Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Errors in management occur frequently in medicine. A notable Institute of Medicine report estimated that 44,000 to 98,000 deaths each year were caused by medical errors. Most of these medical and surgical errors occur in low-intensity, nonemergent scenarios. Obviously, trauma care is a much more difficult setting to perform in an error-free manner. Care of injured patients must occur at a rapid pace and in an emergent fashion. Decisions must be made rapidly, based on limited information. In many instances, interventions must be initiated before a complete clinical and radiographic evaluation is performed. Frequently, the history of the mechanism of injury is incomplete or obscure. Moreover, injured patients are frequently unresponsive or have a decreased level of consciousness for a variety of reasons. Seriously injured patients frequently present with multiple injuries that require the involvement of multiple providers. This increases the complexity of communication.
Routinely, numerous surgeons, surgical subspecialists, emergency medicine physicians, and residents from many specialties must accurately communicate and coordinate care for an optimal outcome. Residents, as members of the trauma team, add complexity to communication, organization, and supervision.
The list of potential causes for errors in trauma care is infinite. Because of these many difficulties, the surgeon who cares for injured patients must pay particular attention to factors that cause errors in management and should make every effort to prevent these errors. In this chapter, a number of common errors in the management of injured patients are discussed. This discussion includes missed diaphragmatic injury, failure to recognize extremity compartment syndrome, failure to prevent or treat abdominal compartment syndrome, delayed damage-control laparotomy, missed hollow viscus injuries, failure to perform a tertiary survey, futile emergency department (ED) thoracotomy, and the dogma of mandatory colostomy.
Traumatic diaphragm injuries (TDIs) have been described since the 16th century, and surgical repair of both penetrating and blunt injuries to the diaphragm were cited in the literature in the 1880s and 1900s, respectively. Incidence of diaphragmatic injuries varies based on mechanism of injury, with blunt trauma having a reported incidence of 0.5% to 8% and penetrating trauma having a 3.4% to 40% incidence; 1% to 2% of patients have bilateral diaphragmatic injury, which is almost exclusively secondary to blunt trauma and is associated with a higher mortality rate. Both solid and hollow organ injuries are common with blunt and penetrating diaphragmatic injuries. Up to 66% of injuries may be missed during the initial diagnostic workup and can result in herniation of solid and hollow viscera, incarceration, and strangulation. Mortality is primarily dependent on mechanism of injury and severity of associated injuries, with the overall high injury severity score being the best predictor of early mortality. Mortality rates from missed TDI are reported in the literature to be as high as 36% when diagnosis is delayed.
Blunt diaphragmatic ruptures are thought to be secondary to significantly increased intra-abdominal pressure (IAP) that can be experienced in motor vehicle collisions, particularly with the use of lap belts. In addition, the negative intrathoracic pressure generated during inspiration contributes to herniation of intraabdominal organs into the thoracic cavity. There is a strong association between thoracic aortic transection and diaphragmatic rupture in deceleration injuries with up to 10% of patients with aortic injuries also having a diaphragmatic injury. Other associated injuries include liver and splenic lacerations, long-bone fractures, and traumatic brain injury. Penetrating thoracoabdominal injuries also often result in diaphragmatic injuries. Because these injuries are typically much smaller than blunt ruptures, often less than 2 cm, the diagnosis may be unrecognized even if appropriate diagnostic modalities are used.
Physical examination is unreliable for diaphragm injuries in most patients. The signs and symptoms are often nonspecific and can include pleuritic chest pain, abdominal and epigastric tenderness, auscultation of bowel sounds in the chest, acute shoulder pain, and hemodynamic instability. Respiratory distress may be present with large injuries causing tension viscerothorax. Diagnostic modalities routinely used for trauma including chest radiograph, focused assessment with sonography in trauma (FAST), and computed tomography (CT) imaging have had varying degrees of success in diagnosing diaphragmatic injuries in the acutely injured patient. In the presence of a herniation through the diaphragm, chest x-ray can be used to diagnose injury 90% of the time. In the absence of herniation, up to 50% of initial chest radiographs in patients with confirmed diaphragmatic injuries are reported as normal. When considering only 10% to 20% of TDI caused by penetrating trauma presents with an initial hernia, one can understand how these can be missed at presentation. In the absence of a hernia, other obscure signs of TDI can include diaphragmatic shadowing, elevated hemidiaphragm, shifting of the mediastinal structures away from the side of injury, and irregular contours. Case reports and small series have suggested that abnormal movement of the diaphragm on FAST supports the diagnosis; however, this technique is not widely accepted as a reliable method of diagnosis. With the use of multidetector CT, sensitivity and specificity have improved, reported to be as high as 82% and 99.7%, respectively, and it is currently considered to be the gold standard for nonoperative diagnosis. As with chest x-ray, there are indirect signs associated with TDI including dependent viscera sign, collar sign, and hump sign. In penetrating injury the most direct sign is the presence of injury on both side of the diaphragm. Unfortunately, there is no reliable imaging modality in the absence of a hernia.
Despite technological advances, operative evaluation (laparotomy, laparoscopy, thoracotomy, or video-assisted thoracoscopy) of the diaphragm remains the diagnostic tool with the highest sensitivity, specificity, and accuracy. Nontherapeutic operations are not without risk; therefore, a high index of suspicion combined with the available diagnostic technologies should be used to evaluate these patients. Patients with equivocal imaging studies and a high index of suspicion warrant further scrutiny. In the recent era of increased use of selective nonoperative management for penetrating injuries, there must be a high index of suspicion for the presence of a TDI especially for those with injuries in thoracoabdominal region. It has been recommended by the Eastern Association for the Surgery of Trauma (EAST) that all patients at risk for TDI, especially those with left thoracoabdominal penetrating injuries, be taken for at least a diagnostic laparoscopy, if not taken to the operating room for other injuries. An aggressive approach to diagnosis and management should be undertaken to avoid the high mortality (80%) that is associated with a missed injury with herniation, presenting with obstruction. Blunt trauma injuries identified by any diagnostic modality should be repaired to avoid the risk of strangulation and perforation. Most injuries may be repaired primarily with permanent sutures. Some large defects may require prosthetic reinforcement. However, there are numerous reports of successful thoracoscopic and laparoscopic repairs of diaphragmatic injuries. The surgical approach should reflect the surgeon’s expertise and experience in treating such injuries, the patient’s clinical condition, and the presence of associated injuries.
Development of a compartment syndrome occurs commonly in patients with injuries to the upper and lower extremities. Compartment syndrome may also occur in any muscular compartment encased by fascia. This includes the hand, shoulder, arm, buttocks, thigh, and foot. Common causes of extremity compartment syndrome can be seen in Table 1 . A commonly held misconception is that patients with open fractures are protected from the development of compartment syndrome. Approximately 10% of patients with open fractures develop a limb-threatening compartment syndrome. The incidence of compartment syndrome can be as high as 5% to 10% in high-energy trauma. Up to 30% of cases occur without associated fractures, and these are at highest risk for delayed diagnosis and treatment when compartment syndrome occurs. In the lower extremity, the anterior compartment followed by the lateral compartment are the most commonly affected.
| Fracture related |
|
| Vascular |
|
| Iatrogenic |
|
| Soft tissue |
|
Compartment syndrome is diagnosed on history and physical findings as well as a few adjunctive evaluations. Physical findings suggestive of compartment syndrome that tend to progress over time include a tense extremity or fullness, pain out of proportion to examination, pain without improvement with analgesia, and pain with passive stretch. Paresthesia indicates advanced ischemia involving nerves. It is an error to assume that a compartment syndrome is not present if a distal pulse is palpable, as it is a late sign of compartment syndrome. In fact, it may indicate irreversible nerve and muscular injury have taken place. Missed compartment syndrome can have serious consequences with permanent muscle dysfunction, ischemic contractures, and worse cases loss of limb.
Compartment syndrome develops in injured extremities secondary to a number of factors. As pressure within the compartment increases and compartment pressure exceeds perfusion pressures, muscle and nerve ischemia will occur. Additionally, venous outflow obstruction results when compartment pressures rise. Compartment syndrome is a well-recognized complication of electrical burns, as well as edema and hemorrhage into compartments after trauma. Ischemia with reperfusion is also a well-known cause of compartment syndrome. Iatrogenic causes of compartment syndrome include misplaced intravenous (IV) catheters into a muscle compartment followed by infusion of fluids into the compartment. Prolonged use of military antishock trousers (MAST) or a tourniquet has also been associated with the development of compartment syndrome.
The diagnosis of compartment syndrome is based on clinical assessment and invasive evaluation of compartment pressure. Measurement of compartment pressure is easily accomplished using a number of techniques. If pressures within a muscular compartment are greater than 30 mm Hg, then compartment syndrome must be considered. A more elegant approach to determining compartment syndrome is measurement of the compartment perfusion pressure. The compartment perfusion pressure is calculated by subtracting the compartment pressure from the mean arterial blood pressure. If the compartment perfusion pressure is less than 40 mm Hg, then compartment syndrome is highly likely.
Definitive therapy for compartment syndrome exists in the form of fasciotomy. Techniques of fasciotomy for both the upper and lower extremities are well known and involve decompression of all compartments of the involved extremity.
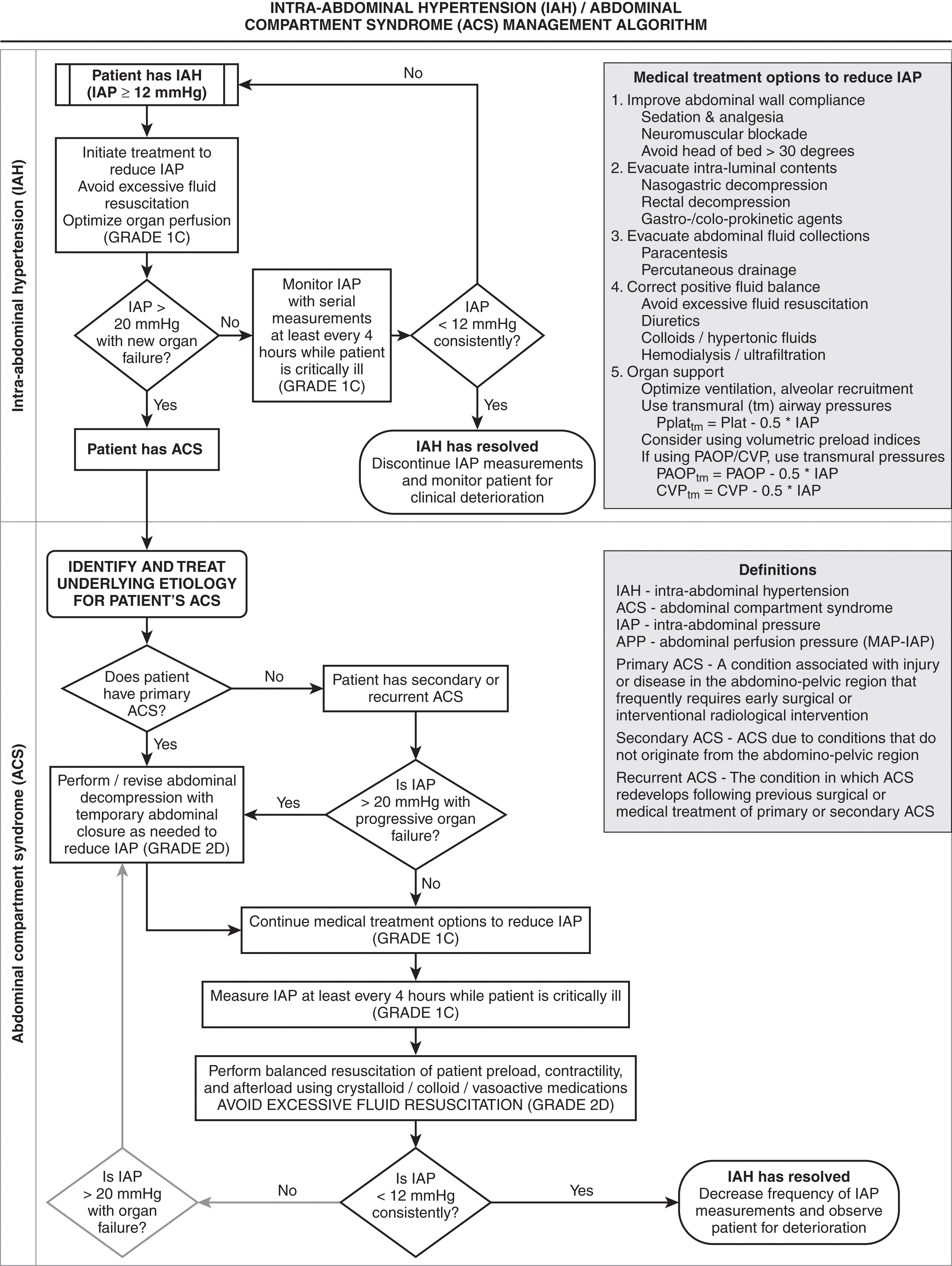
The abdominal compartment syndrome (ACS) is defined as the pathophysiology and organ dysfunction that occurs as a result of intra-abdominal hypertension (IAH). While every organ system can be impacted, the renal, cardiovascular, and pulmonary systems are most commonly affected. The incidence in trauma is 2% to 50%, major abdominal procedures 31.540.7%, and up to 70% in major burns. Over the last decade, recognition of the increased incidence and importance of appropriately diagnosing and treating ACS has led to the development of the World Society of the Abdominal Compartment Syndrome (WCACS) in 2011. ACS is proceeded by intra-abdominal hypertension, which is defined as intra-abdominal pressures > 12 mm Hg and exist on a graded scale based on severity. ACS exist when pressures are >20 mm Hg with resultant new-onset failure of one or more organ systems.
Appreciation of the adverse affects of IAH began in the 19th century. In 1984, Kron described a technique to measure IAP and first used the phrase “abdominal compartment syndrome.” Marey and Burt demonstrated the affects of IAH on respiratory function. Emerson showed the relationship between IAH and adverse cardiovascular affects in 1911. In 1913, Wendt demonstrated the relationship between IAH and renal dysfunction. Later in the century, pediatric surgeons became aware of the adverse physiologic affects of IAH and developed techniques to allow expansion of the abdominal contents. The cardiovascular effects of IAH are consistent and well defined. Cardiac output is reduced as a result of decreased venous return secondary to increased intrathoracic pressure. This phenomenon occurs at IAP greater than 20 mm Hg, although venous return has been shown to be impaired at pressures as low as 15 mm Hg. Elevated intrathoracic pressure also contributes to a reduction in ventricular compliance, which reduces cardiac contractility. The diminished cardiac output seen with IAH has been shown to be exacerbated by hypovolemia and inhalational anesthetics. The respiratory effects of IAH are mechanical. As the diaphragm is displaced cephalad, increased airway pressures are required to maintain adequate ventilation. Ultimately, this leads to ventilation/perfusion mismatch with resultant hypoxia and hypercarbia. The mechanism of renal failure with ACS is multifactorial. Inadequate renal perfusion secondary to poor cardiac output, decreased perfusion, obstruction of renal venous outflow, and compression of the kidney all contribute to the renal failure associated with increasing IAP. Numerous studies have demonstrated that the oliguria and anuria seen with ACS are reversible with timely abdominal decompression.
Very little evidence exists on the effects of ACS on other organ systems. Hepatic artery, portal vein, and microcirculatory perfusion decrease when IAP surpasses 20 mm Hg. Intracranial hypertension and decreased cerebral perfusion pressure consistently improve with abdominal decompression when IAH is present. Decreased blood flow in all abdominal organs occurs when IAP is more than 40 mm Hg.
Many causes exist for the development of ACS. Massive fluid resuscitation with crystalloid solutions, although less commonly encountered with contemporary resuscitation paradigms, plays a prominent role in the development of this syndrome. Any condition associated with intra-abdominal hemorrhage places the patient at risk for ACS. Such conditions include abdominal trauma, ruptured abdominal aortic aneurysm, retroperitoneal hemorrhage, extensive elective abdominal operations, complications of pregnancy, and hepatic transplantation. Table 2 shows some common risk factors for IAH and ACS. In addition to blood, other intraperitoneal fluid collections may contribute to the development of ACS. Edema of the bowel and retroperitoneum, abdominal packing, ileus, ascites, massive volume resuscitation for shock, and inadvisable closure of abdominal fascia all increase the risk of IAH and ACS.
| Decreased abdominal wall compliance |
|
| Increased intra-abdominal contents |
|
| Capillary leak/fluid resuscitation |
|
Diagnosis of ACS can be challenging as patients are often extremely ill, are intubated, and may not be able to express pain. In addition, signs and symptoms may be misinterpreted as being part of or related to the patient’s primary condition or related to those of shock. The diagnosis of ACS is based on clinical parameters and the measurement of IAP. Findings of oliguria (<0.5 mL/kg/hr), hypoxia (oxygen delivery < 600 mL/minute/m 2 ) with increasing airway pressures (peak > 45 cm H 2 O), systemic vascular resistance (SVR) greater than 1000, and a distended abdomen are all suggestive of ACS. Two methods of IAP measurement are clinically useful: (1) intragastric and (2) intravesicular. The latter is the most widely employed as it is easy to perform and inexpensive and is considered the gold standard for diagnosis. First described by Kron et al, the technique involves clamping the bladder catheter, followed by the injection of 50 to 100 mL of sterile saline into the bladder. The catheter is then connected to a pressure manometer.
When IAH is suspected and confirmed, attempts should be made to decrease the intra-abdominal pressures medically ( Fig. 1 ). When this fails, based on the adverse physiologic changes at different IAP levels, most experienced surgeons suggest that the abdomen be decompressed with IAP above 25 mm Hg and that all patients be decompressed above 35 mm Hg. Early decompression, which may be performed in the intensive care unit (ICU), can reverse the pathophysiology of ACS. To avoid hypotension upon decompression, it is important to ensure that adequate intravascular volume resuscitation has been accomplished. Complications of abdominal decompression include hyperkalemia, respiratory alkalosis, hemorrhage, and reperfusion injury. The final step in decompressive laparotomy is to provide temporary abdominal closure that prevents recurrent IAH. Additional concerns include infection, fluid loss, evisceration, enterocutaneous fistula formation, and exposure of the abdominal viscera. Many methods of closure are available, including absorbable mesh, plastic IV (Bogota) bags, and vacuum-assisted closure. The large ventral hernia that results from temporary closure frequently requires delayed repair with nonabsorbable mesh. The mortality rate of ACS, despite decompression, still approaches 50%. Left untreated, it is routinely fatal. Early clinical suspicion in patients at risk, combined with aggressive measurement of IAP, can lead to lifesaving decompression.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here