Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
Hemostasis and thrombosis are critical physiologic mechanisms that neurosurgeons must understand to avoid hemorrhagic and thrombotic complications of surgery and to accurately assess the risks associated with emergent surgery in the era of modern anticoagulant and antiplatelet agents.
Coagulation is not simply a cascade of steps that occurs in the plasma but is a cellular interaction of coagulation factors and receptors that occurs on the surface of platelets to effectively generate a thrombus.
We have listed the antiplatelet and anticoagulants used in clinical practice, described their mechanism of action and where they are available, and provided reversal strategies for these agents for patients who are taking these drugs and present with hemorrhage.
Continued research in developing safer classes of antithrombotic drugs as well as hemostatic agents that can be used intraoperatively will reduce the morbidity and mortality associated with bleeding.
Hemorrhage in neurosurgery can result in devastating morbidity and mortality. This is often exacerbated in patients who are taking anticoagulants, antiplatelets, or a combination of both. Patients who present with intracranial hemorrhage (ICH) while on warfarin (Coumadin) is a familiar scenario to neurosurgeons and one in which there is a formula for anticoagulant reversal. This is now also true for patients who are on direct oral anticoagulants (DOACs) such as dabigatran (Pradaxa), apixaban (Eliquis), and rivaroxaban (Xarelto). Patients on dual antiplatelet therapy, however, remain a persistent challenge for reversal.
In this chapter, we provide an overview of coagulation and platelet aggregation in the brain. We then present a list of anticoagulant and antiplatelet drugs currently used and methods to reverse their activity. Finally, we explore novel approaches to hemostasis and thrombosis that may provide neurosurgeons with new tools to improve outcomes in patients with pathologic hemorrhage.
Normal hemostasis is the product of well-regulated steps that maintain blood in a clot-free state in vessels. When vascular injury occurs, coagulation is initiated by inducing the rapid formation of a localized hemostatic plug within minutes at the site of the injury. Such primary hemostasis is the result of exposure of the extracellular matrix in the injured vessel. This creates a highly thrombogenic environment whereby platelets adhere to the damaged endothelium and become activated from their previously quiescent state. They subsequently release secretory granules, which induce additional platelet aggregation to form a hemostatic plug. There are two theories of coagulation. The classical paradigm presents clotting as two distinct pathways that meet downstream to form a common pathway, known as the clotting cascade. An alternative paradigm describes the role of platelets and proposes that clotting occurs on the surface of tissue factor (TF)-presenting cells and on platelets. The second model is therefore known as the cell-based model of coagulation.
In the 1960s, two groups independently proposed a model consisting of sequential steps of activation resulting in thrombin generation that forms a blood clot. , This model eventually gave rise to a cascade that was divided into extrinsic and intrinsic pathways ( Fig. 18.1 ). The extrinsic pathway starts with TF and factor VIIa and only occurs extrinsically to circulating blood. The intrinsic pathway, an intravascular process, occurs via activation of factors XII, XI, and IX. At this juncture, factor VIIIa is required in both pathways to convert factor X to factors Xa, and Va, which subsequently convert prothrombin (FII) to thrombin (FIIa).
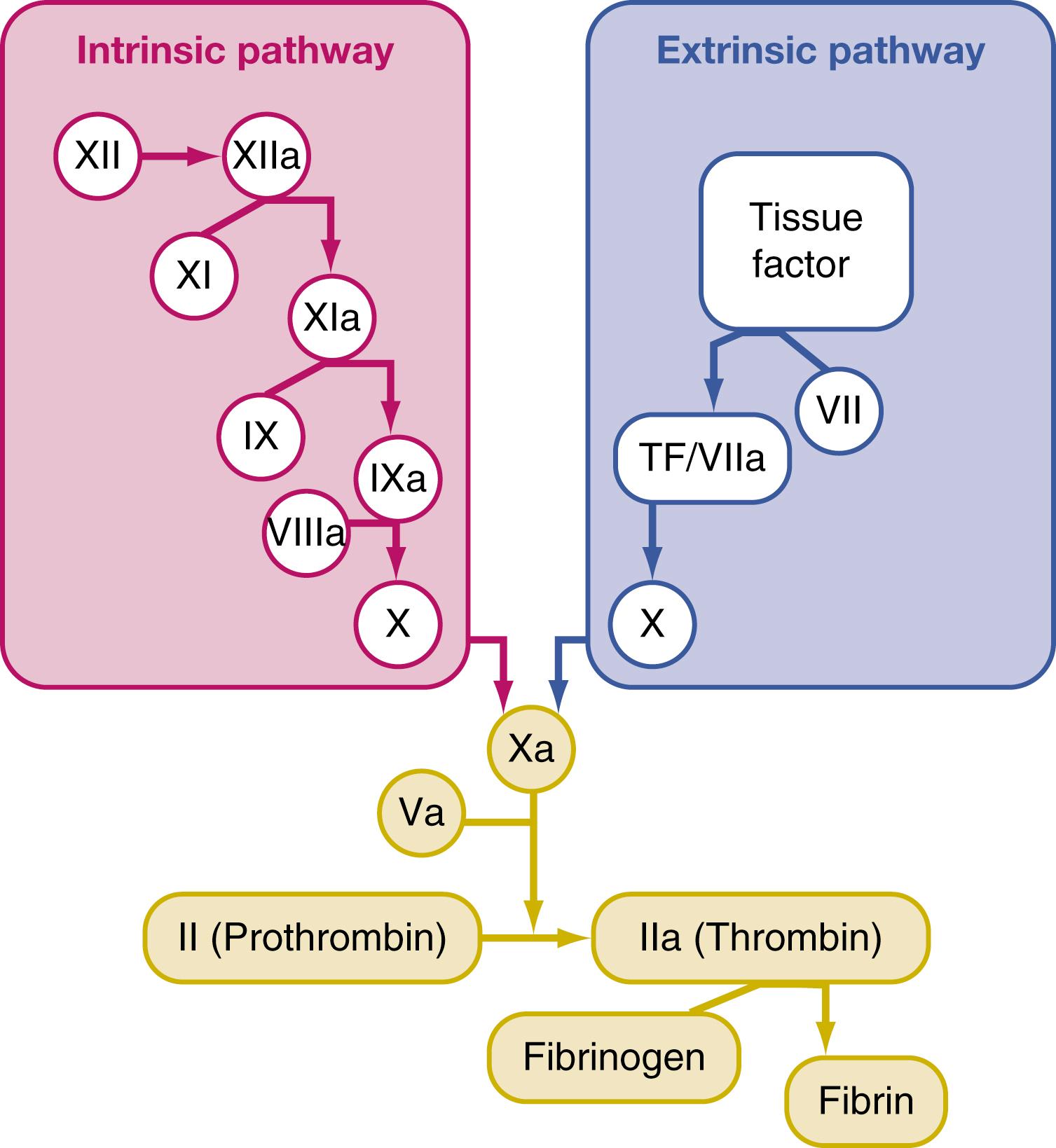
Although the coagulation cascade presents clotting neatly and correlates well with laboratory tests such as prothrombin time (PT) and activated partial thromboplastin time (aPTT), it fails to adequately explain coagulation in vivo because it does not describe coagulation as it occurs on the cell surfaces of fibroblasts, endothelial cells, and platelets. Alternatively, the cell-based model describes blood clotting in three overlapping phases—initiation, amplification, and propagation ( Fig. 18.2 ). The initiation phase occurs on TF-bearing cells such as fibroblasts or endothelial cells. TF is an integral membrane protein localized on the cell in which it was synthesized. When vascular injury occurs, TF binds to factor VII and becomes activated.

The TF/VIIa complex in turn activates factors IX and X. Factor Xa activates factor V, which then converts a small amount prothrombin to thrombin (also referred to as FIIa ). This amount of FIIa is critical to amplification, which is when platelets come into contact with extracellular matrix proteins, principally platelet surface protein gpIb/IX to von Willebrand factor (vWF), at the site of vascular injury. In addition to TF-FVII activation, vascular injury also converts vWF from a globulated inactive conformation to a linear activated molecule. This activation exposes the gpIb-binding site on vWF, an essential and nonredundant step in thrombus formation. Moreover, factor VIII is activated and binds to vWF and is then cleaved by thrombin to release it from vWF. The activated platelet now has factors Va and VIIIa bound to its surface, which sets the stage for a significant amount of thrombin generation. During propagation, the tenase complex (consisting of FVIIIa/IXa) activates factor X on the platelet surface, which binds to its cofactor FVa, generating large amounts of thrombin required to convert fibrinogen to fibrin. Thrombin also activates factor XIII, which in turn stabilizes the fibrin meshwork by cross-linking adjacent fibrin monomers to one another.
As described earlier, the interactions among coagulation factors and between coagulation factors and platelets are critical to thrombus formation. Additionally, the initial thrombin generation from the TF-FVII pathway is important for stimulating the platelet from a quiescent to an active state. Subendothelial vWF, which is exposed as a result of endothelial damage, binds to platelet glycoprotein Ib/IX/V (gpIb/IX/V), resulting in platelet adhesion to the damaged endothelial surface. Thrombin produced from the TF-FVII pathway binds to the protease-activated receptor (PAR) on the platelet surface, resulting in conversion of glycoprotein IIb/IIIa (gpIIb/IIIa) from a quiescent to active state and further recruits internal gpIIb/IIIa molecules to the platelet surface. Activated gpIIb/IIIa binds to fibrinogen, linking activated platelet molecules to one another and resulting in platelet aggregation and formation of a platelet plug. To stabilize platelet aggregation, a number of activated molecules are released including thromboxane A 2 (TX-A 2 ), serotonin, and adenosine diphosphate (ADP). TX-A 2 binds to its receptor on the platelet surface and induces inside-out signal transduction of gpIIb/IIIa, amplifying platelet aggregation. ADP is released from the granules of platelets and has two main functions: it contributes to the signal transduction to activate gpIIb/IIIa, and it binds to the G protein–coupled receptor P2Y12. This ADP-induced platelet aggregation both stabilizes and further stimulates platelet plug formation. This sets off a cascade of events within the activated platelet that generates platelet aggregation and platelet plug formation (see Fig. 18.2 ).
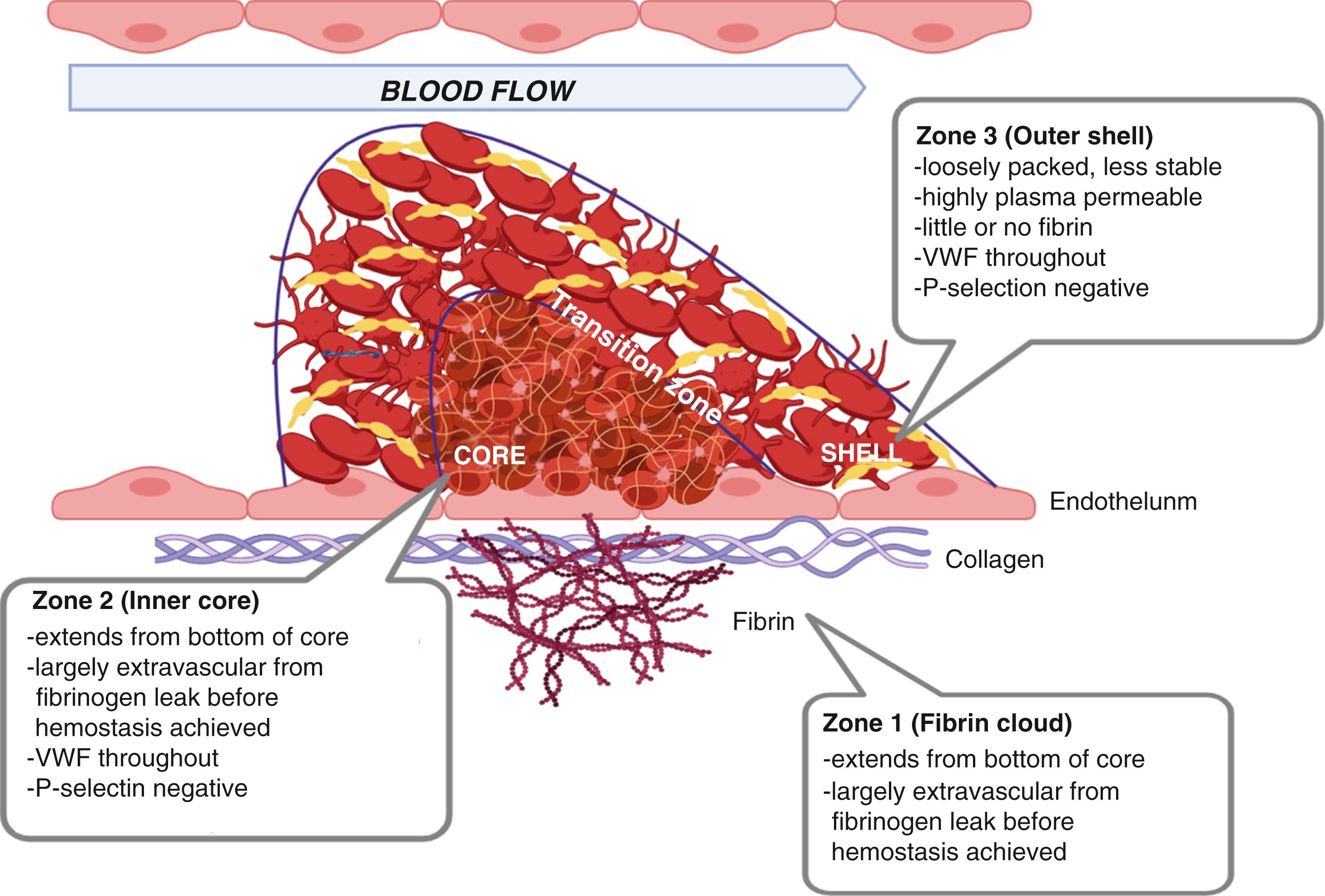
Recent research has demonstrated that rather than a mass of uniformly activated platelets clustered within a fibrin lattice, hemostatic plugs have a regional architecture in which platelets are not uniformly activated and fibrin is localized rather than ubiquitously distributed in the clot. Zone 1 (fibrin cloud) is the base of the thrombus consisting of platelets, VWF, and collagen. Fibrin extends from the base of the core, across the region of endothelium irregularity or damage, and to the extravascular space. Platelets are fully activated, with both vWF- and gpIIb/IIIa-mediated platelet aggregation. Zone 2 (inner core) is a densely packed intraluminal core of platelets. Outside of this dense core is zone 3 (outer shell), which consists of platelets that occlude the vessel, but they are less stable, containing little to no fibrin, and have surface area exposure for receptor-ligand interactions ( Fig. 18.3 ).
Antithrombotic drugs are roughly classified into two groupings: antiplatelet drugs and anticoagulants ( Table 18.1 ). The main classes of antiplatelet drugs include cyclooxygenase-1 (COX-1) inhibitors, phosphodiesterase (PDE) inhibitors, ADP receptor inhibitors, and gpIIb/IIIa inhibitors ( Fig. 18.4 ).
| Drug | Target | Reversal Agent | Dose |
|---|---|---|---|
| Aspirin | Cyclooxygenase-1 |
|
|
| Dipyridimole | Phosphodiesterase type 5 |
|
|
| Cilostizol | Phosphodiesterase type 3 |
|
|
| Clopidogrel | P2Y12 receptor |
|
|
| Prasugrel | P2Y12 receptor |
|
|
| Ticagrelor | P2Y12 receptor |
|
|
| Abciximab | Glycoprotein IIbIIIa |
|
|
| Eptifibatide | Glycoprotein IIbIIIa |
|
|
| Tirofiban | Glycoprotein IIbIIIa |
|
|
| Heparin | IIa; Xa |
|
|
| Enoxaparin | Xa; IIa |
|
|
| Fondaparinux | Direct Xa |
|
|
| Rivaroxaban | Direct Xa |
|
|
| Apixaban | Direct Xa |
|
|
| Edoxaban | Direct Xa |
|
|
| Bivalirudin | Direct IIa |
|
|
| Lepirudin | Direct IIa |
|
|
| Desirudin | Direct IIa |
|
|
| Dabigatran | Direct IIa |
|
|
| Warfarin | II; VII; IX; X; protein C; protein S |
|
|
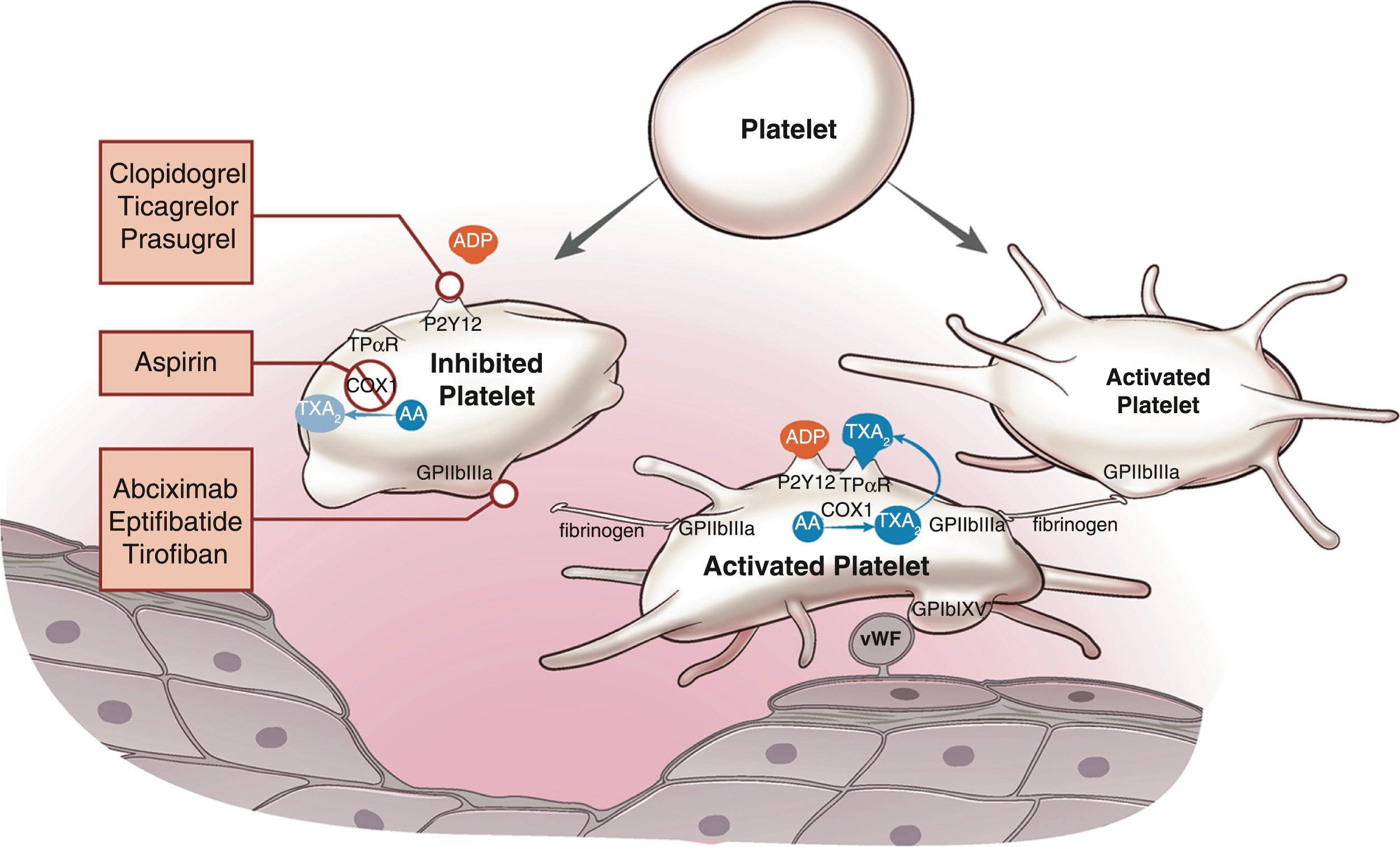
Platelet activation begins with the process of adhesion between vWF exposed on the damaged endothelial surface and glycoprotein IbIX (gpIbIX). This in turn activates gpIIb/IIIa, which binds to fibrinogen to begin the process of platelet aggregation. To perpetuate aggregation, platelets secrete substances to activate receptors on the platelet surface. COX-1 binds to prostaglandin synthase, which subsequently metabolizes arachidonic acid into prostaglandin H 2 (PGH 2 ). PGH 2 , in turn, is a precursor for TX-A 2 , which acts in a positive feedback loop for platelet activation and recruitment. Aspirin (acetylsalicylic acid) is a COX-1 inhibitor. This inhibits PGH 2 , thereby preventing the metabolism of arachidonic acid, inhibiting the production of TX-A 2 . Aspirin irreversibly binds to COX-1, inhibiting TX-A 2 production for the life of the platelet. It is immediately absorbed and has a half-life of 30 minutes. Its prescribed dosages in the United States are 81 mg or 325 mg daily, as stand-alone therapy or in combination with P2Y12 inhibitors like clopidogrel, prasugrel, and ticagrelor.
Aspirin can be monitored using platelet aggregometry, a platelet function analyzer (PFA-100; Siemens Medical Solutions, Malvern, PA), or an aspirin assay (VerifyNow Aspirin Test, Instrumentation Laboratory, Bedford, MA). Platelet aggregometry can be performed using whole blood or platelet-rich plasma (PRP). The platelet aggregometry assay is performed using luminescence or physical impedance measured with a filament. PRP is isolated from whole blood and exposed to an agonist such as platelet-activating factor or thrombin. As platelet agglutinate, they fall to the bottom of the plasma, resulting in increased light transmission. The output measurement is a percentage of maximum inhibition. The PFA-100 platelet function analyzer is a whole-blood point-of-care assay whereby the blood is placed in a cartridge and activated by epinephrine or ADP. The cartridge has a capillary through which the blood is pulled through under high shear to an end aperture 147 μm in diameter filled with either collagen and epinephrine or collagen and ADP. Collagen is the surface on which platelet aggregation occurs, and epinephrine and ADP serve as platelet activators. The time it takes to occlude the aperture is defined as the closing time. The reference closure time is between 80 and 200 seconds for epinephrine-activated cartridges and between 60 and 140 seconds for ADP-activated cartridges. The maximum closing time is 300 seconds, which represents therapeutic effect from platelet inhibition. The assay is useful in determining if the patient is taking aspirin, but closing times between the reference range and the upper limit of the assay are not used to guide therapy. Finally, the VerifyNow Aspirin Test expresses platelet activation in the form of aspirin reactive units (ARU). The reference range is 350 to 700 ARU, with a response to aspirin having an ARU less than 550. The limitations of all of these assays are that they do not measure platelet activity at a physiologic shear rate, and they do not measure platelet aggregation on an endothelial surface. These limitations have relegated these assays to screening tools to determine if patients are taking their medication rather than to assess levels to make decisions about dosing. Beyond screening patients, clinicians point toward “aspirin resistance” as a reason to measure aspirin antiplatelet activity. Interestingly, a study of 400 patients demonstrated no incidence of aspirin resistance with multimodal testing as described in this chapter. Aspirin dosing ranges from 81 to 325 mg, with preference given to 81 mg daily. There is evidence of benefit of twice-daily dosing in patients with diabetes mellitus, obesity, essential thrombocythemia, myeloproliferative neoplasms, and coronary artery bypass surgery, which is explained by higher platelet turnover, but not supported mechanistically.
Aspirin reversal can be performed using two strategies. The first method is administration of deamino- d -arginine-vasopressin (DDAVP), which counteracts the effect of the aspirin by stimulating the release of vWF from endothelial cells rather than specifically reversing the drug. This excess vWF engenders platelet activation and aggregation, thereby counteracting the antiplatelet activity of the aspirin. DDAVP is administered at 0.3 μg per kilogram intravenously over 15 minutes. This can be given every 12 hours to a maximum of six doses. The major side effect of this drug is tachyphylaxis. The second method is by platelet transfusion. Although no randomized trials have been performed to evaluate platelet transfusion therapy, in vitro aggregation studies have demonstrated the effect of normal platelets reversing the effects of drug-treated platelets. In patients with ICH or undergoing emergent neurosurgery, 1 U of platelets, defined as apheresis or pool of whole-blood–derived platelets with a concentration greater than 3 × 10 9 platelets/L can be used. The typical volume of 1 unit of platelets is between 200 and 300 mL. Up to 5 units of platelets are recommended to provide sufficient unaltered platelets to support clot formation ( Table 18.1 lists available reversal strategies for antiplatelet and anticoagulant drugs).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here