Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cerebrospinal fluid is an essential part of a neurologic examination, comprising special attention to cell counts, basic chemical analyses, and microbiological cultures with specific antibody titers.
Determining the etiologic cause of fluid accumulation in various body cavities (synovial fluid in joints, pleural fluid in the chest, pericardial fluid around the heart, and peritoneal fluid in the abdomen) is critical for proper treatment of these disorders.
Serous fluids can be distinguished as either transudates that arise due to an imbalance of hydrostatic pressures or exudates caused by an inflammatory process.
Appropriate laboratory examination of these fluids is critical for the diagnosis of numerous diseases (bacterial, viral, and fungal infections; distinction between various arthritides; primary [e.g., mesothelioma] and metastatic malignancies; among others).
Accurate test interpretation depends on appropriate specimen collection, physician/laboratory communication, analytically sound laboratory methods, and reliable reference values.
Alternative or nonstandard specimens can have an important role in diagnostic or forensic testing; however, performing these tests may require additional method validation to ensure their appropriateness and accuracy.
In adults, approximately 500 mL of cerebrospinal fluid (CSF) is produced each day (0.3–0.4 mL/min). The total adult volume varies from 90 to 150 mL, about 25 mL of which is in the ventricles and the remainder in the subarachnoid space. In neonates, the volume varies from 10 to 60 mL. Thus, the total CSF volume is replaced every 5 to 7 hours ( ). An estimated 70% of CSF is derived by ultrafiltration and secretion through the choroid plexuses. The ventricular ependymal lining and the cerebral subarachnoid space account for the remainder. CSF leaves the ventricular system through the medial and lateral foramina, flowing over the brain and spinal cord surfaces within the subarachnoid space. CSF resorption occurs at the arachnoid villi, predominantly along the superior sagittal sinus.
The CSF has several major functions: (1) It provides physical support because a 1500-g brain weighs about 50 g when suspended in CSF; (2) it confers a protective effect against sudden changes in acute venous (respiratory and postural) and arterial blood pressure or impact pressure; (3) it provides an excretory waste function because the brain has no lymphatic system; (4) it is the pathway whereby hypothalamus releasing factors are transported to the cells of the median eminence; and (5) it maintains central nervous system (CNS) ionic homeostasis.
The blood-brain barrier (BBB) consists of two morphologically distinct components: a unique capillary endothelium held together by intercellular tight junctions; and the choroid plexus, where a single layer of specialized choroidal ependymal cells connected by tight junctions overlies fenestrated capillaries. The CSF ionic components (e.g., H + , K + , Ca ++ , Mg ++ , bicarbonate) are tightly regulated by specific transport systems, whereas glucose, urea, and creatinine diffuse freely but require 2 hours or longer to equilibrate. Proteins cross by passive diffusion at a rate dependent on the plasma-to-CSF concentration gradient and inversely proportional to their molecular weight and hydrodynamic volume ( ). Thus, the BBB maintains the relative homeostasis of the CNS environment during acute perturbations of plasma components.
CSF may be obtained by lumbar, cisternal, or lateral cervical puncture or through ventricular cannulas or shunts. Details of the performance of lumbar puncture are described elsewhere ( ; ).
A manometer should be attached before fluid removal to record the opening pressure. CSF pressure varies with postural changes, blood pressure, venous return, Valsalva maneuvers, and factors that alter cerebral blood flow. The normal opening adult pressure is 90 to 180 mm of water in the lateral decubitus position with the legs and neck in a neutral position. It may be slightly higher if the patient is sitting up and varies up to 10 mm with respiration. However, the pressure may be as high as 250 mm of water in obese patients. In infants and young children, the normal range is 10 to 100 mm of water, with the adult range attained by 6 to 8 years of age ( ). Opening pressures above 250 mm H 2 O are diagnostic of intracranial hypertension, which may be due to meningitis, intracranial hemorrhage, and tumors ( ). If the opening pressure is greater than 200 mm H 2 O in a relaxed patient, no more than 2.0 mL should be withdrawn.
Idiopathic intracranial hypertension is most commonly seen in obese women during their childbearing years. When an elevated opening pressure is noted, CSF must be removed slowly and the pressure carefully monitored. Additional CSF should not be removed if the pressure reaches 50% of the opening pressure ( ).
Elevated pressures may be present in patients who are tense or straining and in those with congestive heart failure, meningitis, superior vena cava syndrome, thrombosis of the venous sinuses, cerebral edema, mass lesions, hypoosmolality, or conditions inhibiting CSF absorption. Opening pressure elevation may be the only abnormality in cryptococcal meningitis and pseudotumor cerebri ( ). Decreased CSF pressure may be present in spinal-subarachnoid block, dehydration, circulatory collapse, and CSF leakage. A significant pressure drop after removal of 1 to 2 mL suggests herniation or spinal block above the puncture site, in which case no further fluid should be withdrawn.
Up to 20 mL of CSF may normally be removed. Prior to collecting the sample, the clinician should be aware of the quantity of CSF required for the requested tests to ensure that a sufficient sample is submitted. In addition, the clinician should always provide an appropriate clinical history to the laboratory. The sample site (e.g., lumbar, cisternal) should be noted because cytologic and chemical parameters vary at different sites. The necessity for a simultaneous serum glucose should also be considered. This is best obtained 2 to 4 hours before lumbar puncture because of the delay in serum-CSF equilibrium.
The CSF specimen is usually divided into three serially collected sterile tubes: tube 1 for chemistry and immunology studies; tube 2 for microbiological examination; and tube 3 for cell count and differential. An additional tube may be inserted in the No. 3 position for cytology if a malignancy is suspected. Under certain conditions, some variations are critical. For example, if tube 1 is hemorrhagic because of a traumatic puncture, it should not be used when protein studies are the most important aspect of the analysis (i.e., suspected multiple sclerosis). Indeed, tube 3 should be examined for the major purpose of CSF collection. Perhaps the only definite statement one can make is that tube 1 should never be used for microbiology because it may be contaminated with skin bacteria. If questions arise, communication between the laboratory and clinician before CSF analysis is critical.
Glass tubes should be avoided because cell adhesion to glass affects the cell count and differential. Specimens should be delivered to the laboratory and processed quickly to minimize cellular degradation, which begins within 1 hour of collection. Refrigeration is contraindicated for culture specimens because fastidious organisms (e.g., Haemophilus influenza , Neisseria meningitidis ) will not survive. It is also contraindicated for samples in which flow cytometry is likely to be needed for detection of leukemia or lymphoma cells, as refrigeration may affect expression and/or detection of certain surface antigens on these cells.
Indications for lumbar puncture can be divided into four major disease categories: meningeal infection, subarachnoid hemorrhage, primary or metastatic malignancy, and demyelinating diseases ( ). Identification of infectious meningitis, particularly bacterial, is the most important indication for CSF examination ( Box 30.1 ). Recommended laboratory tests are directed toward identification of these disorders ( Box 30.2 ). CSF examination for other diseases is generally less helpful but often provides supportive evidence of a clinical diagnosis or helps to rule out other diseases ( ). Limited routine studies followed by reflexive ordering of more focused tests (as needed) on the stored specimen have been advocated as a way of improving test efficiency ( ).
∗ Sensitivity is the ability of a test to detect disease when it is present; specificity is the ability of a test to exclude disease when it is not present (see Chapter 8 ).
Bacterial, tuberculous, viral, and fungal meningitis
Viral encephalitis
Subarachnoid hemorrhage
Multiple sclerosis
Central nervous system syphilis
Infectious polyneuritis
Paraspinal abscess
Meningeal malignancy
Intracranial hemorrhage
Subdural hematoma
Opening CSF pressure
Total cell count (WBC and RBC)
Differential cell count (stained smear)
Glucose (CSF/plasma ratio)
Total protein
Cultures (bacteria, fungi, viruses, Mycobacterium tuberculosis )
Gram stain, acid-fast stain
Fungal and bacterial antigens
Enzymes (LD, ADA, CK-BB)
Lactate
Multiplex polymerase chain reaction (PCR) assay (bacteria, fungi, TB, viruses)
Cytology
Isoelectric focusing and IgG immunoblotting
Proteins (C-reactive, 14-3-3, τ, β-amyloid, transferrin)
VDRL test for syphilis
Fibrin-derivative d -dimer
Tuberculostearic acid
Normal CSF is crystal clear and colorless and has a viscosity similar to that of water. Abnormal CSF may appear cloudy, frankly purulent, or pigment tinged. Turbidity or cloudiness begins to appear with leukocyte (white blood cell [WBC]) counts over 200 cells/μL or red blood cell (RBC) counts of 400/μL. However, grossly bloody fluids have RBC counts greater than 6000/μL. Microorganisms (bacteria, fungi, amebas), radiographic contrast material, aspirated epidural fat, and a protein level greater than 150 mg/dL (1.5 g/L) may also produce varying degrees of cloudiness. Experienced observers may be able to detect cell counts of less than 50 cells/μL with the unaided eye by observing for the Tyndall effect ( ). Here, direct sunlight directed on the tube at a 90-degree angle from the observer will impart a “sparkling” or “snowy” appearance as suspended particles scatter the light.
Clot formation may be present in patients with traumatic taps, complete spinal block (Froin syndrome), or suppurative or tuberculous meningitis. It is not usually seen in patients with subarachnoid hemorrhage. Fine surface pellicles may be observed after refrigeration for 12 to 24 hours. Clots may interfere with cell count accuracy by entrapping inflammatory cells and/or by interfering with automated instrument counting.
Viscous CSF may be encountered in patients with metastatic mucin-producing adenocarcinomas, cryptococcal meningitis due to capsular polysaccharide, or liquid nucleus pulposus resulting from needle injury to the annulus fibrosus.
Pink-red CSF usually indicates the presence of blood and is grossly bloody when the RBC count exceeds 6000/μL. It may originate from a subarachnoid hemorrhage, intracerebral hemorrhage, or cerebral infarct, or from a traumatic spinal tap.
Xanthochromia commonly refers to a pale pink to yellow color in the supernatant of centrifuged CSF, although other colors may be present ( Table 30.1 ). To detect xanthochromia, the CSF should be centrifuged and the supernatant fluid compared with a tube of distilled water. Xanthochromic CSF is pink, orange, or yellow owing to RBC lysis and hemoglobin breakdown. Pale pink to orange xanthochromia from released oxyhemoglobin is usually detected by lumbar puncture performed 2 to 4 hours after the onset of subarachnoid hemorrhage, although it may take as long as 12 hours. Peak intensity occurs in about 24 to 36 hours and then gradually disappears over the next 4 to 8 days. Yellow xanthochromia is derived from bilirubin. It develops about 12 hours after a subarachnoid bleed and peaks at 2 to 4 days but may persist for 2 to 4 weeks.
| CSF Supernatant Color | Associated Diseases/Disorders |
|---|---|
| Pink | RBC lysis/hemoglobin breakdown products |
| Yellow | RBC lysis/hemoglobin breakdown products Hyperbilirubinemia CSF protein >150 mg/dL (1.5 g/L) |
| Orange | RBC lysis/hemoglobin breakdown products Hypervitaminosis A (carotenoids) |
| Yellow-green | Hyperbilirubinemia (biliverdin) |
| Brown | Meningeal metastatic melanoma |
Visible CSF xanthochromia may also be due to the following: (1) oxyhemoglobin resulting from artifactual RBC lysis caused by detergent contamination of the needle or collecting tube, or a delay of longer than 1 hour without refrigeration before examination; (2) bilirubin (bilirachia) in jaundiced patients; (3) CSF protein levels over 150 mg/dL, which are also present in bloody traumatic taps (>100,000 RBCs/μL) or in pathologic states such as complete spinal block, polyneuritis, and meningitis; (4) disinfectant contamination; (5) carotenoids (orange) in people with dietary hypercarotenemia (i.e., hypervitaminosis A); (6) melanin (brownish) from meningeal metastatic melanoma; and (7) rifampin therapy (red-orange).
Although careful gross CSF inspection has good sensitivity ( ), spectrophotometry can also help to differentiate hemoglobin-derived substances from other xanthochromic pigments with different maximal absorption peaks ( ).
A traumatic tap occurs in about 20% of lumbar punctures. Therefore, distinction of a traumatic puncture from a pathologic hemorrhage is of vital importance. Although the presence of crenated RBCs is not useful, the following observations may be helpful in distinguishing the two forms of bleeding.
In a traumatic tap, the hemorrhagic fluid usually clears between the first and third collected tubes but remains relatively uniform in subarachnoid hemorrhage.
Xanthochromia, microscopic evidence of erythrophagocytosis, or hemosiderin-laden macrophages indicate a subarachnoid bleed in the absence of a prior traumatic tap. RBC lysis begins as early as 1 to 2 hours after a traumatic tap. Thus, rapid evaluation is necessary to avoid false-positive results.
A commercially available latex agglutination immunoassay test for cross-linked fibrin derivative d -dimer is specific for fibrin degradation and should theoretically be negative in traumatic taps ( ). However, it has been shown to not effectively distinguish subarachnoid hemorrhage from traumatic lumber puncture ( ).
Although the traditional manual method for cell counting in CSF samples, using undiluted CSF in a manual counting chamber, continues to be a useful approach, because of the low cell counts frequently encountered in CSF, the precision of manual counting in these samples is inherently limited ( , ). Improvements in the hardware and software in flow cytometers now allow reliable use of these instruments in performing automated total WBC counts and WBC differential counts ( ; ), and even in detecting bacteria ( ) in CSF samples. Although these instruments are increasingly used clinically to perform these counts on CSF samples, the low clinical decision levels for total WBC count in CSF and persistent technical limitations of flow cytometers at low WBC levels and with certain WBC types continue to be cause for concern ( ; ; ; ). When issues of turnaround time and cost are considered, however, automated total WBC and WBC differential counts in CSF samples is a reasonable alternative to manual counting ( ; ), with added manual examination of the sample to avoid missing pathologic cell types as a useful cross-check ( ). When considering use of automated cell counters with CSF samples, laboratories should carefully follow manufacturer and Clinical and Laboratory Standards Institute guidelines ( ) when implementing these automated methods.
The normal leukocyte cell count in adults is 0 to 5 cells/μL. It is higher in neonates, ranging from 0 to 30 cells/μL, with the upper limit of normal decreasing to adult values by adolescence. No RBCs should be present in normal CSF. If numerous (except with a traumatic tap), a pathologic process is probable (e.g., trauma, malignancy, infarct, hemorrhage). Although RBC counts have limited diagnostic value, they may give a useful approximation of the true CSF WBC count or total protein in the presence of a traumatic puncture by correcting for leukocytes or protein introduced by the traumatic puncture. To be valid, all measurements (WBC, RBC, protein) must be performed on the same tube. This procedure also assumes that the blood is derived exclusively from the traumatic tap. The corrected WBC count is as follows:
where
and
An analogous formula may be used to correct for added total protein (TP):
In the presence of a normal peripheral blood RBC count and serum protein, these corrections amount to about 1 WBC for every 700 RBCs and 8 mg/dL protein for every 10,000 RBCs/μL. This latter RBC correction factor is reasonably accurate as long as the peripheral WBC count is not extremely high or low.
An observed/expected (added) WBC count ratio greater than 10 has a sensitivity of 88% and a specificity of 90% for bacterial meningitis. When the predicted WBC count is below the observed count, the probability of bacterial meningitis appears to be low ( ; ).
Suggested differential count reference ranges are presented in Table 30.2 . A differential performed in a counting chamber is unsatisfactory because the low cell numbers give rise to poor precision and identifying the cell type beyond granulocytes and “mononuclears” is difficult in a wet preparation. Direct smears of the centrifuged CSF sediment are also subject to significant error from cellular distortion and fragmentation.
| Cell type | Adults, % | Neonates, % |
|---|---|---|
| Lymphocytes | 62 ± 34 | 20 ± 18 |
| Monocytes | 36 ± 20 | 72 ± 22 |
| Neutrophils | 2 ± 5 | 3 ± 5 |
| Histiocytes | Rare | 5 ± 4 |
| Ependymal cells | Rare | Rare |
| Eosinophils | Rare | Rare |
The cytocentrifuge method is rapid, requires minimal training, and allows Wright staining of air-dried cytospins. It is the recommended method for differential cell counts in all body fluids ( ). Cell yield and preservation are better than with simple centrifugation. From 30 to 50 cells can be concentrated from 0.5 mL of “normal” CSF. Variable artifactual distortions may be seen, but they are minimized when the specimen is fresh, albumin is added to the specimen (2 drops of 22% bovine serum albumin), and the cell concentration is adjusted to about 300 WBCs/L prior to centrifugation ( ). Manual differential cell counting on a cytocentrifuge preparation of CSF continues to be the most reliable method, even with low cell numbers. Although automated differential counts may be safely performed on CSF samples using flow cytometers ( ; ; ), confirmation of the automated differential count by manual examination of a cytocentrifuged smear is recommended with some instruments ( ; ) and is a requirement with specimens at risk for containing neoplastic cells. Effective detection of neoplastic cells, such as leukemic cells, using the cytocentrifuge method on CSF appears to be highly instrument dependent ( ).
Filtration and sedimentation methods are too cumbersome for routine use. However, filtration does allow concentration of large volumes of CSF for cytologic examination or culture while retaining the fluid filtrate for additional studies.
In adults, normal CSF contains small numbers of lymphocytes and monocytes in an approximate 70:30 ratio ( Fig. 30.1 ). A higher proportion of monocytes is present in young children, in whom up to 80% may be normal ( ). Erythrocytes due to minor traumatic bleeding are commonly seen, especially in infants. Small numbers of neutrophils (polymorphonuclear leukocytes [PMNs]) may also be seen in “normal” CSF specimens, most likely as a result of minor hemorrhage ( ) and improved cell concentration methods. No general consensus regarding an upper limit of normal for PMNs has been established. Many laboratories accept up to 7% neutrophils with a normal WBC count. Over 60% neutrophils has been reported in high-risk neonates without meningitis ( ). The number of PMNs may be decreased by as much as 68% within the first 2 hours after lumbar puncture owing to cell lysis ( ).
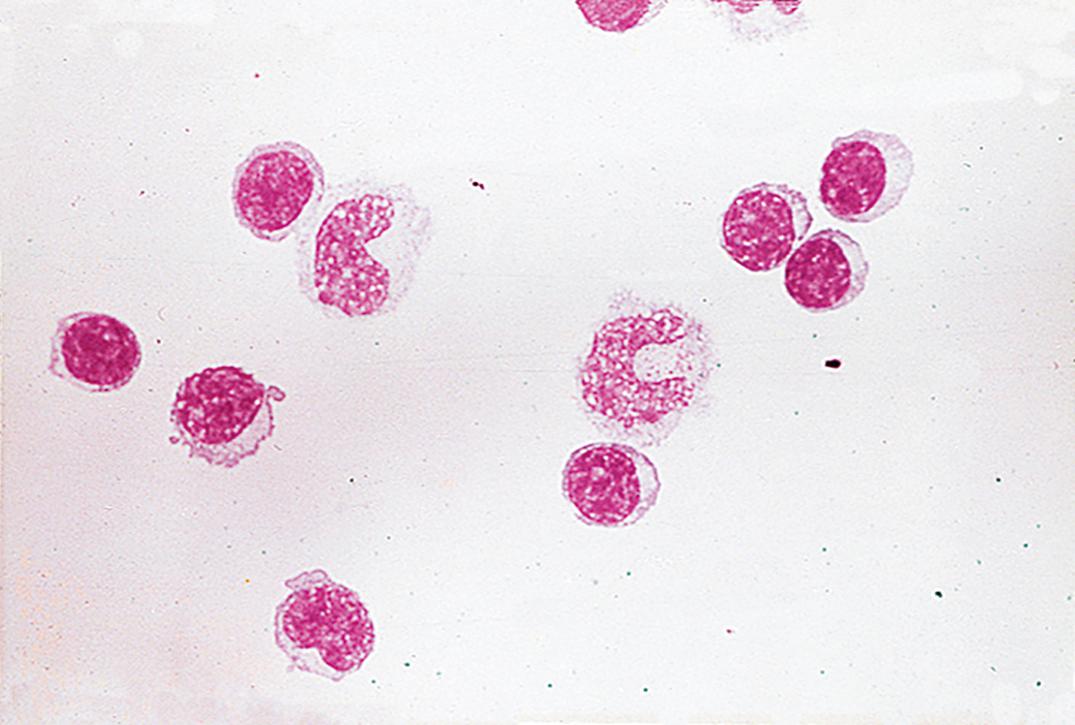
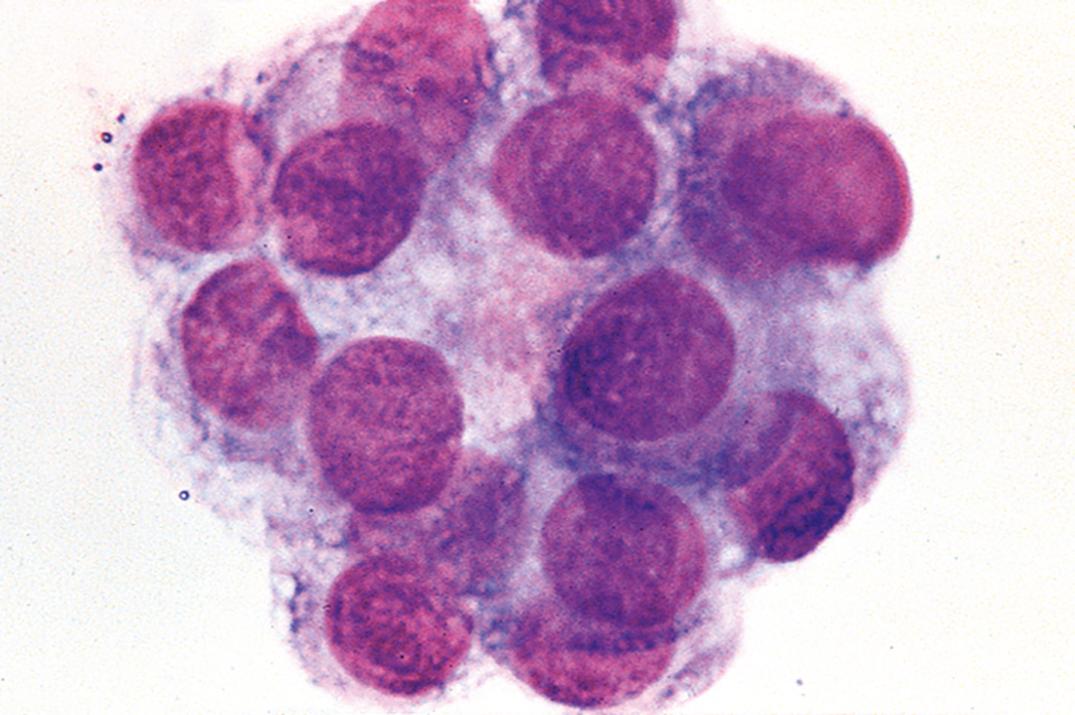
Traumatic puncture may result in the presence of bone marrow cells, cartilage cells, squamous cells, ganglion cells, and soft-tissue elements. In addition, ependymal and choroid plexus cells may rarely be seen ( Fig. 30.2 ). Moreover, blastlike primitive cell clusters, most likely of germinal matrix origin, are sometimes found in premature infants with intraventricular hemorrhage ( Fig. 30.3 ).
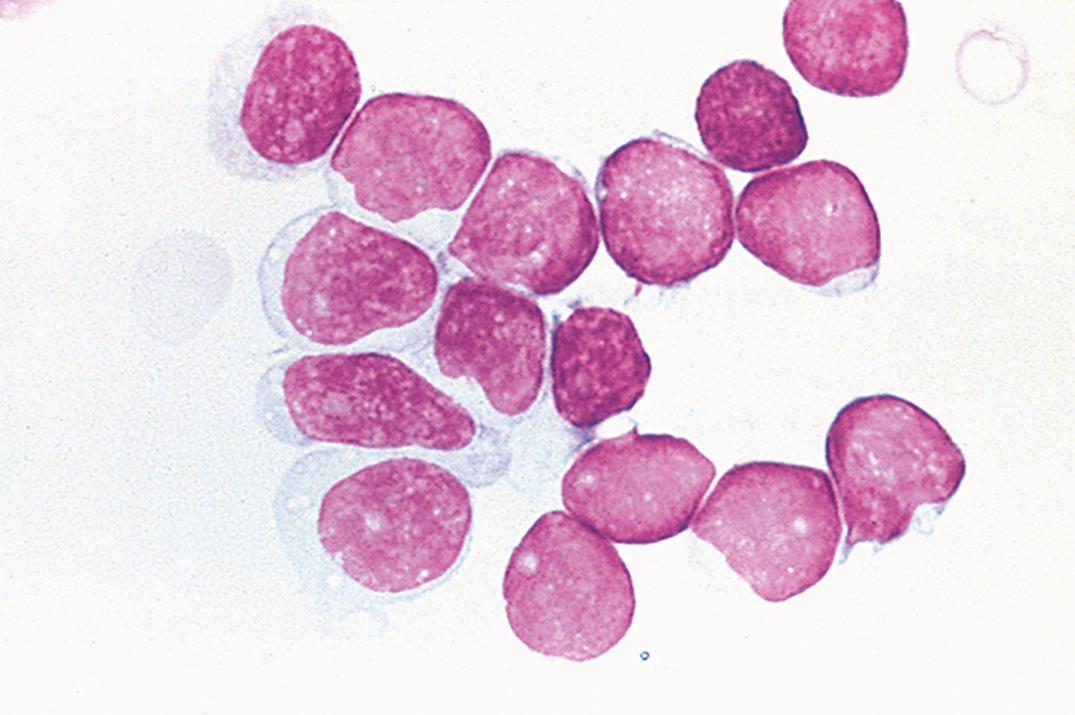
Increased CSF neutrophils occur in numerous conditions ( Box 30.3 ). In early bacterial meningitis, the proportion of PMNs usually exceeds 60%. However, in about one-quarter of cases of early viral meningitis, the proportion of PMNs also exceeds 60%. Viral-induced neutrophilia usually changes to a lymphocytic pleocytosis within 2 to 3 days. A total PMN count of over 1180 cells/μL (or more than 2000 WBCs/μL) has a 99% predictive value for bacterial meningitis ( ). Persistent neutrophilic meningitis (over 1 week) may be noninfectious or due to less common pathogens such as Nocardia , Actinomyces , Aspergillus , and the zygomycetes ( ).
Bacterial meningitis
Early viral meningoencephalitis
Early tuberculous meningitis
Early mycotic meningitis
Amebic encephalomyelitis
Cerebral abscess
Subdural empyema
AIDS-related CMV radiculopathy
Following CNS hemorrhage
Subarachnoid
Intracerebral
Reaction to repeated lumbar punctures
Injection of foreign material in subarachnoid space (e.g., methotrexate, contrast media)
Metastatic tumor in contact with CSF
Increased CSF lymphocytes have been reported in various diseases/disorders ( Box 30.4 ). Lymphocytosis (>50%) may occur in early acute bacterial meningitis when the CSF leukocyte count is under 1000/μL ( ). Reactive lymphoplasmacytoid and immunoblastic variants may be present, particularly with viral meningoencephalitis. Blastlike lymphocytes may be seen admixed with small and large lymphocytes in the CSF of neonates.
Viral meningitis
Tuberculous meningitis
Fungal meningitis
Syphilitic meningoencephalitis
Leptospiral meningitis
Bacterial due to uncommon organisms
Early bacterial meningitis where leukocyte counts are relatively low
Parasitic infestations (e.g., cysticercosis, trichinosis, toxoplasmosis)
Aseptic meningitis due to septic focus adjacent to meninges
Subacute sclerosing panencephalitis
Multiple sclerosis
Drug abuse encephalopathy
Guillain-Barré syndrome
Acute disseminated encephalomyelitis
Handl syndrome (headache with neurologic deficits and CSF lymphocytosis)
Sarcoidosis
Polyneuritis
CNS periarteritis
Plasma cells , not normally present in CSF, may appear in a variety of inflammatory and infectious conditions ( Box 30.5 ), along with large and small lymphocytes, and in association with malignant brain tumors ( ). Multiple myeloma may also rarely involve the meninges ( ).
Acute viral infections
Guillain-Barré syndrome
Multiple sclerosis
Parasitic CNS infestations
Sarcoidosis
Subacute sclerosing panencephalitis
Syphilitic meningoencephalitis
Tuberculous meningitis
Although eosinophils are rarely present in normal CSF, they may be increased in a variety of CNS conditions ( Box 30.6 ). For example, eosinophilia is frequently mild (1%–4%) in a general inflammatory response, but in children with malfunctioning ventricular shunts, it may be marked ( Fig. 30.4 ). A suggested criterion for eosinophilic meningitis is 10% eosinophils ( ); parasitic invasion of the CNS is the most common cause worldwide. Coccidioides immitis is a significant cause of CSF eosinophilia in endemic regions of the United States ( ).
Acute polyneuritis
CNS reaction to foreign material (drugs, shunts)
Fungal infections
Idiopathic eosinophilic meningitis
Idiopathic hypereosinophilic syndrome
Parasitic infections
Bacterial meningitis
Leukemia/lymphoma
Myeloproliferative disorders
Neurosarcoidosis
Primary brain tumors
Tuberculous meningoencephalitis
Viral meningitis
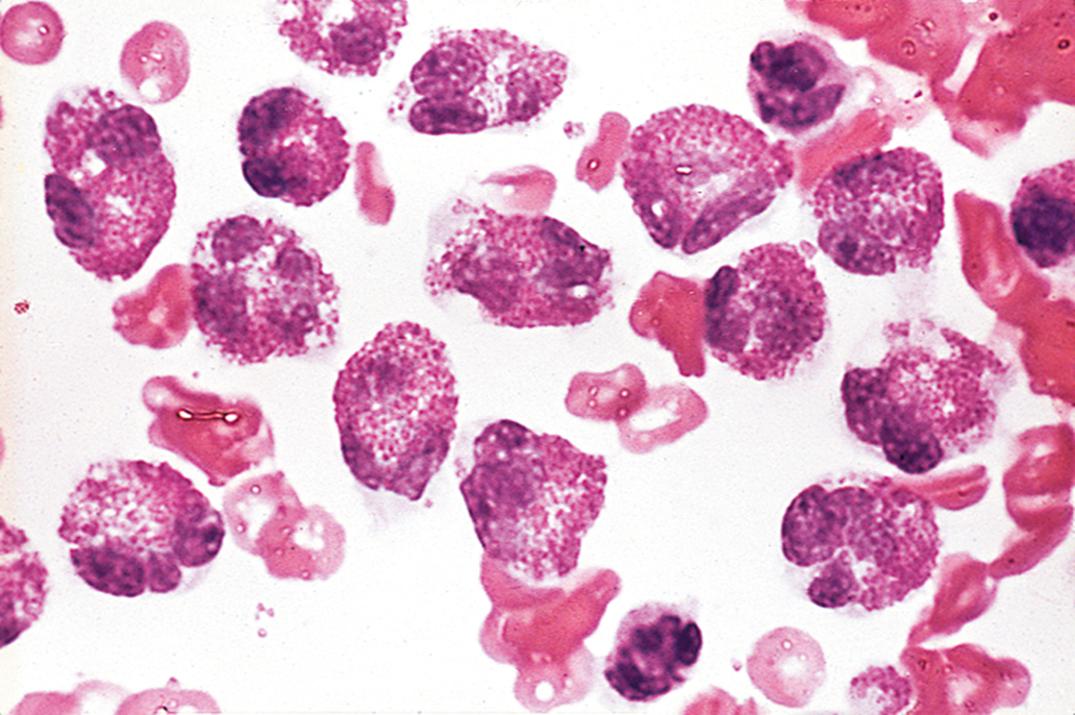
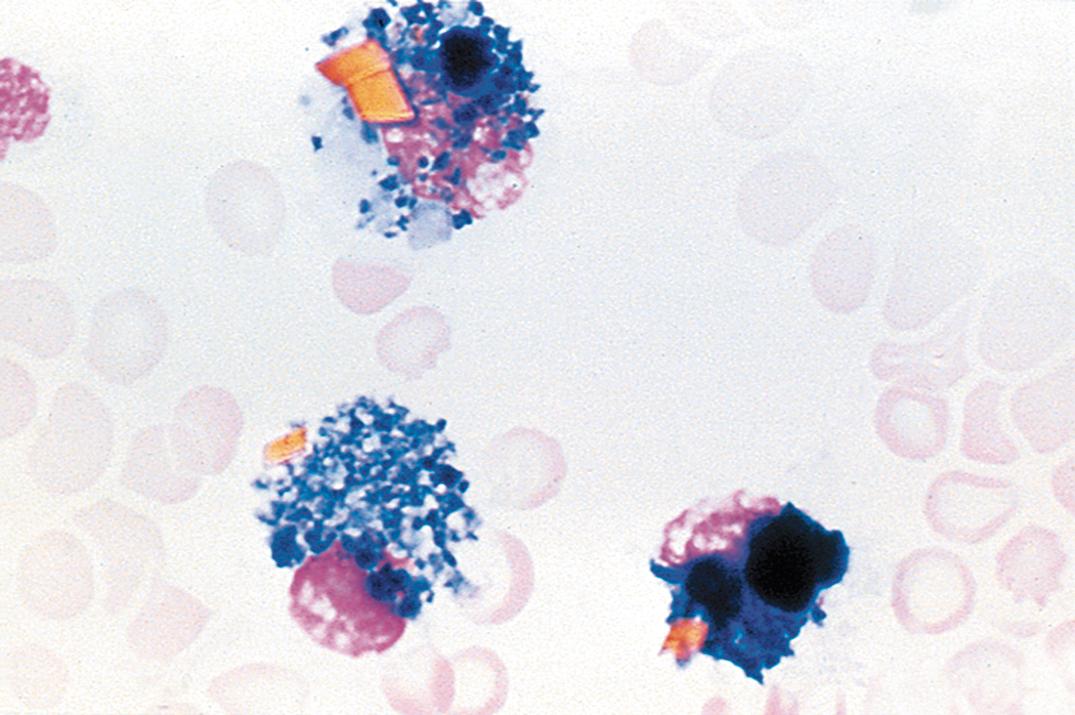
Increased CSF monocytes lack diagnostic specificity and are usually part of a “mixed-cell reaction” that includes neutrophils, lymphocytes, and plasma cells. This pattern is seen in tuberculous and fungal meningitis, chronic bacterial meningitis (i.e., Listeria monocytogenes and others), leptospiral meningitis, ruptured brain abscess, Toxoplasma meningitis, and amebic encephalomeningitis. A mixed-cell pattern without neutrophils is characteristic of viral and syphilitic meningoencephalitis. Macrophages with phagocytosed erythrocytes ( erythrophages ) appear from 12 to 48 hours following a subarachnoid hemorrhage or traumatic tap. Hemosiderin-laden macrophages ( siderophages ) appear after about 48 hours and may persist for weeks ( Fig. 30.5 ). Brownish yellow or red hematoidin crystals may form after a few days.
Morphologic CSF examination for tumor cells has moderate sensitivity and high specificity (97%–98%) ( ). Sensitivity depends on the type of neoplasm. CSF examination of leukemic patients has the highest sensitivity (about 70%), followed by metastatic carcinoma (20%–60%) and primary CNS malignancies (30%). Sensitivity may be optimized by using filtration methods with larger fluid volumes or by performing serial punctures in patients in whom a neoplasm is strongly suspected. Processing of CSF samples using liquid-based thin-layer methods also increases sensitivity in the detection of neoplastic cells and enhances preservation of these cells for potential immunocytochemical analysis ( ). These liquid-based methods are now commonly used for cytopathologic examination of CSF and other body cavity fluid specimens.
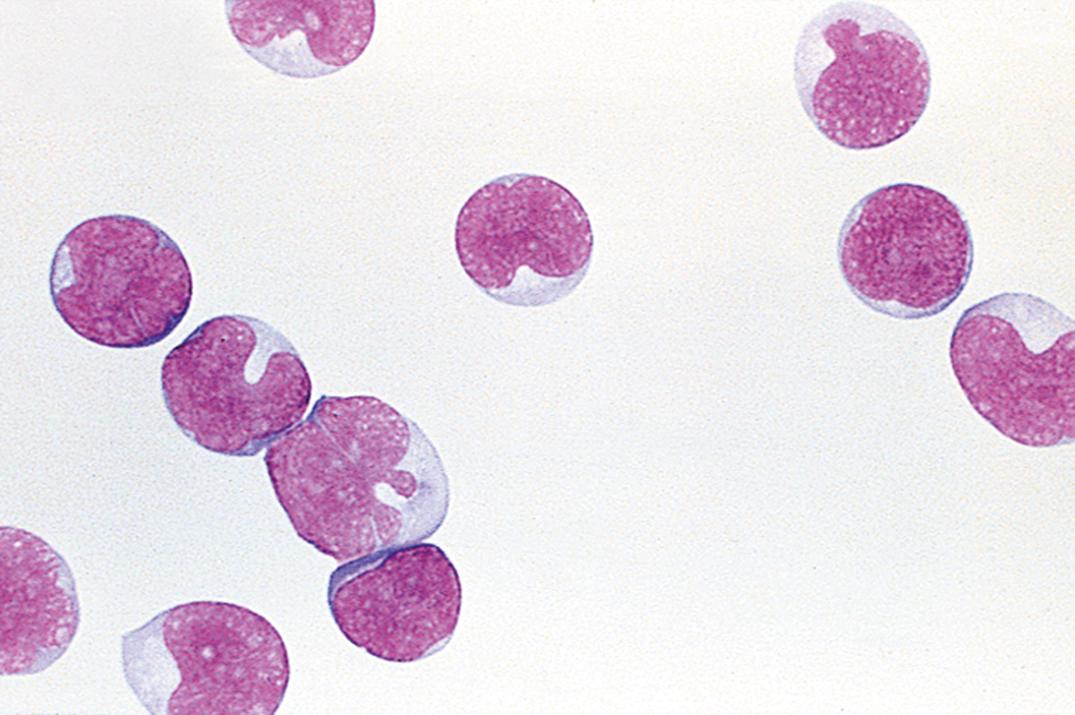
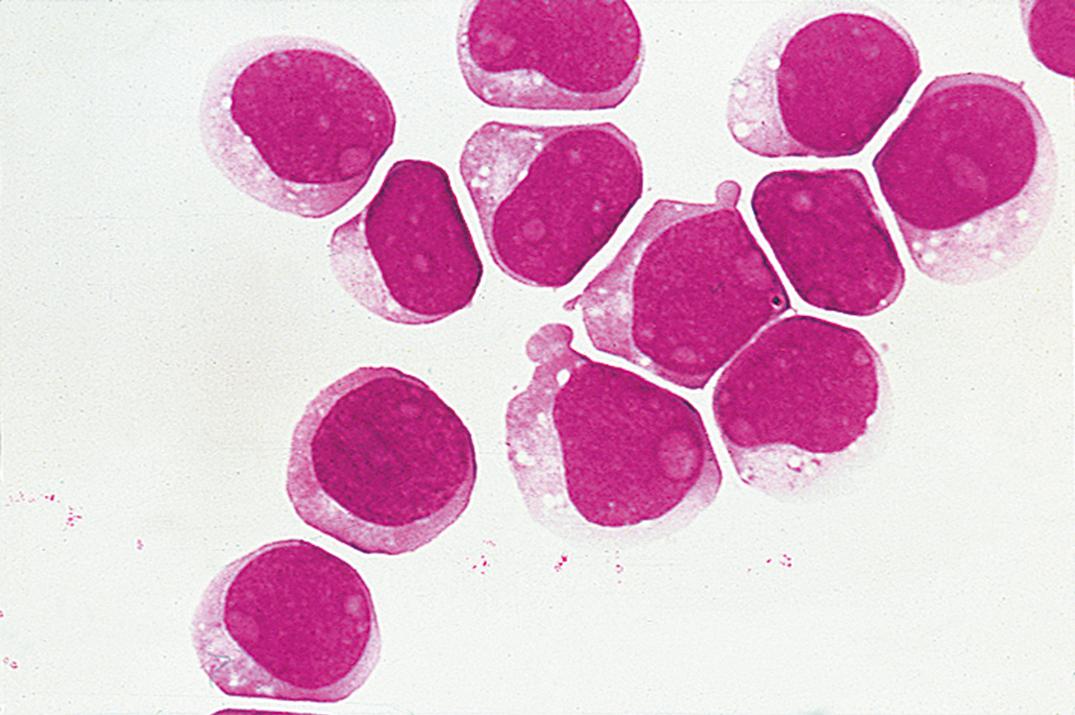
Leukemic involvement of the meninges is more frequent in patients with acute lymphoblastic leukemia ( Fig. 30.6 ) than in those with acute myeloid leukemia ( Fig. 30.7 ). Both are significantly more common than CNS involvement in the chronic leukemias. A leukocyte count over 5 cells/μL with unequivocal lymphoblasts in cytocentrifuged preparations is commonly accepted as evidence of CSF involvement. The incidence of CNS relapse in children with lymphoblasts but cell counts lower than 6 cells/μL appears to be low and is not significantly different from cases in which no blasts are identified ( ; ; ). As noted previously, the ability to detect leukemic cells in CSF using the cytocentrifuge method is highly instrument dependent; low levels of these cells may be missed with certain instruments ( ).
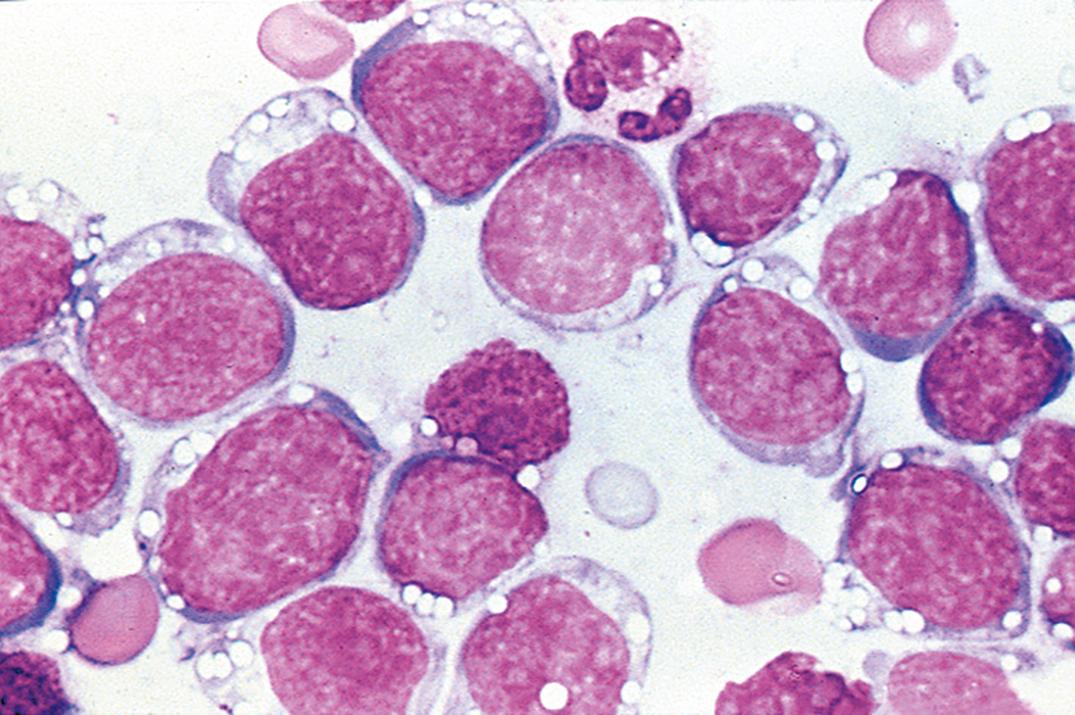
Non-Hodgkin lymphomas involving the leptomeninges are usually high-grade tumors (lymphoblastic, large-cell immunoblastic, and Burkitt lymphomas; Fig. 30.8 ); low-grade lymphomas and Hodgkin lymphoma are significantly less common ( ; ). T cells predominate in normal and inflammatory conditions, whereas most lymphomas, especially those occurring in immunocompromised hosts, are of B-cell lineage. Lymphoblastic lymphoma, the most common T-cell lymphoma to involve the CSF, can be detected by terminal deoxynucleotidyl transferase stain.
Multiparameter flow cytometric immunophenotypic studies, deoxyribonucleic acid (DNA) analysis by polymerase chain reaction (PCR), and, more recently, DNA sequence analysis have been shown to significantly improve diagnostic sensitivity and specificity in CSF samples involved by leukemic or lymphoma cells ( ; ; ; ; ; ; , ). Considering their greater sensitivity in detecting leukemia or lymphoma cells in CSF samples, whenever available, these advanced technologies should be part of the complete evaluation in patients being worked up for these conditions.
Amebas, fungi (especially Cryptococcus neoformans ), and Toxoplasma gondii organisms may be present on cytocentrifuge specimens but may be difficult to recognize without confirmatory stains.
Reference values for lumbar CSF in adults are listed in Table 30.3 .
| Analyte | Conventional Units | SI Units |
|---|---|---|
| Protein | 15–60 mg/dL | 0.15–0.60 g/L |
| Prealbumin | 2%–7% | |
| Albumin | 56%–76% | |
| α 1 -Globulin | 2%–7% | |
| α 2 -Globulin | 4%–12% | |
| β-Globulin | 8%–18% | |
| γ-Globulin | 3%–12% | |
| Electrolytes | ||
| Osmolality | 280–300 mOsm/L | 280–300 mmol/L |
| Sodium | 135–150 mEq/L | 135–150 mmol/L |
| Potassium | 2.6–3.0 mEq/L | 2.6–3.0 mmol/L |
| Chloride | 115–130 mEq/L | 115–130 mmol/L |
| Carbon dioxide | 20–25 mEq/L | 20–25 mmol/L |
| Calcium | 2.0–2.8 mEq/L | 1.0–1.4 mmol/L |
| Magnesium | 2.4–3.0 mEq/L | 1.2–1.5 mmol/L |
| Lactate | 10–22 mg/dL | 1.1–2.4 mmol/L |
| pH | ||
| Lumbar fluid | 7.28–7.32 | |
| Cisternal fluid | 7.32–7.34 | |
| pCO 2 | ||
| Lumbar fluid | 44–50 mm Hg | |
| Cisternal fluid | 40–46 mm Hg | |
| pO 2 | 40–44 mm Hg | |
| Other Constituents | ||
| Ammonia | 10–35 μg/dL | 6–20 μmol/L |
| Glutamine | 5–20 mg/dL | 0.3–1.4 mmol/L |
| Creatinine | 0.6–1.2 mg/dL | 45–92 μmol/L |
| Glucose | 50–80 mg/dL | 2.8–4.4 mmol/L |
| Iron | 1–2 μg/dL | 0.2–0.4 μmol/L |
| Phosphorus | 1.2–2.0 mg/dL | 0.4–0.7 mmol/L |
| Total lipid | 1–2 mg/dL | 0.01–0.02 g/L |
| Urea | 6–16 mg/dL | 2.0–5.7 mmol/L |
| Urate | 0.5–3.0 mg/dL | 30–180 μmol/L |
| Zinc | 2–6 μg/dL | 0.3–0.9 μmol/L |
More than 80% of the CSF protein content is derived from blood plasma in concentrations of less than 1% of the plasma level ( Table 30.4 ).
| Protein | CSF, mg/L | Plasma/CSF Ratio |
|---|---|---|
| Prealbumin | 17.3 | 14 |
| Albumin | 155.0 | 236 |
| Transferrin | 14.4 | 142 |
| Ceruloplasmin | 1.0 | 366 |
| Immunoglobulin (Ig) G | 12.3 | 802 |
| IgA | 1.3 | 1346 |
| α 2 -Microglobulin | 2.0 | 1111 |
| Fibrinogen | 0.6 | 4940 |
| IgM | 0.6 | 1167 |
| β-Lipoprotein | 0.6 | 6213 |
Prealbumin (transthyretin), transferrin, and small quantities of nerve tissue–specific proteins are the major qualitative differences that normally exist between CSF and plasma proteins. Although some authors have argued against routine measurement of total protein ( ), it is the most common abnormality found in CSF. Thus, an increased CSF protein serves as a useful, albeit nonspecific, indicator of meningeal or CNS disease.
CSF total protein reference values vary considerably among laboratories owing to differences in methods, instrumentation, and type of reference standard used. CSF protein levels of 15 to 45 mg/dL have long been accepted as the “normal” reference range ( ). Other studies using different methods have shown generally higher reference ranges, approximately 15 to 60 mg/dL ( ).
Although discrepancies in gender and in those older than 60 years of age have been reported, the differences are probably not significant. However, infants have significantly higher CSF protein levels than older children and adults. Thus, mean levels of 90 mg/dL for term infants and 115 mg/dL for preterm infants were reported; the upper levels were 150 mg/dL and 170 mg/dL, respectively ( ). Others have noted that the CSF protein concentration falls rapidly from birth to 6 months of age (mean levels, 108 mg/dL to 40 mg/dL), plateaus between 3 and 10 years of age (mean, 32 mg/dL), and then rises slightly from 10 to 16 years of age (mean, 41 mg/dL) ( ).
Elevated CSF protein levels may be caused by increased permeability of the BBB, decreased resorption at the arachnoid villi, mechanical obstruction of CSF flow due to spinal block above the puncture site, or an increase in intrathecal immunoglobulin (Ig) synthesis. Common conditions associated with elevated lumbar CSF protein values (>65 mg/dL) are summarized in Box 30.7 .
Arachnoiditis (e.g., following methotrexate therapy)
Meningitis (bacterial, viral, fungal, tuberculous)
Hemorrhage (subarachnoid, intracerebral)
Endocrine/metabolic disorders
Milk-alkali syndrome with hypercalcemia
Diabetic neuropathy
Hereditary neuropathies and myelopathies
Decreased endocrine function (thyroid, parathyroid)
Other disorders (uremia, dehydration)
Ethanol, phenothiazines, phenytoin
Mechanical obstruction (tumor, abscess, herniated disk)
Loculated CSF effusion
Multiple sclerosis
Subacute sclerosing panencephalitis
Guillain-Barré syndrome
Collagen vascular diseases (e.g., lupus, periarteritis)
Chronic inflammatory demyelinating polyradiculopathy
Low lumbar CSF total protein levels (<20 mg/dL) normally occur in some young children between 6 months and 2 years of age and in patients with conditions associated with increased CSF turnover. These include (1) removal of large CSF volumes; (2) CSF leaks induced by trauma or lumbar puncture; (3) increased intracranial pressure, probably due to an increased rate of protein resorption by the arachnoid villi; and (4) hyperthyroidism ( ).
Protein electrophoresis of concentrated normal CSF reveals two distinct differences from serum: a prominent transthyretin (prealbumin) band and two transferrin bands. Transthyretin is relatively high because of its dual synthesis by the liver and choroid plexus. The second transferrin band, referred to as β 2 -transferrin, migrates more slowly than its serum equivalent owing to cerebral neuraminidase digestion of sialic acid residues.
Turbidimetric methods, commonly based on trichloroacetic acid (TCA) or sulfosalicylic acid and sodium sulfate for protein precipitation, are popular because they are simple, rapid, and require no special instrumentation. However, they are temperature sensitive and require much larger specimen volumes (about 0.5 mL). A false protein elevation may be observed using TCA methods in the presence of methotrexate ( ). Benzethonium chloride and benzalkonium chloride have been used as precipitating agents in automated methods and micromethods ( ; ).
Colorimetric methods include the Lowry method, dye-binding, methods using Coomassie brilliant blue (CBB) or Ponceau S, and the modified Biuret method. The CBB method is rapid and highly sensitive and can be used with small sample sizes. Immunochemical methods measure specific proteins, require only 25 to 50 μL of CSF, and are relatively simple to perform once conditions and reagents have been standardized. Automated methods are now almost always used and show good correlation with traditional standard methods ( ).
The permeability of the BBB may be assessed by immunochemical quantification of the CSF albumin/serum albumin ratio in grams per deciliter (g/dL). The normal ratio of 1:230 ( ) yields an unwieldy decimal of 0.004, which prompted the use of the CSF/serum albumin index , which is arbitrarily calculated as follows:
An index value less than 9 is consistent with an intact barrier. Slight impairment is considered with index values of 9 to 14, moderate impairment with values of 14 to 30, and severe impairment with values greater than 30 ( ). The index is slightly elevated in infants up to 6 months of age, reflecting the immaturity of the BBB, and increases gradually after 40 years of age. A traumatic tap invalidates the index calculation.
Increased intrathecal IgG synthesis is reflected by an increase in the CSF/serum IgG ratio:
The normal ratio is 1/390 or 0.003 ( ). Similar to the albumin index, the CSF/serum IgG index may be obtained by using milligrams per deciliter for the CSF IgG value. The CSF/serum IgG index normal range is 3.0 to 8.7.
The CSF/serum IgG index can be elevated by intrathecal IgG synthesis or increased plasma IgG crossover from breakdown of the BBB. Ig derived from plasma crossover may be corrected by dividing the CSF/serum IgG index by the CSF/albumin index to yield the CSF IgG index.
or
The reference range for the IgG index varies, reflecting variations in determination of the four index components. A reasonable normal upper limit is 0.8 ( ). However, each laboratory should determine its own critical ratio.
The IgG synthesis rate is calculated by an empirical formula ( ):
All protein concentrations are expressed in milligrams per deciliter. The first bracketed term represents the difference between measured CSF IgG and the IgG expected from diffusion across a normal BBB; 369 is the normal serum/CSF ratio. The second bracketed term represents the difference between measured CSF albumin and expected albumin if the blood-brain barrier is intact; 230 is the normal serum/CSF albumin ratio. The CSF albumin excess is multiplied by the IgG/albumin ratio and the molecular weight ratio of IgG to albumin (0.43) to correct for changes in CSF IgG due to increased barrier permeability. The number 5 converts the result from a concentration to a daily amount, assuming an average daily CSF production of 500 mL (i.e., 5 dL). The formula does not consider variations in CSF production or Ig consumption. It assumes that the IgG/albumin ratio remains constant over various degrees of BBB impairment—a concept that may lead to variable error ( ). The reference interval for the synthesis rate is –9.9 to +3.3 mg/day. Values greater than 8.0 mg/day indicate an increased rate ( ).
CSF IgG is normally 3% to 5% of total CSF protein. However, in multiple sclerosis (MS), the concentration approaches that of plasma (15%–18%) ( ). The CSF IgG index and the IgG synthesis rate have a sensitivity of 90% in patients with definite MS, but the sensitivity is lower in patients with possible MS, in whom accuracy is most needed ( ). In addition, the specificity for MS is only moderate because increased intrathecal IgG synthesis occurs in many other inflammatory neurologic diseases.
The Ig index and synthesis rate calculations may also be applied to IgM, IgA, Ig light chains, and specific antibodies to infectious microorganisms. For example, increased synthesis of IgM and free κ light chains have been suggested as markers for MS ( ; ).
Although the diagnosis of MS is ultimately a clinical one, significant advances have been made in laboratory testing for this disorder. CSF total protein is increased in less than 50% of patients with MS. Indeed, if the CSF protein exceeds 100 mg/dL, the patient probably does not have MS. However, the γ-globulin fraction, as determined by CSF electrophoresis, is often increased in MS. Thus, the CSF total protein/γ-globulin ratio exceeds 0.12 in about 65% of cases ( ). Using electroimmunodiffusion , a CSF IgG/albumin ratio greater than 0.25 is present in about 75% of cases ( ). Levels greater than the mean CSF IgG index + 3SD are present in 80% to 85% of MS cases, although the upper reference level varies significantly among laboratories.
High-resolution agarose gel electrophoresis of concentrated CSF from patients with MS often shows discrete populations of IgG, the oligoclonal bands . Although these discrete IgG populations are normally absent, two or more bands are necessary to support the diagnosis of MS; a single band is not considered a positive result. Using this technique, oligoclonal bands have been reported in 83% to 94% of patients with definite MS, 40% to 60% of those with probable MS, and 20% to 30% of possible MS cases. However, they are also frequently present in patients with subacute sclerosing panencephalitis, various viral CNS infections, neurosyphilis, neuroborreliosis, cryptococcal meningitis, Guillain-Barré syndrome, transverse myelitis, meningeal carcinomatosis, glioblastoma multiforme, Burkitt lymphoma, chronic relapsing polyneuropathy, Behçet disease, cysticercosis, and trypanosomiasis, among others ( ; ; ; ). Subsequent studies have shown that agarose gel electrophoresis sensitivity for MS is less than previously reported (see later discussion).
Oligoclonal light chains (both κ and λ) are present in about 90% of MS patients ( ; ). They have also occasionally been identified in the CSF of those who are negative for IgG oligoclonal bands. Detection of free light chains in CSF and comparison to serum has been shown to be a sensitive marker for intrathecal immunoglobulin synthesis ( ).
Coomassie brilliant blue or paragon violet stains can resolve oligoclonal bands in only 5 μg of IgG ( ). However, silver staining is 20 to 50 times more sensitive than CBB and can be used on unconcentrated CSF. It is important to note that these electrophoretic techniques must be simultaneously carried out on the patient’s serum to be certain that a polyclonal gammopathy is not present (e.g., liver disease, systemic lupus, rheumatoid arthritis [RA], chronic granulomatous disease) because these disorders may be accompanied by Ig diffusion into the CSF, yielding false-positive results.
Immunofixation electrophoresis (IFE) is more sensitive than agarose gel electrophoresis and does not require CSF concentration ( ). Another study reported a sensitivity of 74% using this technique compared with 57% for agarose gel electrophoresis ( ). More recently, using a semiautomated immunofixation-peroxidase technique, the sensitivity was 83% and the specificity was 79% in patients with clinically definite MS ( ).
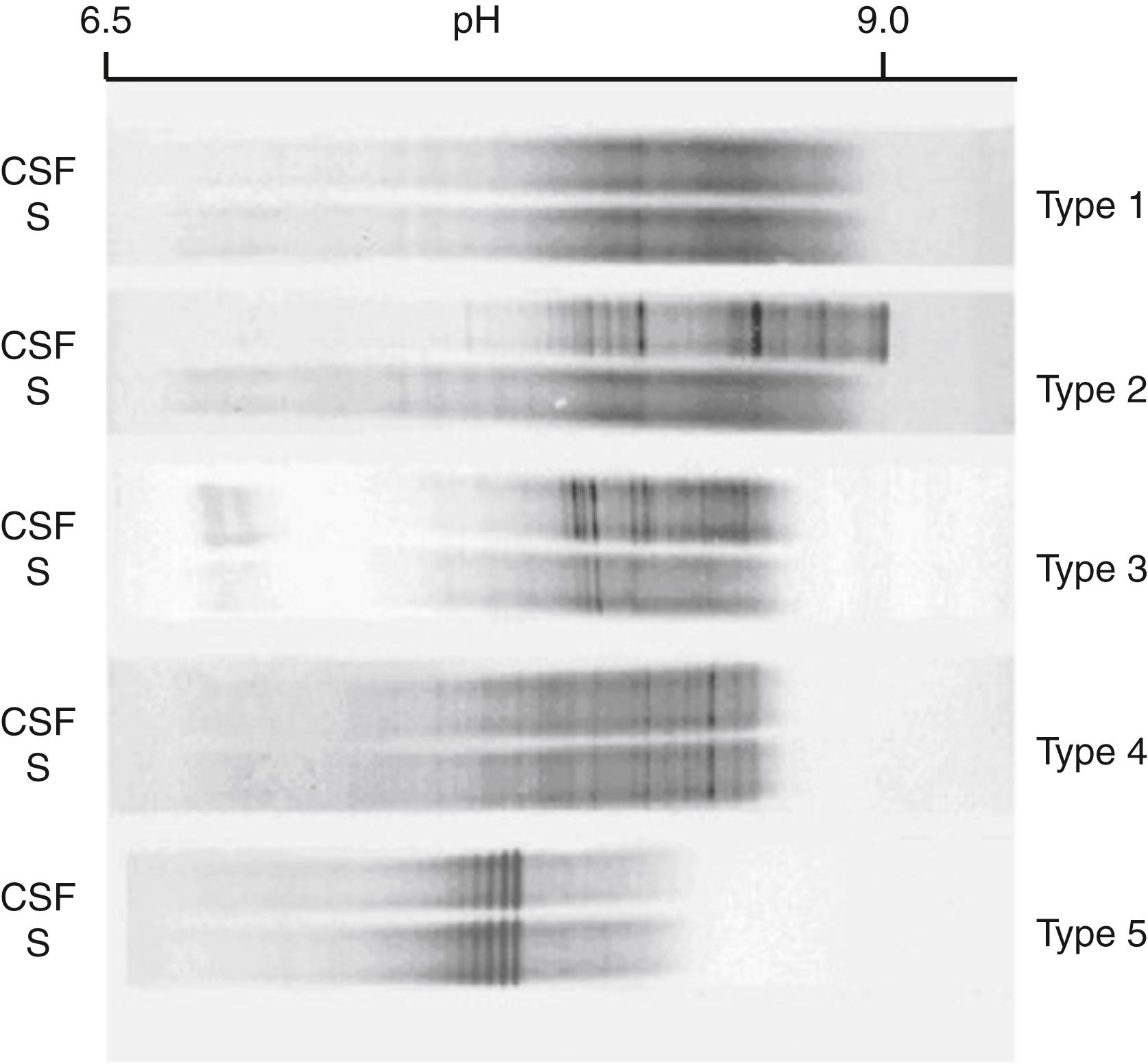
Isoelectric focusing and IgG immunoblotting (IgG-IEF) performed on paired CSF and serum samples is the most sensitive and now the recommended method for the detection of oligoclonal bands ( ; ; ). One study showed that IgG-IEF detected 100% of definite MS, but only about 50% were positive by agarose electrophoresis ( ). Others detected 91% of MS cases but only 68% with agarose ( ). Similarly, a semiautomated IgG-IEF technique identified 90% of MS cases compared with 60% for agarose electrophoresis ( ). In 2005, an international consensus standard for the diagnosis of MS established IgG-IEF as the method of choice for qualitative detection of oligoclonal IgG bands as evidence of intrathecal synthesis of IgG ( ). More recent studies have reinforced the utility of IgG-IEF in diagnosing MS and distinguishing this disease from other disorders ( ). Figure 30.9 shows the different patterns seen on IgG-IEF in paired CSF and serum samples from patients with MS and other conditions.
Oligoclonal bands (OCBs) in CSF have a high sensitivity but low specificity for MS; thus, they are useful for screening but not for diagnosis. The overlap of positive OCB findings in infectious and other inflammatory conditions has stimulated the search for new biomarkers in CSF that are more specific for MS (Gastaldi et al., 2017) and that have potential prognostic relevance. Candidate markers showing promise include the following:
Immunoglobulin free light chains (FLCs) as an indicator of B-cell activity are found in serum as well as other body fluids and could potentially be useful for diagnosing true MS patients who happen to be OCB negative. Early studies indicate utility for FLC measurements in CSF (especially because such measurements are automated); however, larger patient groups should be evaluated for wide acceptance ( ).
Oligoclonal IgM bands in CSF appear to be a very powerful marker for more severe and more aggressive MS ( ). Unfortunately, hydrophobicity of IgM requires its chemical reduction prior to electrophoresis, which may still be affected by artifacts from reassociation of monomeric IgM. Nevertheless, oligoclonal IgM in CSF (which is directed against myelin lipid, usually phosphatidylcholine) is considered a very promising prognostic test for MS but will require more developmental work for standardization ( ).
The measles-rubella-zoster (MRZ) reaction consists of measuring antibody titers to those viruses in CSF and serum. The MRZ reaction is positive if at least two of those indexes (CSF titer/serum titer) are positive. MRZ is considered highly specific for MS and could be recommended for patients with suspected MS who are OCB negative ( ).
Neurofilaments are released into CSF following neuron and axon injury. They are also found in serum and, as such, could be used to monitor progression of MS as a highly prognostic marker once measurements become well established and are widely practiced ( ).
Further advances in CSF proteomic testing whereby literally hundreds of individual protein species can be identified is likely to provide more diagnostic power for neurologic diseases in coming years ( ; ).
In summary, the diagnosis of MS, as with many other neurologic disorders, is ultimately a clinical one based on neurologic history and physical examination. Nevertheless, advanced laboratory results in CSF—such as elevated IgG indices, particularly the detection of oligoclonal IgG bands and neuroimaging techniques—have proved invaluable in the diagnosis of MS.
Approximately 300 different proteins have been identified in CSF using two-dimensional electrophoresis, the first dimension being isoelectric focusing and the second polyacrylamide gel in the presence of sodium dodecyl sulfate ( ). Using this technique, four abnormal proteins were identified in patients with Creutzfeldt-Jakob disease (CJD). Two of these proteins (molecular mass about 40 kilodaltons [kDa] each) were present in some, but not all, patients with herpes simplex encephalitis, Parkinson disease, Guillain-Barré syndrome, and schizophrenia. They were not present in various other neurologic disorders or in 100 normal CSF control specimens. However, these and two other proteins (molecular masses about 26 and 29 kDa) were present in all cases of CJD and in 5 of 10 cases of herpes simplex encephalitis. Neither of these latter proteins was present in any other neurologic disease or in controls.
Increased concentrations of various specific CSF proteins have been associated with several CNS diseases ( Table 30.5 ).
| Protein | Major Diseases/Disorders |
|---|---|
| α 2 -Macroglobulin | Subdural hemorrhage, bacterial meningitis |
| β-Amyloid and τ proteins | Alzheimer disease |
| β 2 -Microglobulin | Leukemia/lymphoma, Behçet syndrome |
| C-reactive protein | Bacterial and viral meningitis |
| Fibronectin | Lymphoblastic leukemia, AIDS, meningitis |
| Methemoglobin | Mild subarachnoid/subdural hemorrhage |
| Myelin basic protein | Multiple sclerosis, tumors, others |
| Protein 14-3-3 | Creutzfeldt-Jakob disease |
| Transferrin | CSF leakage (otorrhea, rhinorrhea) |
Myelin basic protein (MBP), a component of the myelin nerve sheath, is released during demyelination as a result of various neurologic disorders, especially MS. Thus, MBP has been shown to positively correlate with CSF leukocyte count, intrathecal IgG synthesis, and the CSF/serum albumin concentration quotient ( ). These results supported the use of MBP in CSF as a surrogate disease marker during acute MS exacerbations. Others have found that analysis of antibody against MBP in patients with a clinically isolated syndrome is a rapid and precise method for predicting early conversion to clinically definite MS ( ). However, increased CSF levels have also been reported in Guillain-Barré syndrome, lupus erythematosus (LE), subacute sclerosing panencephalitis, and various brain tumors, and following CNS irradiation and chemotherapy ( ; ). Measurement of CSF MBP levels has also been proposed as a prognostic marker in patients with serious head injury ( ). More recently, the clinical utility of MBP measurement in patients with MS has been questioned based on similarity of abnormal results to other established laboratory methods, such as presence of OCBs on protein electrophoresis, for diagnosing MS ( ).
Except for a small amount transported across the BBB in pinocytic vesicles, α 2 -macroglobulin (A2M) is normally excluded from the CSF because of its large size. The number of these vesicles is increased in certain polyneuropathies, resulting in an increased CSF A2M level. Significant elevation reflects subdural hemorrhage or breakdown of the BBB, as occurs in bacterial meningitis. A2M measurement alone, or in relationship to albumin and IgG, may assist in the evaluation of neurologic disorders and increased CSF protein, and in the rapid differentiation between bacterial and aseptic meningitis ( ; ).
This protein is part of the human leukocyte antigen class I molecule on the surfaces of all nucleated cells. CSF levels above 1.8 mg/L are associated with leptomeningeal leukemia and lymphoma but are not highly specific ( ) in that they have a maximal positive predictive value of 78% in cases with a positive cytology ( ). β 2 -Microglobulin (B2M) has also been shown to be a marker for neuro-Behçet syndrome ( ). Viral infections, including human immunodeficiency virus (HIV)-1, other inflammatory conditions, and various malignancies have also been associated with elevated levels. The measurement of B2M remains primarily investigational.
Early studies indicated that CSF C-reactive protein (CRP) is useful in differentiating viral (aseptic) meningitis from bacterial meningitis ( ; ; ). Others reported that CSF CRP is a more useful screening test for viral versus bacterial meningitis, especially in children ( ). A meta-analysis of CRP studies since 1980 suggested that a normal CSF or serum CRP has a high probability of ruling out bacterial meningitis (i.e., negative predictive value about 97%) ( ). Another study found not only that CSF CRP levels were increased in bacterial meningitis, but that these levels were significantly higher in patients with gram-negative bacterial meningitis than in those with gram-positive bacterial meningitis ( ). This assay has been found to be more specific than blood CRP levels in the laboratory diagnosis of meningitis in children ( ).
This large glycoprotein (molecular mass ≈420 kDa) is normally present in essentially all tissues and body fluids. Its primary function is its role in cell adhesion and phagocytosis ( ). Thus, cell adhesion allows leukocytes to adhere to and pass through the vascular endothelia and migrate to the inflammatory site.
In children with acute lymphoblastic leukemia, elevated CSF fibronectin levels are associated with a poor prognosis, presumably due to leukemic involvement of the CNS ( ). Significant CSF elevations have also been reported in Burkitt lymphoma ( ), some metastatic solid tumors, astrocytomas, and bacterial meningitis ( ; ). Decreased levels have been reported in viral meningitis and in the acquired immunodeficiency syndrome (AIDS)–dementia complex ( , ). Detection of fibronectin with alternatively spliced extra domain A is found in lower amounts in CSF from children with bacterial meningitis as opposed to viral meningitis or without meningitis ( ).
The diagnosis of Alzheimer disease (AD) is based on the presence of dementia and a specific clinical profile (i.e., from medical history, clinical examination) suggestive of AD, together with the exclusion of other causes of dementia. Pathologically, the disease is characterized by the presence of neurofibrillary tangles and amyloid plaques.
Studies indicate that measurement of biochemical markers increases diagnostic accuracy, especially early in the course of the disease, when clinical symptoms are mild and vague and overlap with cognitive changes that accompany aging and ischemic dementia. Thus, increased CSF levels of microtubule-associated tau (τ) protein and decreased levels of β-amyloid protein ending at amino acid 42 (β-amyloid protein 42) have been shown to significantly increase the accuracy of AD diagnosis ( ; ; ). The positive predictive value for early AD is greater than 90% ( ). Others have found that the calculated ratio of phosphorylated τ protein (P-tau) to β-amyloid peptide is superior to either measure alone ( ). The results were as follows: distinguishing patients with AD from healthy controls—sensitivity 96%, specificity 97%; patients with AD from those with non-AD dementia—sensitivity 80%, specificity 73%; and patients with AD from those with other neurologic disorders—sensitivity 80%, specificity 89%. These assays all appear to have a place in the biochemical diagnosis of early AD ( ), although development of a highly effective CSF assay to diagnose AD remains elusive.
The transmissible spongiform encephalopathies constitute a group of uniformly fatal neurodegenerative diseases. Of these, CJD is the major spongiform disease in humans. Two proteins, designated 130 and 131, have been detected in low concentrations in CSF from CJD patients. These proteins have the same amino acid sequence as protein 14-3-3 ( ). In patients with dementia, a positive immunoassay for the 14-3-3 protein in CSF strongly supports a diagnosis of CJD. In a subsequent study of patients with suspected CJD, the sensitivity of the 14-3-3 protein determined by immunoassay was 97%, and the specificity was 87% ( ). False-positive results were seen primarily in patients with stroke and meningoencephalitis.
Others, using a modified Western blot technique, reported a 94.7% positive predictive value and a 92.4% negative predictive value for CJD ( ). False-positive results were seen in patients with herpes simplex encephalitis, atypical encephalitis, metastatic lung cancer, and hypoxic brain damage.
Detecting elevation of protein 14-3-3 in combination with elevation of other brain-derived proteins, such as τ protein, in CSF appears to be useful in the diagnosis of CJD ( , ).
CSF leakage usually presents as otorrhea or rhinorrhea following head trauma, in some cases beginning months to years after the injury. Recurrent meningitis is a serious complication, making accurate identification of the leaking fluid very important. In this regard, protein and glucose measurements are too nonspecific to be of value. Transferrin, an iron-binding glycoprotein with a molecular mass of about 77 kDa, is synthesized primarily in the liver. However, two transferrin isoforms are present in the CSF; the major isoform (β 1 -transferrin) is present in all body fluids. The second isoform (β 2 -transferrin), present only in the CNS, is produced in the CNS by the catalytic conversion of β-1-transferrin by neuraminidase. Immunofixation electrophoresis readily identifies both isoforms (see Chapter 20 ).
Detection of β 2 -transferrin in CSF by protein electrophoresis with transferrin immunofixation is a noninvasive, rapid, and inexpensive test of high sensitivity and specificity that requires as little as 0.1 mL of fluid ( ; ). Several reports have demonstrated the value of this technique in the diagnosis of CSF otorrhea and rhinorrhea—conditions in which both isoforms are readily identified ( ; ; ). Others have stressed the importance of β 2 -transferrin identification in both CSF and inner ear perilymphatic leakage, as well as possible sources of error due to the presence of a transferrin allelic variant ( , ; ). β 2 -transferrin is sufficiently stable in CSF stored at room temperature and/or when mixed with nasal mucus that a negative result in a patient-collected nasal fluid specimen up to 1 week old is a reliable negative finding for CSF leakage ( ).
Although most cases of subarachnoid and intracerebral hemorrhage are readily identified by computed tomography (CT), patients with mild subarachnoid hemorrhage, small subdural or cerebral hematomas, and blood seepage from an aneurysm or neoplasm and from small cerebral infarcts often are not identified by this technique. In these cases, CSF spectrophotometric analysis has been shown to detect methemoglobin in colorless CSF (<0.3 μmol/L) ( ). An increase in CSF bilirubin is also recognized as a key finding supporting the diagnosis of subarachnoid hemorrhage ( ). A single net bilirubin absorbance cutoff point of >0.007 absorbance units is recommended in the decision tree for interpretation and reporting of results. Guidelines for the use of CSF spectrophotometric analysis in the detection of hemoglobin breakdown products have been developed ( ).
Derived from blood glucose, fasting CSF glucose levels are normally 50 to 80 mg/dL (2.8–4.4 mmol/L)—about 60% of plasma values. Rigorous evaluation of CSF and serum glucose levels in a large number of children confirmed that the CSF glucose level is normally approximately 60% of the serum glucose level during childhood ( ). Results should be compared with plasma levels, ideally following a 4-hour fast, for adequate clinical interpretation. The normal CSF/plasma glucose ratio varies from 0.3 to 0.9, with fluctuations in blood levels caused by the lag in CSF glucose equilibration time. A good rule of thumb is that CSF glucose is about two-thirds of the level of serum glucose. This enables the detection of low CSF glucose in patients with abnormal glucose levels. Thus in a diabetic patient with a serum glucose level of 250 mg/dL, the expected CSF glucose would be around 167 mg/dL in CSF. If the patient were found to have a CSF glucose of, say, 100 mg/dL, there would be evidence for a possible microorganism-induced lowering of CSF glucose even though the absolute value of CSF glucose is elevated.
CSF values below 40 mg/dL (2.2 mmol/L) or ratios below 0.3 are considered to be abnormal. Hypoglycorrhachia is a characteristic finding of bacterial, tuberculous, and fungal meningitis. However, sensitivity can be as low as 55% for bacterial meningitis ( ); thus, a normal level does not exclude these conditions. Some cases of viral meningoencephalitis also have low glucose levels but generally not to the degree seen in bacterial meningitis. Meningeal involvement by a malignant tumor, sarcoidosis, cysticercosis, trichinosis, ameba ( Naegleria ), acute syphilitic meningitis, intrathecal administration of radioiodinated serum albumin, subarachnoid hemorrhage, symptomatic hypoglycemia, and rheumatoid meningitis may also produce low CSF glucose levels ( ).
Decreased CSF glucose results from increased anaerobic glycolysis in brain tissue and leukocytes and from impaired transport into the CSF. Bacteria are usually present in insufficient quantities to be a major contributor to the use of glucose in CSF. CSF glucose levels normalize before protein levels and cell counts during recovery from meningitis, making it a useful parameter in assessing response to treatment.
Increased CSF glucose is of no clinical significance, reflecting increased blood glucose levels within 2 hours of lumbar puncture. A traumatic tap may also cause a spurious increase in CSF glucose.
CSF and blood lactate levels are largely independent of each other. The reference interval for older children and adults is 9.0 to 26 mg/dL (1.0–2.9 mmol/L) ( ). Newborns have higher levels, ranging from about 10 to 60 mg/dL (1.1–6.7 mmol/L) for the first 2 days and from 10 to 40 mg/dL (1.1–4.4 mmol/L) for days 3 to 10 ( ). Elevated CSF lactate levels reflect CNS anaerobic metabolism due to tissue hypoxia.
Lactate measurement has been used as an adjunctive test in differentiating viral meningitis from bacterial, partially treated bacterial, mycoplasma, fungal, and tuberculous meningitis, in which routine parameters yield equivocal results ( ). In patients with viral meningitis, lactate levels are usually below 25 mg/dL (2.8 mmol/L) and are almost always less than 35 mg/dL (3.9 mmol/L), whereas bacterial meningitis typically has levels above 35 mg/dL ( ; ). Using 30 to 36 mg/dL as the cutoff value for bacterial meningitis, the sensitivity and specificity are about 80% and 90%, respectively. Viral meningitis, partially treated bacterial meningitis, and tuberculous meningitis often have intermediate lactate levels that overlap each other, limiting the use of lactate measurements in this differential diagnosis.
Persistently elevated ventricular CSF lactate levels are associated with a poor prognosis in patients with severe head injury ( ).
Isoprostanes are prostaglandin-like compounds resulting from non-enzymatic free radical-initiated peroxidation of arachidonic acid. A subclass of these compounds have a characteristic so-called F2 ring structure and are therefore called F2-isoprostanes. F2-isoprostanes are increased in diseased regions of the brain in patients with AD ( ) and are markers of free radical brain injury associated with advanced age and latent AD ( ). Compared with age-matched controls, CSF F2-isoprostanes are elevated in patients with probable AD ( ). Therefore, in conjunction with CSF τ and β-amyloid protein, the measurement of CSF F2-isoprostanes appears to enhance the accuracy of the laboratory diagnosis of AD ( ) and by itself appears to correlate with the degree of cognitive decline in some AD patients ( ).
A wide variety of enzymes derived from brain tissue, blood, or cellular elements have been described in the CSF. Although CSF enzyme assays are not commonly used in the diagnosis of CNS diseases, there are diseases/disorders in which they may prove useful.
Adenosine deaminase (ADA) catalyzes the irreversible hydrolytic deamination of adenosine to produce inosine. Because ADA is particularly abundant in T lymphocytes, which are increased in tuberculosis, its measurement has been recommended in the diagnosis of pleural, peritoneal, and meningeal tuberculosis. Higher ADA levels are present in tuberculous infections than in viral, bacterial, and malignant diseases ( ; , ), and measuring ADA in CSF appears to be a sensitive and specific assay in diagnosing tuberculous meningitis ( , ). ADA appears to have limited utility in HIV-associated neurologic disorders ( ).
Brain tissue is rich in creatine kinase (CK) because it participates in maintaining an adequate supply of adenosine triphosphate. Increased CSF CK activity has been reported in numerous CNS disorders, including hydrocephalus, cerebral infarction, various primary brain tumors, and subarachnoid hemorrhage, among others ( ). In patients with head trauma, CSF CK levels correlate directly with the severity of the concussion ( ).
CSF CK-MM and CK-MB are not normally present; when identified, they are due to blood contamination (CK-MM) and an equilibrium between CK-BB and CK-MM to produce CK-MB. Because the CK-BB isoenzyme accounts for about 90% of brain CK activity and mitochondrial CK (CKmt) the other 10%, CK isoenzyme measurements are more specific than total CK for CNS disorders ( ).
CSF CK-BB is increased about 6 hours following an ischemic or anoxic insult. Global brain ischemia following respiratory or cardiac arrest results in diffuse cerebral injury with peak CK-BB levels in about 48 hours ( ). Here, CSF CK-BB activity less than 5 U/L (upper normal level) indicates minimal neurologic damage; 5 to 20 U/L indicates mild to moderate CNS injury; and levels between 21 and 50 U/L are commonly correlated with death. Death occurs in essentially all patients with levels above 50 U/L.
Increased CSF CK-BB levels also correlate with the outcome following a subarachnoid hemorrhage ( ). A CK-BB level greater than 40 U/L increases the chance of an unfavorable early or late outcome to 100%.
Lactate dehydrogenase (LD) activity is high in brain tissue, with a predominance of the electrophoretically fast-moving isoenzyme fractions LD 1 and LD 2 . Total LD activity of 40 U/L is a reasonable upper limit of normal for adults and 70 U/L for neonates ( ; ). LD is useful in differentiating a traumatic tap from intracranial hemorrhage because a current traumatic tap with intact RBCs does not significantly elevate the LD level ( ). Sensitivity and specificity are about 70% to 85% depending on the cutoff value. As with lactate, LD activity is significantly higher in bacterial meningitis than in aseptic meningitis ( ; ). Using a cutoff of 40 U/L, the sensitivity is about 86% and the specificity about 93%.
Total CSF LD levels are also increased in patients with CNS leukemia, lymphoma, metastatic carcinoma, bacterial meningitis, and subarachnoid hemorrhage ( ). CSF LD isoenzymes have been shown to add considerable specificity in the evaluation of various metastatic brain tumors ( ). The LD 5 /total LD ratio is increased (i.e., above 10%–15%) in patients with leptomeningeal metastases from carcinoma of the breast and lung and malignant melanoma. LD isoenzyme analysis is also useful in detecting CNS involvement by leukemias and lymphomas ( ). Isoenzyme analysis shows a distinct pattern in young children with infantile spasms ( ) and febrile convulsions ( ). Compared with controls, both disorders are characterized by decreased LD 1 , increased LD 2 and LD 3 , and no changes in LD 4 and LD 5 .
CT is of limited value in estimating recovery potential and neurologic outcome during the early stages of ischemic brain injury. However, compared with controls (mean LD, 11.2 U/L), patients with an early stroke had a mean level of 40.9 U/L, and those with a transient ischemic attack (TIA) had a mean value of 11.8 U/L ( ). In patients with hypoxic brain injury, an increased LD level 72 hours following resuscitation indicates a poor prognosis ( ).
Lysozyme (muramidase) catalyzes the depolymerization of mucopolysaccharides. Because the enzyme is particularly rich in neutrophil and macrophage lysosomes, its activity is very low in normal CSF. However, CSF lysozyme activity is significantly increased in patients with both bacterial and tuberculous meningitis. Thus, discriminant analysis demonstrated that 97% of patients with bacterial meningitis had increased lysozyme levels ( ). Others found that patients with tuberculous meningitis had significantly higher CSF lysozyme levels than those with bacterial meningitis, partially treated bacterial meningitis, and controls ( ). The diagnostic sensitivity and specificity for tuberculous meningitis were 93.7% and 84.1%, respectively. Increased levels are also present in cerebral atrophy, various CNS tumors, multiple sclerosis, intracranial hemorrhage, and epilepsy ( ).
CSF ammonia levels vary from 30% to 50% of blood values. Elevated levels are generally proportional to the degree of existing hepatic encephalopathy but are difficult to quantify. Moreover, because hepatic encephalopathy generally correlates with blood ammonia levels, the measurement of CSF ammonia has little, if any, clinical value. However, cerebral glutamine, synthesized from ammonia and glutamic acid, serves as the means for CNS ammonia removal. Thus, CSF glutamine levels reflect the concentration of brain ammonia. Glutamine reference intervals are method dependent; the upper reference level is about 20 mg/dL. Values over 35 mg/dL are usually associated with hepatic encephalopathy ( ). Elevated CSF glutamine levels have also been reported in patients with depression ( ) and encephalopathy secondary to hypercapnia and sepsis ( ). Measurement of a variety of biogenic amines in CSF has been shown to assist in the diagnosis of bacterial and viral meningitis ( ).
A major etiologic theory of schizophrenia involves dopamine. The cornerstone for this theory is the fact that neuroleptic drugs that block dopamine receptors are effective in the treatment of this disorder. Thus, it has been reported that CSF levels of homovanillic acid (HVA), a metabolite of the biogenic amines, are related to the severity of schizophrenic psychosis ( ). However, HVA concentration varied as a function of psychosis rather than being related to the diagnosis of schizophrenia per se. Others have reported decreased CSF levels of 5-hydroxyindoleacetic acid, a metabolite of serotonin, in schizophrenic patients with suicidal behavior ( ). This report adds support for a possible relationship between suicide and CNS serotonin metabolism.
Although free CSF amino acids are relatively high in infants younger than 30 days of age, their concentration is further increased in those with febrile convulsions and bacterial meningitis. γ-Aminobutyric acid (GABA), a major inhibitory brain transmitter, is significantly decreased in basal ganglia neurons and is very low or undetectable in the CSF of patients with AD and Huntington disease ( ; ). CSF GABA was detected in all patients with migraine attacks, but not in those with tension headaches or in the control group without headaches ( ). Infants with startle disease, a rare inherited autosomal-dominant disorder characterized by seizures or the so-called stiff baby syndrome , have significantly decreased CSF GABA levels ( ; ).
There are no clinically useful indications for the measurement of CSF sodium, potassium, chloride, calcium, or magnesium. Measurements of CSF pH, partial pressure of carbon dioxide (pCO 2 ), and bicarbonate are also not practical for patient care ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here