Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Chronic thromboembolic pulmonary hypertension (CTEPH) is an increasingly recognized clinical entity. Pulmonary embolism (PE) is a common disease, with an annual incidence of about 100/100,000 in the United States. Many episodes of PE, however, are silent; from 30% to 50% of patients with CTEPH have no history of a PE. Patients with a prior PE who do not have complete fibrinolysis of their clot may go on to have the residual clot remodel into a scar, which can occlude or narrow segmental, lobar, and even main pulmonary arteries with a fibrous plug, leading to pulmonary hypertension. The incidence of CTEPH after a documented PE has been reported in several studies, and a reasonable estimate is in the 4% to 5% range. The classic study by Pengo and colleagues has suggested a 3.8% incidence of CTEPH after a PE. A more recent study has suggested a 4.8% incidence of CTEPH after a PE. Risk factors for CTEPH are listed in Table 34.1 . Although many hypercoagulable states have been documented in CTEPH patients (e.g., antithrombin III deficiency, protein C, protein S, factor V Leiden, prothrombin gene mutation), they are not more prevalent than in patients with primary pulmonary hypertension. However, antiphospholipid antibody syndrome is more prevalent in CTEPH patients.
| RISK FACTOR | ODDS RATIO |
|---|---|
| Previous PE | 19 |
| Younger age | 1.8/decade |
| Larger defect on perfusion scan | 2.2/decile decrease in perfusion |
| Unprovoked PE | 5.7 |
| Splenectomy | 18 |
| VA shunt or infected pacemaker | 76 |
| Chronic inflammation | Increased |
| Antiphospholipid antibody syndrome | Increased |
The diagnostic evaluation process is relatively straightforward; the most important factor is to consider this process when a patient presents with unexplained dyspnea with a clear chest radiograph and no obvious cardiopulmonary disease. Common presenting symptoms include dyspnea on exertion, atypical exertional chest discomfort, exercise intolerance, exertional presyncope or syncope, and hemoptysis. Common signs include signs of right heart failure with lower extremity edema, hypoxemia, and occasionally pulmonary artery (PA) flow murmurs.
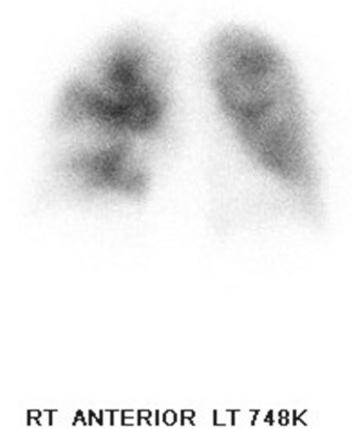
A ventilation-perfusion (V̇/Q̇) scan will show substantial segmental defects in CTEPH, whereas a normal V̇/Q̇ scan essentially eliminates CTEPH from further diagnostic consideration ( Fig. 34.1 ). An echocardiogram can then confirm significant pulmonary hypertension and screen for other cardiac disease. Complete pulmonary function tests are usually performed in older patients to screen for other pulmonary disease. In the United States, contrast-enhanced, pulmonary embolism (PE) protocol, chest computed tomography angiography (PE-CTA) is also performed to screen for other pulmonary disease and to demonstrate the PA anatomy. Findings on CT suggestive of CTEPH include a mosaic perfusion pattern, peripheral infarcts, a clot within the PA, narrowed pulmonary arteries, webs, and PA cutoffs. Dual-energy CTA is a promising imaging modality that may help better identify good operative candidates and can potentially offer a quantitative estimate of the degree of PA obstruction. Magnetic resonance angiography (MRA) is often used in European centers instead of CTA to evaluate CTEPH patients.
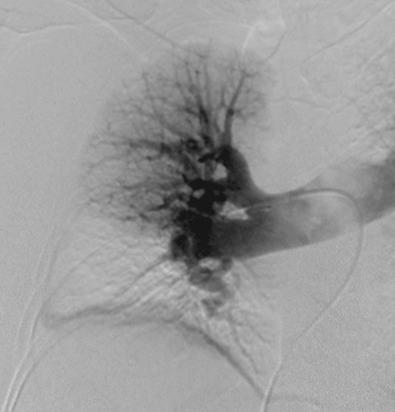
A right heart catheterization is finally performed to measure the hemodynamics of the pulmonary circulation and obtain a two-plane pulmonary angiogram. Typical findings include delayed filling of vessels, branch occlusions, webs, pouches, and narrowed vessels ( Fig. 34.2 ). A left heart catheterization is performed in those with suspected or reasonably possible coronary disease based on age and atherosclerosis risk factors. Ideally, an experienced multidisciplinary CTEPH team reviews the evaluation and makes an operability decision. In general, the degree of identified PA obstruction should match the pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR). The vast majority of patients are operable in experienced centers if there are at least several segmental PA obstructions because imaging studies usually underestimate the degree of disease found at pulmonary thromboendarterectomy (PTE). True distal disease should be avoided but is uncommon. Particularly in patients with acute or chronic PE, it is important to determine if there is still clotting in the deep venous systems of the legs. If vascular ultrasound suggests this, addition of an inferior vena cava (IVC) filter should be considered. Although IVC filters are accompanied by some degree of controversy, if used in this setting, consideration should be given to removing them within 6 postoperative months. Although routine IVC filters used to be advocated for all patients with CTEPH, this is an area of unresolved controversy, and we usually do not place them in the typical patient. We have occasionally proceeded to surgery based solely on the V̇/Q̇ scan and echocardiographic estimation of PA pressure in patients with classic histories who present acutely and are very ill.
The indications to perform a PTE are straightforward—a symptomatic patient, significant pulmonary hypertension, segmental or proximal obstructive disease amenable to clearance with PTE, and the absence of severe comorbid disease. A controversial indication is symptomatic patients with only exercise-induced pulmonary hypertension. The reasons for performing a PTE include not only improving exercise capacity, but also stabilizing the longer term prognosis by preventing progression of distal PA arteriopathy and continued loss of right ventricular (RV) function. Patients with atrial level shunts can also become desaturated, particularly with exercise.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here