Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although there have been significant improvements in the survival of infants with congenital heart disease (CHD), they remain at high risk for neurodevelopmental impairments.
Brain dysmaturation and brain injury are key brain changes contributing to adverse neurodevelopmental outcomes in CHD.
Fetal neuroimaging studies have found that brain dysmaturation begins in utero and may be linked to altered cerebral blood flow and oxygenation in fetuses with CHD.
With advances in genetic and genomic studies, there has been an expansion of our understanding of genetic risk factors for CHD. The associations between genetic abnormalities and neurodevelopmental outcomes in CHD require further study.
Modifying environmental risk factors such as socioeconomic status and the home environment may be potential opportunities to optimize brain maturation and neurodevelopmental outcomes in CHD.
Congenital heart disease (CHD) is the most common birth defect and occurs in approximately 1% of live births. A recent systematic review and meta-analysis showed an increase in the birth prevalence of CHD globally from 1970 to 2017 with the highest prevalence seen in Asia (9.3 per 1000 live births) and lowest in Africa (2.3 per 1000 live births). This increase was mainly driven by a change in the prevalence of mild CHD lesions (e.g., atrial septal defect, ventricular septal defect, patent ductus arteriosus) during the study period, likely reflecting improvements in the screening and detection of these lesions over time, although other studies have not observed a similar increase in prevalence. , This variation may be due to differences in definitions of CHD and study periods that were included across studies. Approximately one-third of infants with CHD have severe malformations requiring surgical interventions within the first year of life. This was previously associated with significant mortality, however, with advances in cardiac surgical and intensive care, there has been an increase in the survival of infants with CHD with now >85% surviving into adulthood. , , This has led to an increase in the lifetime prevalence of CHD, and >65% of the entire CHD population are adults. As a result, there has been an expanding body of research focused on understanding long-term outcomes in patients with CHD.
Neurodevelopmental impairments are common in severe CHD; they are present in over 50% of children. The typical neurodevelopmental profile of children with CHD consists of mild but highly prevalent deficits across multiple domains: visual-spatial skills, executive function, memory, language, motor skills, social interactions, and behavior. Although IQ scores in children with CHD on a group level are typically within the normal range, they are significantly lower when compared to population normative data or healthy control children. Neurodevelopmental impairments emerge early in childhood with predominantly motor delays seen in infants with CHD. , However, as children with CHD become older, abnormalities in cognition, adaptive skills, and behavior become more apparent as cognitive and social expectations change with increasing age. , This highlights the importance of long-term neurodevelopmental follow-up in this population. These cognitive impairments persist throughout adolescence and adulthood. , , Interestingly, a recent cohort study observed an increased risk of dementia, particularly early-onset dementia, in CHD adults compared to the general population, with the highest risk seen in severe CHD. Adults with CHD are also more likely to be unemployed and achieve lower levels of education. Further longitudinal studies following patients into late adulthood are needed to understand long-term neurological outcomes and psychosocial functioning in individuals with CHD.
Although mild, the highly prevalent neurodevelopmental and cognitive abnormalities in CHD have important functional consequences. Children and adolescents with CHD have an increased need for educational support, and lower employment rates are seen in adults with CHD. , , Understanding the biologic basis of neurodevelopmental abnormalities in CHD and their key contributing factors are critical in developing effective interventions and management strategies that support optimal neurodevelopmental and cognitive outcomes. Neuroimaging studies have observed that brain dysmaturation , which begins antenatally, and brain injury are the key brain changes that underlie adverse neurodevelopmental outcomes in CHD. Several risk factors for neurodevelopmental impairments in CHD have been identified and include innate (genetic) as well as acquired and potentially modifiable (prenatal diagnosis, perioperative management, socioeconomic status [SES]) factors. This chapter reviews abnormalities in brain maturation and common types of brain injury observed in infants with CHD, as well as key contributors to these brain changes. Neuromonitoring and neuroprotective strategies that are currently in use or are under investigation to promote optimal brain health and neurodevelopment in CHD are also discussed. Finally, we highlight key knowledge gaps and areas in need of further study to improve neurodevelopmental outcomes in this population.
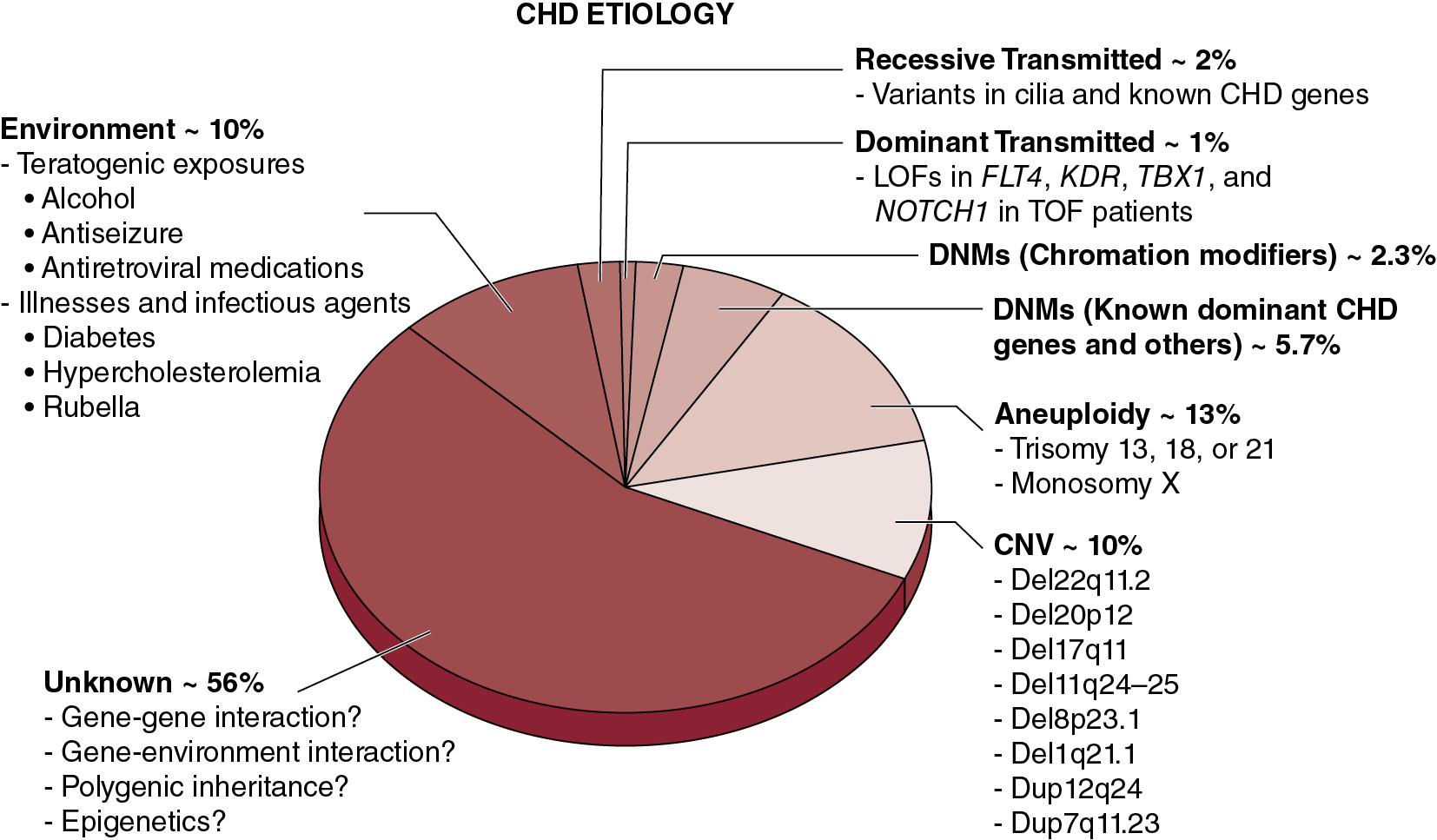
The etiology of CHD is multifactorial with both environmental and genetic predisposing factors as shown in Fig. 16.1 . Environmental contributors to CHD include antenatal exposures to infections (e.g., rubella), teratogens (e.g., retinoic acid, phenytoin, lithium), and maternal chronic conditions (e.g., obesity, diabetes mellitus, phenylketonuria). The genetics of CHD is complex and heterogeneous; both inherited and de novo genetic alterations cause CHD. Several genetic syndromes have been associated with CHD including aneuploidy syndromes (e.g., Down syndrome or trisomy 21), copy number variants (e.g., DiGeorge syndrome or 22q11.2 deletion), and single gene mutations (e.g., Noonan syndrome). , With advances in genetic and genomic technologies, recent studies have reported de novo single nucleotide variants across hundreds of genes involving multiple biological pathways that contribute to CHD, including in isolated (nonsyndromic) CHD. Moreover, recent studies have shown gene-environment interactions between Notch signaling and maternal hyperglycemia and hypoxia resulting in an increased incidence of CHD in mice. , The complex interactions between environmental and genetic risk factors for CHD are an area that warrants further study.
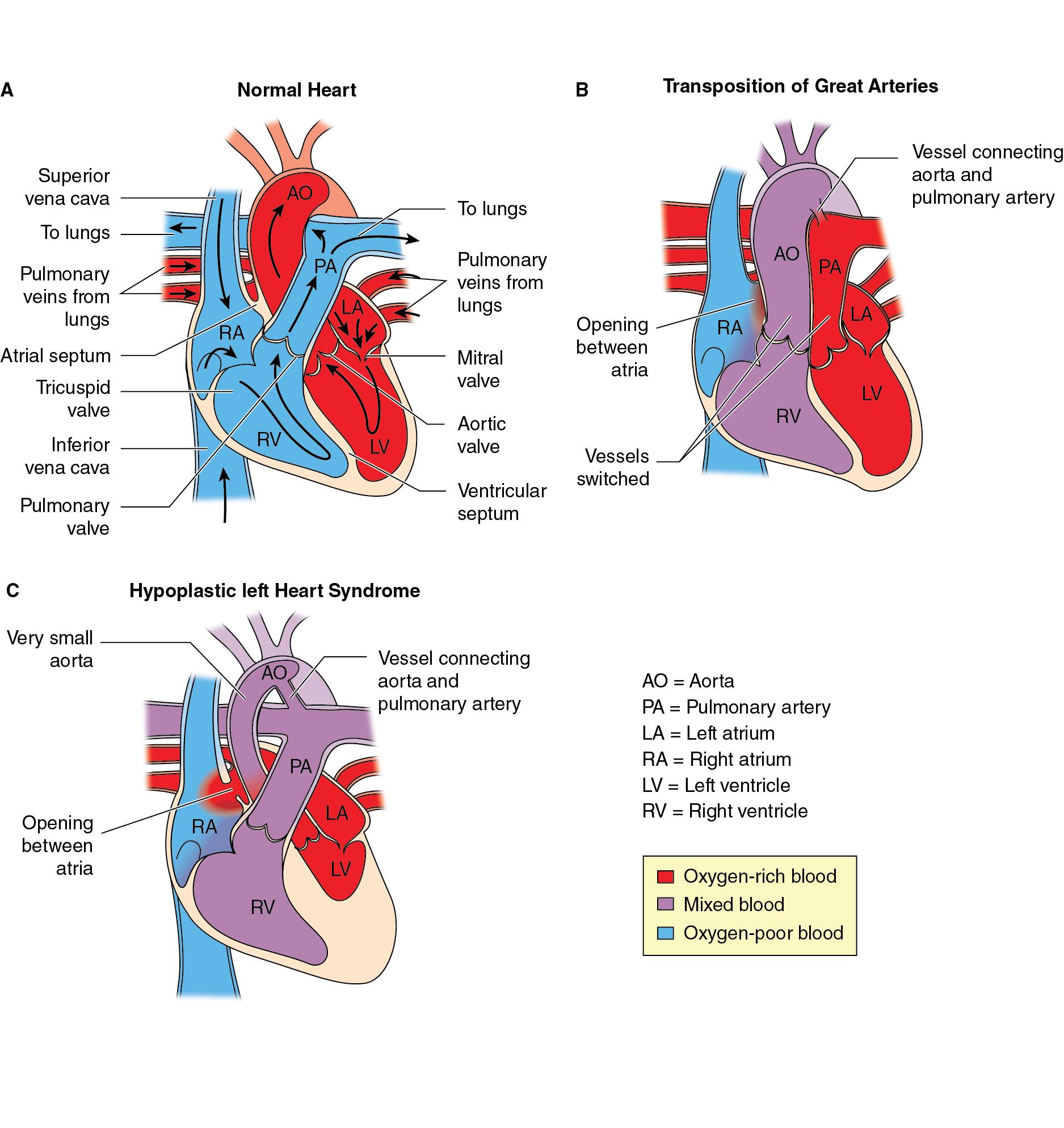
Severe CHD is often categorized as single ventricle or biventricular lesions, and may be associated with aortic arch obstruction such as aortic coarctation and aortic valve atresia or stenosis. Severe CHD also frequently includes intracardiac (e.g., atrial septal defect or ventricular septal defect) or extracardiac (e.g., patent ductus arteriosus) shunts. The presence of shunts, along with abnormal cardiac connections, malformed valves, and obstructions to blood flow may result in reduced systemic oxygen delivery due to the mixing of venous and arterial blood. Two common forms of severe CHD accounting for a large proportion of surgeries performed in the neonatal period are transposition of the great arteries (TGA) and patients with single ventricle physiology, including those with hypoplastic left heart syndrome (HLHS), with much of the literature of neurodevelopment, brain maturation, and brain injury in infants with CHD focusing on these two high-risk populations.
TGA ( Fig. 16.2 ) results from ventriculoarterial discordance when the aorta arises from the right ventricle and the pulmonary artery from the left ventricle. This leads to the systemic circulation, being supplied with deoxygenated venous blood returning from the body, and the pulmonary circulation, being supplied with oxygenated blood returning from the lungs, to be in parallel rather than in series as they are in a normal heart. This results in the affected infant being cyanosed. TGA can be associated with other cardiac abnormalities, such as ventricular septal defects, left ventricular outflow tract obstruction, or coarctation of the aorta. TGA can be difficult to diagnose antenatally with ultrasound, while affected infants can present with cyanosis and tachypnea. The severity of symptoms depends on the presence of other cardiac anomalies and the degree of mixing between the two parallel circulations.
The initial management of TGA is to stabilize the infant until the corrective surgery is performed. This includes maintaining the patency of the ductus arteriorus using prostaglandin E1 infusion to optimize circulatory mixing. Balloon atrial septostomy may also be performed to improve oxygenation and survival in neonates with d-TGA. In this intervention, a balloon is passed into the left atrium, inflated, and pulled vigorously across the atrial septum to create a larger atrial septal defect. The arterial switch operation is now the standard corrective procedure for TGA.
HLHS ( Fig. 16.2 ) describes a spectrum of cardiac malformations characterized by underdevelopment of the left heart with normally related great arteries, leaving the right ventricle to perfuse both the pulmonary and systemic circulations. In HLHS, there is significant hypoplasia of the left ventricle which is associated with atresia, stenosis, or hypoplasia of the aortic and/or mitral valves, and hypoplasia of the ascending aorta and arch. The anatomic spectrum varies from almost complete absence of left ventricle combined with aortic and mitral atresia to milder hypoplasia of the left ventricle combined with aortic and mitral valve hypoplasia but without stenosis or atresia. Survival is dependent on a patent ductus arteriosus and nonrestrictive atrial septal defect to ensure adequate systemic perfusion and mixing of oxygenated and deoxygenated blood. Affected infants become symptomatic when the ductus arteriosus closes and pulmonary vascular resistance decreases as expected after birth, progressing to cardiogenic shock and respiratory failure. Infants with a restrictive or intact atrial septal defect present with severe cyanosis and respiratory distress at birth because of pulmonary blood flow. Fortunately, a prenatal diagnosis is made in approximately 50% to 75% of cases with routine obstetrical ultrasound, typically between 18 and 24 weeks gestation, and is associated with improved survival and decreased morbidity.
The initial management of infants with HLHS is focused on ensuring adequate systemic perfusion, which is achieved with intravenous prostaglandin E1 infusion to maintain patency of the ductus arteriosus. Balloon atrial septostomy may also be used in patients with a restrictive or intact atrial septum. The surgical palliation approach usually consists of a three-staged approach: (1) Norwood procedure (neonatal period), (2) bidirectional Glenn procedure (around 3–6 months of age), and (3) Fontan procedure (typically 2–5 years of age). In the Norwood procedure, a neoaorta is created by using the proximal pulmonary artery and homograft material, which is then connected to the native ascending aorta. A source of pulmonary blood flow is established either via a right ventricle to pulmonary artery conduit (Sano) or by connecting the innominate artery with the proximal right pulmonary artery via a Gore Tex tube (called modified Blalock-Taussig shunt). By 6 months of age, the arterial shunt can usually be substituted with a bidirectional cavo-pulmonary shunt (SVC to pulmonary artery anastomosis), which results in diminished volume loading for the ventricle. As the child grows further, the Fontan circulation can be completed. In this third stage, the inferior vena cava is connected to the pulmonary arteries, allowing the entire systemic venous return to pass through the lungs, driven by the suction force of the heart and finally achieving near normal arterial oxygen saturations.
Table 16.1 summarizes common neuroimaging and neuromonitoring studies performed in CHD infants and children, their utility, and expected findings.
| Investigation | Indications and Typical Findings |
|---|---|
| Cranial ultrasound |
|
| CT |
|
| MRI |
|
| MRS |
|
| DTI |
|
| fMRI |
|
| Aeeg |
|
| Cerebral NIRS |
|
Abnormalities in brain maturation and growth are common brain changes seen in CHD and are key contributors to adverse neurodevelopmental outcomes. Neonates with severe CHD have preoperative abnormalities in brain microstructural and metabolic maturation when compared to healthy controls. , Smaller head circumferences, decreased total and regional brain volumes, reduced cortical folding, , and alterations in structural and functional brain network connectivity , , are also present at birth, even before neonates with CHD undergo surgery ( Table 16.2 ). Some studies have observed a link between brain dysmaturation in the neonatal period and adverse neurodevelopmental outcomes later in childhood in CHD, although additional studies are required to further elucidate this relationship. These alterations in brain maturation persist through childhood and adolescence and are also associated in long-term neurodevelopmental outcomes in CHD. , Recent studies have observed smaller brain volumes and altered white matter microstructure in adults with CHD, which are associated with cognitive function. , Table 16.3 summarizes a selected list of studies that have observed associations between brain abnormalities and neurodevelopment and cognition in CHD.
| Study: First Author (Year) | Study Design | Neuroimaging Modality | Risk Factors | Brain Abnormalities |
|---|---|---|---|---|
| Lynch et al. (2021) |
|
MRI, diffuse optical spectroscopy, diffuse correlation spectroscopy | Cerebral oxygen extraction during DHCA | Larger decreases in cerebral oxygen saturation during DHCA associated with new postoperative WMI ( p = 0.02). |
| Peyvandi et al. (2021) |
|
Fetal MRI, neonatal MRI | CHD lesion type | Lower brain volumes with more severe WMI in TGA ( p = 0.04) but not HLHS. |
| Schlatterer et al. (2021) |
|
MRI | Autonomic dysfunction | Lower autonomic tone associated with preoperative brain injury ( p < 0.01). |
| Feldmann et al. (2020) | MRI, DTI, tractography | — |
|
|
| Ng et al. (2020) |
|
|
— | Volume reduction in basal ganglia, thalami, corpus callosum, and cortical regions, and volume expansion in CSF in CHD. |
| Ni Bhroin et al. (2020) |
|
MRI, DTI, tractography | — | Reduced structural connectivity in a cortico-striatal-thalamic subnetwork in CHD. |
| Claessens et al. (2019) |
|
MRI, DTI | SVP | Increased preoperative fractional anisotropy in TGA and mean diffusivity highest in SVP. |
| Kelly et al. (2019) |
|
MRI, NODDI | Impaired cerebral oxygen delivery |
|
| Kelly et al. (2019) |
|
MRI | BAS | Preoperative brain injury in 39%. Strokes (4%) were only seen in patients who had BAS. |
| De Asis-Cruz et al. (2018) |
|
fMRI | — | Intact global network topology but reduced preoperative regional functional connectivity involving subcortical areas and brainstem. |
| Peyvandi et al. (2018) |
|
MRI | HLHS |
|
| Schmithorst et al. (2018) |
|
MRI, DTI, tractography | — | Reduced global network efficiency and nodal efficiency in CHD pre- and postoperatively. |
| Fogel et al. (2017) |
|
MRI | Surgical stage |
|
| Peyvandi et al. (2016) |
|
MRI, DTI | Postnatal diagnosis of CHD |
|
| von Rhein et al. (2015) |
|
MRI | — | Lower total brain and regional volumes in CHD ( p < 0.001). |
| Andropoulos et al. (2010) |
|
MRI, NIRS | SVP |
|
| Miller et al. (2007) |
|
MRI, MRS, DTI | — | Lower preoperative NAA:choline ( p < 0.01) and white matter fractional anisotropy ( p < 0.001) in CHD. |
| McQuillen et al. (2006) |
|
MRI | BAS | 41% had focal preoperative brain injury, associated with BAS (number needed to harm 1.6). |
| Mahle et al. (2002) |
|
MRI, MRS | — |
|
| Study: First Author (Year) | Study Design | Assessment Modality | Neuroimaging Modality | Key Findings |
|---|---|---|---|---|
| Bonthrone et al. (2021) |
|
Bayley-III at 22 months | MRI | Cognitively stimulating parenting associated with cognitive outcomes at 2 years. |
| Ehrler et al. (2021) |
|
Extensive test battery | MRI, DTI |
|
| Kuhn et al. (2021) |
|
PSOM, Glasgow Outcome Scale-Extended (Pediatric version) between 5 and 23 months of age | MRI |
|
| Stegeman et al. (2021) |
|
Bayley-III at 3, 6, 18 months. | MRI |
|
| Verrall et al. (2021) |
|
Cogstate battery | MRI |
|
| Noorani et al. (2020) |
|
MoCA, Wide Range Assessment of Memory and Learning-2 | MRI |
|
| Cabrera-Mino et al. (2020) |
|
MoCA, Wide Range Assessment of Memory and Learning-2 | MRI |
|
| Ehrler et al. (2020) |
|
Weschler Intelligence Scale for Children-IV | MRI, DTI |
|
| Hottinger et al. (2020) |
|
Bayley-III at 1 year | MRI | Lower pre- ( p = 0.01) and postoperative ( p = 0.03) brain maturation scores in CHD. Brain maturation not associated with Bayley-III scores. |
| Morton et al. (2020) |
|
Weschler Intelligence Scale for Children-IV, Delis-Kaplan Executive Function System | MRI | Differences in sulcal patterns in CHD that are associated with cognitive outcomes. |
| Lim et al. (2019) |
|
Bayley-III at 18 months | MRI |
|
| Meuwly et al. (2019) |
|
Bayley-III at 12 months | MRI |
|
| Claessens et al. (2018) | Prospective cohort CHD neonates with aortic arch obstruction (n = 34) | Bayley-III at 2 years, WPPSI at 6 years | MRI |
|
| Peyvandi et al. (2018) |
|
Bayley-II at 12 and 30 months | MRI | WMI associated with motor outcomes at 30 months ( p ≤ 0.05). |
| Watson et al. (2018) |
|
Weschler Intelligence Scale for Children, Weschler Adult Intelligence Scale | MRI, DTI |
|
| Rollins et al. (2017) |
|
Bayley-II, MacArthur-Bates Communicative Development Inventories (CDI) at 1 year | MRI |
|
Brain dysmaturation in CHD begins during fetal brain development ( Table 16.4 ). A fetal neuroimaging study of fetuses with CHD observed smaller total brain volumes and lower NAA:choline ratios (reflective of metabolic maturation) compared to controls. In this study, the differences in metabolic maturation and brain volumes between CHD and control fetuses widened over the third trimester during a period when brain growth and maturation is expected to accelerate, suggesting that the impairments in brain maturation observed in fetuses with CHD progress throughout gestation. Fetuses with CHD also have smaller regional brain volumes, slower head growth, , and delayed cortical development. , Interestingly, fetuses with CHD had less pronounced reductions in regional brain volumes when compared to fetuses with a family history of CHD rather than when compared to control fetuses with no family history of CHD, suggesting that genetic or shared environmental factors may contribute to brain dysmaturation in fetuses with CHD.
| Study: First Author (Year) | Study Design | Neuroimaging Modality | Brain Abnormalities |
|---|---|---|---|
| Paladini et al. (2021) | Case-control CHD (101/522) | Fetal ultrasound | Smaller frontal lobe anteroposterior diameter/occipitofrontal diameter ratio ( p < 0.001) in CHD fetuses. |
| Peyvandi et al. (2021) | Prospective cohort of TGA (n = 37) and HLHS (n = 26) | Fetal MRI, neonatal preoperative MRI | Lower total brain volume (fetal and neonatal scans) associated with increased risk of postnatal moderate-severe WMI in TGA, but not HLHS. |
| Ren et al. (2021) | Case-control CHD (40/160) | Fetal MRI | Smaller gray matter, subcortical brain tissue, cerebellar and brainstem volumes and larger CSF and ventricular volumes in CHD fetuses. |
| Ren et al. (2021) | Case-control CHD (50/150) | Fetal MRI, DWI | ADC values lower in frontal and periventricular white matter and pons and higher in thalamus in CHD fetuses. |
| Rollins et al. (2021) | Case-control CHD: HLHS/TGA (24/179), other CHD (50/179) | Fetal MRI |
|
| Inversetti et al. (2020) | Case-control CHD (79/229) | Fetal ultrasound |
|
| Jaimes et al. (2020) | Case-control CHD (48/69) | Fetal MRI | Lower fetal total (brain) maturation score ( p < 0.01) in CHD fetuses. |
| Wu et al. (2020) | Case-control CHD (48/140) | Fetal MRI | Maternal psychological distress associated with smaller hippocampal and cerebellar volumes ( p < 0.05) in CHD fetuses. |
| Claessens et al. (2019) | Prospective cohort CHD (n = 61) | Fetal MRI, neonatal pre- and postoperative MRI |
|
| Ortinau et al. (2019) | Case-control CHD (17/36) | Fetal MRI | Altered global sulcation pattern of left hemisphere of CHD fetuses. |
| Olshaker et al. (2018) | Retrospective CHD cohort (n = 46) | Fetal MRI | Smaller cerebellar volumes in CHD compared to population norm ( p < 0.05) |
| Rajagopalan et al. (2018) | Prospective cohort of biventricular (n = 7) and single ventricle (n = 10) CHD | Fetal MRI | Slower cerebral regional ( p < 0.05) and cerebellar growth ( p < 0.01) trajectories in single ventricle fetuses. |
| Ruiz et al. (2017) | Prospective cohort CHD (n = 119) | Fetal ultrasound | Smaller biparietal diameter and head circumference throughout gestation compared to normative data. |
| Wong et al. (2017) | Case-control CHD (11/62) | Fetal MRI, neonatal pre- and postoperative MRI | Delayed internal closure of bilateral opercula, enlargement of bilateral lateral ventricles, and smaller cerebellar vermis height in CHD fetuses and neonates (all p < 0.05). |
| Masoller et al. (2016) | Case-control CHD (58/116) | Fetal MRI, MRS, fetoplacental Doppler ultrasound |
|
| Sun et al. (2015) | Case-control CHD (30/60) | Fetal MRI | Smaller total brain volume correlated with reduced cerebral oxygen delivery and consumption in CHD ( p < 0.001). |
| Clouchoux et al. (2013) | Case-control HLHS (18/48) | Fetal MRI |
|
| Limperopoulos et al. (2010) | Case-control CHD (55/105) | Fetal MRI, MRS | Lower total brain volumes ( p < 0.001) and NAA/choline ( p < 0.001) in CHD fetuses |
Findings of animal studies suggest that hypoxia-ischemia plays a key role in fetal neuronal and white matter dysmaturation in CHD, possibly mediated by the hypoxia-inducible-factor signaling pathway and impaired angiogenesis. This is of particular importance given alterations in cerebral blood flow and oxygenation that are observed in fetuses with CHD. In particular, the immature white matter in the developing brain is especially vulnerable to hypoxia-ischemia. The primary myelinating cells in the brain are oligodendrocytes which undergo expected maturation from neural stem cells to oligodendrocyte precursor cells to immature pre-oligodendrocytes and finally into mature myelinating oligodendrocytes. Hypoxia-ischemia causes pre-oligodendrocyte cell injury following which the injured pre-oligodendrocyte cells degenerate. , New pre-oligodendrocytes regenerate from the population of oligodendrocyte progenitor cells that are resistant to hypoxia-ischemia. However, these new pre-oligodendrocytes experience maturational arrest and do not differentiate into mature myelinating oligodendrocytes, resulting in impaired myelination and white matter microstructural abnormalities.
Imaging studies of human fetuses with CHD in vivo have found an association between brain dysmaturation and altered fetal cerebral oxygenation and blood flow. Fetuses with CHD have decreased umbilical blood flow and streaming of oxygenated blood from the placenta to the ascending aorta, which is associated with lower oxygen saturations in the ascending aorta. These hemodynamic changes correlate with less cerebral oxygen delivery and smaller brain volumes in fetuses with CHD. A recent study using a novel fetal imaging technique to quantify fetal cerebral oxygenation (measuring T2* decay) observed a similar association between reduced cerebral oxygenation in fetuses with CHD compared to control, which was associated with reduced brain volumes. Reduced regional brain volumes in important structures containing maturing neurons and glial cells (subplate, intermediate zone, ventricular zone) in fetuses with CHD is also associated with less cerebral substrate delivery. These studies support the hypothesis that altered fetal hemodynamics and cerebral oxygenation play a critical role in brain dysmaturation in fetuses with CHD. Thus, current studies are investigating the use of maternal hyperoxygenation as a potential neuroprotective strategy in CHD.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here