Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Chlamydia trachomatis imposes a tremendous burden on human health because it is one of the most common causes of bacterial infection. Worldwide in 2011, an estimated 21 million people had active trachoma, which is an ocular C. trachomatis infection that is the leading cause of infectious blindness. The World Health Organization (WHO) estimated that in 2016, 1.9 million individuals were blind or had severe visual impairment as a long-term consequence of trachoma. This disease has disappeared from the developed world coincident with improved sanitation and access to clean water. However, an estimated 190 million people live in endemic areas, mainly in poor, rural regions of 41 low-income countries, and require interventions to prevent blinding disease.
C. trachomatis genital tract infection is the most common bacterial sexually transmitted infection (STI) in the world, with an estimated 131 million new cases in 2012. Women disproportionately suffer the major complications, including pelvic inflammatory disease (PID), ectopic pregnancy, and infertility, and can transmit the bacterium to their newborns, who develop conjunctivitis and pneumonia. In 2016, 1.59 million new cases of C. trachomatis infection were reported to the Centers for Disease Control and Prevention (CDC), making it the most commonly reported infectious disease in the United States. Not all C. trachomatis infections are diagnosed, however, and the actual incidence is higher. For example, in 2008 the annual incidence of C. trachomatis genital infections in the United States was estimated to be 2.86 million cases, which was more than twice the number that were reported. In a population-based assessment, chlamydial prevalence in the United States in the 14- to 39-year-old age group was estimated to be 2.0% for women and 1.4% for men for 2007 to 2012, with stable overall prevalence during this period. Women have had consistently higher rates, which may be due to increased screening and diagnosis compared to men. Higher chlamydial prevalence is reported when screening targeted populations, who tend to be higher-risk groups. For example, in women 15 to 24 years old, chlamydial prevalence ranging from 6.6% to 15.6% has been reported, with the highest prevalence in STI clinics and juvenile detention centers. In the United States, reported case rates continue to rise, largely as a result of increased screening and improved diagnostic tests ( Fig. 180.1A ), but population prevalence appears stable, with notable disparities by age and race/ethnicity ( Fig. 180.1B ). Data from 2016 demonstrate that the disease is most common in adolescents and young adults ages 15 to 24 years, with higher rates in women and among African Americans compared with Hispanics/Latinos and non-Hispanic whites. C. trachomatis infections thus continue to be a major public health problem.
Human C. trachomatis infections are associated with ocular, genitourinary, and respiratory disease syndromes, and although clinically distinct, they share common characteristics. These include the propensity to cause (1) infections that are often long-lasting in the absence of treatment; (2) repeated infections after natural clearance or antibiotic treatment of an initial infection; (3) infections that are often asymptomatic or minimally symptomatic; and (4) infections that produce inflammatory and scarring sequelae in the absence of treatment, often with few or no symptoms until end-organ dysfunction is manifest.
Chlamydiae are obligate intracellular bacteria that replicate within eukaryotic cells. C. trachomatis, like all other Chlamydia spp., has one of the smallest bacterial genomes. Its 1.04-Mb genome encodes approximately 900 genes, which is less than a fifth of the genes in Escherichia coli. The large majority of these genes (668) are shared among all Chlamydia, representing a core set of genes necessary for the intracellular chlamydial lifestyle. The small genome size is due to reductive evolution in which C. trachomatis has lost enzymes and metabolic pathways for nutrients that are readily available from the host cell. As a consequence, however, Chlamydia is dependent on its host cell to complete its developmental cycle. Other common obligate intracellular bacteria include Rickettsia, which also has a small genome, and Mycobacterium leprae, which is undergoing active genome reduction.
Chlamydia is unusual among bacteria in having two developmental forms, which are morphologically different and functionally specialized. The reticulate body (RB) is the vegetative form that grows and divides by binary fission. Chlamydiae lack many enzymes and pathways for biosynthesis of amino acids and nucleotides and thus are auxotrophic for many essential metabolites. The RB compensates by having numerous membrane transport systems to acquire nutrients—including amino acids, oligopeptides, nucleotides, ATP, carbohydrates, and metal ions—from the host cell. The RB thus resembles a typical bacterium, but it occupies an intracellular niche in which it can obtain many nutrients from its host cell.
The spore-like elementary body (EB) is a unique chlamydial adaptation that is the transmission vehicle to infect another host cell. It is environmentally stable, with a rigid envelope of cross-linked membrane proteins, and DNA condensed into chromatin by two Chlamydia -specific histone-like proteins, Hc1 and Hc2. The most abundant of its membrane proteins is the major outer membrane protein (MOMP), which is also the immunodominant antigen. Extracellular EBs have limited metabolic and biosynthetic activity. However, EBs contain the machinery for central metabolism and glucose catabolism, and large stores of ATP, which prime it for a burst of metabolic activity upon entry into a host cell.
For many years, it was not clear if chlamydiae have peptidoglycan, which is a major structural component of the bacterial cell wall. It has now been shown that C. trachomatis RBs form a narrow peptidoglycan ring at the septum during chlamydial cell division. However, EBs lack peptidoglycan, and the classical mesh-like exoskeleton of peptidoglycan found in most other bacteria is not present in pathogenic Chlamydia spp.
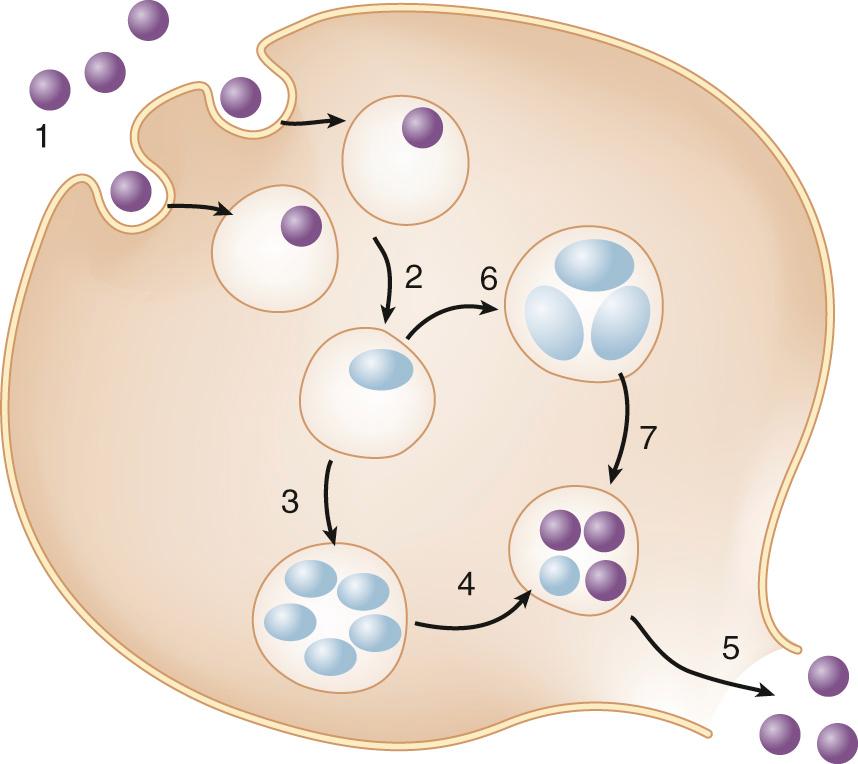
All Chlamydia spp. share an unusual biphasic developmental cycle in which there is conversion between RBs and EBs inside an infected host cell ( Fig. 180.2 ). An EB binds and enters a susceptible epithelial cell, within a membrane-bound vacuole called the chlamydial inclusion, and converts into an RB within 2 to 8 hours. RBs divide repeatedly by binary fission and then begin converting into EBs at about 24 hours after entry. The delayed and asynchronous timing of RB-to-EB conversion has been proposed to be controlled by RB size, which progressively decreases by sixfold as the RB population in an infected cell expands via successive rounds of replication. By 48 hours, several hundred to 1000 EBs are released by lysis of the host cell, or by extrusion of the inclusion from an intact host cell.
EB attachment to an epithelial host cell occurs through a two-step process. The first step is a low-affinity interaction between an EB surface protein OmcB, and heparan sulfate proteoglycan associated with the host cell surface. The second step is an irreversible, high-affinity interaction between chlamydial ligands, including MOMP and the Pmp family of polymorphic membrane proteins, and specific host receptors. C. trachomatis has been reported to bind a number of host receptors, which may contribute to strain-specific differences in tissue tropism, but could also represent a strategy to increase the chance of binding to a host cell.
This bacterium plays an active role in promoting its own entry into a host cell, which allows it to infect nonphagocytic cells. Upon contact, the EB induces actin cytoskeletal rearrangements in the host cell by secreting preformed protein effectors via a polarized, type III secretion (T3S) mechanism. For example, a chlamydial effector called Tarp (translocated actin-recruiting phosphoprotein) nucleates actin polymerization directly, while also activating the host actin-nucleating machinery, and another effector (CT694) binds the actin-binding protein AHNAK. In addition, binding of the EB to receptor tyrosine kinases, such as epidermal growth factor receptor, fibroblast growth factor receptor, and platelet-derived growth factor receptor, activates the MEK-ERK and phosphoinositide 3-kinase signaling pathways, promoting chlamydial entry and host cell survival.
The inclusion provides a protected intracellular niche within which chlamydiae grow and replicate. Maintenance of this intracellular compartment depends on inhibition of phagolysosomal fusion and selective recruitment of vesicles carrying nutrients such as lipids and iron. Chlamydia inserts inclusion membrane proteins into the inclusion membrane, where they interact with host regulators of vesicular trafficking and fusion, including Rab guanosine triphosphatases and SNARE proteins. There may initially be multiple small inclusions in a host cell, from infection by more than one EB, but they coalesce into a single inclusion through fusion mediated by an inclusion membrane protein called IncA (see Fig. 180.2 ).
The inclusion has interactions with a number of organelles in the host cell that help promote the intracellular infection. Early in the infection, the inclusion migrates along microtubules from the cell periphery to the microtubule organizing center in the perinuclear region of the host cell. Chlamydiae acquire host lipids from the Golgi apparatus via exocytic vesicles, from the endoplasmic reticulum by recruiting the host lipid biosynthetic machinery to the inclusion, and via lipid-rich organelles called lipid droplets. These host lipids are necessary for the enlarging inclusion membrane and may contribute to the chlamydial cell membrane. The inclusion also recruits recycling endosomes and mitochondria to facilitate acquisition of iron and ATP, respectively.
Chlamydial genes are expressed as three major temporal groups that correspond to three main stages of the developmental cycle. Early genes are first transcribed during EB-to-RB conversion and have a likely role in establishing the chlamydial inclusion. The largest temporal group consists of midcycle genes, which include most housekeeping genes as well as virulence genes that are expressed during RB replication. Data indicate that midcycle genes and a subset of early genes are activated by higher levels of negative DNA supercoiling in midcycle. This novel use of its DNA topology to regulate developmental gene expression makes Chlamydia dependent on enzymes such as DNA gyrase that modulate supercoiling levels. It also provides a Chlamydia -specific target for the fluoroquinolones, including ofloxacin and levofloxacin, which are DNA gyrase inhibitors used to treat chlamydial infections. Late genes, which are the third and last temporal group, have roles in RB-to-EB conversion and EB function. They are negatively regulated by the transcription factor EUO, providing a mechanism to prevent their premature expression.
Chlamydia actively promotes the survival of its host cell so that it can complete its developmental cycle. At the time of entry, binding of the EB to receptor tyrosine kinases and ephrin receptors activates prosurvival pathways involving MEK-ERK and phosphoinositide 3-kinase. In addition, chlamydiae block intrinsic apoptosis by downregulating and sequestering proapoptotic factors, and by upregulating and stabilizing antiapoptotic factors. Chlamydia also inhibits extrinsic apoptosis by blocking caspase activation.
Chlamydiae grown in cell culture can enter an altered growth state of viable but noncultivable bacteria that is called in vitro persistence. Infectious progeny cannot be recovered because there is no RB-to-EB conversion. Persistence can be induced by exposing Chlamydia -infected cells to interferon (IFN)-γ, which induces host indoleamine 2,3,-dioxygenase to deplete tryptophan. It can also be induced by penicillin treatment, iron or nutrient starvation, herpesvirus coinfection, or host cell differentiation. In vitro persistence is reversible because removal of the inducing agent causes reactivation back to the normal developmental cycle and production of EBs. The relevance of in vitro persistence to clinical infection and disease is not clear.
Chlamydia causes centrosomal and DNA defects that may explain its association with cervical cancer. Chlamydia alters centrosomal function by inducing the formation of extra centrosomes, centrosome clustering, and mitotic spindle defects, each of which may lead to chromosome segregation errors. Chlamydia causes DNA damage in an infected cell by inducing DNA double-strand breaks and impairing the DNA damage response. In addition, Chlamydia can transform infected cells in cell culture and cause cervical dysplasia in mice.
C. trachomatis has been historically classified into 15 serovars on the basis of antigenic cross-reactivity in the microimmunofluorescence test of Wang and Grayston. The serovars can be grouped into two biovariants that are associated with different clinical presentations. The trachoma biovar consists of serovars A, B, Ba, and C, which cause trachoma, and serovars D through K, which cause urogenital disease and inclusion conjunctivitis. The lymphogranuloma venereum (LGV) biovar includes serovars L1, L2, and L3, which cause LGV and proctocolitis in men who have sex with men (MSM). Occasionally, serovars B and Ba have been isolated from the genital tract, but A and C have not. Alternatively, serovars can be divided into two subgroups on the basis of cross-reactivity patterns: the B complex consists of serovars B, Ba, D, E, L1, and L2, and the C complex is composed of serovars A, C, H, I, J, K, and L3. Serovars F and G bridge the two complexes, although they are more closely related to the B complex. Additional serovars have been proposed (Da, Ia, and L2a), as well as serovars such as L2b that have caused a recent outbreak of LGV proctitis. When full-length sequencing of ompA is used for strain identification, additional stable variants have been described.
Species-, subspecies-, and serovar-specific antibodies recognize epitopes located in four variable sequence regions of MOMP. Serovar-specific epitopes are mostly found in variable sequence regions 1 and 2, whereas the more broadly shared epitopes cluster in variable region 4. However, some serovars contain more than one serovar-specific epitope, and serovar-specific epitopes are found in variable region 4 as well. The immunodominant genus-reactive epitope is in lipopolysaccharide (LPS), which is closely related to LPS of other gram-negative bacteria, particularly the deep rough (Re) mutants of enterobacteria. Chlamydial LPS is technically a lipooligosaccharide composed of a 3-deoxy- d -manno-octulosonic acid trisaccharide in a 2,4 linkage and a Chlamydia -specific 2,8 linkage. Additional genus-reactive epitopes have been identified in MOMP, the 60-kDa cysteine-rich protein (OmcB), and the 60-kDa heat shock protein..
With the advent of affordable whole-genome sequencing, chlamydial strains are increasingly being typed by genetic differences on a genomic scale. Genomic comparison has confirmed that C. trachomatis can be divided into three lineages, with the urogenital and ocular clades more closely related to each other than to the LGV clade. There is strong conservation of gene content and order between C. trachomatis strains, with only 4860 single nucleotide polymorphisms, out of just over 1 million bases, between the trachoma and LGV biovars. This overall genetic similarity has led to a search for specific genes that contribute to differences in tropism and clinical presentation. A hot spot of genetic diversity called the plasticity zone is located at the replication terminus of the chlamydial genome, which is a site of large-scale rearrangements in many bacterial genomes. Between Chlamydia spp., there is considerable variation in the size of the plasticity zone, from 18 kb to 81 kb, and the presence or absence of genes encoding virulence factors, such as membrane attack complex/perforin, phospholipase D, cytotoxin, and tryptophan synthetase. Notably, only C. trachomatis serovars that cause urogenital disease, and not trachoma strains, encode a functional tryptophan synthase. Urogenital strains may have retained this enzyme to convert indole, produced by vaginal microbiota, into tryptophan as a defense mechanism to counter IFN-γ–mediated tryptophan depletion. Another difference is that the enzymatic portion of the cytotoxin gene is only present in urogenital, but not ocular or LGV, strains. A family of autotransporters, called the polymorphic membrane proteins, has a high concentration of single nucleotide polymorphisms, which appear to correlate with tissue tropism. Genetic differences have also been identified between urogenital and rectal clinical isolates of C. trachomatis . This identification of strain-specific differences will be made easier by new methods for whole-genome sequencing of chlamydial clinical isolates without cultivation.
Recombination between C. trachomatis strains is another source of genetic diversity. It occurs in nature, most commonly between strains that infect the same tissue site, providing evidence for mixed infections with more than one C. trachomatis strain. In addition recombination has been documented between urogenital and ocular strains, and even between trachoma and LGV biovars. As a dramatic example, a hypervirulent C. trachomatis LGV strain isolated from a patient with severe hemorrhagic proctitis was found to be a recombinant of an invasive L2 strain and a noninvasive urogenital D strain. Nevertheless, the high degree of genomic conservation among C. trachomatis strains suggests that most recombination events are not fixed as gene gain or loss. Recombination events have been fixed at a higher rate near the MOMP gene ( ompA ), most likely because of selective pressure from the host immune system. ompA has transferred between phylogenetically unrelated strains on multiple occasions, leading to serovar switching and chimerism. These findings explain why MOMP is not a good phylogenetic marker and why MOMP strain typing does not correlate well with clinical manifestations.
Experimental methods to genetically manipulate chlamydiae have recently become available. These methods include transformation of chlamydiae, generation of mutants by chemical mutagenesis, and, in a few instances, targeted gene disruption and replacement. Chlamydiae appear to be naturally competent because recombinant progeny can be readily obtained by coinfecting the host cell with two chlamydial strains. The phenotypes of mutants can be studied with linkage analysis, conditional mutants, and complementation. These approaches have been aided by the use of whole-genome sequencing to identify genetic alterations that can then be related to phenotypes in cell culture and infection models. These methodologic breakthroughs provide the potential for forward and reverse genetic approaches to study chlamydial gene function. However, their widespread use has been limited by the low efficiency of EB transformation and the time (in months) to produce and isolate mutants. The downsized C. trachomatis genome is expected to contain a high proportion of essential genes that cannot be deleted.
The target cells of the trachoma biovar of C. trachomatis are the squamocolumnar epithelial cells of the endocervix and upper genital tract in women, the epididymis and perhaps the prostate in men, and the conjunctiva, urethra, rectum, and to a lesser extent the pharynx in both women and men. In infants, the columnar epithelial cells of the respiratory tract are also commonly infected. C. trachomatis strains exhibit strong tissue tropism during natural infection, thereby producing very different clinical presentations; genital serovars preferentially infect genital tract epithelial cells, ocular serovars favor conjunctival epithelial cells, and LGV serovars infect macrophages and spread to lymph nodes.
Regardless of the site, the initial response to infection appears to be primarily a polymorphonuclear leukocyte response. Infected epithelial cells produce proinflammatory cytokines, such as the chemokine interleukin (IL)-8, that direct the initial innate and acquired responses to chlamydial infection and drive inflammatory responses that both produce disease and resolve the infection. LPS may be the predominant chlamydial antigen capable of inducing proinflammatory cytokines. In immortalized human cells, IL-8 induction depends on host cell signaling, in part via the MEK-ERK pathway. Independently, IL-8 is also induced via the pattern response receptor nucleotide-binding oligomerization domain 1, which is a peptidoglycan sensor. C. trachomatis pathogenesis is often studied with the related species Chlamydia muridarum, which offers a convenient mouse model of human genital infection. C. muridarum infection of mouse oviduct epithelial cell lines induces a variety of cytokines, including IL-1α, IL-6, tumor necrosis factor-α, and cytokines that augment IFN-γ production, including type I interferons IFN-α/β and IL-12p70. In this mouse model of chlamydial genital infection, Toll-like receptor 2 (TLR2) is the principal pattern response receptor responsible for induction of acute-phase mediators and chronic inflammatory pathology. Chlamydiae can also be detected by the intracellular nucleotide sensors cyclic GMP-AMP synthase (cGAS) and STING (stimulator of interferon genes), which induce expression of type I interferons. Mice deficient in receptors for type I interferons (α and β) have reduced duration and shedding of organisms during infection, less oviduct pathology, and enhanced CD4 recruitment to cervical tissue compared with wild-type mice, suggesting that induction of type I interferons exacerbates infection by inhibiting the specific CD4 response to chlamydiae.
The initial neutrophilic infiltration is followed by tissue infiltration with lymphocytes, macrophages, plasma cells, and eosinophils. In ocular and genital infections, plasma cells are generally present in large numbers, whereas in infant pneumonia, eosinophils and neutrophils predominate. In ocular and genital disease with the trachoma biovar, lymphoid follicles (aggregates of lymphocytes and macrophages in the submucosa) form as the acute inflammation begins to subside. There is thinning or loss of epithelium overlying the follicles, and they may become necrotic as the disease progresses. In the conjunctiva, these follicles are clinically apparent as raised avascular lesions. Epithelial proliferation leads to formation of papillae and papillary hypertrophy. As the infection then begins to resolve, fibrosis and scarring occur.
Initial infection of the eye in humans and of the eye and genital tract in monkeys resolve with little or no residual tissue damage. However, in the human eye and the monkey genital tract, as few as two to three repeated infections produce an accelerated and more intense inflammatory response with scarring and tissue damage; multiple rechallenges amplify the effect in the primate eye. The potentially important role of reinfection in chlamydial disease was first recognized during human trachoma vaccine trials in which volunteers were immunized and then subsequently challenged with live organisms. In these and other studies in humans and monkeys, limited serovar-specific protection against infection could be induced. However, when infection did occur after immunization in humans, it was more severe than in unvaccinated people and the increased severity was not serovar specific.
Studies have raised questions about the relative contributions of conjunctival infection by C. trachomatis and other bacterial pathogens to trachoma development. It was previously thought that a chlamydial component, the chlamydial 60-kDa heat shock protein (cHSP60), was driving disease expression by hypersensitivity mechanisms. Although serologic data show an association between antibody response to cHSP60 and risk of PID and scarring trachoma, these responses are likely due to complicated or severe disease and are not reflective of a causal link. In early follicular and intense trachoma, the inflammatory response and early disease expression are driven and sustained by Chlamydia -infected nonimmune epithelial cells. However, trachomatous scarring in adults shows a greater association with other bacteria than with C. trachomatis infection. It has therefore been proposed that C. trachomatis plays an initiating role but that chronic conjunctival inflammation and tissue damage may be due to the host immune response to other bacterial pathogens after the C. trachomatis infection.
Chlamydial infection induces production of IFN-γ, which inhibits chlamydial replication and in animal models shortens the duration of infection. In cell culture, it induces a dose-related persistent infection as described in “ Chlamydial Biology ” earlier. However, subsequent removal of IFN-γ allows the organisms to be rescued and to resume replication and conversion into infectious EBs. Long-lasting infections are known to occur relatively frequently in the absence of treatment in women and in men. The in vivo bacterial and inclusion morphology of these long-lasting infections in people is unknown. However, it is possible that cyclic changes in inhibitory cytokines, chlamydial replication, and antigen production and the resulting continued inflammatory signaling from nonimmune epithelial cells could explain the chronic inflammation and scarring often associated with chlamydial infections.
The LGV biovar of C. trachomatis is distinctive in being able to cause a systemic infection. This organism gains entrance through breaks in the skin or infects epithelial cells of the mucous membranes of the genital tract or rectum. It has tropism for the lymphatic system and is carried by lymphatic drainage to the regional lymph nodes, where it multiplies inside mononuclear phagocytes. Bacteremic spread may also occur, and the central nervous system may be infected. The characteristic histopathology is that of granuloma formation with development of small abscesses that may become necrotic or coalesce into suppurative foci.
Almost all strains of C. trachomatis carry a conserved 7.5-kb plasmid that has a role in pathogenesis. It encodes eight proteins, including Pgp3, which is an effector that is secreted into the host cytosol, and an immunodominant antigen that is being used as a serologic marker. Another plasmid gene, pgp4, regulates transcription of chromosomal genes involved in virulence and glycogen synthesis. Rare C. trachomatis clinical isolates lacking this plasmid have been described. Studies with chlamydial strains experimentally cured of the plasmid show that the plasmid modulates chlamydial exit from host cells, infectivity, and virulence. For example, a plasmid-cured C. muridarum strain was able to infect the lower genital tract but did not produce upper tract disease in the mouse oviduct because it failed to activate TLR2-dependent cytokine production. These results suggest that the plasmid controls expression of the chlamydial TLR2 ligand, which has not yet been identified. Plasmid exchange between different C. trachomatis strains only occurs infrequently.
There is evidence that chlamydial infections may involve a mixed population of genetic variants. These variants may differ in pathogenicity, such as their ability to cause lower versus upper genital tract disease. Experimental work with animal models indicates that these variants can compete or cooperate, and that mixed infections may differ from monoinfection with a single variant.
Natural infection with C. trachomatis appears to confer little protection against reinfection, and this limited protection is short-lived. Multiple or long-lasting infections are an essential factor in the pathogenesis of trachoma. Rates of repeated infection are also high in young, sexually active individuals with genital tract infections: 29% of men and women attending an STI clinic during a  -year period, as well as 38.4% of adolescent women observed prospectively for up to 2 years, had repeated infections. However, other data suggest that genital tract infections confer at least partial immunity against reinfection. In women with endocervical infection, the presence of secretory immunoglobulin (Ig) A correlated inversely with the numbers of organisms shed. Men experiencing their first episode of nongonococcal urethritis (NGU) are more likely to have C. trachomatis recovered from their urethra than men with a prior history of NGU. A study of commercial sex workers demonstrated reduced chlamydial detection with increasing duration of sex work. In another study of female sex workers in Nairobi, protective immunity against incident infection was observed and correlated with cHSP60-specific IFN-γ production in peripheral blood mononuclear cells. In the trachoma vaccine trials mentioned previously, partial serovar-specific immunity could be elicited, but protection lasted for only 1 or 2 years. Repeated genital infections in adolescent women are common after effective treatment, and it has even been suggested that treatment may inhibit the development of protective immunity. In support of this intriguing hypothesis, women who spontaneously resolved an uncomplicated chlamydial infection before they could be treated were less likely to have reinfection at 1 to 12 months than women whose infections were ended by treatment.
-year period, as well as 38.4% of adolescent women observed prospectively for up to 2 years, had repeated infections. However, other data suggest that genital tract infections confer at least partial immunity against reinfection. In women with endocervical infection, the presence of secretory immunoglobulin (Ig) A correlated inversely with the numbers of organisms shed. Men experiencing their first episode of nongonococcal urethritis (NGU) are more likely to have C. trachomatis recovered from their urethra than men with a prior history of NGU. A study of commercial sex workers demonstrated reduced chlamydial detection with increasing duration of sex work. In another study of female sex workers in Nairobi, protective immunity against incident infection was observed and correlated with cHSP60-specific IFN-γ production in peripheral blood mononuclear cells. In the trachoma vaccine trials mentioned previously, partial serovar-specific immunity could be elicited, but protection lasted for only 1 or 2 years. Repeated genital infections in adolescent women are common after effective treatment, and it has even been suggested that treatment may inhibit the development of protective immunity. In support of this intriguing hypothesis, women who spontaneously resolved an uncomplicated chlamydial infection before they could be treated were less likely to have reinfection at 1 to 12 months than women whose infections were ended by treatment.
In mouse models of C. muridarum infection, CD4 lymphocytes of the Th1 type that traffic to the genital mucosa are crucial for restriction of intracellular growth and resolution of infection. Antibodies directed at epitopes on MOMP are neutralizing in cell culture and may play a role in reducing acquisition of infection. In the mouse genital infection model, antibodies against MOMP or LPS partially protect against reinfections but not primary infections, indicating that the protective effect is dependent on CD4-mediated adaptive immunity acquired at the initial infection. Antibodies may also influence the severity of upper genital tract pathology in the mouse. IgA antibodies are not absolutely required for protective immunity in this model. Both antibody and cell-mediated mechanisms are important in protective immunity in the guinea pig model. It is possible that antigen presentation in natural mucosal infection may be relatively ineffective in producing strong protective immunity, because dendritic cells pulsed in vitro with inactivated chlamydiae are capable of conferring protective immunity in the mouse model. The current consensus is that CD4 + T cells and B cells are most critical in mediating recall immunity to C. trachomatis infection and CD8 + T cells are less important. The latter may exert antichlamydial activity by production of IFN-γ rather than by cytotoxic activity. In C. muridarum genital tract infections in the mouse, two independent subsets of Chlamydia -specific CD4 T cells exhibit different effector functions, one dependent on inducible nitric oxide synthase (iNOS) and the other requiring expression of Plac8. If Plac8 -deficient mice are treated with an iNOS inhibitor, the mice are unable to resolve genital tract infections.
The natural history of human infection is not well defined, but we know that many infections resolve without treatment, while many are long-lasting. In one of the few studies of its kind, Molano and colleagues found that 46% of untreated women had persistent infection with identical strains at 1 year. These infections were likely to represent long-lasting infection rather than reinfection because most of these women were older than 30 years of age, and 83% reported a single lifetime sexual partner. It is not clear how C. trachomatis is able to avoid immune clearance for prolonged intervals or why an effective response is so slow to develop. One hypothesis is that C. trachomatis may utilize nutrients produced by the genitourinary microbiota to evade inhibitory effects of IFN-γ, which restricts chlamydial replication by inducing tryptophan starvation. C. trachomatis is auxotrophic for tryptophan, but urogenital serovars can use indole to synthesize tryptophan. The microbiomes of the vagina in women and the urethra in men sometimes contain Prevotella species and other bacteria that produce indole. In vitro studies indicate that vaginal secretions, and bacterial supernatants containing indole, mitigate the inhibitory effect of IFN-γ on chlamydial growth. Additional chlamydial genes not involved in the tryptophan biosynthetic pathway appear to contribute to IFN-γ resistance.
Vaccine development efforts over the past 20 years have been directed at defining relevant epitopes for use as components in a synthetic or genetically engineered vaccine. This subunit approach is important because of the combination of protective and deleterious effects produced by infection or vaccination with the whole organism. Studies of DNA vaccines utilizing the ompA gene of C. muridarum showed reduced organism burden and mortality in a mouse pneumonia model, but similar studies have not demonstrated an influence on the course of experimental genital infection in mice. Vaccines composed of native conformations of trimeric MOMP of C. muridarum compounded with human vaccine adjuvants have been shown to induce partial protection in genital tract challenge in mice. Chlamydial protease–like activity factor (CPAF) is a possible vaccine candidate because it is an immunodominant antigen, and mice immunized with recombinant CPAF, together with IL-12 or CpG, are protected against genital challenge. Promising recent data have established that strains of C. trachomatis and C. muridarum lacking the 7.5-kb plasmid are attenuated for pathology in the primate eye and in the mouse oviduct even in the absence of sterilizing immunity. Such plasmidless strains hold promise as live-attenuated vaccines against both trachoma and complications of genital infection, including PID.
Efforts are also underway to improve vaccine response by developing mucosal vaccines. For example, a vaccine composed of ultraviolet light–inactivated C. trachomatis conjugated to synthetic adjuvant nanoparticles induced a robust systemic memory T-cell response and long-lived protection from genital infection in mice immunized via the intrauterine or intranasal route.
Among C. trachomatis infections, only trachoma can be diagnosed on clinical grounds alone, given the proper epidemiologic setting in a high-prevalence area. Other chlamydial infections are often associated with specific clinical syndromes but require laboratory confirmation for definitive diagnosis. Detection of C. trachomatis indicates the presence of an infection because the organism is intrinsically invasive as an intracellular pathogen and there is no extracellular colonization state. The mainstay of modern diagnostic testing of C. trachomatis is the nucleic acid amplification test (NAAT). Other laboratory methods for detection of chlamydial antigens and nucleic acids have been largely superseded as routine diagnostic tests. Microbiologic identification by isolation of C. trachomatis in cell culture remains important but in a specialized role.
NAATs are the preferred diagnostic and screening test for C. trachomatis genital infection in the United States because they are sensitive and can be used for noninvasive testing without the need for a pelvic examination or a urethral swab. The optimal specimens are a vaginal swab in women, which can be obtained by either a clinician or the patient, and first-catch urine in men. Some NAATs have been cleared by the US Food and Drug Administration (FDA) for use on liquid-based cytology specimens collected for Papanicolaou smears. NAATs target and amplify conserved nucleic acid sequences that are present in almost all clinical strains of C. trachomatis, including the urogenital, LGV, and ocular serovars. However, NAATs are only approved by the FDA for diagnosis of C. trachomatis urogenital infections and have not been cleared to detect the organism in extragenital sites, such as the rectum, oropharynx, and eye. Some laboratories have performed in-house validations for NAAT testing of extragenital specimens in order to conform to Clinical Laboratory Improvement Amendments (CLIA) regulations. Some NAATs can also detect Neisseria gonorrhoeae, providing a convenient means to test for chlamydial infection and gonorrhea in the same clinical specimen.
NAATs are considerably more sensitive and nearly as specific as culture, which was the historical gold standard for identifying C. trachomatis. The high sensitivity is due to the amplification step, which allows detection of chlamydial nucleic acids from a single infected host cell. NAATs are 15% to 20% more sensitive than culture performed in an experienced laboratory and may be up to 40% to 50% more sensitive than culture or enzyme immunoassay tests in other settings ( Table 180.1 ). NAATs can be used to detect C. trachomatis in traditional urogenital specimens, including endocervical swabs in women and urethral swabs in men, which require an invasive procedure for collection. However, a major advantage is that NAATs have a comparable high sensitivity for noninvasive specimens, such as a vaginal swab or first-catch urine. Thus noninvasive testing of chlamydial infections is now possible, allowing innovations such as self-collected vaginal swabs that make widespread screening and prevention programs feasible. NAATs have also been shown to be sensitive and specific for detection of C. trachomatis infection of the eyes in patients with trachoma and in the rectum and oropharynx in MSM.
| TEST | SENSITIVITY RELATIVE TO EXPANDED GOLD STANDARD (%) a | SPECIFICITY (%) | DETECTABILITY LEVEL (ELEMENTARY BODIES) |
|---|---|---|---|
| Enzyme immunoassay | 40–60 | 99.5 b | 1000–10,000 |
| Nonamplified genetic probe | 40–65 | 99.0 | 1000–10,000 |
| Direct fluorescent antibody | 50–80 | 99.8 | 50–1000 |
| Cell culture | 50–90 | 99.9 | 10–100 |
| Nucleic acid amplification tests | Cervix: 81–100 Vagina: 91–98 Male urine: 90–96 |
99.7 | 1–10 |
a Defined using a combination of different test methodologies, including culture, direct fluorescent antibody, and polymerase chain reaction (PCR) or ligase chain reaction (LCR) directed against a target sequence distinct from that used in the routine PCR or LCR assays.
Although NAATs are sensitive, a number of disadvantages have been discussed. They are expensive and thus may not be affordable by health departments for comprehensive screening. In addition, they may be technically demanding for some routine laboratory settings, resulting in reproducibility issues and false-positive and false-negative rates that are higher than the reported rates. Another disadvantage is that the current FDA-approved NAATs do not distinguish between LGV and non-LGV strains, which is important because the duration of treatment is longer for LGV infections. Nonapproved NAATs that can distinguish between LGV and non-LGV strains have been described but are not widely available. Another issue is that NAATs detect chlamydial DNA or RNA rather than live organisms, and positive NAATs are not uncommon 3 weeks after completion of antibiotic therapy. Thus the NAAT should not be used as a test-of-cure assay, except in pregnant women in whom it is justified to document cure at 3 to 4 weeks after completion of therapy in efforts to prevent infection in the infant.
Commercially available NAATs differ in the method used to amplify chlamydial nucleic acids from a clinical sample. Although the tests also amplify different DNA or RNA sequences, these targets are not intrinsic to the amplification method and can be altered if necessary. Transcription-mediated amplification is sensitive because it amplifies C. trachomatis –specific 23S ribosomal RNA, which is present in high copy number. The DNA strand displacement assay amplifies sequences on the chlamydial plasmid. It has excellent sensitivity because the chlamydial plasmid is present in all clinical isolates, with rare exceptions, and there are approximately four to eight copies of the plasmid in each organism. Polymerase chain reaction (PCR) is another commonly used amplification method that amplifies different sequences on the chlamydial plasmid or chromosome. Ligase chain reaction was a chlamydial NAAT method that was used for many of the initial validation studies, but it is no longer available commercially.
In 2006, a new variant strain of C. trachomatis that was not detectable with a PCR-based NAAT was discovered in Sweden. This strain has a 377-bp deletion in the region of the chlamydial plasmid that was amplified by two different PCR-based NAATs (AMPLICOR [CT/NG] Test [Roche Diagnostics, Indianapolis, IN] and m 2000 RealTi m e [Abbott Laboratories, Abbott Park, IL]), resulting in false-negative tests. Its discovery was prompted by an unexpected decrease in chlamydial prevalence in Sweden due to underreporting from counties that used these two NAATs compared with other counties that used a strand displacement NAAT assay that targeted a different region on the plasmid. Once recognized, the strain was found to be highly prevalent in counties where it was not being detected, demonstrating that missed diagnosis and treatment of infected individuals can confer a diagnostic selective advantage that promotes the spread of a particular strain. It also provides an important lesson about the limitation of detecting a pathogen by only targeting a single small region of DNA or RNA. In addition, the plasmid may not be an ideal NAAT target because other deletion and recombination events within the plasmid have been reported. Improved versions of the Roche and Abbott PCR-based NAATs targeting two regions of the C. trachomatis genome are now available. However, there are still other FDA-approved Chlamydia NAATs that only target a single sequence on the C. trachomatis plasmid or chromosome. The Swedish new variant strain of C. trachomatis has been largely restricted to the Nordic countries, where its prevalence has decreased with the use of NAATs that detect both wild-type and variant strains. So far, this clonal strain has not been detected in the United States.
Before the introduction of NAATs, a number of molecular tests were developed to detect chlamydial antigens or nucleic acids in clinical samples without the need to culture the organism. The three main nonculture tests are direct fluorescent antibody (DFA) staining and enzyme-linked immunoassay (EIA), which both detect chlamydial antigen, and nucleic acid hybridization, which uses a DNA probe to detect chlamydial ribosomal RNA without an amplification step. These tests have sensitivities of 60% to 80% and specificities of 99% at best, which provide acceptable positive and negative predictive values, except in low-prevalence populations (<5% infected) where the false-positive rate is high. However, this level of sensitivity can only be achieved with invasive specimens, such as a cervical swab in women or a urethral swab in men. In general, the sensitivity is higher in patients who are symptomatic and shedding large numbers of chlamydiae compared with asymptomatic patients shedding fewer organisms. The C. trachomatis nonculture tests have been largely replaced because of the superior performance of NAATs even when performed on noninvasive specimens.
Culture has been supplanted by NAATs as the routine method for detecting C. trachomatis in clinical samples because it has lower sensitivity and is more difficult to perform. Even under optimal conditions, the sensitivity of culture is estimated at between 70% and 80% on the basis of the comparison to the more sensitive NAAT and may be as low as 40% to 50% outside experienced laboratories. Many of the relative disadvantages of culture stem from the need to grow the organism in cell culture, which requires expertise, proper handling and storage of specimens, and a long turnaround time. C. trachomatis is most commonly cultivated in HeLa or McCoy cells, but the bacterium grows well in a variety of cell lines. The most sensitive and specific way to identify intracytoplasmic inclusions is by direct immunofluorescent staining with antichlamydial monoclonal antibodies, which is usually performed after 36 to 72 hours of incubation. The chlamydial inclusion can also be detected by staining with Giemsa stain. C. trachomatis differs from other Chlamydia spp. in containing glycogen, which can be stained with iodine.
The isolation rate of cell culture depends on the number of viable organisms in the clinical specimen. For example, the highest concentrations of chlamydiae, and thus the highest isolation rates, are with ocular infections such as active trachoma and neonatal or adult inclusion conjunctivitis. In urogenital infections, many more organisms are recovered from the endocervix compared with the male urethra, and even fewer from the female urethra. Infections associated with symptoms and signs, as well as infections in younger individuals, generally have higher isolation rates.
Chlamydial culture is still used as a specialized diagnostic method and for research purposes. One important advantage is that it allows recovery of the clinical isolate, which molecular methods such as NAATs do not. Culture can be important for public health issues, including antibiotic susceptibility testing and identification of outbreak or mutant strains. The high specificity of culture, which approaches 100%, makes it the recommended test for medicolegal evaluation of child abuse, although the NAAT can be used for C. trachomatis detection from the urine or vagina in girls. In sexual assault in adults and adolescents, NAATs are recommended because they are more sensitive and widely available.
Typical intracytoplasmic inclusions and free chlamydiae can be identified in Giemsa-stained cell scrapings from the eye. Stained conjunctival scrapings are positive in 90% of infants with neonatal conjunctivitis and 50% of adults with inclusion conjunctivitis. It is less sensitive for active trachoma, in which only 10% to 30% of conjunctival scrapings are positive. Cytology has also been used to evaluate endocervical scrapings, including those obtained for Papanicolaou smears, but the sensitivity and specificity are low.
There has been great interest in the development of rapid tests for C. trachomatis infection, to shorten the time between sample collection and test results. Currently, the best rapid test for C. trachomatis is the Xpert CT/NG (Cepheid, Sunnyvale, CA) rapid PCR test, which can produce results in approximately 90 minutes. This test is a rapid NAAT for C. trachomatis and N. gonorrhoeae, and it has performance in both low- and high-prevalence populations similar to existing NAATs, which typically have a processing time of 1 to 2 days. The CT/NG GeneXpert has been approved by the FDA as a “moderate complexity” test, which means that it can be performed in many more clinic and hospital laboratories than other NAATs, which are classified as “high complexity” tests. It has been approved for use on endocervical and vaginal swabs and for urine.
The ideal rapid Chlamydia test is a point-of-care (POC) test that is performed while the patient is being evaluated, producing immediate, yet sensitive and specific, results. POC Chlamydia tests have been developed that use a rapid immunoassay to detect chlamydial antigens in a patient specimen. These tests are similar in principle to a rapid group A streptococcus or pregnancy test and have the potential to be simple to perform and to interpret. However, current non-NAAT C. trachomatis POC tests have low sensitivities compared with NAATs, and reported performances have been highly variable from study to study. These Chlamydia POC tests are commercially available in some countries but have not been approved for use in the United States. A portable NAAT that can produce results in 1 hour has been developed and evaluated as a POC test in a hospital emergency department setting. It utilizes a microfluidic cartridge to capture and process chlamydial nucleic acids, and a mobile phone to operate the cartridge and to collect the optical signal with its built-in camera.
Serologic tests are not recommended for diagnosis of most common oculogenital C. trachomatis infections. Anti- Chlamydia IgM is infrequent in adults with genital tract infection, whereas anti- Chlamydia IgG is long lived and cannot distinguish past from current infection. Although a rise in convalescent titers can be used to diagnose an acute infection, it is not a practical option given the wide availability of Chlamydia NAATs. Serology can be used to support a diagnosis of LGV infection because commercial NAATs do not distinguish between LGV and non-LGV serovars of C. trachomatis, but this role is limited because reliable serologic testing for LGV is not widely available.
The complement fixation test has been used to diagnose LGV, but it is technically demanding and performed in few clinical laboratories. It measures complement-fixing antibodies against the genus-specific LPS antigen, which is present in C. trachomatis as well as Chlamydia pneumoniae and Chlamydia psittaci. Thus it lacks specificity because it can also be positive in patients with recently acquired C. pneumoniae infections and some patients with oculogenital infections, although titers tend to be higher with LGV because it is a more invasive disease. A confirmed result requires a fourfold or greater rise in titer between acute and convalescent sera, but patients with LGV often present 3 or 4 weeks into their illness when their antibody titers are stable. Complement fixation serology has no diagnostic utility for C. trachomatis urethritis, cervicitis, or conjunctivitis because most patients with these infections have low or nonexistent titers.
The microimmunofluorescence (MIF) test is the historical gold standard for detection of chlamydial antibodies because of its sensitivity, and species and serovar specificity. It is not widely used because it is primarily available in research laboratories, although less well-characterized immunofluorescence serologies are commercially available. In its most common format, elementary bodies from each of the 15 C. trachomatis serovars are used as antigens to detect antibodies against chlamydial cell wall components. This test reliably differentiates among C. trachomatis, C. pneumoniae, and C. psittaci, and occasionally, but not reliably, among C. trachomatis serovars. In an experienced laboratory it is more sensitive than the complement fixation test. Many adult patients with symptomatic eye or genital infection have a fourfold rise in antibody titers between acute and convalescent sera. Titers are especially high in women with PID or perihepatitis, in whom its diagnostic sensitivity may be highest. However, among C. trachomatis infections, the MIF test is most useful for diagnosing neonatal chlamydial pneumonia because almost all affected infants test positive, with an IgM titer of 1 : 32 or greater. Anti- Chlamydia IgM is also present in approximately 30% of infants with neonatal inclusion conjunctivitis.
The limited availability of the MIF test and the interlaboratory variability in its interpretation emphasize the need for more clinically practical serologic tests. As an example of an assay in development, an enzyme-linked immunosorbent assay test using whole EBs as antigens has been reported to be more sensitive than a commercially available MOMP peptide-based assay and able to distinguish between C. trachomatis and C. pneumoniae infection. This and other assays may prove useful in defining seroprevalence and duration of antibody positivity after infection in advance of future vaccine trials. In addition, reproducible assays will be necessary to document preexisting antibodies and the response after vaccination.
C. trachomatis infections can be divided into four clinical categories: (1) trachoma, (2) oculogenital diseases in adults, (3) perinatal infections, and (4) LGV.
Trachoma is a chronic keratoconjunctivitis caused by repeated infections with serovars A, B, Ba, and C of C. trachomatis. The disease has been recognized as a cause of blindness since antiquity, with therapy for trachoma and its complications described in China in the 27th century bc and in Egypt in the 19th century bc . The countries with the highest prevalence are in sub-Saharan Africa, but about half of all active trachoma cases are located in just five countries: Ethiopia, India, Nigeria, Sudan, and Guinea. In high-prevalence areas, active trachoma is present in more than 50% of children younger than 10 years of age. The economic burden from blindness and lost productivity has been estimated to be $5.3 billion annually.
Active C. trachomatis infection mostly occurs in children, but a small proportion of individuals go on to have complications of impaired vision and blindness in adulthood. In areas where trachoma is endemic, the first infection usually occurs in the first 2 years of life, and active infection and inflammation persist for several months. Although initial infections may resolve spontaneously, they are frequently followed by reinfection or by superimposed bacterial conjunctivitis. This active stage of trachoma manifests as a chronic follicular conjunctivitis with papillary hypertrophy and inflammatory infiltration. Individuals are frequently asymptomatic, but can have mild symptoms of chronic conjunctivitis with redness, discomfort, photophobia, and mucopurulent discharge. Many individuals will clear the infection and inflammation without sight-threatening consequences. However, in a significant minority there is scarring of the tarsal conjunctiva lining the eyelid. Over years, this distortion of the eyelid causes the eyelashes to turn in and abrade the cornea (trichiasis), resulting in ulceration, scarring, corneal opacification, and loss of vision as an adult, typically at 30 to 40 years of age. Trachoma is a clinical diagnosis, and laboratory detection of C. trachomatis is not used for diagnosis. The WHO simplified grading scheme for evaluating trachoma by direct examination of the conjunctiva is presented in Table 180.2 . The assessment can be easily performed in the field, and the only equipment necessary is a pair of binocular loupes, which are simple magnifying glasses.
| Trachomatous inflammation—follicular (TF): ≥5 follicles in the upper tarsal conjunctiva (follicles must be at least 0.5 mm in diameter). Trachomatous inflammation—intense (TI): Pronounced inflammatory thickening of the tarsal conjunctiva, which obscures half of the normal deep tarsal vessels. Trachomatous conjunctival scarring (TS): The presence of easily visible scars in the tarsal conjunctiva. Trachomatous trichiasis (TT): At least one eyelash rubs on the eyeball. Evidence of recent removal of in-turned lashes was also graded as trichiasis. Corneal opacity (CO): Easily visible corneal opacity present over the pupil, which was so dense that at least part of the pupil margin was blurred when seen through the opacity. |
In endemic areas, children with ocular infection are the primary reservoir. Transmission occurs by hand-to-eye contact between children and their caregivers, by contact with fomites contaminated with ocular or nasal secretions, and via the feet of flies. Flies feed on the exudate from children with active conjunctivitis and act as a mechanical vector. Hygienic factors important for control of disease include face washing, access to clean water, improvements in sanitation, and reduction of household fly density. Although global estimates of trachoma prevalence have limitations, a downward trend has been noted in both active trachoma and trachoma-related blindness since 1981.
Individuals can be successfully treated with systemic antibiotics, but in high-incidence areas they will be easily reinfected, and treatment of the entire community is necessary to have an impact. WHO recommends mass drug administration in communities in which more than 10% of children have follicular trachoma. More than 85 million people worldwide received single-dose azithromycin for trachoma in 2016. Trials of mass treatment with azithromycin at the village level indicate that both infection and clinical disease are markedly decreased at 6 and 12 months, but multiple rounds of treatment over 3 to 5 years are necessary in hyperendemic areas. Of the regimens recommended by WHO, single-dose oral azithromycin is the preferred treatment. Topical application of tetracycline ointment to the eyes is an alternative, but adherence is difficult because of the 6-week course. In addition, topical treatment does not clear the bacterium from extraocular sites, such as the nasopharynx and rectum, that are also infected in children with trachoma. Surgery for trichiasis is performed to correct in-turned eyelashes and has some value in preventing progression to corneal opacity and blindness, but recurrence of trichiasis after surgery is common, occurring in about 20% of persons at 1 year and in up to 60% at 3 years. An added benefit of trachoma treatment programs is an associated reduction in childhood mortality, diarrhea, and respiratory infections.
WHO has initiated a program to eliminate blinding trachoma by 2020. The objective is not necessarily to eliminate the trachoma serovars of C. trachomatis, but to eliminate or at least markedly reduce clinically active disease. The key elements of the S-A-F-E strategy are S urgery for deformed eyelids, A ntibiotics administered as periodic mass treatment of villages with single-dose azithromycin, F ace washing and hygiene, and E nvironmental improvements to control flies, using approaches such as building latrines outside villages. In some areas, trachoma appears to be disappearing coincident with economic gains and without the introduction of specific control programs, illustrating the strong historic link between poor socioeconomic conditions and endemic trachoma.
C. trachomatis serovars D through K, and occasionally B and Ba, produce a wide variety of oculogenital infections ( Fig. 180.3 ).

Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here