Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The generation of the action potential is based on an exquisite orchestration of many ion channels acting in concert with a balance of depolarizing and repolarizing forces such that the system (the heart) generates an impulse and returns to its baseline. Despite decades of research into ion channel biology, it is currently impossible to predict how genetic variants in key ion channels will impact the heartbeat. One reason this has been so challenging is that the organ-level proarrhythmic consequences of disease states and genetic variants are fundamentally because of nanoscale changes that affect protein conformational dynamics. Naturally, these molecular details extend to understanding the therapeutic benefits of antiarrhythmic drugs as well. Examples of such critical molecular level interactions include channel binding by therapeutic antiarrhythmic drugs, off-target proarrhythmic effects of drugs binding to other ion channels and organs, and altered channel function by genetic variants that subtly change channel structure. More recently, many different experimental and theoretical approaches have been developed to probe how genetic variation and drug interactions affect protein structure and dynamics. In addition, computational methods have been developed to understand how molecular-level interactions will propagate across spatial and temporal scales to affect cardiac conduction and arrhythmia. By connecting conformations of ion channels to tissue-level dynamics, molecularly detailed models have the potential to be used as high-throughput in silico drug screenings to rapidly assess drug channel interactions and predict their ability to stabilize heart rhythm disturbances or, in the case of off-target effects, provide a screen of cardiac toxicity.
The aim of this chapter is to provide an overview of the methods, the insight that has been gained by their application, and the future advances that are required to improve arrhythmia patient outcomes. It begins with a description of new insights from structural biology that provide high-resolution snapshots of key proteins, goes to functional techniques that connect structural data to protein dynamics, and concludes with theoretical approaches that are being developed to connect this molecular level insight to the cellular and whole heart physiology.
Cardiac electrical conduction is facilitated by a class of transmembrane proteins called voltage-gated ion channels. Voltage-gated ion channels control the myocyte membrane electrical potential by opening and closing to regulate ionic current flow across the cell membrane. Distinct channels selective for specific ions (e.g., K + , Na + , Ca 2+ ) are responsible for various parts of the cardiac action potential. Given their central role in cardiac electrophysiology, these ion channels are targets for pro- and antiarrhythmic small molecules. Accordingly, the structural basis of ion channel function and their pharmacologic modulation represent an active area of cardiac electrophysiology basic science research.
Over the last decade, the field of structural biology has evolved at an incredibly rapid pace, with protocols to resolve high-resolution structures of membrane proteins that had been elusive for many decades. These new techniques have been essential in determining the structures of the key ion channel proteins responsible for cardiac conduction. Initial insight came from x-ray structures of bacterial orthologues, followed by detailed structures of mammalian Ca 2+ , Na + , and K + channels, including cardiac orthologs, that were obtained because of the emergence of high-resolution cryo-electron microscopy (Cryo-EM) protocols. This complement of ion channel structures now reveals the molecular details of the voltage-gated channels that initiate the cardiac action potential (cardiac Na + channel, Na V 1.5), allow entry of Ca 2+ ions to initiate contraction (skeletal muscle Ca V 1.1), and the two components of the delayed rectifier that enable action potential repolarization (the slow component, K V 7.1, and the rapid component, K V 11.1 or hERG). ,
The K V 1.2 channel was the first voltage-gated mammalian channel to be structurally resolved with x-ray crystallography, which revealed modular structural domains responsible for voltage-sensing and ionic current conduction. Each channel subunit contains six membrane-spanning segments numbered from S1 to S6 ( Fig. 19.1A ), with the first four segments (S1–S4) forming the voltage-sensing domain (VSD), and the last two segments contributing to the ion-selective pore domain (S5–S6). The S4 segment within the VSD contains multiple positively charged amino acids that move in response to changes in membrane potential to open and close the channel. Four identical subunits tetramerize to form the full K + channel, yielding four flanking VSDs surrounding a central pore domain (see Fig. 19.1B ). A major surprise from the structure was that the pore domain of one subunit interacted with the VSD of the adjacent subunit and not its own (see Fig. 19.1B ). This “domain-swapped” architecture was also found to hold for the Na + and Ca 2+ channels that can be formed by monomers. For mammalian Na + and Ca 2+ channels, each channel contains four homologous domains (domains I–IV or DI–DIV; see Fig. 19.1C ). Each homologous domain consists of six membrane-spanning segments, resembling a single subunit within a K + channel. The four domains within Na + and Ca 2+ channels are connected by intracellular linkers.
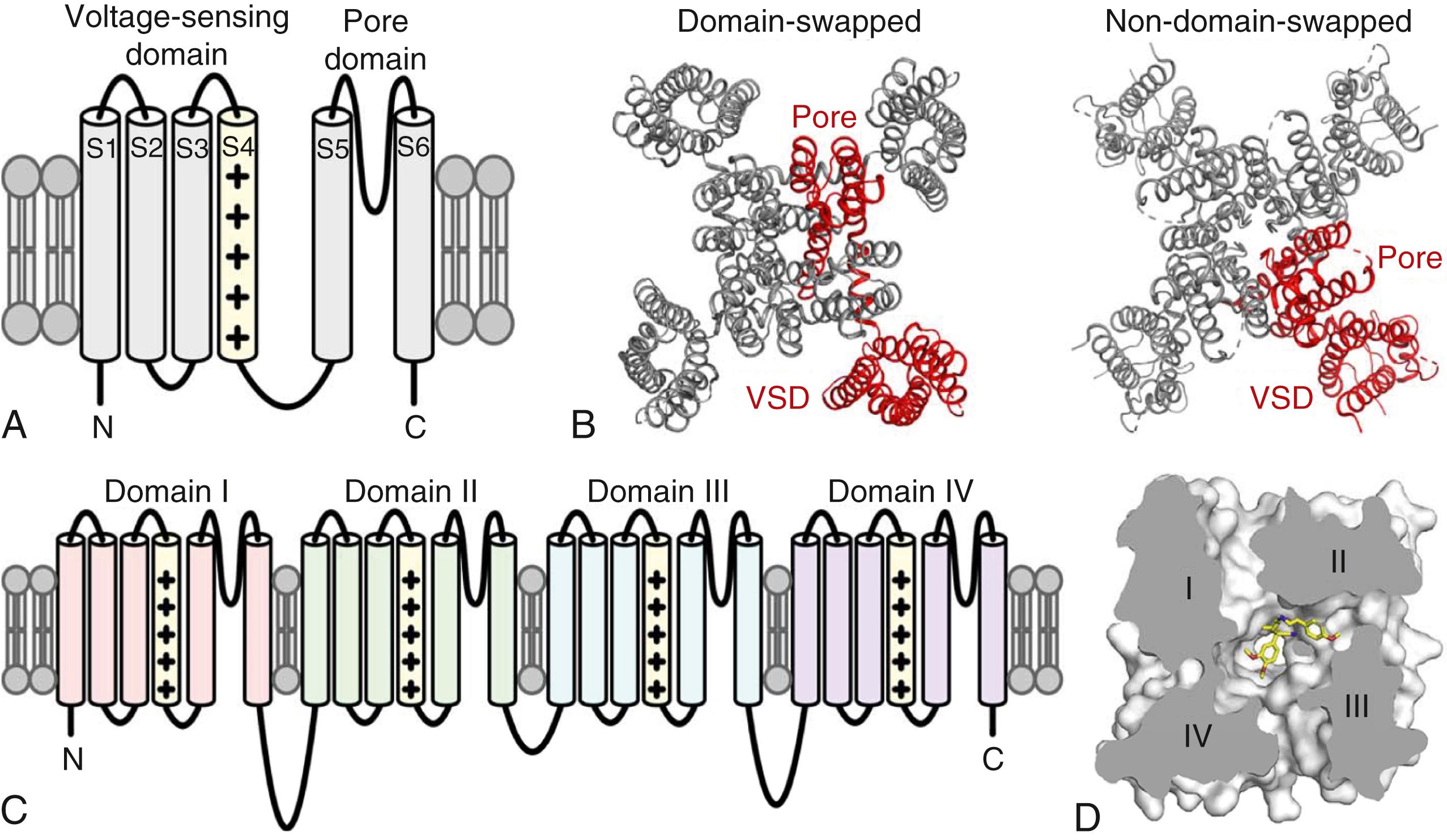
Although structures for the ventricular repolarizing K + channels K V 7.1 and K V 11.1 were expected to be resolved quickly, they were not published at high resolution until Cryo-EM methods were available. The structure of Kv7.1 revealed a domain-swapped architecture, like K V 1.2. K V 11.1, however, adopted a “non-domain-swapped” architecture such that the VSD of each subunit interacted with its own pore domain (see Fig. 19.1B ). These two classes of ion channel architectures suggest distinct activation mechanisms. The structure of K V 11.1 was also highly sought because of its block by both class III antiarrhythmic molecules and many noncardiac drugs that have off-target interactions with the channel that impair repolarization, causing the acquired long QT syndrome (LQTS). Close examination of the K V 11.1 pore showed a hydrophobic pocket that is likely to facilitate channel blockade by many of these molecules.
The bacterial Na + channel (Na V Ab) was the first Na + -selective channel to be resolved using x-ray crystallography. Intriguingly, it displays kinetics and drug sensitivity that are reminiscent of the mammalian Na + channels, even though functional channels are formed by homomeric tetramers, instead of a monomer that comprises four homologous domains (DI–DIV), which is characteristic of mammalian channels. One of the key features that was noted immediately upon the publication of the structure was the presence of fenestrations in the side of the pore that could allow hydrophobic molecules to enter from the membrane to block the channel. Many class I antiarrhythmic drugs, including lidocaine and flecainide, have a significant uncharged fraction at physiologic pH and are therefore likely to use this side door to exert a therapeutic effect. Intriguingly, more recent mammalian Na + channel structures, obtained via Cryo-EM, also reveal the presence of fenestrations, demonstrating that this route of entry is likely operative in cardiac Na + channels.
Class IV antiarrhythmic Ca 2+ channel antagonists, including the phenylalkamines (e.g., verapamil) and benzothiazepines (e.g., diltiazem), have been modeled onto the bacterial Ca + channel, Ca V Ab. Based on functional data in combination with the structure, verapamil blocks the intracellular-facing pore, with diltiazem binding a partially overlapping site. A subsequent structure of the skeletal muscle Ca 2+ channel, Ca V 1.1, was consistent with these results, showing that both verapamil and diltiazem bound to the intracellular side of the channel pore at overlapping sites.
Although the focus of this section has been on the primary voltage-gated channels, it should be noted that many additional structures have been published that represent key action potential-forming proteins, including the ryanodine receptor that releases Ca 2+ from the sarcoplasmic reticulum and the inward rectifying K + channels that maintains the resting potential, among others. We expect that the methods that are used to connect the details of voltage-gated channel function to normal heart function and arrhythmia will also be applicable to these proteins.
Even though many structures of a variety of channels have been published, there is still much structural study to be done, including on different channel states. Most ion channel structures capture the protein conformation at 0 mV, which, for most channels, is open and inactivated. There is a distinct lack of information about the noninactivated closed and open states. Moreover, in native cells, channels interact with many other proteins, including β-subunits, the cytoskeleton, kinases, and therapeutic molecules. It will be some time before each of these key interactions is understood with appropriate molecular detail.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here