Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Physiologic hemostasis consists of endothelium, platelets, plasma coagulation proteins, natural anticoagulants, and the fibrinolytic system.
Primary hemostasis involves platelets and von Willebrand factor. Secondary hemostasis is initiated by the tissue factor pathway and then amplified and propagated by the intrinsic pathway to generate thrombin that converts fibrinogen to fibrin.
Routine coagulation tests (prothrombin time and activated partial thromboplastin time) may help identify a hemostatic defect in a bleeding patient, but mild to moderate abnormalities of these tests do not predict a bleeding tendency in a nonbleeding patient.
Acquired coagulopathy often reflects multiple coagulation defects rather than specific protein defects.
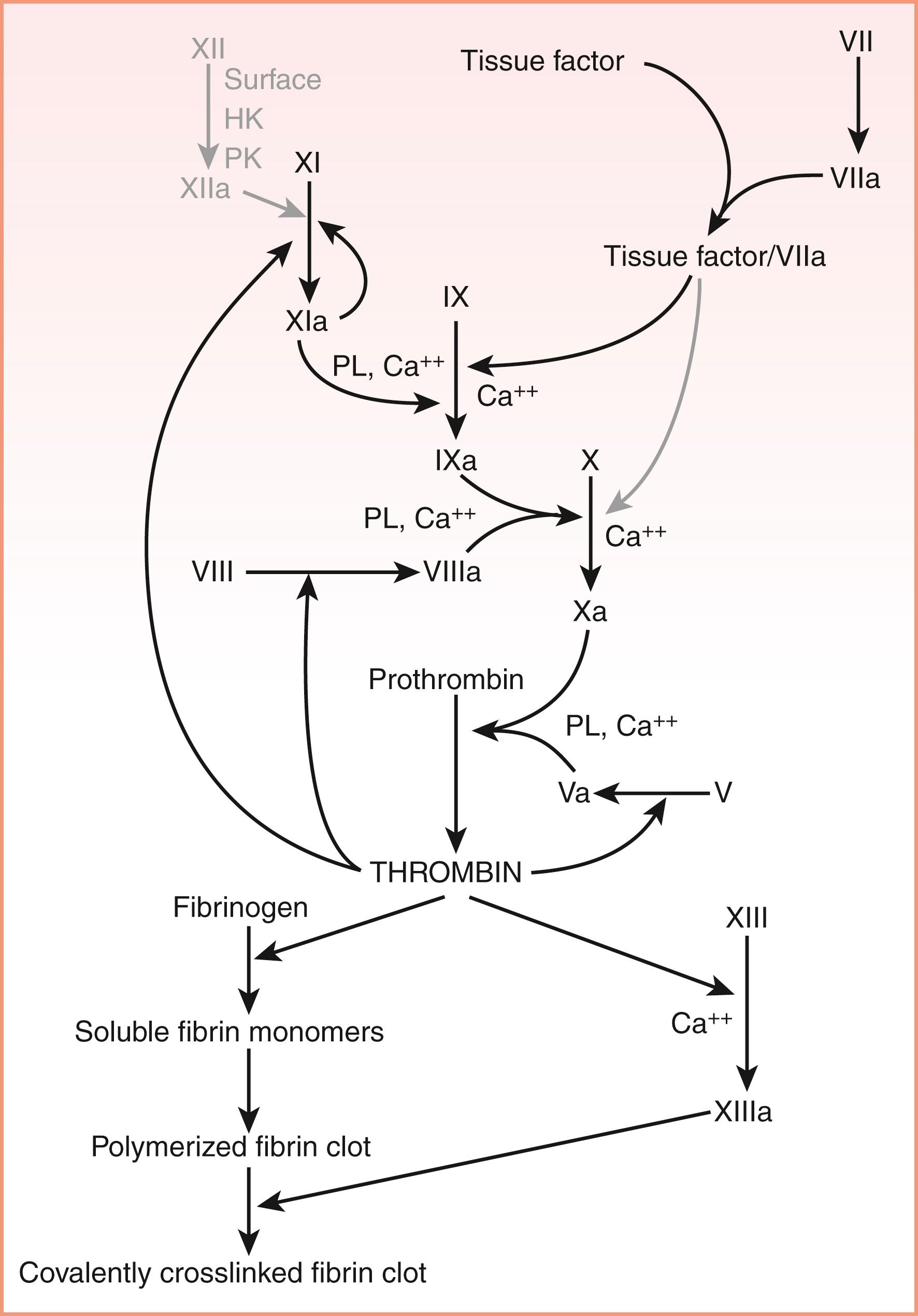
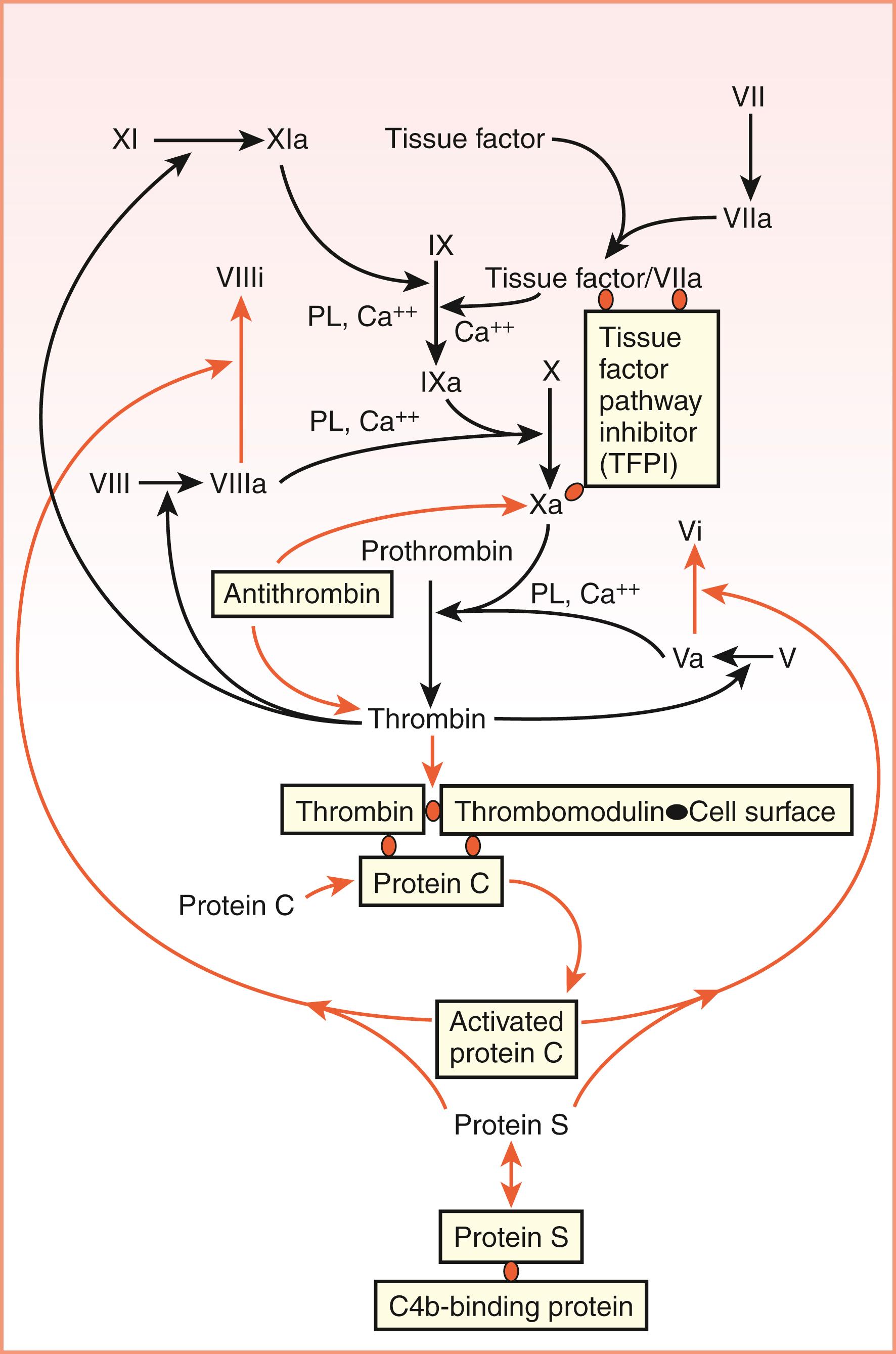
Hemostasis is a physiologic response to a vascular injury to limit blood loss. Therefore, it is initiated rapidly, is localized, and is well regulated. Recently, there has been an evolution in our understanding of the physiologic hemostatic system ( Fig. 40.1 ). Initially, the hemostatic system was referred to as the coagulation cascade based on the waterfall model of Ratnoff and Davies; MacFarland published simultaneously a sequence of proteolytic reactions beginning with factor XII (FXII; Hageman factor) activation and leading to thrombin formation that proteolyzes fibrinogen to form a clot ( ; ). However, the physiologic basis of this pathway was questioned because patients with FXII deficiency did not bleed. Furthermore, in the mid-1970s, the cofactors for FXII activation—prekallikrein and high-molecular-weight kininogen—were identified and their deficiencies also did not cause bleeding ( ; ; ). Later, the physiologic role of tissue factor (TF) and FVIIa was recognized along with its regulator tissue factor pathway inhibitor (TFPI) ( ). Thus, the physiologic hemostatic system is a cell-based model in which cellular elements such as endothelium, platelets, neutrophils, monocytes, and macrophages express TF and provide phospholipid (PL) surface for various coagulation proteins for attachment and sequential activation of coagulation factors to generate thrombin at the site of injury. Thrombin converts fibrinogen to a fibrin clot. Natural anticoagulants regulate thrombin generation, and the fibrinolytic system dissolves the clot once it has served its purpose.
The physiologic hemostatic system, a tightly regulated balance between formation and dissolution of hemostatic plugs modulated by a series of enzymes and scaffolding proteins, has two major parts. The first part is its cellular component, which consists mostly of platelets and endothelial cells but also includes neutrophils and monocytes. The second part is a large group of plasma proteins, which participate in clot formation (coagulation), dissolution of clots (fibrinolysis), and the action of naturally occurring serine protease inhibitors (anticoagulation) that terminate the activity of several enzymes of the coagulation and fibrinolytic systems.
Normal hemostasis involves interplay between the cellular components and proteins involved in clot formation and lysis. Intact endothelium that lines the vascular wall in a physiologic state exerts a hemostatic balance. When vascular integrity is breached, blood coagulation is initiated. Intact endothelial cells secrete an ectonucleotidase, CD39, that degrades adenosine diphosphate (ADP) ( ). Endothelial cells also secrete prostacyclin and nitric oxide (NO), which prevent platelet activation and aggregation, thereby reducing the risk of arterial thrombosis ( ; ; ). Endothelial cells additionally bind plasminogen, tissue plasminogen activator, and single-chain urokinase, all of which contribute to fibrinolysis and maintenance of hemostatic balance ( ; ). The glycosaminoglycans present on endothelium bind antithrombin (AT), which then neutralizes thrombin and FXa ( ). Thrombomodulin on endothelium binds to thrombin; that complex then converts protein C to activated protein C (APC) ( ).
When a vessel wall is injured, subendothelial collagen is exposed and platelets adhere to the site of injury ( ). Von Willebrand factor helps platelets adhere to the injured vessel wall by binding to exposed collagen at the A3 domain and to the platelet receptor GP Ib/IX/V complex ( ). This adhesion event activates platelets, initiating a signaling cascade within them ( ). The stimulated platelets release the contents of their granules, recruit additional platelets to aggregate to those already adherent and provide a phospholipid surface for the activation of multiple coagulation factors. Furthermore, in flowing blood, FXII autoactivates if exposed to collagen or in the milieu of activated platelets, leading to the cascade of proteolytic events producing thrombin ( ).
Once prothrombin has been activated to thrombin in the intravascular compartment, the resulting thrombin stimulates endothelium to upregulate TF, and TF forms a complex with FVIIa. The complex of FVIIa–TF activates FIX to FIXa, which converts zymogen FX to enzymatically active FXa (see Fig. 40.1 ). In the presence of FVa from liver or injured endothelium, FXa activates prothrombin (FII) to thrombin (FIIa). Under normal circumstances, the rate-limiting component of prothrombinase complex formation and the ultimate generation of thrombin activity is the concentration of FXa ( ). Thrombin then proteolyzes fibrinogen to form fibrin. In static in vitro systems, as little as 5 to 10 pM TF is sufficient to induce clot formation, leading to a 1000- to 2000-fold amplification of the process, which increases the concentration of thrombin to 10 to 20 nM, which is sufficient to initiate clot formation ( ). In a static model of blood coagulation, the addition of 5 pM TF results in an average clot time of approximately 5 minutes—sufficiently fast for physiologic hemostasis. Thrombin also is a major physiologic activator of platelets together with other agonists, such as collagen, ADP, platelet-activating factor, and epinephrine. Inactivated platelets probably circulate without promoting coagulation. When platelets are activated (see Chapter 41 ), their membranes become a site for additional thrombin formation. According to a cell-based model of coagulation ( ), activated platelets offer the most important source of negatively charged phospholipids (mainly, phosphatidylserine) and procoagulant membranes ( ) needed for coagulation factor action in the propagation phase. This pathway for thrombin formation conjoins with FXII autoactivation on exposed vessel collagen and downstream elaboration of thrombin as well ( ). Critical reactions to form FXa and thrombin are accelerated 300,000-fold on the platelet surface. Platelets control three important phases of coagulation: thrombin generation, fibrin formation, and clot retraction ( ).
The proteins that constitute the coagulation system consist of zymogens and cofactors ( Table 40.1 ). The zymogens (proenzymes) of the coagulation system can be grouped into the phospholipid-bound and the surface-bound categories. Phospholipid-bound zymogens make up the physiologically important hemostatic system. These proteins are vitamin K dependent. Vitamin K is required for an essential γ-carboxylation reaction that takes place on the glutamic acid residue of each of these proteins located in their amino-terminal regions (see Chapter 43 , Fig. 43.1 ). This carboxylation reaction allows these proteins to bind to phospholipid and cell membranes, where they are activated. Without this carboxylation reaction, these proteins do not function normally in the hemostatic system. Proenzymes include FX, FIX (Christmas factor), FVII, and FII (prothrombin). Protein C is a vitamin K–dependent phospholipid-bound zymogen; however, when activated (APC), it functions as an anticoagulant by inactivating FVa and FVIIIa (see Chapter 42 ). Protein S is another natural anticoagulant that is also vitamin K dependent and acts as a cofactor for APC.
| Surface-Bound Zymogens | Vitamin K–Dependent Zymogens | Cofactors/Substrates |
|---|---|---|
| Factor XII | Factor VII | High-molecular-weight kininogen |
| Prekallikrein | Factor IX | Factor VIII |
| Factor XI | Factor X | Factor V |
| Factor II | Fibrinogen | |
| Protein C | Protein S |
The surface-bound proenzymes are proteins of the plasma kallikrein/kinin system (see Table 40.1 ). Surface-bound proenzymes include FXII (Hageman factor), prekallikrein (Fletcher factor), and FXI. These protein zymogens are also known as the contact system because FXII autoactivates when associated with a negatively charged surface, such as a glass tube and several physiologic substances ( ; ). The autoactivation phenomenon of FXII allows for a common laboratory test, activated partial thromboplastin time (APTT), which is used to assess the integrity of the coagulation system. Although absolutely essential for a normal APTT, the proteins of the plasma kallikrein/kinin system do not have a role in hemostasis. These proteins (FXII, prekallikrein, and high-molecular-weight kininogen) may participate in blood pressure regulation, fibrinolysis, inflammation, angiogenesis, and thrombosis ( , , ; ; ; ).
Although deficiencies of FXII, prekallikrein, and high-molecular-weight kininogen are not associated with bleeding, investigations indicate that they may have a role in arterial and venous thrombosis. Recently, activation of the contact factor coagulation pathway has been shown to occur in vivo from negatively charged surfaces such as polyphosphate, extracellular RNA, and collagen. In detail, polyphosphate (PolyP) in humans consists of 60 to 100 phosphate units, and it is released from platelet dense granules upon platelet activation. PolyP has prohemostatic, prothrombotic, and proinflammatory properties. It has been identified as an activator of the contact phase ( ). It is a potent activator of thrombin and coagulation FXI, and it accelerates FV activation and abolishes the inhibitory effect of TFPI.
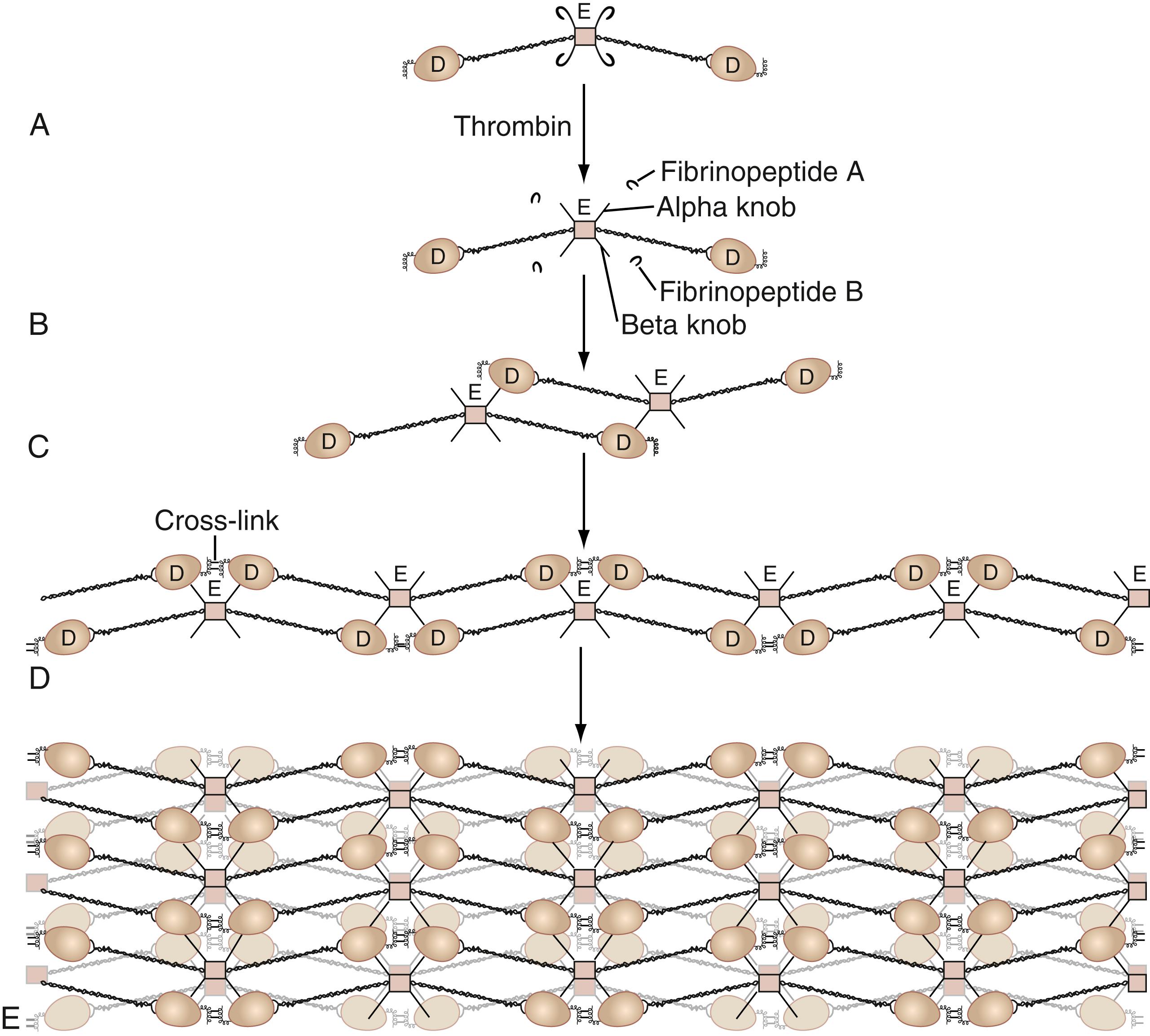
TF (47-kDa protein) is an essential cofactor for activated FVIIa. It is found in most tissues and cells. Upregulation of TF results in the formation of complexes with FVII that produce the initiation of hemostatic reactions. FVIII (antihemophilic factor) is a 330-kDa protein. When activated to FVIIIa, it is a cofactor for FIXa in the activation of FX. Its absence is associated with the most severe clinically recognized bleeding disorder, hemophilia A. FV is also a 330-kDa protein with homology to FVIII. When activated to FVa, it serves as a cofactor for FXa in the activation of FII (prothrombin) to thrombin. Fibrinogen is a 330-kDa protein that not only is the main substrate of thrombin (FIIa) but is also the principal adhesive molecule for platelet aggregation. When fibrinogen is proteolyzed by thrombin, a fibrin monomer is formed. These monomers associate end to end and side to side to form a polymerized fibrin clot. The clot is stabilized by activated FXIII, a tissue transglutaminase that cross-links the strands of associating fibrin ( Fig. 40.2 ).
Certain critical protein assemblies in hemostatic reactions accelerate these proteolytic events. The proteins of the coagulation system that are essential for hemostasis or control of bleeding were originally identified by observation of affected patients and, more recently, through mouse knockout studies. Deficiencies in coagulation factors VIII and IX are the most prominent bleeding disorders that occur in patients who survive gestation and birth. Rare patients who have congenital deficiencies of coagulation factors VII, X, V, and II usually do not have severe bleeding states. In contrast, murine models have demonstrated that mouse embryos containing complete genetic knockouts of factor VII, X, V, or II die of massive hemorrhage during gestation or at birth ( ; ; ; ). This information suggests that the human patient who has a deficiency of factor VII, X, V, or II may have some factor, albeit less than 1%, to allow for a milder clinical phenotype than that seen in mouse models. Alternatively, other compensatory mechanisms may be operative in the human patient. All of these proteins participate in two critically important assemblies that are essential for normal hemostasis: tenase and prothrombinase complexes.
Extrinsic tenase involves TF and FVIIa complex on the PL surface, converting FX to FXa. The intrinsic tenase complex comprises the assembly of FIXa and FVIIIa on phospholipid surfaces to convert FX to FXa. When all of these components are present, the rate of FX activation by FIXa is increased 1.4 × 10 8 -fold over the rate of FX activation by FIXa alone. The prothrombinase complex comprises the assembly of FXa and FVa on phospholipid membranes or cell membranes in an ordered structure with FII (prothrombin) to accelerate its activation to FIIa (thrombin). When all of these components are present, the rate of FII activation by FXa is increased 1.7 × 10 8 -fold over the rate of FII activation by FXa alone. Because these coagulation reactions occupy critical regulatory points within the physiologic hemostatic system, they are also the target of a number of anticoagulant agents for venous thrombosis that are currently in use, that is, direct FXa and thrombin inhibitors.
The six peptide chains of the fibrinogen molecule are organized into a structure described as having a central E domain and two terminal D domains. When thrombin is formed, it cleaves fibrinopeptide A from the Aα chain and fibrinopeptide B from the Bβ chain of fibrinogen in the E domain region ( ). The remainder of this thrombin-proteolyzed fibrinogen is called a soluble fibrin monomer . Soluble fibrin monomers then assemble with an end-to-end and side-to-side association to form a noncovalent fibrin polymer (see Fig. 40.2 ). Activated FXIII, a transglutaminase, cross-links fibrin monomeric subunits into an insoluble, cross-linked fibrin clot. When insoluble, cross-linked fibrin is made, a new linkage between the D domains of two adjacent fibrin monomers occurs. In the process, a neo-epitope of interaction is formed (see Fig. 40.2 ).
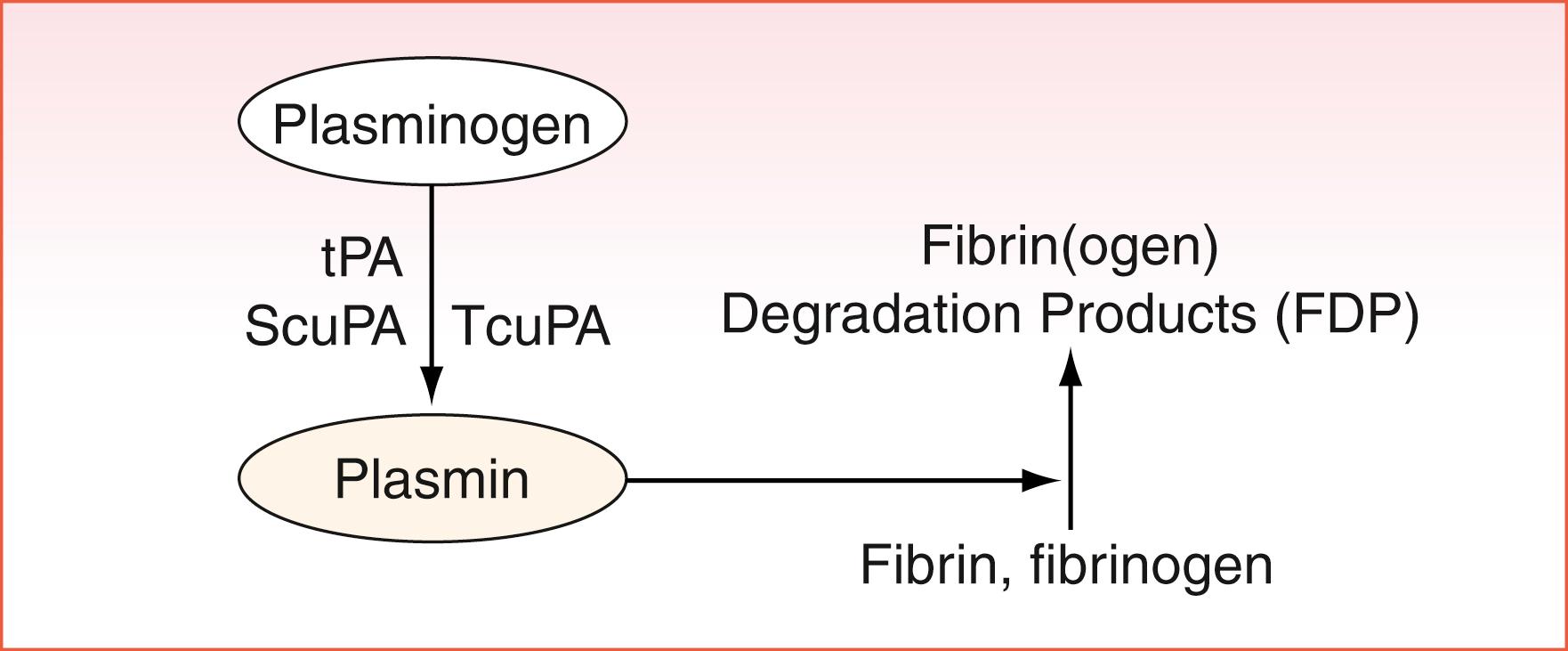
The fibrinolytic system regulates fibrin clot degradation and clot dissolution. In fibrinolysis, the circulating zymogen plasminogen is converted to its active form plasmin mainly by endogenous tissue plasminogen activator (tPA) ( ). Two other plasminogen activators are single-chain urokinase plasminogen activator (ScuPA) and two-chain urokinase plasminogen activator (TcuPA). These activators are found in the endothelium as well as in neutrophils and monocytes. The natural plasminogen activators tPA, ScuPA, and TcuPA convert zymogen plasminogen to the active enzyme plasmin ( Fig. 40.3 ). tPA is produced constitutively, and ScuPA is increased in inflammatory states. Plasminogen activator inhibitor-1 (PAI-1) is the major inhibitor of tPA and TcuPA. α 2 -Antiplasmin, a serine protease inhibitor (serpin), is the most potent and highly selective inhibitor of plasmin. However, the plasma concentration of α 2 -antiplasmin, even if high (approximately 1 mM), is only about half the plasma concentration of plasminogen . The fibrinolytic system is also regulated by thrombin-activatable fibrinolysis inhibitor (TAFI) ( ). TAFI is a carboxypeptidase able to remove lysine residues from fibrin, such that it indirectly controls plasmin by interfering with the binding of plasminogen and tPA to fibrin.
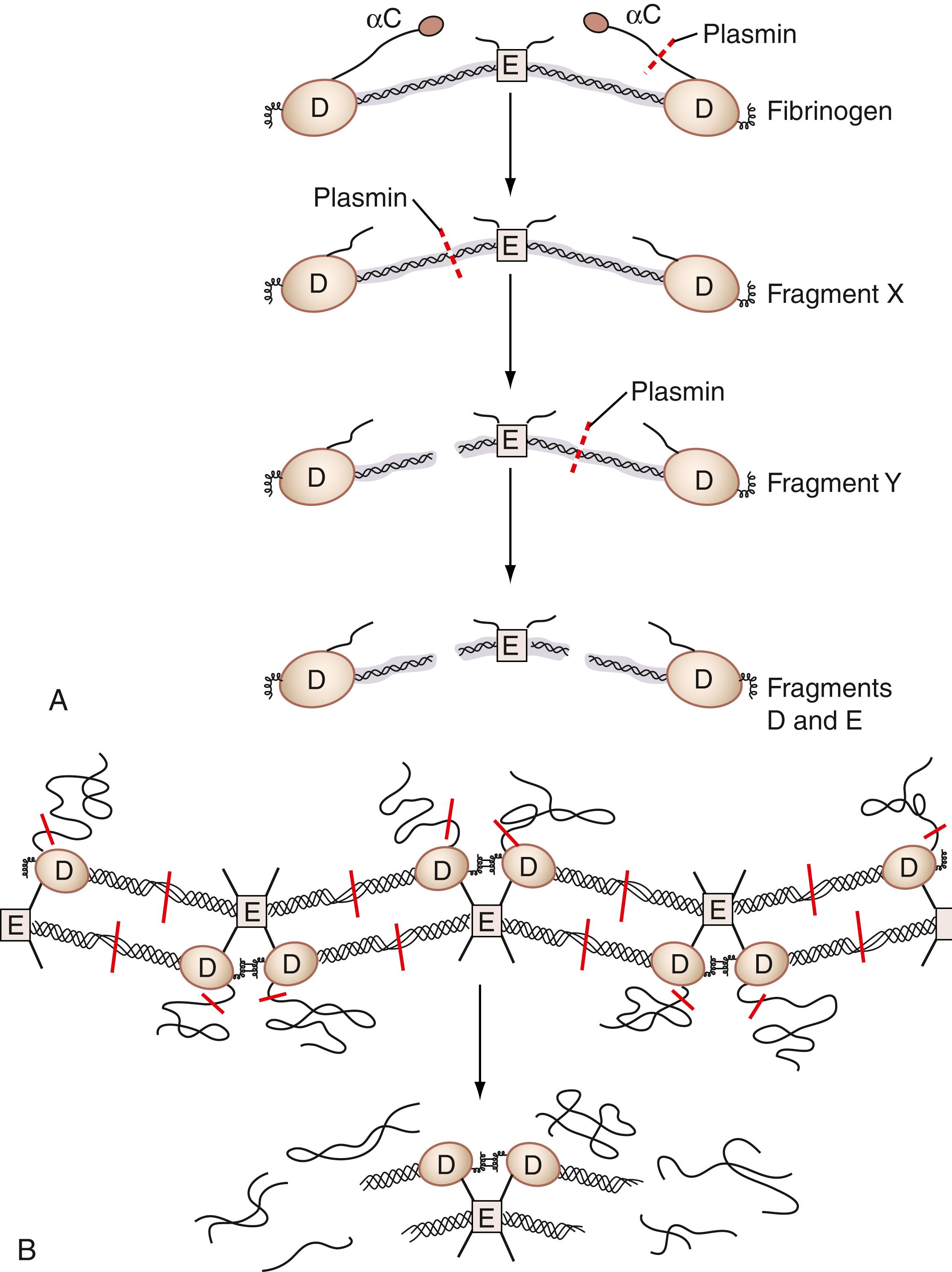
The binding of both plasminogen and tPA to fibrin can increase plasmin generation by two orders of magnitude. The active plasmin molecule is a potent protease, and it recognizes multiple substrates. Plasmin will degrade soluble fibrinogen to produce fibrinogen degradation products ( Fig. 40.4A ). Plasmin cleaves fibrinogen into an X fragment of similar molecular mass by eliminating portions of the α-chain ( ). Plasmin then cleaves the X fragment asymmetrically between the D and E domains to produce fragment Y. Plasmin further degrades the Y fragment to produce soluble D and E domains that are called soluble fibrinogen degradation products or, in the case of fibrin digestion by plasmin, fibrin degradation products . Their presence indicates that plasmin has been formed. Plasmin will also degrade insoluble, crossed-linked fibrin ( Fig. 40.4B ). When it does, it liberates a D-D-dimer domain formed as a result of the neo-epitope between these two domains (see Fig. 40.4B ) ( ). The presence of soluble D-dimer indicates that, first, thrombin has been formed, followed by clotting, then the clot has been cross-linked by FXIIIa, and, finally, plasmin has been formed and has cleaved the insoluble, cross-linked fibrin clot.
Coagulation is tightly regulated by natural anticoagulants and the fibrinolytic system to avoid thrombus formation and extension. Three major anticoagulant systems regulate the enzymes of the coagulation protein system to help to inhibit clot formation. These systems are the protein C/protein S system, the plasma serine protease inhibitor system, and TFPI, a Kunitz-type serine protease inhibitor. AT is the main serine protease inhibitor of coagulation enzymes of the plasma serine protease inhibitor system. Each of these systems plays a critical role in the proper regulation of the coagulation system. Moreover, in murine deletion models, complete deficiencies of protein C, protein S, AT, and TFPI are incompatible with the survival of mammalian gestation, delivery, or life ex utero ( ; ; ; Burstyn-Cohen et al., 2009).
When activated, protein C, a 62-kDa vitamin K–dependent protein, is an enzyme that functions as an inhibitor. When free thrombin binds to thrombomodulin on endothelium, this complex then converts protein C to APC. Its activation is increased when protein C binds to the endothelial protein C receptor (EPCR). The complex formed by APC+EPCR needs to dissociate in order to allow binding of APC with protein S to inactivate FVa and FVIIIa ( ). Protein S, a 69-kDa vitamin K–dependent protein, is a cofactor, or receptor, for APC on cell membranes. It allows APC to bind to cell surfaces in such a manner as to orient itself to inactivate FVa and FVIIIa. The enzyme uses this cofactor as a receptor to localize its activity to perform its inhibitory function. Plasma protein S is in equilibrium between the free form (40%) and a bound form (60%) that is complexed to C4b-binding protein; only the free form functions as a cofactor for APC. APC also binds to three receptors on endothelial cells: (1) endothelial cell protein C receptor to activate (2) protease-activated receptor (PAR) 1 and (3) apolipoprotein E receptor 2 (ApoER2) ( ; ; ). Activation of PAR1 on endothelial cells may contribute to the anticoagulant function of activated protein C by liberating tPA. APC on endothelial cells also has an inflammatory effect via activation of sphingosine-1-phosphate receptor transactivation and activation of ApoER2 ( ; ). Thus, APC reduces thrombin formation, stimulates fibrinolysis, and initiates inflammation to reduce thrombosis risk.
This serpin is a 58-kDa protein that inhibits each of the following hemostatic enzymes: IIa, Xa, VIIa, IXa, XIa, kallikrein, and XIIa. It exerts its anticoagulant effect primarily by inhibiting FIIa and FXa ( Fig. 40.5 ). Antithrombin has two functional sites: the heparin-binding site (at the amino terminus of the protein) and the reactive site (Arg329-Ser394). The ability of antithrombin to function as an inhibitor of coagulation protein enzymes is potentiated by endogenous and exogenous heparins. In fact, it is the presence of antithrombin that gives heparin its anticoagulant properties. In the presence of heparins, antithrombin undergoes a conformational change that increases from 1000-fold to 4000-fold its inhibitor effect on FIIa ( ). Trace amounts of circulating TF or TF transiently exposed by damaged vessels contribute to FVIIa inactivation by antithrombin ( ).
In addition to antithrombin, other serpins regulate the enzymes of the hemostatic and inflammatory systems. Heparin cofactor II is a serpin that specifically inhibits thrombin in the presence of dermatan sulfate. Protein Z inhibitor is a serpin that specifically inhibits factor Xa in the presence of its cofactor protein Z—a vitamin K–dependent protein. C1 inhibitor (C1 esterase inhibitor) is the most potent inhibitor of FXIIa, kallikrein, and FXIa in plasma. Its main function is to regulate the amount of free bradykinin in the intravascular compartment and reduce inflammatory events ( ; ; ). The absence of C1 inhibitor is the causative factor for types I and II hereditary angioedema, a disorder associated with excessive bradykinin delivered to tissues.
In addition to the serpins, there is the Kunitz-type serine protease inhibitor, TFPI (Crawley & Lane, 2008). TFPI is the most potent inhibitor of the FVIIa–tissue factor complex. Under physiologic conditions, TFPI exerts its inhibitory effects by forming a quaternary complex with FVIIa, TF, and FXa (see Fig. 40.5 ). It is noteworthy that the murine TFPI knockout is embryonically lethal ( ). TFPI is produced by microvascular endothelium, and it seems mainly associated with glycosaminoglycans on the endothelial wall. Only approximately 20% of TFPI circulates in plasma associated with lipoproteins ( ). Another family of Kunitz-type serine protease inhibitors, the amyloid β-protein precursor (AβPP) and related members, is present in platelets and brain and regulates factors XIa, IXa, Xa, VIIa/TF, and plasmin ( ; , ; , ). This inhibitor does not inhibit thrombin. The exact function of this inhibitor is not completely known, but it is believed to be a cerebral anticoagulant ( ). AβPP and amyloid precursor-like/protein 2 gene–deleted mice survive gestation but have an accelerated rate of thrombosis in a carotid artery thrombosis model ( ).
In physiologic conditions, endothelial cells produce vasoactive hormones able to control the primary phase of hemostasis. Endothelial cells express the cyclooxygenase-2 enzyme that produces prostacyclins (PGI 2 ) from arachidonic acid ( ). PGI 2 is a vasodilator and inhibits platelet aggregation. The actions of prostacyclins are mediated by cell membrane prostacyclin (IP) receptors and intracellular peroxisome proliferator-activated receptors (PPARβ and PPARγ). Endothelial cells also express the NO synthase enzyme (eNOS, NOS3), and they release NO derived from l -arginine. NO is a potent vasodilator that also inhibits platelet adhesion and aggregation. NO activates guanylate cyclase and cGMP. Activated platelets adhering to collagen seem also able to produce NO under shear flow ( ), thus, controlling platelet adhesion and vasoconstriction directly at the sites of vessel damage.
Thrombospondin-5, also known as cartilage oligomeric matrix protein ( COMP ), is an extracellular protein able to control vascular tone. COMP is released by a variety of cartilage and muscle tissues and activated platelets. COMP inhibits thrombin and acts as a natural anticoagulant in mice ( ). It determines a dose-dependent prolongation of thrombin time.
More proteins participate in coagulation reactions in vitro than are critical for hemostatic reactions in vivo. The original coagulation cascade waterfall hypothesis initiated by FXII has been replaced by one whereby TF and FVIIa are initiators (Gailani & Broze, 1993). TF is ubiquitous throughout the body, although it is rich in the brain, lung, and placenta. Its expression is upregulated after injury. Regulation of the expression of TF is a major control mechanism for the initiation of hemostasis. TF is not fully active on intact cells; it becomes able to activate coagulation when cell membrane properties are modified. This process is called decryption of encrypted TF and is still not completely understood. Different mechanisms of TF decryption have been proposed: an exposition of phosphatidylserine, dimerization of TF, and oxidation of a specific disulfide bond in TF ( ). In conditions in which a vessel wall injury is limited to endothelial activation, blood-borne TF has been characterized on microparticles released by leukocytes and involved in the initiation of coagulation ( ). Activated macrophages have been shown to express TF on their cell membrane and microparticles involved in physiologic hemostasis and thrombosis ( ). There is a developing notion that endothelial cell TF is the initial nidus that leads to hemostasis and thrombosis ( ; ).
The FVIIa – TF complex activates FIX, leading, in turn, to FX activation. It must be noted that at the concentrations of factors ordinarily present in the body, FVIIa/TF does not directly activate appreciable amounts of FX because of the presence of TFPI—a point emphasized diagrammatically in Figure 40.1 by the graying of this reaction. Once FX has been activated to FXa, this FXa, in turn, contributes to the activation of thrombin from prothrombin. Thrombin then proteolyzes fibrinogen to form fibrin. Although TF-dependent coagulation reactions are rapidly inhibited by TFPI, if the stimulus for thrombin formation is sufficiently strong, coagulation is maintained through the activation of FXI by thrombin ( ). Activation of FXI to FXIa results in increased activation of FIX and eventually increases the formation of thrombin. Further modulation of hemostatic balance in the direction of clot formation is exerted by TAFI (also known as carboxypeptidase U ). Among its actions, TAFI cleaves C-terminal lysine residues from fibrin, thereby decreasing the binding of plasminogen to the clot and diminishing clot lysis ( ).
At the site of endothelial cell injury, subendothelial collagen and von Willebrand factor (vWF) are exposed. The disrupted endothelium alters laminar blood flow at the site of injury; thereby, platelets undergo shape change from discoid to spherical and produce pseudopodia that will breach the site of endothelial damage by binding to vWF via GPIb/IX/V receptors. This step is called platelet adhesion (attachment of platelets to a nonplatelet surface), which is followed by secretion . During secretion, contents of alpha and dense granules from platelets are released along with the generation of thromboxane A2 (TXA2) via the cyclooxygenase-1 (COX-1) pathway. TXA2 causes vasoconstriction and activates platelets via the TXA receptor. During the next step of platelet activation, the signal transduction within platelets leads to a conformational change in GPIIb/IIIa, allowing fibrinogen (acts as a ligand) to bring adjacent platelets together, causing aggregation (attachment of one platelet to another). Activation of platelets leads to exposure of phosphatidylserine (PS) similar to altered endothelium that also exposes PS for secondary hemostasis that begins simultaneously to generate thrombin.
Secondary hemostasis can be divided into two different phases: (1) the TF pathway (previously called the extrinsic pathway ), which is the initiator of coagulation; and (2) the intrinsic pathway, which amplifies and propagates thrombin generation. The TF pathway begins with endothelial injury and expression of TF on the cell surface, which binds the small amount of FVIIa present in circulation to form a TF+FVIIa complex. This complex is known as extrinsic tenase because this will activate FX to FXa on the cell surface in the presence of Ca ++ . This FXa along with FVa on the cell surface (PL) and Ca ++ forms a prothrombinase complex , converting prothrombin to thrombin. As soon as a small amount of thrombin is generated by the TF pathway, this thrombin activates the TFPI, which shuts down the TF pathway by inhibiting the TF+FVIIa and FXa complex. The same thrombin also initiates the intrinsic pathway by activating FXI to FXIa and FIX to FIXa on the cell surface (PL) with Ca ++ . FIXa along with FVIIIa on the cell surface (PL) and Ca ++ forms the intrinsic tenase and converts FX to FXa, which, along with FVa, forms the prothrombinase complex converting prothrombin to thrombin. This intrinsic pathway is the primary pathway of thrombin formation. Thus, the TF pathway is the initiator and the intrinsic pathway is the amplifier and propagator of thrombin, and these two pathways are interrelated. The previous concept of independent extrinsic and intrinsic pathways is not physiologic because if these two were independent pathways producing thrombin, then patients with hemophilia A and B would not have bleeding tendencies. The independence of extrinsic and intrinsic pathways to produce thrombin is “true only in the test tubes” where prothrombin time (PT) and activated partial thromboplastin time (APTT), respectively, assess them. Thrombin is the main enzyme in the coagulation system because it activates FV and FVIII to their activated forms to amplify further thrombin generation and converts fibrinogen to fibrin monomers. It also activates FXIII to FXIIIa to cross-link these fibrin monomers to stabilize the clot.
Laboratory evaluation of primary hemostatic defects presenting as mucocutaneous type bleeding due to platelets and vWF is described in Chapter 41 . Patients with bleeding tendency due to secondary hemostasis defects often present with ecchymoses, soft tissue, and joint bleeds. Bleeding tendencies could be congenital or acquired. Therefore, an elaborate personal and family history of bleeding is the prerequisite for appropriate laboratory testing. Most congenital bleeding disorders often are diagnosed early in childhood; however, mild bleeding disorders may not be revealed until later in life when there is a robust hemostatic challenge.
Routine coagulation screening tests are useful to identify a hemostatic defect in a bleeding phenotype. However, performing these tests to assess hemostasis in a nonbleeding patient is not clinically useful—it often may lead to unnecessary expensive further testing and could delay surgeries.
Prothrombin time (PT), APTT, fibrinogen, thrombin time and D-dimers are considered screening coagulation tests along with platelet count for initial evaluation of a bleeding patient. Whole blood is collected in 3.2% sodium citrate (blue top) in a 9:1 ratio (blood:citrate) by a single needlestick without trauma or prolonged stasis and mixed well by gently inverting 3 to 5 times (avoid vigorous mixing that may activate platelets) ( ). The specimen should be transported to the laboratory at room temperature, and the testing should be completed within 4 hours of collection. However, if the testing cannot be performed within 4 hours of collection, then ideally the specimen should be centrifuged immediately to separate plasma from cellular component and frozen at −20°C and then transported on dry ice for testing to the laboratory. The separated plasma ideally should be platelet free (<10 K) before freezing because frozen plasma that is rich in platelets upon thawing will cause disruption of platelets releasing platelet factor 4, which binds to heparin and may give false low anti-FXa activity, or phospholipids released from platelet membranes bind to lupus anticoagulant and may give false-negative results. The specimen should not be held at room temperature for more than 4 hours to avoid loss of FVIII (and, to some extent FV, both being heat-labile factors) to avoid getting spuriously long APTT and low FVIII (and vWF) that might result in a further unnecessary investigation. Improper sample handling is often encountered in office clinics that send out their specimens to remote reference laboratories for coagulation testing. Thus, the preanalytic part of coagulation testing is crucial for accurate diagnosis and management.
PT is a simple test in which the patient plasma is mixed with a PT reagent that contains tissue thromboplastin and CaCl2. Tissue thromboplastin consists of tissue factor and phospholipid. The source of tissue thromboplastin is important because nonhuman sources (e.g., rabbit brains) may have lower sensitivity to various vitamin K–dependent coagulation factors as compared with human sources (e.g., placenta) or recombinant tissue thromboplastin. The international normalized ratio (INR) was developed to harmonize PT results for monitoring vitamin K antagonist (VKA) therapy. The INR is a calculated value obtained by first calculating the prothrombin time ratio (PTR) by dividing the patient PT by the control PT, and then raising this PTR by an international sensitivity index (ISI) [INR = (PTR) ISI ]. Ideally, the INR should be used only for monitoring VKA therapy. However, due to the inclusion of the INR in the model of end-stage liver disease (MELD) score calculation, it is often also used to assess hemostasis in patients not on VKA for no good reason other than the convenience. When the INR was used for MELD score calculation, the PT reagents most likely had a higher ISI with less sensitivity for vitamin K–dependent factor deficiencies than most current PT reagents, especially recombinant tissue thromboplastin. With different ISI, the INR can be different; hence, the MELD score can also be significantly different.
Isolated prolonged PT is usually due to an FVII deficiency and may also be prolonged due to mild deficiencies of common pathway FII, FV, and FX depending on the reagent’s sensitivity. Recombinant tissue thromboplastin is very sensitive to even mild reduction of FVII (40%–45% activity), which is not clinically relevant since most patients with mild to moderate FVII deficiency are clinically asymptomatic and are often diagnosed when there is an incidental prolonged PT. Hemostatic levels of FVII for major surgery are 10% to 20%; however, even mild prolongation of PT/INR often results in unnecessary plasma transfusion to correct a laboratory value that is not clinically relevant. Because of rebalanced hemostasis in patients with cirrhosis (discussed later), several societies have recommended ignoring PT/INR values obtained preprocedurally in cirrhotics.
APTT is another routinely performed test to assess clinical hemostasis with poor sensitivity and specificity in a nonbleeding patient. APTT generally assesses coagulation factors in the intrinsic pathway as well as the common pathway. In APTT, a contact activator (e.g., ellagic acid, silica, kaolin) is added to the patient’s plasma to initiate the activation of contact factors (prekallikrein, HMWK, and FXII) in the presence of PLs. After incubation for 3 to 5 minutes, the plasma is recalcified and the clotting time is recorded (usual range, 23–35 sec). Unfortunately, APTT reagents are not well standardized; hence, there is significant variation from one reagent to another and when we include coagulation instruments from different companies, the variability in APTT is even more significant. The APTT reagents come with three different quantities of PL with different sensitivities for various needs. APTT-factor sensitive (APTT-FS) has the highest amount of PL, making this reagent very sensitive to detect even slightly lower levels of coagulation factors in the intrinsic pathway. APTT-LA (lupus anticoagulant sensitive) reagent has the smallest amount of PL, making it very sensitive to detect even a weak LA. APTT-FSL (factor and lupus sensitive) has an intermediate amount of PL; hence, it should be used in daily practice because this will detect clinically relevant decreased amounts of coagulation factors and lupus anticoagulant.
Isolated prolonged APTT is a common clinical finding. Further investigation should be dictated by a personal and family history of bleeding if that is the presenting symptom, whereas in a nonbleeding patient it is essential to check previous APTTs. If the previous APTTs were normal, then the current prolongation is due to an acquired defect, most likely lupus anticoagulant. However, if the previous APTTs are also prolonged, then the contact factor deficiency should be considered and investigated because FXII-, HMWK-, and PK-deficient patients do not bleed.
In a bleeding patient, if APTT is prolonged, unfractionated heparin should be excluded. If the previous APTTs were also prolonged, then a congenital bleeding disorder of intrinsic pathway should be considered and investigated by measuring FVIII first (common thing first!) followed by FIX or, rarely, FXI. If the previous APTTs were normal and current presentation includes soft tissue hematomas, easy bruising, and other conditions, with a prolonged APTT, then acquired hemophilia should be considered due to autoantibody against FVIII.
Thrombin time (TT) is usually not a commonly ordered test because of its limited utility in laboratory assessment of most bleeding disorders. In the TT test, bovine thrombin (3–5 U/mL) is added to the patient’s plasma and the clotting time recorded. The upper limit of the normal range should be adjusted close to 25 sec to make TT more sensitive to detect dysfibrinogenemia. The most frequent cause of prolonged TT is heparin followed by hypofibrinogenemia and then dysfibrinogenemia. Direct thrombin inhibitors (oral and parenteral) significantly prolong TT.
Fibrinogen assay should be measured routinely in a bleeding patient and patients with cirrhosis. The fibrinogen assay is based on TT (Clauss method) in which a standard curve is generated by using known calibrators and bovine thrombin at a very high concentration of 50 U/mL (for TT 3–5 U/mL) so that the clotting time is independent of thrombin concentration. Bovine thrombin is added to the patient’s plasma and the clotting time obtained is used to extrapolate fibrinogen value from the standard curve. A very high amount of the direct thrombin inhibitor will give false low fibrinogen levels.
If there is clinical suspicion for hypofibrinogenemia, the direct fibrinogen assay should be performed rather than relying on PT and APTT to be prolonged because, in the majority of patients with mild to moderate hypofibrinogenemia, PT and APTT are likely to be normal. When PT and APTT were performed manually in the past, the endpoint was a solid, firm clot formation on the side of the test tube. However, with automation, the endpoint of PT/APTT is the change in optical density or turbidity due to formation of fibrin strands. Therefore, the endpoint is often sooner than the manual method; hence, automated PT and APTT are usually normal, with mild to moderate hypofibrinogenemia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here