced by RFA and MWA due to reduced complication rates and improved oncologic outcomes. ,
Hepatocellular carcinoma (HCC) is the sixth most common new cancer diagnosis in the world with 841,080 new cases in 2018 and the fourth most common cause of cancer-related death in the world with 781,631 deaths in 2018. Incidence and mortality vary by geographic location with highest incidence and mortality in Eastern Asian and North African countries. Viral hepatitis, mainly hepatitis B, is a key risk factor for HCC and the incidence of hepatitis B mirrors that of HCC. In the United States, hepatitis C is much more common and has likely driven the rising incidence of HCC (see Chapter 68 ).
HCC is typically preceded by cirrhosis (see Chapter 74 ), hence a curative resection is typically reserved for those patients with early-stage cancers and preserved liver function. Unfortunately, only 5% to 10% of patients present with resectable disease. Ablation can be curative in early stage disease and as such, ablative techniques are typically used for patients with very early stage HCC (single nodules, <2 cm, Child-Pugh A, ECOG Performance Status: 0) and patients with early stage HCC (up to 3 nodules <3 cm, Child-Pugh A-B, ECOG Performance Status: 0). Survival after ablation in Child-Pugh A patients has been shown to be 50% to 75% at 5 years, paralleling survival after resection.
The most common site of metastatic disease for gastrointestinal malignancies is the liver. Colorectal cancer liver metastases (CRLM) represent the most common metastatic tumors to the liver both in the United States and worldwide (see Chapter 90 ). Although locoregional therapies for most secondary liver tumors have not translated into a survival benefit, CRLM are the exception. Of the 20% to 35% of patients with CRLM who present with metastatic disease confined to the liver, only a fraction are truly candidates for liver resection. Patients who are not candidates for liver resection may be candidates for regional ablative therapies as a means to control their disease. Patients with bilobar CRLM present a unique challenge as their disease may be resectable in a staged fashion. Ablative techniques can be useful in this setting as well in combination with liver resection.
A majority of patients with either primary liver cancers or metastatic liver cancers present with unresectable disease, largely because these tumors are relatively asymptomatic. Disease can be technically unresectable due to tumor involvement of both the right and left portal inflow pedicles as well as involvement of multiple hepatic veins such that any attempt at resection would result in an inadequate liver remnant. Disease can also be unresectable due to oncologic factors such as aggressive tumor biology, extent of disease in the liver and elsewhere in the body, and risk of recurrence, as these reduce the risk of long-term survival and/or cure. Patients with HCC have the added complexity of compromised liver function (cirrhosis, portal hypertension, or both) rendering safe liver resection unfeasible (see Chapter 74 ). Numerous techniques have been developed to help treat unresectable liver tumors, including chemoembolization, radioembolization, irreversible electroporation, and ablation. Many of these can be used alone, in conjunction with resection, or, in the case of HCC, as bridging therapy for a curative liver transplant. It is important to always keep in mind that ablated liver is nonfunctional and future liver remnant calculations must be adjusted accordingly.
Cryotherapy has been used to treat both primary and metastatic liver tumors. Although mostly of historical significance due to the improved outcomes and lower cost of RFA and MWA, favorable long-term outcomes are still reported. In relation to other ablative techniques, there are no specific absolute indications and contraindications, and which modality to use is dependent on what technology is available and what the proceduralist has the most experience with.
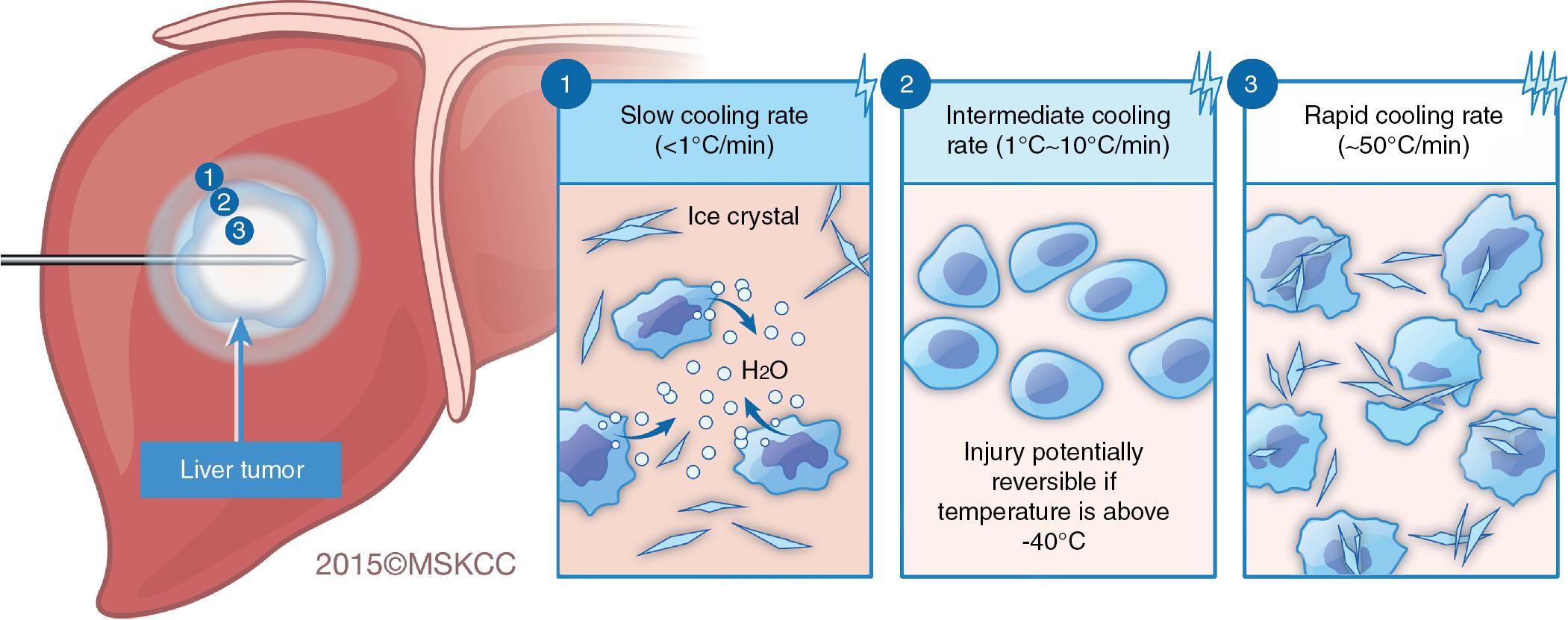
Neither cryotherapy nor any of the other ablative techniques can distinguish normal liver tissue from neoplastic tissue. Whereas RFA and MWA generate heat to trigger cellular death, cryotherapy, as the name suggests, cools tissue to subzero temperatures, thereby causing tissue necrosis. Cellular death is highly dependent on the rate of cooling, depth of hypothermia, rate of thawing, number of freeze-thaw cycles used, and delayed effects of post-thaw ischemia. When a cryoprobe is inserted into the liver, three overlapping zones of injury develop within the ice ball. Rapid tissue freezing occurs closest to the cryoprobe. The rate of freezing decreases in proportion to the distance from the probe, creating zones of intermediate and slow cooling. Similarly, a gradient of temperature develops in the ice ball, decreasing 3°C/mm to 10°C/mm from −170°C near the probe to just below 0°C at the periphery of the cryolesion. The dynamics of the freezing process cause different mechanisms of injury in these three idealized zones. ,
The rate at which the tissue cools directly affects the degree of cell death. Slow cooling results in cellular dehydration whereas rapid cooling induces ice crystallization, which disrupts cellular membranes ( Fig. 96D.1 ). Both slow and rapid cooling induce maximal cell death. Intermediate cooling rates, however, are associated with the greatest degree of cellular survival.
Extracellular and intracellular fluid are composed of varying concentrations of electrolytes, proteins, other macromolecules, and water. Various homeostatic mechanisms maintain iso-osmolarity between the two fluid compartments. The presence of solute in water depresses the freezing point such that supercooling occurs rather than crystallization at below-freezing temperatures.
The actual composition of each fluid compartment differs so greatly that the freezing point of the intracellular compartment is depressed further than the freezing point of the extracellular compartment. Therefore, when tissues are cooled, the extracellular fluid freezes first. When ice forms in the extracellular space, solutes are excluded, making the remaining fluid hyperosmolar. Cellular dehydration occurs as the unfrozen intracellular water flows out of the cell across the osmotic gradient. At a critical level of dehydration, no further fluid can be extracted from the cell because the intracellular macromolecules become concentrated enough to equalize the osmotic gradient across the cell membrane. The ion concentration across the membrane becomes deranged, however, allowing ions to flow into the cell from the hypertonic extracellular fluid to reestablish the Gibbs-Donnon equilibrium. As a consequence of cellular dehydration, the intracellular pH and ion concentrations are altered, proteins denature, and membranes and membrane-bound enzyme systems are disrupted. Some cells will die as a direct result of dehydration, but others require the additional insult provided during the thaw cycle of cryotherapy. When the cryolesion thaws, the extracellular fluid thaws, creating a hypo-osmotic environment. Water flows across the osmotic gradient back into the cells, causing them to swell and ultimately lyse. This type of injury predominates in the slowly cooled zone at the periphery of the cryolesion.
With intermediate cooling rates (1°–10°C/min), cells dehydrate as the extracellular fluid turns to ice. The temperature falls fast enough, however, to freeze the intracellular water before cellular dehydration reaches the critical level to produce irreversible cell injury. Intracellular ice formation excludes solutes, increasing the intracellular osmotic concentrations, equalizing the osmotic gradient across the cell membrane, and stopping further cellular dehydration. As a result, the critical level of dehydration allowing influx of solutes is not reached, protecting the cell from the lethal injury caused by water influx secondary to isotonic rehydration during the thaw cycle. Cells in the zone of intermediate cooling do not suffer the consequences of the cellular dehydration and their survival is improved. Cells located in the intermediate zone may survive, which is a limitation to the efficacy of this therapy.
Rapidly frozen tissue is destroyed by an altogether different mechanism. Cooling rates on the order of 50°C/min, found only in close proximity to the cryoprobe, cause intracellular fluid to freeze before cellular dehydration occurs. Intracellular ice is particularly lethal. Small ice crystals coalesce, causing a physical grinding action that disrupts organelles and cellular membranes. This leads to reproducible and certain cell death.
Regardless of the cooling rate, temperatures less that −20°C result in extensive tissue injury. Moreover, temperatures below −40°C are lethal for nearly all cells. At these temperatures, intracellular fluid freezes and ice crystals are formed, resulting in irreversible cellular damage as described previously. Unfortunately, only tissues near the cryoprobe reliably reach these temperatures, rendering cryotherapy less consistent at inducing cellular death at the periphery of the ablation zone.
The sensitivity of different tissues to hypothermia varies considerably. Most normal hepatocytes die at −15° to −20°C, whereas at −10°C, most hepatocytes survive. Bile ducts, connective tissue, and vascular structures tolerate slightly lower temperatures than hepatocytes. In contrast, liver tumors tend to require deeper hypothermia to −40°C for complete and reliable cell death. As a general rule, the −40°C isotherm is located approximately three-quarters the distance from the probe to the edge of the ice ball, as seen at intraoperative sonography. To reach this level of hypothermia and obtain reliable ablation at the tumor margin, the ice ball is extended 1 cm beyond the peripheral edge of the tumor.
The argon-helium system uses a high-pressure freezing gas (argon) system based on the Joule-Thompson effect. This effect relies on the physical principle that gas changes temperature by expansion through a narrow port into the low-pressure zone that occurs at the tip of a probe. While compressed argon gas passes through the cryoablation needle, the tip is cooled, forming an ice ball that destroys tumor cells. To thaw the tissue, a high-pressure gas (helium) is converted to a warm, low-pressure gas.
Thawing of frozen tissues induces cellular damage and is dependent on the rate of thaw. Rapid thawing is associated with increased survival whereas slow thawing is more destructive than either rapid or slow cooling. In slowly thawed tissue, the extracellular ice melts before the intracellular ice, briefly making the extracellular fluid relatively hypo-osmolar compared with the intracellular fluid. Free water flows down this osmotic gradient into the cells, causing them to swell and ultimately burst. Simultaneously, the ice within the cell undergoes recrystallization, especially in the temperature range of −20° to −25°C. Recrystallization is a process during which the ice crystals reform, coalesce, and enlarge, mechanically disrupting the cellular membranes. The effects of thawing are potentiated by allowing the entire lesion to reach ambient temperature slowly and passively.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here