Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Chronic pulmonary thromboembolism is an uncommon entity resulting from an incomplete resolution of thrombi, leading to complex restructuring processes within pulmonary arteries. Extensive clinical experience from the literature suggests that failure of thromboembolic resolution after a single embolic event or after recurrent thromboembolic events represents the predisposing condition in most patients with the disease. Although the clinical signs and symptoms are nonspecific, a confident diagnosis can usually be made on computed tomography angiography (CTA).
It has been estimated that in the United States approximately 600,000 episodes of pulmonary embolism occur each year. Although the natural history of adequately treated acute pulmonary embolism is not well characterized, data based predominantly on clinical follow-up suggest that thromboembolic resolution occurs in the overwhelming majority of patients who experience an acute embolic event ( Fig. 51.1 ). Evolution toward chronic thromboembolic disease has been estimated to range between 2% and 18% of patients when serial perfusion scans have been performed, and it has been reported in 13% of patients with CT follow-up ( Fig. 51.2 ). The basis for this alternative natural history has not been clearly established. Despite extensive investigation the only identifiable thrombotic predisposition has been the presence of lupus anticoagulant in approximately 10% of patients. Less than 1% of patients have had deficiencies of antithrombin III, protein C, or protein S. In a subset of patients, long-standing increase in pulmonary artery pressure resulting from the chronic obstruction of the pulmonary arterial bed leads to chronic thromboembolic pulmonary hypertension, and can be complicated by cor pulmonale and right-sided heart failure. At this stage there is a poor prognosis of the disease with a 5-year survival rate of only 30%.
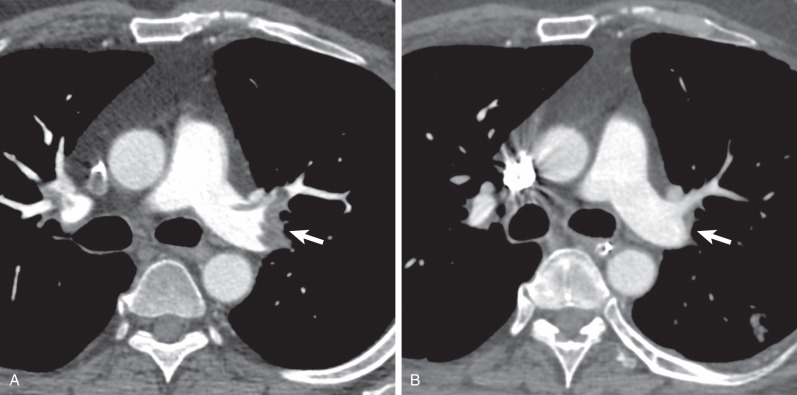
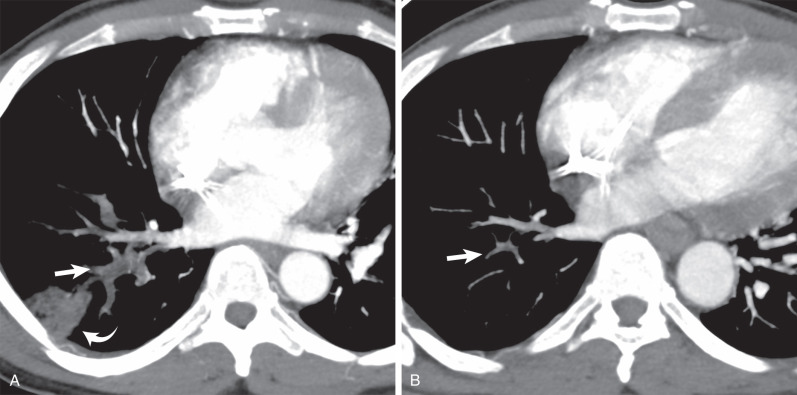
The spectrum of symptoms associated with chronic pulmonary thromboembolism depends on the percentage of the pulmonary arterial bed chronically obstructed and the development of pulmonary hypertension. In the absence of pulmonary hypertension the symptoms are nonspecific, including progressive dyspnea and exercise intolerance. A diagnostic delay, particularly in the absence of an acute history of venous thromboembolism, is common. The mean time from onset of symptoms to diagnosis usually exceeds 3 years.
The symptomatic history has been well described. After a documented venous thromboembolic event, symptomatic recovery occurs, although often not to a level equivalent to that before the acute event. In patients without a documented acute thromboembolic event, several data confirm the often subtle clinical manifestations of a venous thromboembolic event and the frequency with which misdiagnosis occurs. After a period of clinical stability that may range from months to years, increasing exertional dyspnea, hypoxemia, and right ventricular dysfunction ultimately ensue.
Chronic thromboembolic pulmonary hypertension (CTEPH) can develop in up to 3.8% of patients after an acute episode and in up to 10% of patients with recurrent pulmonary embolism. CTEPH is classified as group IV pulmonary hypertension according to the Fifth World Symposium of Pulmonary Hypertension (Nice, France, 2013). The diagnosis can be made in the setting of elevated pulmonary artery pressure (mean pressure ≥25 mm Hg, with wedge pressure ≤15 mm Hg), with evidence of chronic emboli as assessed by a ventilation-perfusion (VQ) scan or cross-sectional imaging, such as CT or magnetic resonance imaging (MRI).
Fresh thrombus may fragment and disperse into smaller pulmonary arteries, but within a few days, thrombotic emboli undergo organization and become firmly adherent to the vessel wall. In 4 to 6 weeks they are converted into fibrous tissue, often with recanalization. Some emboli may disappear, and it is presumed that they are destroyed by plasmin. Thin fibrous bands stretching across the lumen of major pulmonary arteries are sometimes the only evidence of previous thromboembolism. More often, the lumen of smaller arteries is divided into multiple channels by the usual process of recanalization. A mixed pattern of vascular stenosis, intraluminal webs, and abnormal tapering of vessel diameter can be seen. As a consequence of the presence of pulmonary hypertension, a secondary, small-vessel arteriopathy, also described as plexogenic arteriopathy, may develop.
Early resolution of pulmonary vascular obstruction occurs by two mechanisms: mechanical changes in thrombus location and endogenous thrombolysis. After these early events, organization and recanalization further alleviate the degree of pulmonary vascular obstruction. However, significant organized residual disease may persist, and therefore abnormal pulmonary hemodynamics at rest or with exercise may be present in patients whose perfusion scans (i.e., the most frequent means of follow-up) return to normal.
Chronic pulmonary thromboembolism may show a silent clinical period lasting months or years, followed by clinical deterioration that parallels the loss of right ventricular functional capacity. This decline may be due to recurrent thromboembolism, in situ pulmonary artery thrombosis, and a hypertensive pulmonary arteriopathy similar to that encountered in patients with pulmonary hypertension from other causes.
The results of spirometry, often performed as part of the evaluation of the patient's dyspnea, are usually within normal limits. Approximately 20% of patients show mild to moderate restriction, to a large extent caused by parenchymal scarring related to prior infarcts. A reduction in single-breath diffusing capacity for carbon monoxide (DLCO) can be observed, ranging from mild to severe; a normal value does not exclude the diagnosis.
Chest radiography may be normal or may demonstrate findings of pulmonary hypertension. Asymmetry in size of the central pulmonary arteries can be seen in conjunction with areas of relative hypoperfusion and hyperperfusion. The asymmetry of the central pulmonary arteries may be so dramatic that it may suggest pulmonary artery agenesis, whereas areas of increased perfusion may suggest consolidation with pneumonia or interstitial lung disease. Chest radiography may also reveal parenchymal or pleural scars, consistent with prior infarcts, and enlargement of the right ventricle in the advanced forms of the disease.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here