Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The field of pathology is dedicated to understanding the causes of disease and the changes in cells, tissues, and organs that are associated with development of disease. Thus, pathology provides the scientific foundation for the practice of medicine. There are two important terms that students will encounter throughout their study of pathology and medicine:
Etiology is the origin of a disease, including the underlying causes and modifying factors. Notably, many common diseases, such as hypertension, diabetes, and cancer, are caused by a combination of inherited genetic susceptibility and various environmental triggers. Elucidating the genetic and environmental factors underlying diseases is a major goal of modern medicine.
Pathogenesis refers to the steps in the development of disease, from the initial etiologic trigger to the cellular and molecular changes that give rise to the specific functional and structural abnormalities which characterize any particular disease. Thus, etiology refers to why a disease arises and pathogenesis describes how a disease develops ( Fig. 1.1 ).
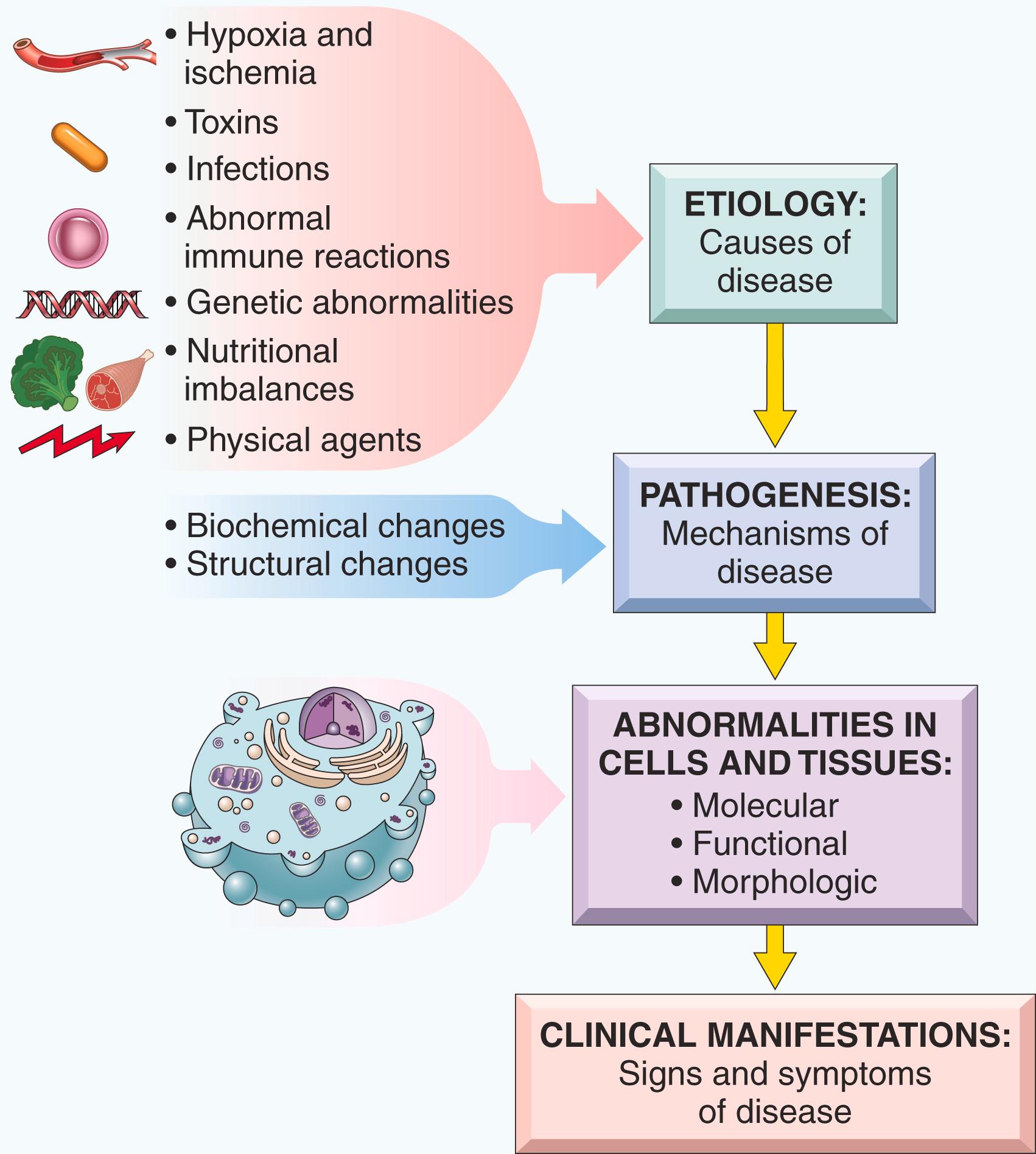
Defining the etiology and pathogenesis of disease is essential not only for understanding disease but is also the basis for developing rational treatments. It is now appreciated that even diseases that present with similar morphologic features (e.g., cancer of a particular organ) show important molecular differences (e.g., mutations, epigenetic modifications) from case to case. This realization has launched the field of precision (or personalized ) medicine , in which therapies are designed for each individual’s disease rather than the diseases as a whole.
To render diagnoses and guide therapy in clinical practice, pathologists identify changes in the gross or microscopic appearance (morphology) of cells and tissues and in their constituents (e.g., genes and proteins), as well as biochemical alterations in body fluids (such as blood and urine). Defining these alterations in diseased tissues aids in diagnosis as well as in predicting outcomes and optimal therapies.
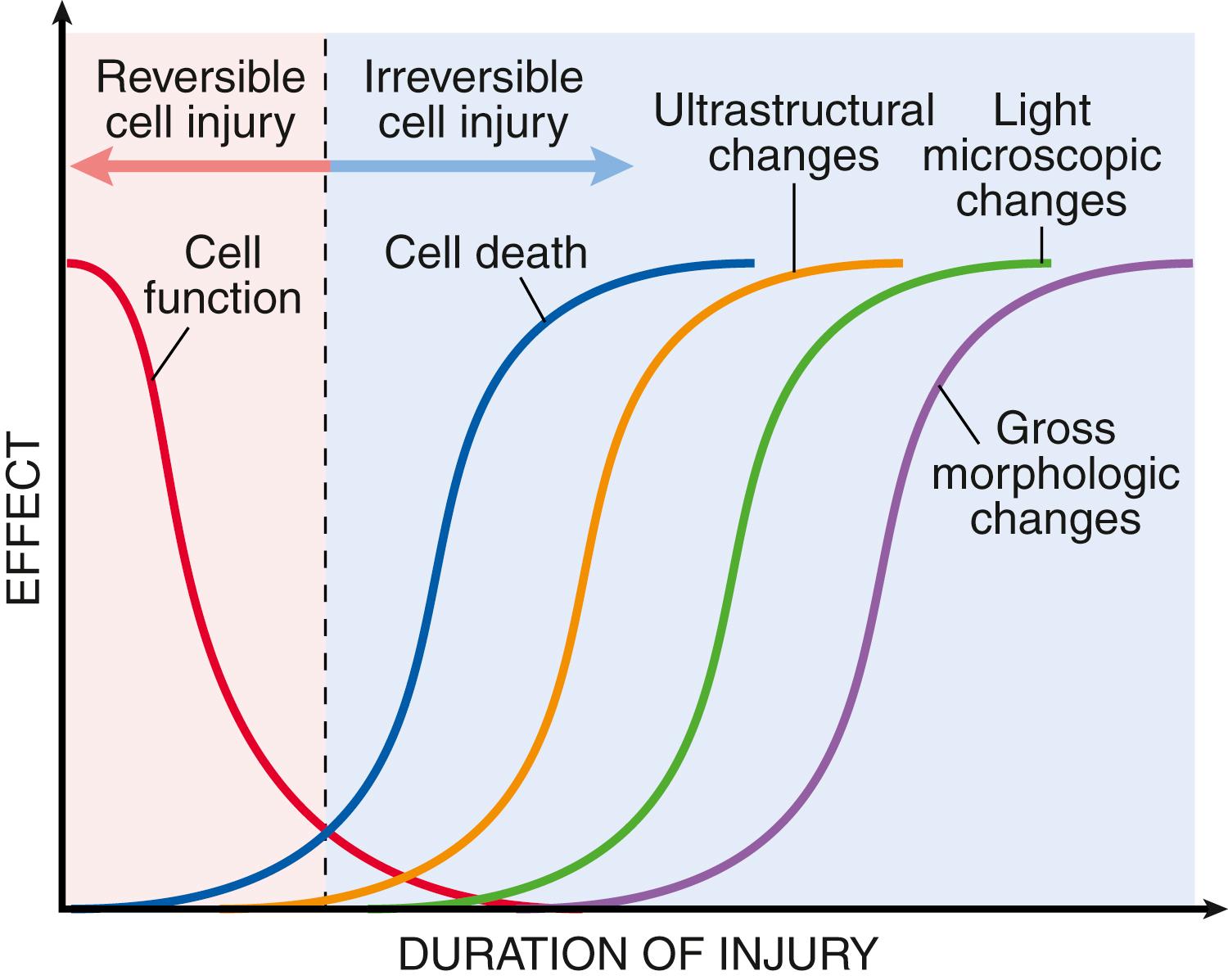
Cells actively interact with their environment, constantly adjusting their structure and function to accommodate changing demands and extracellular stresses in order to maintain a steady state, a process called homeostasis . As cells encounter physiologic stresses or injurious stimuli, they can undergo adaptation , achieving a new steady state and preserving viability and function. If the adaptive capability is exceeded or if the external stress is inherently harmful, cell injury occurs ( Fig. 1.2 ). Within certain limits, injury is reversible , and homeostasis is restored; however, if the stress is severe or persistent, it results in irreversible injury and death of the affected cells. Cell death is a crucial event in the development of many diseases.
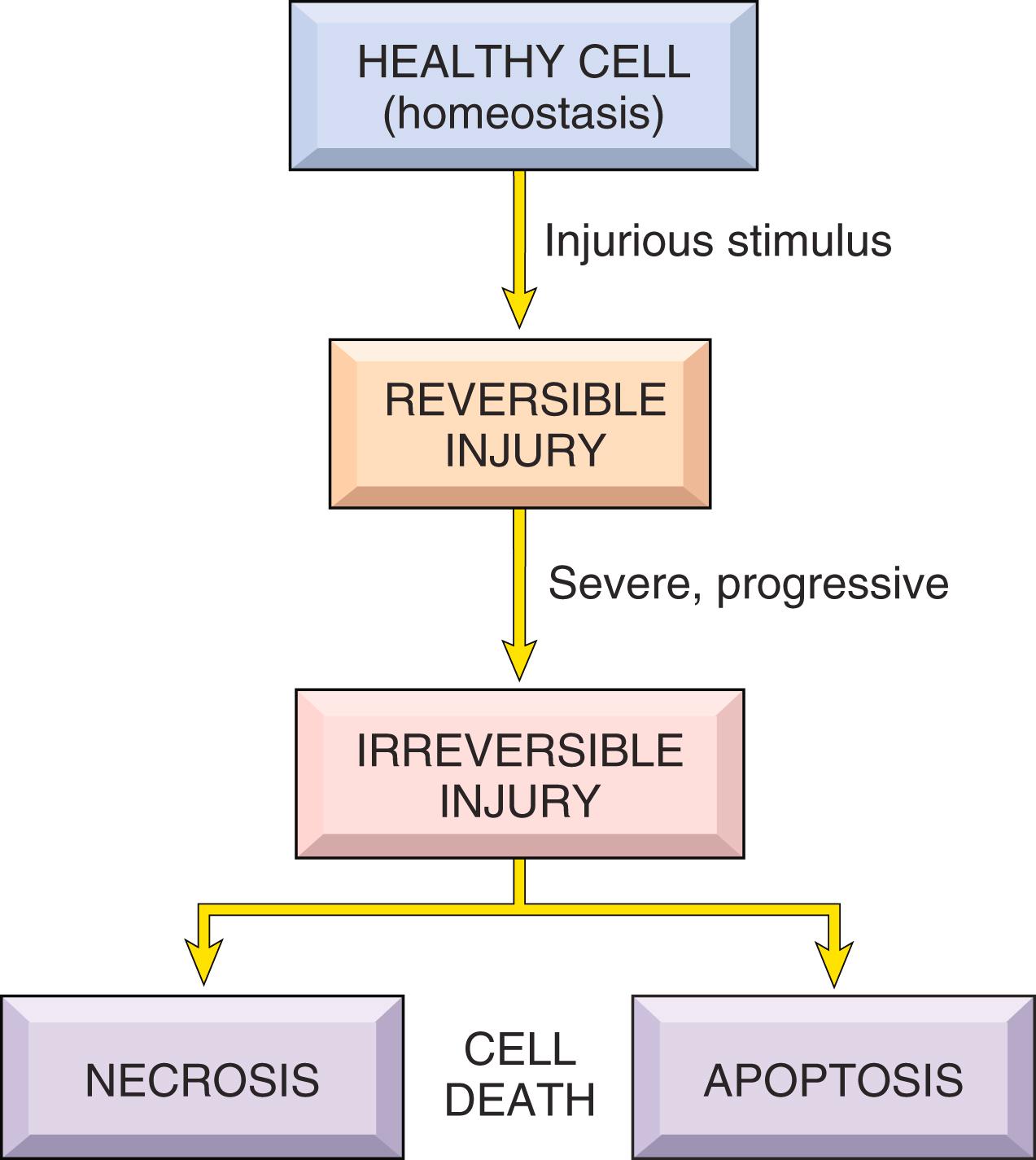
Cell injury is the basis of all disease, and in this chapter we discuss the causes, mechanisms, and consequences of reversible injury and cell death. We then consider cellular adaptations to stress and conclude with two other processes that affect cells and tissues, the deposition of abnormal substances and cell aging.
The major causes of cell injury can be grouped into the following categories.
Hypoxia and ischemia. Hypoxia refers to oxygen deficiency, and ischemia means reduced blood supply. These are among the most common causes of cell injury. Both deprive tissues of oxygen, the essential molecule for generating energy for cell function and survival, and ischemia also reduces the supply of nutrients. The most common cause of hypoxia is ischemia resulting from blockage of an artery, but it can also result from inadequate oxygenation of the blood, as in diseases of the lung, or from reduction in the oxygen-carrying capacity of the blood, as with anemia of any cause.
Toxins. Potentially toxic agents are encountered daily in the environment; these include air pollutants, insecticides, carbon monoxide, asbestos, cigarette smoke, ethanol, and drugs. Many therapeutic drugs can cause cell or tissue injury in a susceptible patient or if used excessively or inappropriately ( Chapter 7 ).
Infectious agents . All types of infectious pathogens, such as viruses, bacteria, fungi, and parasites, can injure cells by diverse mechanisms, including liberation of toxins and eliciting harmful immune responses.
Immunologic reactions. Although the immune system defends the body against pathogenic microbes, immune reactions can also result in cell and tissue injury. Examples are autoimmune reactions against one’s own tissues, allergic reactions against environmental substances, and excessive or chronic immune responses to microbes ( Chapter 5 ). In all these situations, the immune responses elicit inflammatory reactions, and inflammation is often the cause of damage to cells and tissues.
Genetic abnormalities. Some chromosomal abnormalities or mutations can result in pathologic changes as conspicuous as the congenital malformations associated with Down syndrome or as subtle as the single amino acid substitution in hemoglobin that gives rise to sickle cell anemia ( Chapter 4 ). Mutations may cause cell injury as a consequence of a decrease (e.g., enzymes in inborn errors of metabolism) or an increase in the function of a protein, or through the accumulation of damaged DNA or misfolded proteins, which can trigger cell death. Mutations also have a central role in cancer development ( Chapter 6 ).
Nutritional imbalances. Protein-calorie insufficiency remains a major cause of cell injury, and specific vitamin deficiencies occur frequently even in countries with plentiful resources ( Chapter 7 ). On the other hand, excessive dietary intake may result in obesity, which is an important underlying factor in many common diseases, such as type 2 diabetes and atherosclerosis.
Physical agents. Trauma, extremes of temperature, radiation, electric shock, and sudden changes in atmospheric pressure all have damaging effects on cells ( Chapter 7 ).
With this introduction, we proceed to a discussion of the process and morphologic manifestations of cell injury and then the biochemical mechanisms of injury caused by different noxious stimuli.
Although injurious stimuli damage cells through diverse biochemical mechanisms, all tend to induce a stereotypic sequence of morphologic and structural alterations in most cell types.
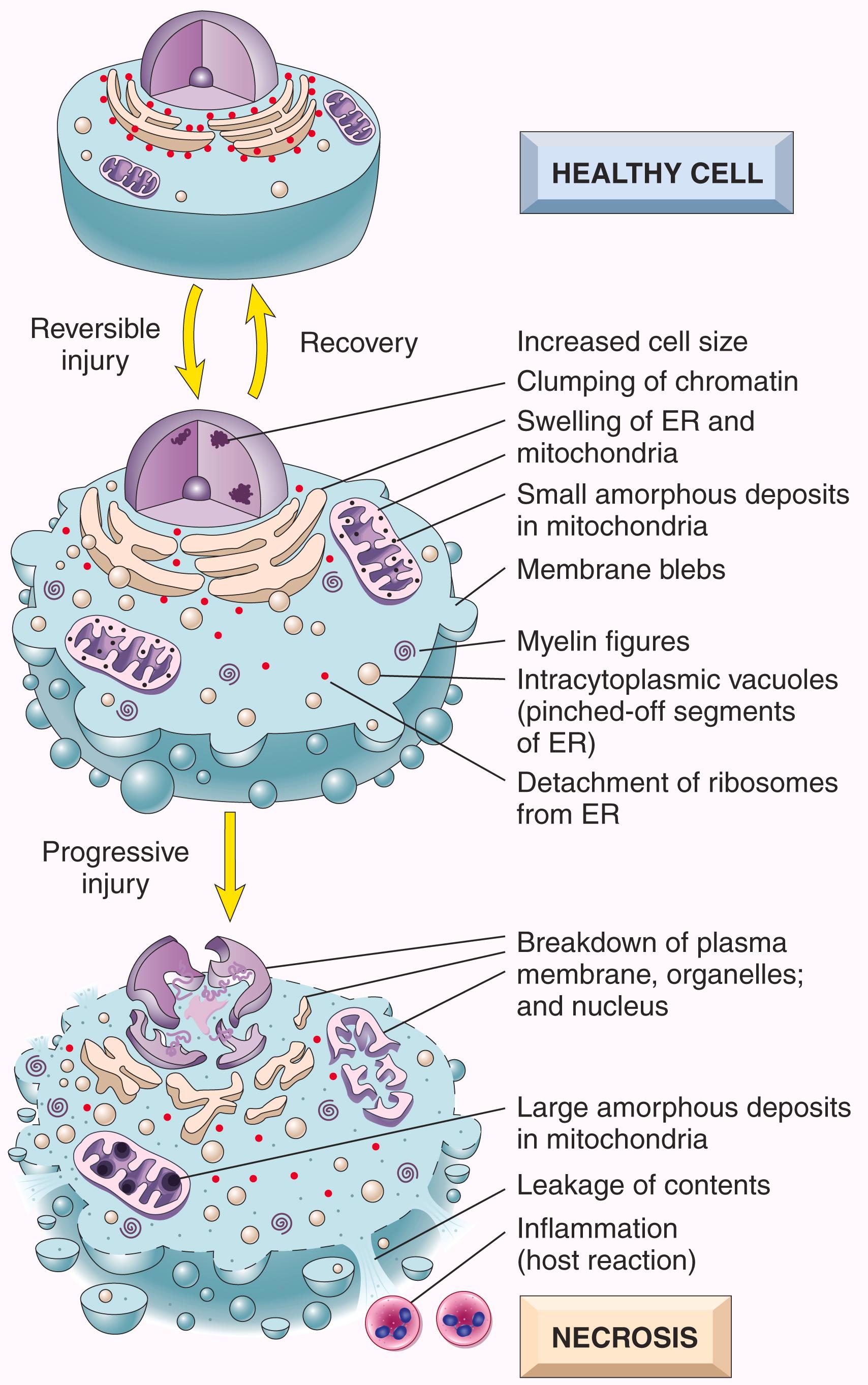
Reversible cell injury is defined as a derangement of function and morphology that cells can recover from if the damaging stimulus is removed ( Fig. 1.3 ). In reversible injury, cells and intracellular organelles become swollen because they take in water as a result of the failure of energy-dependent ion pumps in the plasma membrane. In some forms of injury, degenerated organelles and lipids accumulate inside the injured cells.
The two most consistent morphologic correlates of reversible cell injury are cellular swelling and fatty change.
Cellular swelling ( Fig. 1.4B ) is commonly seen when cells are injured by hypoxia, toxins, and other causes. It may be difficult to appreciate with the light microscope (since fluid from cells is extracted during tissue processing) but is often apparent grossly when the whole organ is examined. When many cells in an organ are affected, there can be pallor (due to compression of capillaries), increased turgor, and increased organ weight. Microscopic examination may reveal small, clear vacuoles within the cytoplasm; these represent distended and pinched-off segments of the endoplasmic reticulum (ER). This pattern of nonlethal injury is sometimes called hydropic change or vacuolar degeneration.
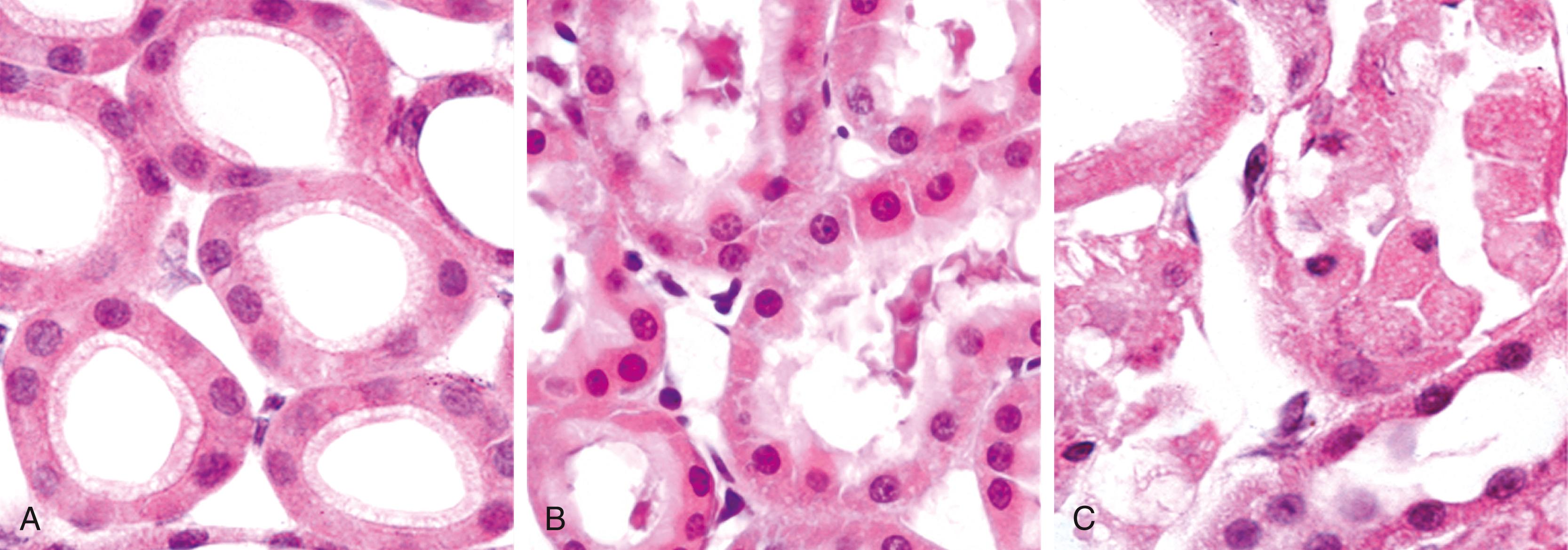
Fatty change is manifested by the appearance of lipid vacuoles in the cytoplasm. It is principally encountered in organs that are involved in lipid metabolism, such as the liver, and hence it is discussed in Chapter 14 .
The cytoplasm of injured cells also may become redder (eosinophilic, meaning stained red by the dye eosin—the E in the hematoxylin and eosin [H&E] stain)—a change that becomes more pronounced with progression to necrosis (described later). Other intracellular changes associated with cell injury, which are best seen by electron microscopy ( eFig. 1.1 ), include: (1) plasma membrane alterations such as blebbing, blunting, or distortion of microvilli, and loosening of intercellular attachments; (2) mitochondrial changes such as swelling and the appearance of phospholipid-rich amorphous densities; (3) dilation of the ER with detachment of ribosomes and dissociation of polysomes; and (4) nuclear alterations, with clumping of chromatin. The cytoplasm may contain so-called myelin figures, collections of phospholipids resembling myelin sheaths that are derived from damaged cellular membranes.
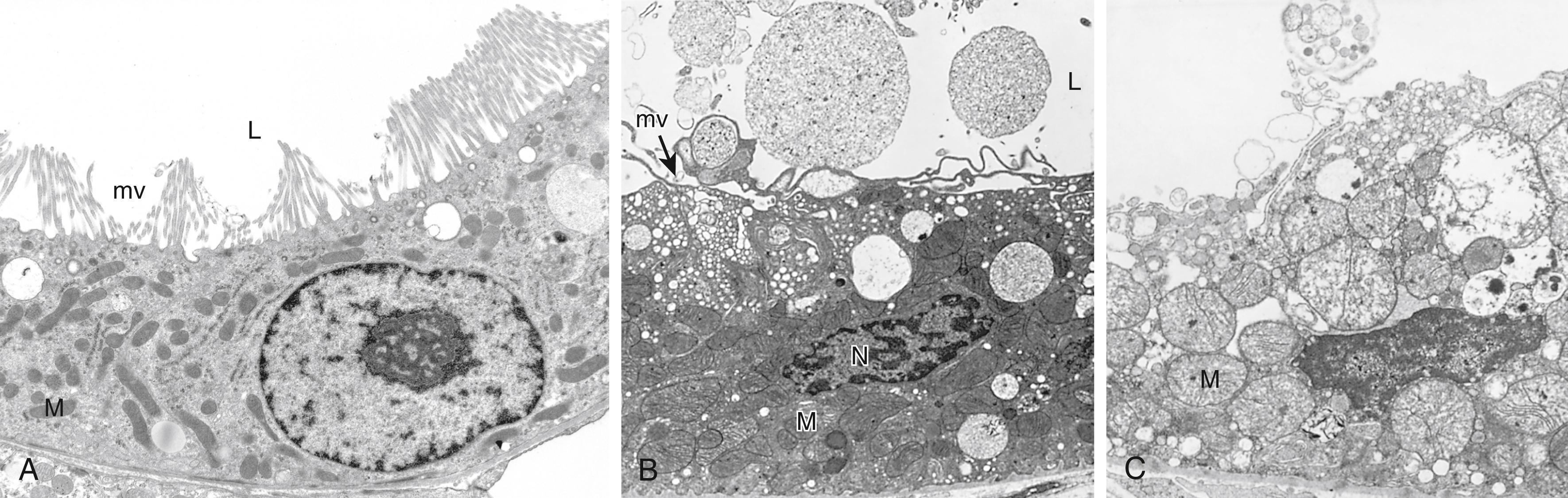
In some situations, potentially injurious insults induce specific alterations in cellular organelles, like the endoplasmic reticulum (ER). The smooth ER is involved in the metabolism of various chemicals, including alcohol and drugs such as barbiturates ( Chapter 7 ). Cells exposed to these chemicals show hypertrophy of the smooth ER as an adaptive response that may have important functional consequences. Cells adapted to one drug may have increased capacity to metabolize other compounds handled by the same system. Thus, if patients taking phenobarbital for epilepsy increase their alcohol intake, they may experience a drop in blood concentration of the antiseizure medication to subtherapeutic levels because of increased activity of the smooth ER in response to the alcohol.
Persistent or excessive injury causes injured cells to pass the nebulous “point of no return” and undergo cell death, typically by the process of necrosis. Although there are no definitive morphologic or biochemical correlates of irreversible injury, it is consistently characterized by three phenomena: the inability to restore mitochondrial function (oxidative phosphorylation and adenosine triphosphate [ATP] generation) even after resolution of the original injury; altered structure and loss of function of the plasma membrane and intracellular membranes; and the loss of structural integrity of DNA and chromatin. As discussed in more detail later, injury to lysosomal membranes results in the enzymatic digestion of the injured cell, which is the culmination of necrosis.
When cells are injured, they die by different mechanisms, depending on the nature and severity of the insult ( Table 1.1 ).
Necrosis . Severe disturbances, such as loss of oxygen and nutrient supply and the actions of toxins, cause a rapid and uncontrollable form of death that has been called “accidental” cell death. The morphologic manifestation of accidental cell death is necrosis (Greek, necros = death). Necrosis is the major pathway of cell death in many commonly encountered injuries, such as those resulting from ischemia, exposure to toxins, various infections, and trauma. Necrosis is considered the inevitable end result of severe damage that is beyond salvage and is not thought to be regulated by specific signals or biochemical mechanisms; necrosis occurs because the injury goes beyond what a cell can repair or survive.
Apoptosis . By contrast, when cells must be eliminated without eliciting a host reaction, a precise set of molecular pathways is activated in the cells that produce a form of cell death called apoptosis (see Table 1.1 ). Apoptosis relies on defined genes and biochemical pathways and must be tightly controlled because once it starts, it is irreversible; thus, it is referred to as “regulated” cell death. The discovery of regulated cell death was a revelation, since it showed that cell death can be an intentional, highly controlled process. Apoptosis serves to eliminate cells with a variety of intrinsic abnormalities and promotes clearance of the fragments of the dead cells without eliciting an inflammatory reaction. This “clean” form of cell suicide occurs in pathologic situations when a cell’s DNA or proteins are damaged beyond repair or the cell is deprived of necessary survival signals. But unlike necrosis, which is always an indication of a pathologic process, apoptosis also occurs in healthy tissues and is not necessarily associated with pathologic cell injury. For example, it serves to eliminate unwanted cells during development and to maintain constant cell numbers. This type of physiologic cell death is also called programmed cell death.
| Feature | Necrosis | Apoptosis |
|---|---|---|
| Cell size | Enlarged (swelling) | Reduced (shrinkage) |
| Nucleus | Pyknosis → karyorrhexis → karyolysis | Fragmentation into nucleosome-sized fragments |
| Plasma membrane | Disrupted | Intact; altered structure, especially orientation of lipids |
| Cellular contents | Enzymatic digestion; may leak out of cell | Intact; may be released in apoptotic bodies |
| Adjacent inflammation | Frequent | Absent |
| Physiologic or pathologic role | Invariably pathologic (culmination of irreversible cell injury) | Often physiologic; means of eliminating unnecessary cells; may be pathologic after some forms of cell injury, especially DNA and protein damage |
It is important to recognize that cellular function may be lost long before cell death occurs and that the morphologic changes of cell injury (or death) lag behind loss of function and viability ( Fig. 1.5 ). For example, myocardial cells become noncontractile after 1 to 2 minutes of ischemia but may not die until 20 to 30 minutes of ischemia have elapsed. Morphologic features indicative of the death of ischemic myocytes appear by electron microscopy within 2 to 3 hours after the death of the cells but are not evident by light microscopy until 6 to 12 hours later.
In necrosis, cellular membranes fall apart, cellular enzymes leak out and ultimately digest the cell, and there is an accompanying inflammatory reaction (see Fig. 1.3 ). The local host reaction, called inflammation , is induced by substances released from dead cells and serves to eliminate debris and start the subsequent repair process ( Chapter 2 ). The enzymes responsible for digestion of the dead cells come from leukocytes that are recruited as part of the inflammatory reaction and from the disrupted lysosomes of the dying cells themselves.
The biochemical mechanisms of necrosis vary with different injurious stimuli and are described later.
Necrosis is characterized by changes in the cytoplasm and nuclei of the injured cells (see Figs. 1.3 and 1.4C ).
Cytoplasmic changes. Necrotic cells show increased eosinophilia, attributable in part to increased binding of eosin to denatured cytoplasmic proteins and in part to loss of basophilic ribonucleic acid (RNA) in the cytoplasm (basophilia stems from binding of the blue dye hematoxylin—the H in “H&E”). Compared with viable cells, necrotic cells may have a glassy, homogeneous appearance, mostly due to the loss of glycogen particles. When enzymes have digested cytoplasmic organelles, the cytoplasm becomes vacuolated and appears “moth-eaten.” By electron microscopy, necrotic cells are characterized by discontinuities in plasma and organelle membranes, marked dilation of mitochondria associated with large amorphous intramitrochondrial densities, disruption of lysosomes, and intracytoplasmic myelin figures, which are more prominent in necrotic cells than in cells with reversible injury ( eFig. 1.1 ).
Nuclear changes. Nuclear changes assume one of three patterns, all caused by breakdown of DNA and chromatin. Pyknosis is characterized by nuclear shrinkage and increased basophilia; the DNA condenses into a dark, shrunken mass. The pyknotic nucleus can subsequently undergo fragmentation; this change is called karyorrhexis . At the same time, the nucleus may undergo karyolysis , in which the basophilia fades due to digestion of deoxyribonucleic acid (DNA) by DNase. In 1 to 2 days, the nucleus in a dead cell may undergo complete dissolution.
Fates of necrotic cells. Necrotic cells may persist for some time or may be digested by enzymes and disappear. Dead cells may be replaced by myelin figures, which are either phagocytosed by other cells or further degraded into fatty acids. These fatty acids bind calcium salts, which may result in the dead cells ultimately becoming calcified ( dystrophic calcification , see later).
Some severe injuries result in the death of many or all cells in a tissue or even an entire organ. This may happen in severe ischemia, infections, and inflammatory reactions. There are several morphologically distinct patterns of tissue necrosis, which may provide clues about the underlying cause. Although the terms that describe these patterns do not reflect underlying mechanisms, such terms are commonly used and their implications are understood by pathologists and clinicians. Most of the types of necrosis have distinctive gross appearances; fibrinoid necrosis is detected only by microscopic examination.
In coagulative necrosis, the underlying tissue architecture is preserved for at least several days after the injury ( Fig. 1.6 ). The affected tissues take on a firm texture. Presumably, the injury denatures not only structural proteins but also enzymes, limiting the proteolysis of the dead cells; as a result, eosinophilic, anucleate cells may persist for days or weeks. Ultimately, the dead cells are digested by the lysosomal enzymes of recruited leukocytes and the cellular debris is removed by phagocytosis. Coagulative necrosis is characteristic of infarcts (areas of necrosis caused by ischemia) in all solid organs except the brain.
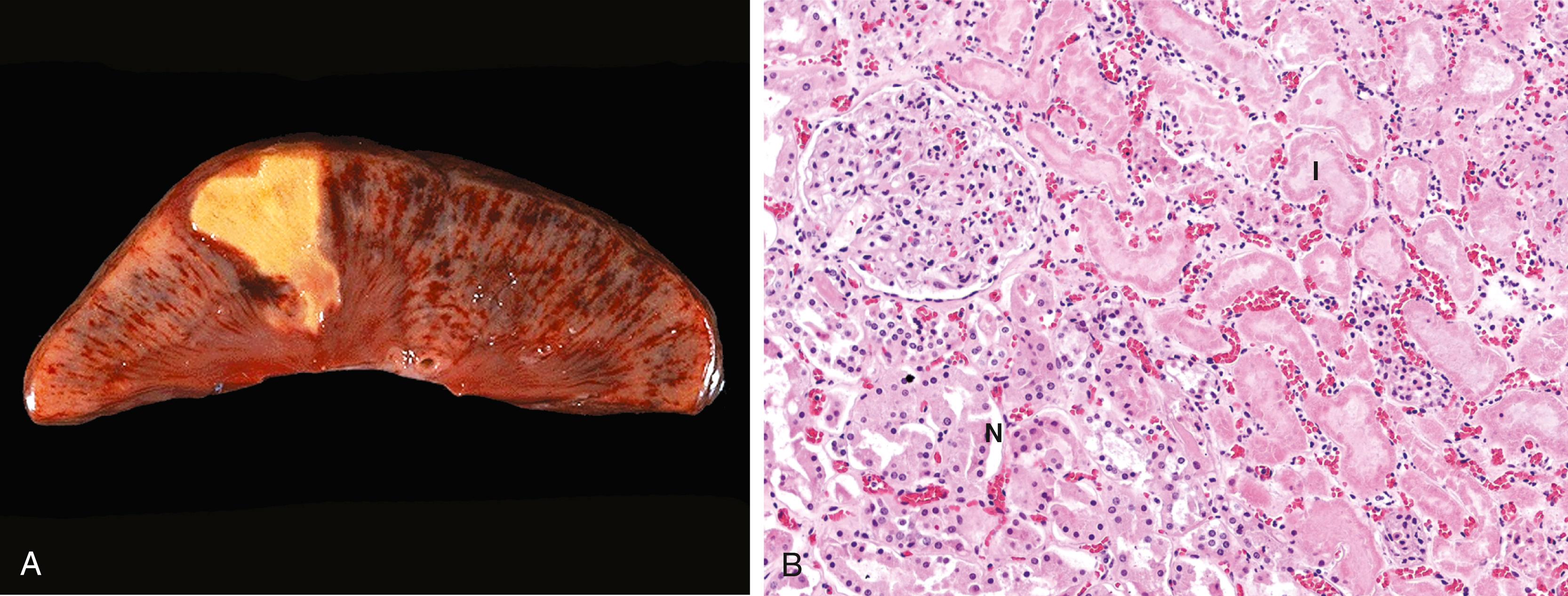
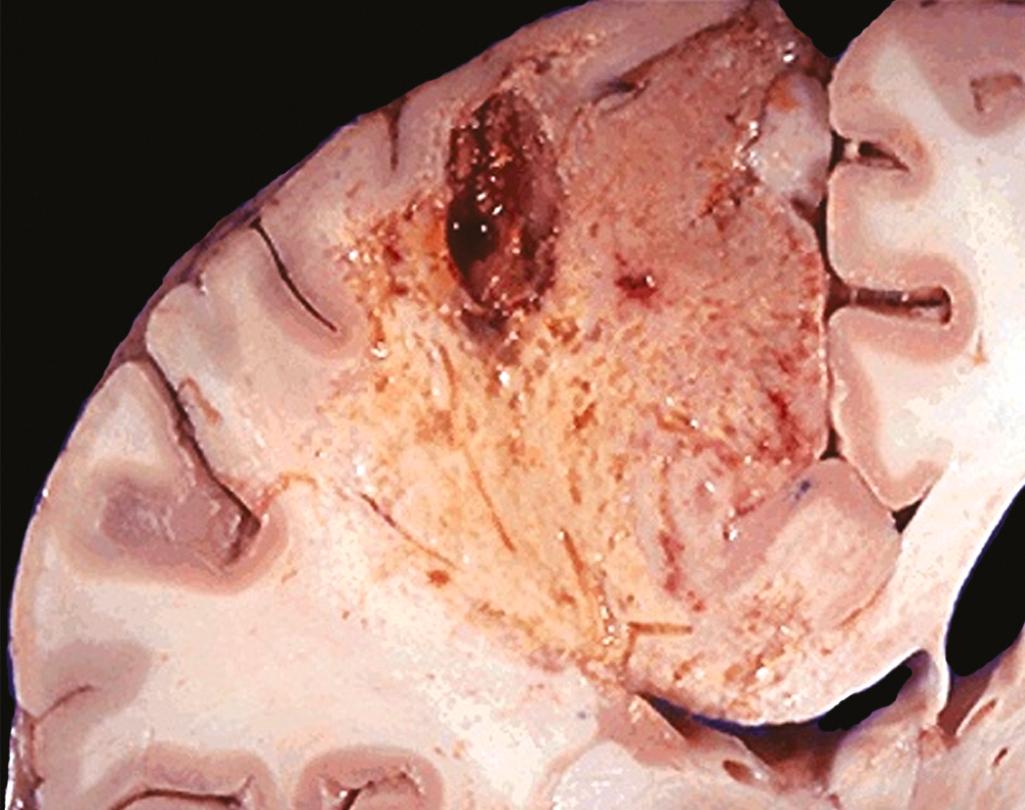
Liquefactive necrosis is seen at sites of bacterial or, occasionally, fungal infections, because microbes stimulate the accumulation of inflammatory cells and the enzymes of leukocytes digest (“liquefy”) the tissue. For obscure reasons, hypoxic death of cells within the central nervous system often causes liquefactive necrosis ( Fig. 1.7 ). In this form of necrosis, the dead cells are completely digested, transforming the tissue into a viscous liquid that is eventually removed by phagocytes. When the process is initiated by acute inflammation, as in a bacterial infection, the material is frequently creamy yellow and is called pus . A localized collection of pus is called an abscess ( Chapter 2 ).
Although gangrenous necrosis is not a distinctive pattern of cell death, the term is still commonly used in clinical practice. It usually refers to the condition of a limb (generally the lower leg) that has lost its blood supply and has undergone coagulative necrosis involving multiple tissue layers. When bacterial infection is superimposed, the morphologic appearance is often liquefactive because of destruction mediated by the contents of the bacteria and the attracted leukocytes (resulting in so-called wet gangrene ).
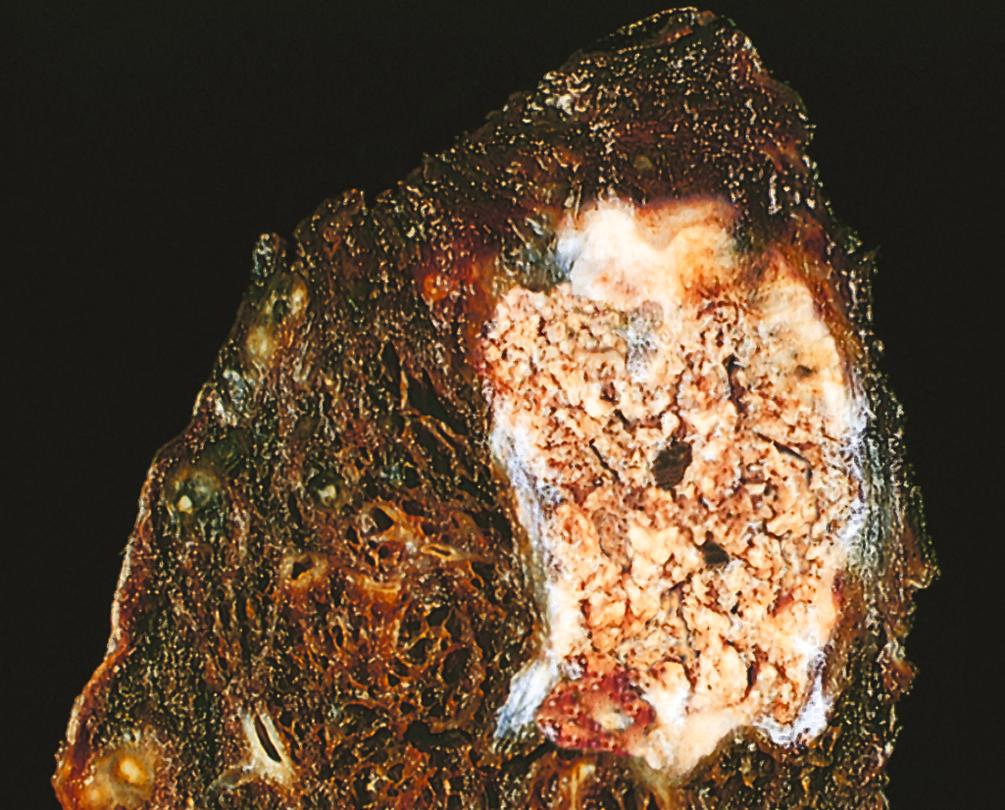
Caseous necrosis is encountered most often in foci of tuberculous infection. Caseous means “cheese-like,” referring to the friable yellow-white appearance of the area of necrosis ( Fig. 1.8 ). On microscopic examination, the necrotic focus appears as a collection of cellular debris with an amorphous granular pink appearance in H&E-stained tissue sections. Unlike coagulative necrosis, the tissue architecture is obliterated and cellular outlines cannot be discerned. Caseous necrosis is often surrounded by a collection of macrophages and other inflammatory cells; this appearance is characteristic of a nodular inflammatory lesion called a granuloma ( Chapter 2 ).
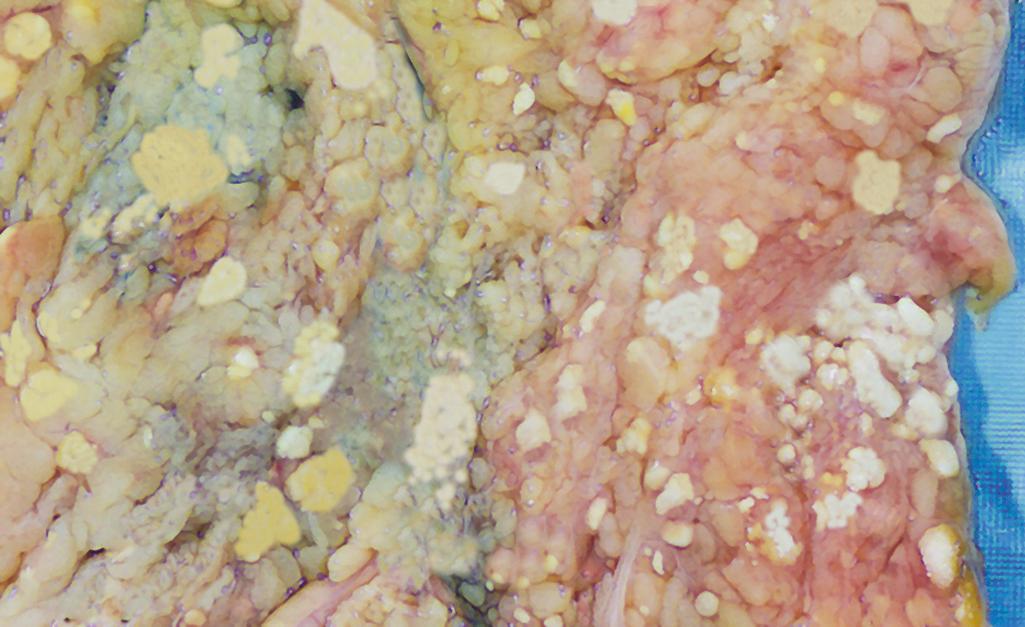
Fat necrosis refers to focal areas of fat destruction, which can be due to abdominal trauma or acute pancreatitis ( Chapter 15 ), in which enzymes leak out of damaged pancreatic acinar cells and ducts and digest peritoneal fat cells and their contents, including stored triglycerides. The released fatty acids combine with calcium to produce grossly identifiable chalky white lesions ( Fig. 1.9 ). On histologic examination, the foci of necrosis contain shadowy outlines of necrotic fat cells surrounded by granular basophilic calcium deposits and an inflammatory reaction.
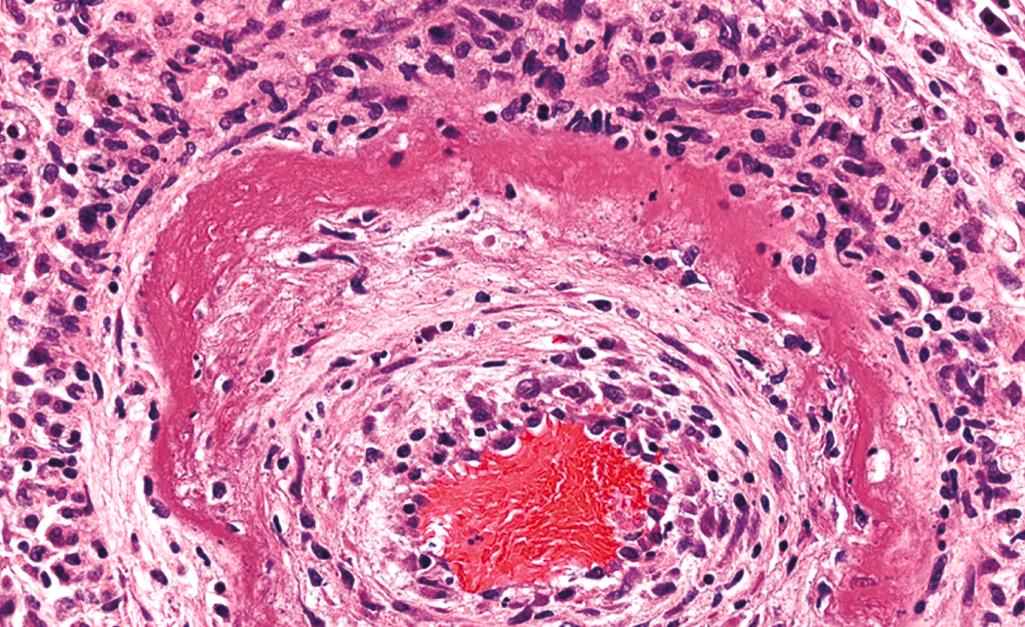
Fibrinoid necrosis is a special form of necrosis, visible by light microscopy. It may be seen in immune reactions in which complexes of antigens and antibodies are deposited in the walls of blood vessels, and in severe hypertension. Deposited immune complexes and plasma proteins that have leaked into the walls of injured vessels produce a bright pink, amorphous appearance on H&E preparations called fibrinoid (fibrin-like) by pathologists ( Fig. 1.10 ). Fibrinoid necrosis is seen most often in certain forms of vasculitis ( Chapter 3 ) and in transplanted organs undergoing rejection ( Chapter 5 ).
Leakage of intracellular proteins through the damaged cell membrane provides a means of detecting tissue-specific necrosis using blood or serum samples. Cardiac muscle, for example, contains a unique isoform of the contractile protein troponin, whereas hepatic bile duct epithelium contains a temperature-resistant isoform of the enzyme alkaline phosphatase, and hepatocytes contain transaminases. These proteins leak out of necrotic cells into the blood, where they serve as clinically useful markers of damage in the corresponding tissues.
Apoptosis is a pathway of cell death in which cells activate enzymes that degrade the cells’ own nuclear DNA and nuclear and cytoplasmic proteins ( Fig. 1.11 ). Fragments of the apoptotic cells then break off, giving the appearance that is responsible for the name ( apoptosis , “falling off”). The plasma membrane of the apoptotic cell remains intact, but the membrane is altered in such a way that the fragments, called apoptotic bodies, are recognized and rapidly phagocytosed by macrophages. In contrast to necrosis (see Table 1.1 ), the apoptotic cell and its fragments are cleared before cellular contents have leaked out, so apoptotic cell death does not elicit an inflammatory reaction in the host.
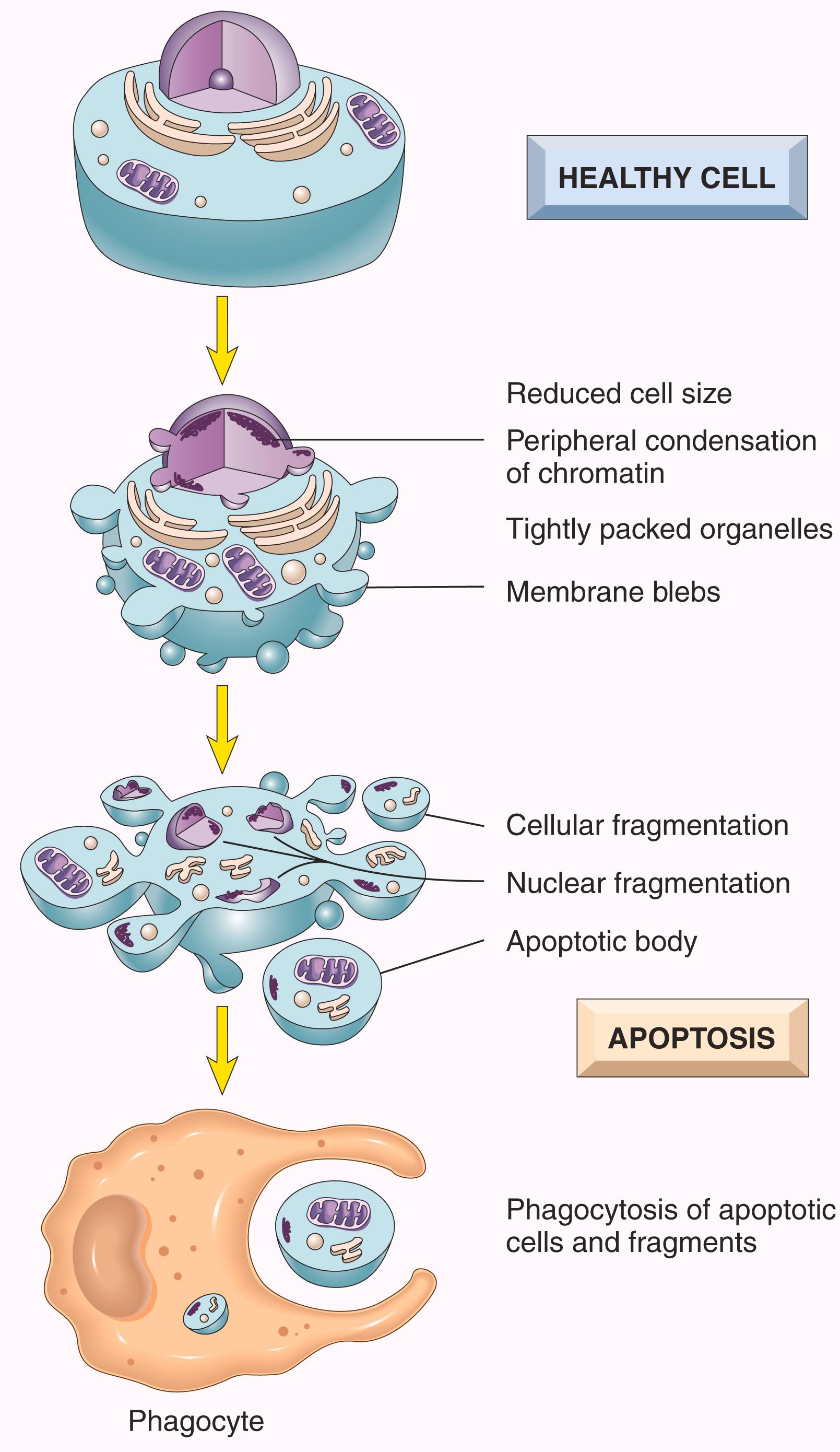
Apoptosis occurs in many physiologic conditions and serves to eliminate potentially harmful cells and cells that have outlived their usefulness ( Table 1.2 ). It also occurs as a pathologic event when cells are damaged beyond repair, especially when the damage affects the cells’ DNA or proteins.
Physiologic apoptosis. During the normal development of an organism, some cells die and are replaced by new ones. In mature organisms, highly proliferative and hormone-responsive tissues undergo cycles of proliferation and cell loss that are often determined by the levels of growth factors or survival signals. In these situations, the cell death is always by apoptosis, ensuring that unwanted cells are eliminated without eliciting potentially harmful inflammation. In the immune system, apoptosis removes excess leukocytes following immune responses, B lymphocytes in germinal centers that fail to produce high-affinity antibodies, and lymphocytes that recognize self antigens that could cause autoimmune diseases if they were to survive ( Chapter 5 ).
Apoptosis in pathologic conditions. Apoptosis eliminates cells with certain types of irreparable damage, such as severe DNA damage, e.g., after exposure to radiation and cytotoxic drugs. The accumulation of misfolded proteins also triggers apoptotic death; the underlying mechanisms of this cause of cell death and its significance in disease are discussed later, in the context of ER stress. Certain infectious agents, particularly some viruses, also induce apoptotic death of infected cells.
| Condition | Mechanism of Apoptosis |
|---|---|
| Physiologic | |
| During embryogenesis | Loss of growth factor signaling (presumed mechanism) |
| Turnover of proliferative tissues (e.g., intestinal epithelium, lymphocytes in lymph nodes and thymus) | Loss of growth factor signaling or survival signals (presumed mechanism) |
| Involution of hormone-dependent tissues (e.g., endometrium) | Decreased hormone levels lead to reduced survival signals |
| Decline of leukocyte numbers at the end of immune and inflammatory responses | Loss of survival signals as stimulus for leukocyte activation is eliminated |
| Elimination of potentially harmful self-reactive lymphocytes | Strong recognition of self antigens induces apoptosis by both the mitochondrial and death receptor pathways |
| Pathologic | |
| DNA damage | Activation of proapoptotic BH3-only proteins |
| Accumulation of misfolded proteins | Activation of proapoptotic BH3-only proteins, possibly direct activation of caspases |
| Infections, especially certain viral infections | Activation of proapoptotic proteins or caspases by viral proteins; killing of infected cells by cytotoxic T lymphocytes (CTLs), which activate caspases |
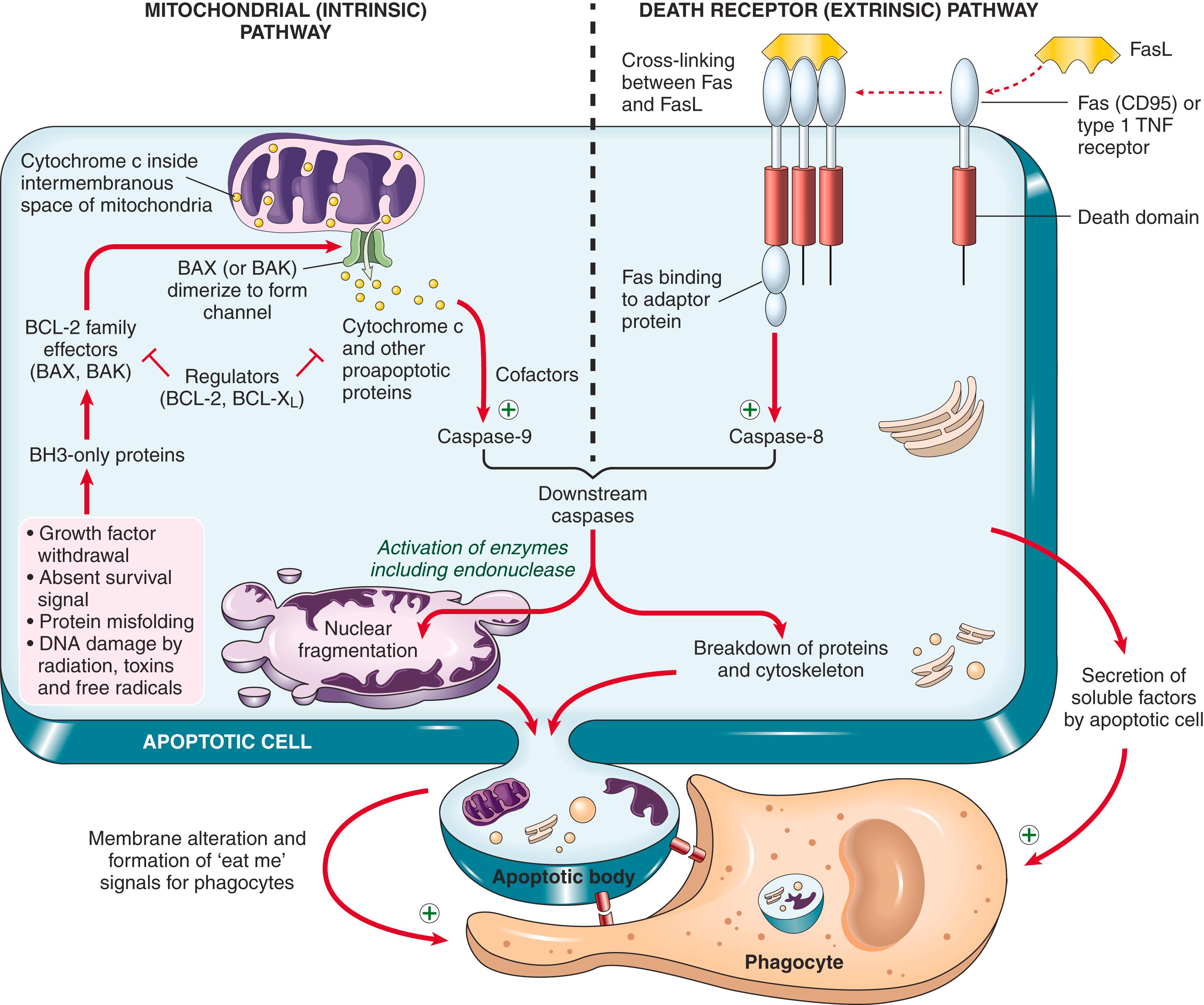
Apoptosis is regulated by biochemical pathways that control the balance of death- and survival-inducing signals and ultimately the activation of enzymes called caspases , so named because they are cy steine prote ases that cleave proteins after asp artic acid residues. Two distinct pathways converge on caspase activation: the mitochondrial pathway and the death receptor pathway ( Fig. 1.12 ). Although these pathways can intersect, they are generally induced under different conditions, involve different molecules, and serve distinct roles in physiology and disease.
The mitochondrial (intrinsic) pathway is responsible for apoptosis in most physiologic and pathologic situations. Mitochondria contain several proteins that are capable of inducing apoptosis, including cytochrome c. When mitochondrial membranes become permeable, cytochrome c leaks into the cytoplasm, triggering caspase activation and apoptotic death. The permeability of mitochondria is controlled by a family of more than 20 proteins, the prototype of which is BCL-2. In healthy cells, BCL-2 and the related protein BCL-X L are produced in response to growth factors and other stimuli that keep cells viable. These antiapoptotic proteins maintain the integrity of mitochondrial membranes, in large part by holding two proapoptotic members of the family, BAX and BAK, in check. When cells are deprived of growth factors and survival signals, are exposed to agents that damage DNA, or accumulate unacceptable amounts of misfolded proteins, a number of sensors are activated. The most important of these sensors are called BH3-only proteins because they contain the third homology domain of the BCL-2 family. These sensors shift the balance in favor of BAK and BAX, which dimerize, insert into the mitochondrial membrane, and form channels through which cytochrome c and other mitochondrial proteins escape into the cytosol. At the same time, the deficiency of survival signals leads to decreased levels of BCL-2 and BCL-X L , further compromising mitochondrial permeability. Once in the cytosol, cytochrome c interacts with certain cofactors and activates caspase-9, leading to the activation of a caspase cascade.
The death receptor (extrinsic) pathway of apoptosis. Many cells express surface molecules, called death receptors, which trigger apoptosis. Most of these are members of the tumor necrosis factor (TNF) receptor family, which contain in their cytoplasmic regions a conserved “death domain,” so named because it mediates interaction with other proteins involved in cell death. The prototypic death receptors are the type I TNF receptor and Fas (CD95). Fas ligand (FasL) is a membrane protein expressed mainly on activated T lymphocytes. When these T cells recognize Fas-expressing targets, Fas molecules are crosslinked by FasL and bind adaptor proteins via the death domain (see Fig. 1.12 ). These recruit and activate caspase-8, which in turn activates downstream caspases. The death receptor pathway is involved in elimination of self-reactive lymphocytes and in killing of target cells by some cytotoxic T lymphocytes that express FasL.
Terminal phase of apoptosis. Activated caspase-8 and caspase-9 act through a final common series of reactions that first involve the activation of additional caspases, which through numerous substrates ultimately activate enzymes that degrade the cell’s proteins and nucleus. The end result is the characteristic cellular fragmentation of apoptosis.
Clearance of apoptotic cells. Apoptotic cells and their fragments entice phagocytes by producing a number of “eat-me” signals. For instance, in normal cells phosphatidylserine is present on the inner leaflet of the plasma membrane, but in apoptotic cells this phospholipid “flips” to the outer leaflet, where it is recognized by tissue macrophages. Cells that are dying by apoptosis also secrete soluble factors that recruit phagocytes. Numerous macrophage receptors are involved in the binding and engulfment of apoptotic cells. This process is so efficient that the dead cells disappear without leaving a trace, and there is no accompanying inflammation.
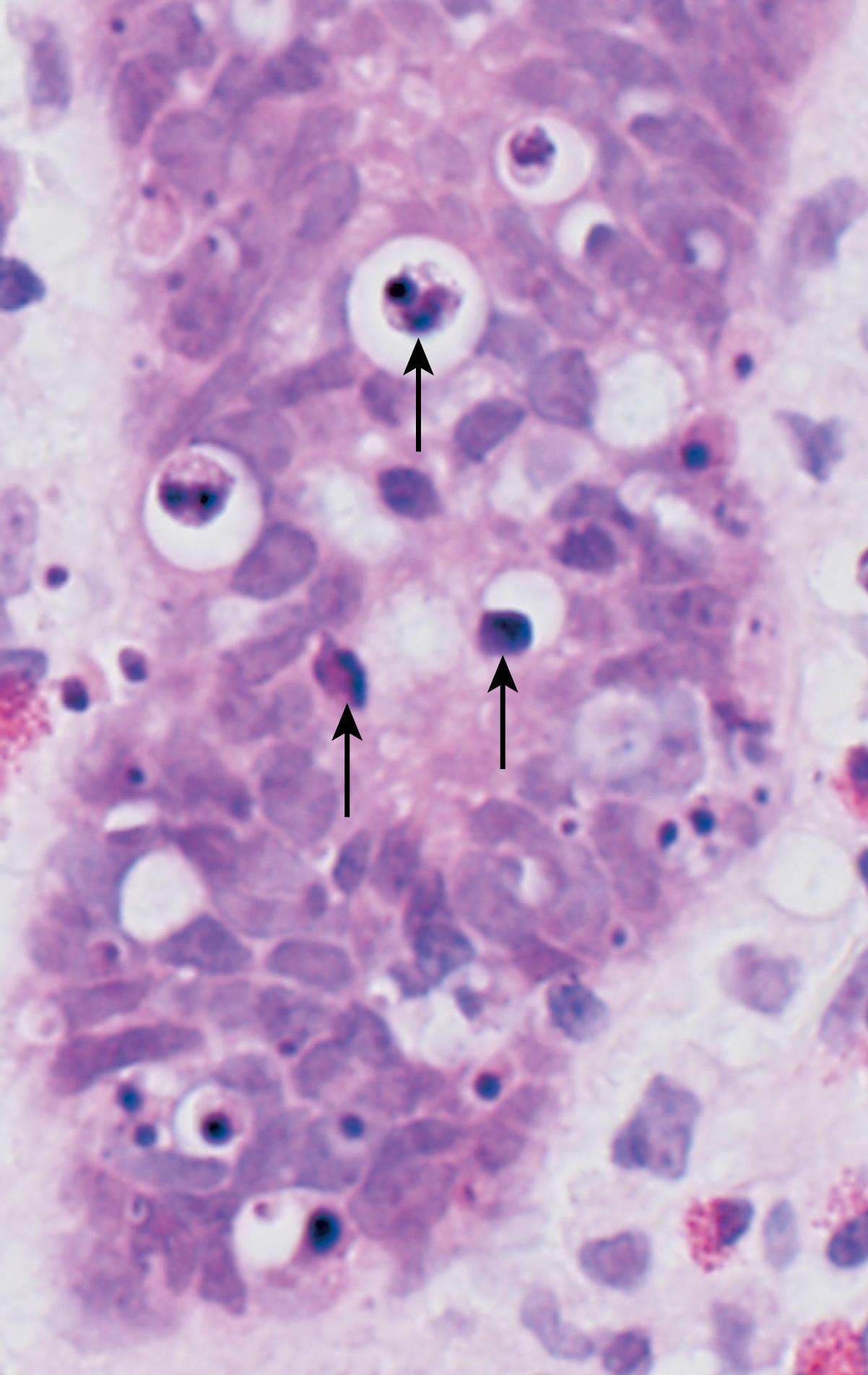
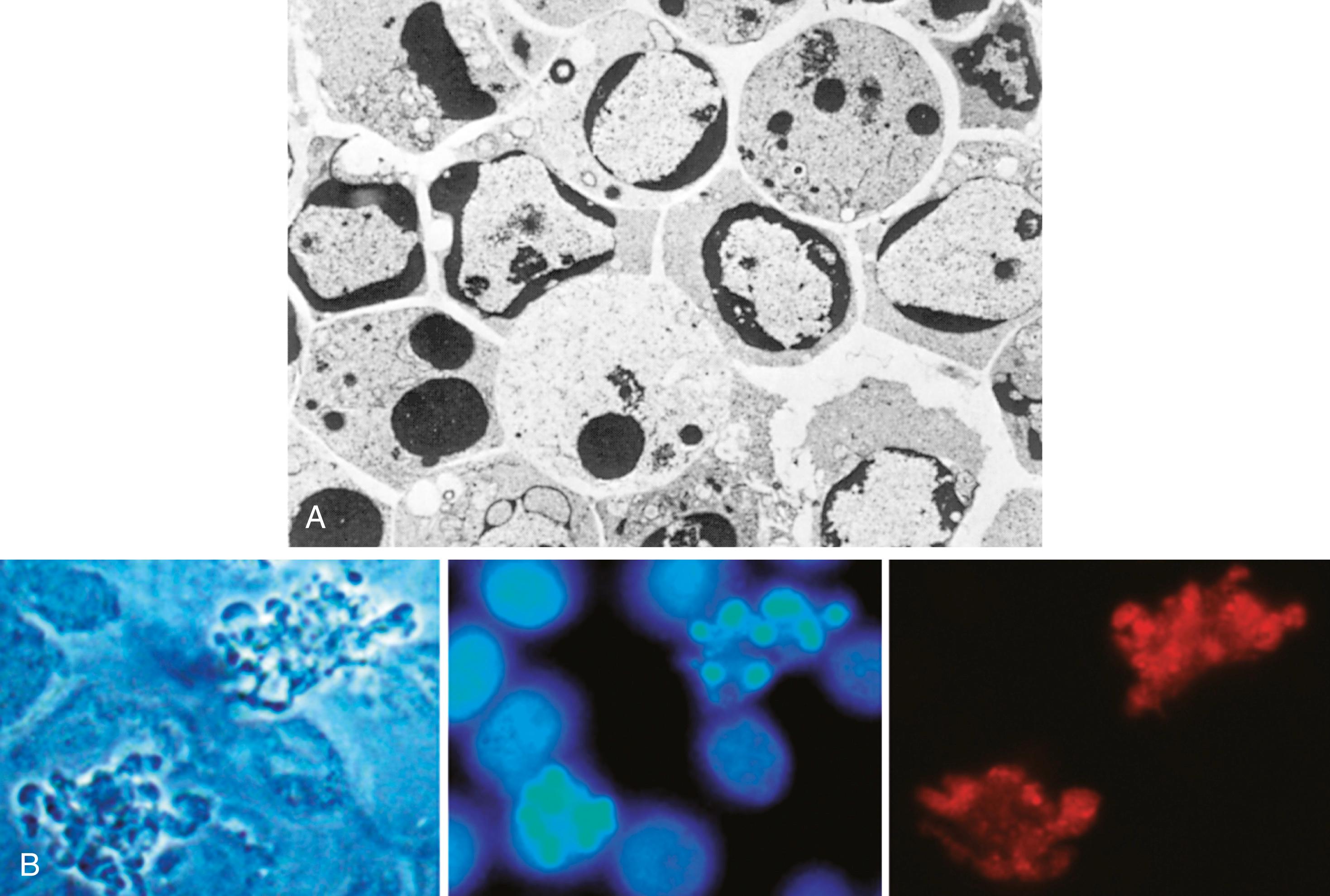
In H&E-stained tissue sections, the nuclei of apoptotic cells show various stages of chromatin condensation, aggregation, and, ultimately, karyorrhexis ( Fig. 1.13 ). At the molecular level this is reflected in fragmentation of DNA into nucleosome-sized pieces. The cells rapidly shrink, form cytoplasmic buds, and fragment into apoptotic bodies that are composed of membrane-bound pieces of cytosol and organelles ( eFig. 1.2 ; also see Fig. 1.11 ). Because these fragments are quickly shed and phagocytosed without eliciting an inflammatory response, even substantial apoptosis may be histologically undetectable.
Other pathways of cell death, in addition to necrosis and apoptosis, have been described. Necroptosis is a form of cell death caused by the cytokine tumor necrosis factor (TNF) that shows features of both necrosis and apoptosis, hence its name. Pyroptosis ( pyro , fever) is induced by activation of inflammasomes ( Chapter 5 ), which releases the cytokine interleukin-1 (IL-1), which cause inflammation and fever. Ferroptosis depends on levels of cellular iron. The roles of these mechanisms of cell death in normal physiology and pathologic states are not clearly established and remain topics of investigation.
Autophagy (“self-eating”) refers to lysosomal digestion of the cell’s own components. It is a survival mechanism in times of nutrient deprivation that enables the starved cell to live by eating its own contents and recycling these contents to provide nutrients and energy. In this process, intracellular organelles and portions of cytosol are first sequestered within an ER-derived double membrane (phagophore), which matures into an autophagic vacuole. The formation of this autophagosome is initiated by cytosolic proteins that sense nutrient deprivation ( Fig. 1.14 ). The vacuole fuses with lysosomes to form an autophagolysosome, and lysosomal enzymes digest the cellular components. In some circumstances, autophagy may be associated with atrophy of tissues (discussed later) and represent an adaptation that helps cells survive lean times. If, however, the starved cell can no longer cope by devouring its contents, autophagy may also signal cell death by apoptosis.
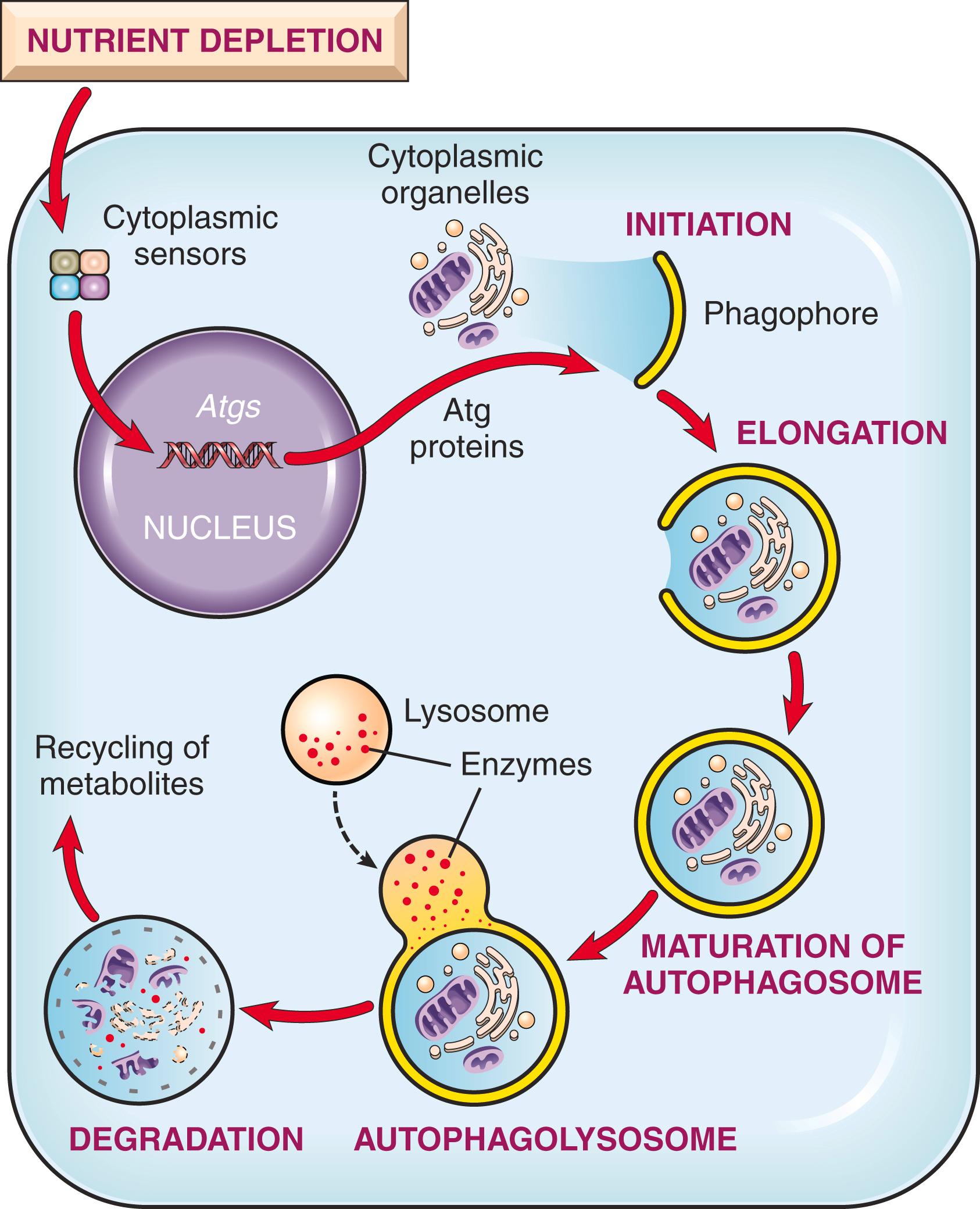
Extensive autophagy is seen in ischemic injury and some types of myopathies. Autophagic vacuoles may also form around microbes in infected cells, leading to destruction of these infectious pathogens. Cancer cells acquire the ability to survive even in times of stress without autophagy ( Chapter 6 ). Thus, a once little-appreciated survival pathway in cells may prove to have wide-ranging roles in human disease.
There are numerous and diverse extrinsic causes of cell injury and cell death, so it is not surprising that there are many intrinsic biochemical pathways that can initiate the sequence of events that lead to cell injury and culminate in cell death. Before discussing individual pathways of cell injury and their mechanisms, some general principles should be emphasized.
The cellular response to injurious stimuli depends on the type of injury and its duration and severity. Thus, low doses of toxins or a brief duration of ischemia may lead to reversible cell injury, whereas larger toxin doses or longer ischemia times may result in irreversible injury and necrosis.
The consequences of an injurious stimulus also depend on the type of cell and its metabolic state, adaptability, and genetic makeup. For instance, skeletal muscle in the leg can survive complete ischemia for 2 to 3 hours, whereas more metabolically active cardiac muscle dies after only 20 to 30 minutes. Genetically determined diversity in metabolic pathways can contribute to differences in responses to injurious stimuli. For instance, when exposed to the same dose of a toxin, individuals who inherit variants in genes encoding cytochrome P-450 may catabolize the toxin at different rates, leading to different outcomes.
Cell injury usually results from functional and biochemical abnormalities in one or more essential cellular components ( Fig. 1.15 ). Deprivation of oxygen and nutrients (as in hypoxia and ischemia) primarily impairs energy-dependent cellular functions, while damage to proteins and DNA triggers apoptosis. Because any one injurious insult may trigger multiple, overlapping biochemical pathways, it has proved difficult to prevent cell injury from any cause by targeting an individual pathway.
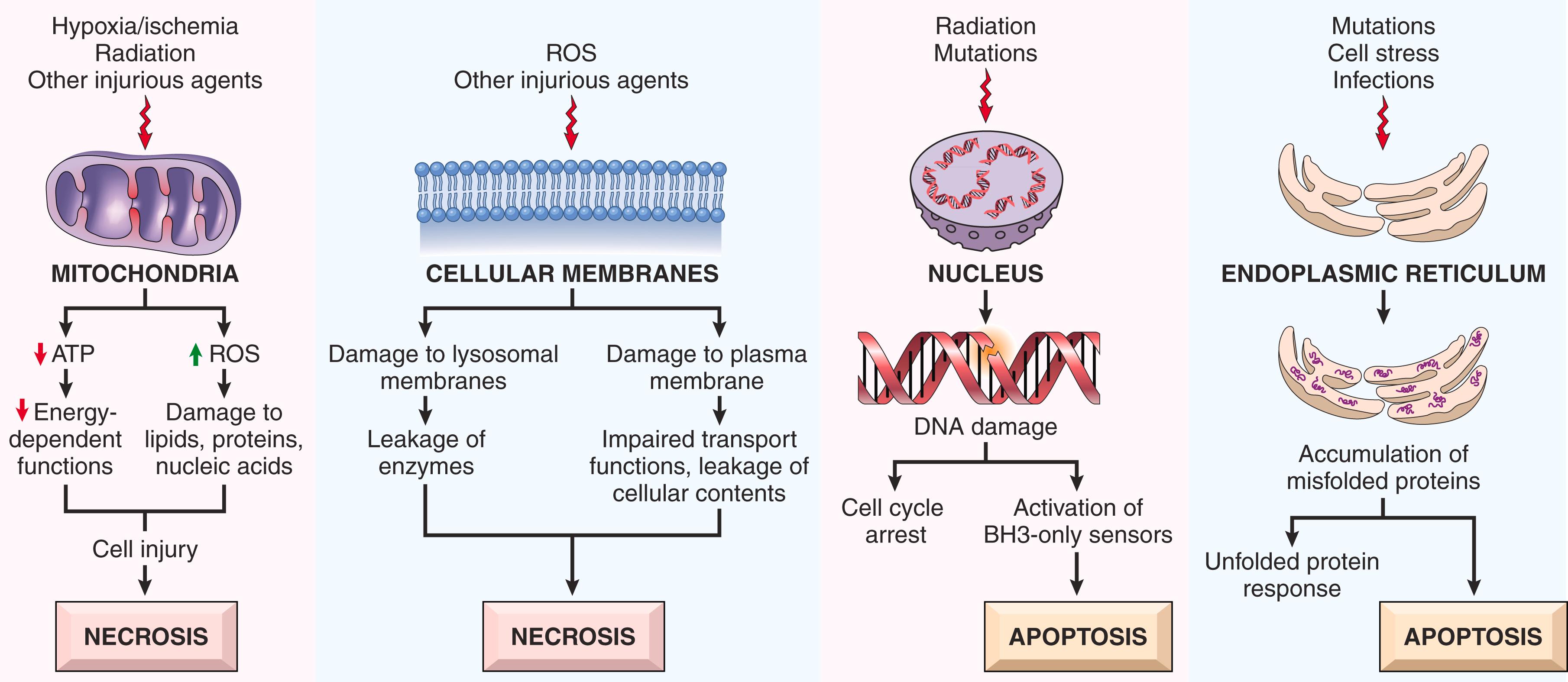
In the following sections, we discuss the mechanisms that lead to cell injury and death. While each of the mechanisms tends to cause cell death predominantly by necrosis or apoptosis, the two pathways may intersect. For instance, ischemia and the production of free radicals are typically associated with necrotic cell death, but they can also trigger apoptosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here