Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Carpal injuries represent 3% to 9% of all sports-related ailments, with carpal fractures comprising 8% to 19% of hand injuries. Carpal fractures, ligament injury, and instability can occur as a result of acute injury or overuse. Contact sports, such as football, have the highest incidence of carpal injury. These injuries are also frequently seen in gymnasts, mixed martial artists, and boxers, although any participant in sport will be at risk. These injuries may be career-threatening for the high-level athlete; therefore timely diagnosis is critical. One major challenge in management is to balance the desire to allow early return to play (RTP) where appropriate, while minimizing the risk of re-injury, future pain, and disability that may result when injuries are treated too aggressively. Treatment is tailored to the individual, accounting for injury severity, concomitant injury, demands of the sport, and career goals. Open communication among the athlete, family, coaches, trainers, and the physician is paramount. This chapter reviews carpal anatomy and kinematics, patterns of injury, and management options with the specific needs of the athlete in mind.
The wrist is a diarthrodial joint composed of eight carpal bones arranged in two rows. The geometry of the carpal bones and their attachments allows motion in two primary planes: flexion-extension and radial-ulnar deviation. The flexion-extension arc is derived from roughly equal motion through both the radiocarpal and the midcarpal articulations. Radial-ulnar deviation is similarly distributed, with the radiocarpal joint contributing 40% and the midcarpal joint contributing 60%. Meanwhile, axial load bearing at the wrist occurs 80% through the radiocarpal joint and 20% through the ulnocarpal articulation.
Several theories have been proposed to explain carpal kinematics and function. Navarro proposed that the carpus was arranged in three rigid, vertical columns. In 1984, Weber introduced the longitudinal column theory. The column theories provided a model to account for axial load transmission from hand to forearm but did not account for the coupled motion between the proximal and distal rows and the small but significant independent motion present between the carpal bones.
The row theory was introduced in the 1980s and suggested that the proximal and distal rows behave as separate functional units. The interosseous ligaments and the articular geometry allow the carpal bones to move in unison during wrist motion. Linscheid and Dobyns noted that as the wrist deviates radially, the scaphoid and proximal row flex. They believed that scaphoid flexion occurs in response to the pressure exerted by the trapezium and trapezoid. Weber postulated that it is the helicoid geometry of the triquetrohamate articulation that forces the distal row to translate palmarly during radial deviation, creating a flexion moment on the proximal row. Conversely, during ulnar deviation, the distal row is forced to translate dorsally, creating an extension moment on the proximal row.
Different theories on kinematics have provided a greater understanding of the coordinated motion between rows and the tendencies for abnormal motion when the system breaks down. Given that no one concept adequately describes the complexity of carpal motion, most investigators agree that carpal kinematics can best be explained by a combination of these theories. It is evident that in the intact wrist, a complex synchrony exists between the carpal bones and between the carpal rows.
Intrinsic and extrinsic ligaments of the wrist link and stabilize the carpal bones. Intrinsic ligaments, also known as interosseous ligaments, connect carpal bones to one another. Extrinsic ligaments connect the carpal bones to the radius and ulna and tend to be stronger than their intrinsic counterparts. With the exception of the pisiform, tendons do not directly insert on the carpal bones but help to stabilize their motion when actively contracting. Wrist motion is initiated by the distal carpal row and metacarpals, which move as a unit. Radioulnar motion occurs primarily through the midcarpal joint. Radial deviation of the wrist causes the distal row to incline radially, extend, and supinate while the proximal row remains flexed with minimal translation. Conversely, during ulnar deviation, the distal row inclines ulnarly, flexes, and pronates while the proximal row extends with minimal translation.
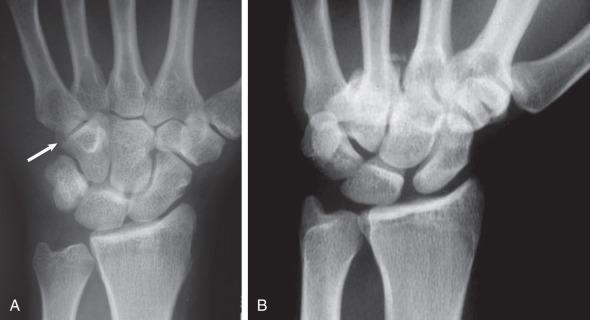
The scaphoid is the most commonly injured carpal bone, accounting for nearly 70% of carpal bone fractures. A scaphoid fracture is typically seen after a fall onto an outstretched, pronated, and ulnar-deviated hand but can also be seen after a direct blow or as an occult injury. Patterns of injury include waist (the most common), distal pole, or proximal pole fractures. Any athlete presenting with radial-sided wrist pain should be considered to have a scaphoid fracture until proven otherwise.
Scaphoid fractures classically present with anatomic snuffbox swelling and tenderness. Palpation over the volar scaphoid tubercle and scaphoid compression with axial load of the thumb metacarpal may also elicit pain. Duckworth et al. found that the combination of snuffbox tenderness and pain with ulnar wrist deviation was 91% specific for scaphoid fracture in males with sports-related injuries.
Radiographs, including posteroanterior (PA), lateral and oblique views, should be obtained. Additionally, a scaphoid-specific view taken as a PA at 45 degrees of pronation and ulnar deviation should be taken. False-negative results on initial plain radiographs have been estimated to be as high as 25%. Additional studies early in an acutely injured athlete can help define the injury and can be cost-effective. Magnetic resonance imaging (MRI) is the gold standard for diagnosing occult scaphoid fractures. Computed tomography (CT) scans can be helpful in providing detail of the fracture and, when obtained, should be done in 1-mm cuts in the longitudinal axis of the scaphoid.
Fractures of the distal pole and nondisplaced fractures of the waist are considered stable and demonstrate a high rate of healing with nonoperative treatment. Non-skill position players (e.g., a football player who does not have to handle the ball) can be treated with a padded short arm thumb spica cast for most nondisplaced fractures, with the caveat that this may increase the risk of nonunion. Athletes who require wrist motion to perform (e.g., a quarterback, running back, or wide receiver in football) may be able to RTP earlier with early internal fixation.
Proximal pole fractures require a longer time to union and have higher rates of nonunion due to interruption of the integrity of the dorsal vascular supply and retrograde intraosseous blood supply. Therefore early fixation may be recommended for nondisplaced proximal pole fractures. Ultimately the weakness and stiffness that result from extended immobilization are significant setbacks for the athlete; therefore early surgical intervention for scaphoid fractures in the athlete should be considered.
Nonoperative treatment is carried out with cast immobilization for 6 to 12 weeks. Whether to use a long arm, short arm, or thumb spica cast has been debated. However, it is reasonable to consider a long arm cast in any patient with concern for noncompliance. Surgical techniques include percutaneous, open, or arthroscopic-assisted approaches to fixation with a headless compression screw.
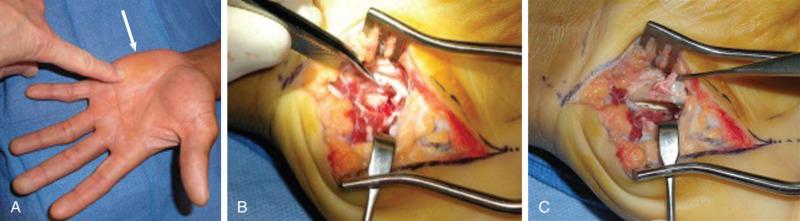
For nondisplaced scaphoid fractures, we prefer a minimally invasive approach with a cannulated, headless compression screw. This approach has the advantage of minimizing soft tissue disruption and damage to the blood supply, which may optimize conditions for achieving union and maximizing range of motion (ROM). For displaced fractures, however, anatomic reduction requires direct visualization through an open approach. For proximal pole fractures, this is achieved through a dorsal approach. ( Fig. 70.1 ). Distal pole fractures and any humpback deformities are addressed through a volar approach, which affords superior visualization. Waist fractures may be addressed from either approach.
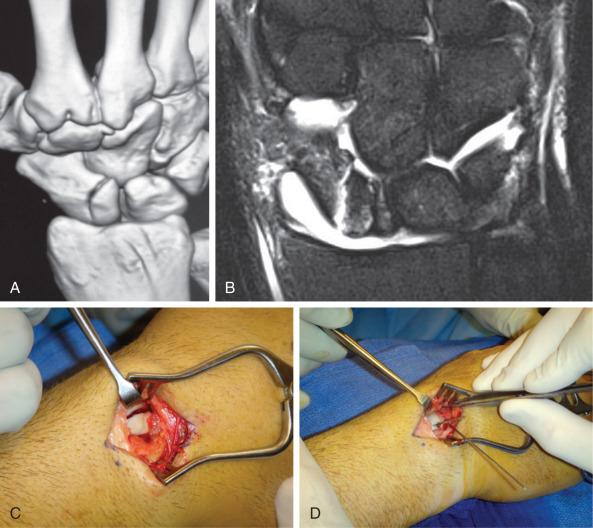
Patients are placed in a thumb spica splint following surgery and transitioned to a short arm cast at their 2-week clinic follow up. A playing cast can be considered for noncontact athletes at this juncture. For contact athletes, the cast is removed at 6 weeks postoperatively if union is achieved. For proximal pole fractures or where there is a concern for incomplete union, the cast may be continued for up to 12 weeks postoperatively. If early RTP is critical, CT scans should be obtained at 6-week intervals to assess for union. Once the fracture has achieved at least 50% bony bridging and the patient is free of pain with at least 80% of preoperative grip strength and good motion, RTP is considered. These recommendations are strictly guidelines and the most appropriate postoperative plan should be individualized to the athlete.
Surgical fixation of scaphoid fractures achieves excellent rates of union with a shorter period of immobilization and faster gains in strength and motion compared with nonoperative treatment in a cast. Whether percutaneous techniques in particular allow for shorter time to union compared with nonoperative treatment remains an area of debate. However, it is clear that surgical treatment is associated with an increased risk of complications.
Complications of scaphoid fractures include avascular necrosis (up to 40%), malunion (up to 50% of displaced fractures), nonunion (up to 5% of nondisplaced and 55% of displaced fractures), carpal instability and posttraumatic arthritis or development of scaphoid nonunion with advanced collapse. Complications of surgical treatment include displacement of nondisplaced fractures, injury to the radial artery, and over- or underdrilling, which can lead to poor screw purchase or distraction across the fracture site, respectively.
The triquetrum is the second most commonly injured carpal bone, representing up to 18% of all carpal fractures. Fracture patterns include dorsal cortical avulsion fractures, fractures through the body, and volar cortical fractures. Dorsal cortical avulsion fractures are most prevalent, accounting for 93% of all triquetral fractures. The mechanism of injury for dorsal cortical fractures primarily involves either palmar flexion and radial deviation or dorsiflexion and ulnar deviation of the wrist. During wrist hyperflexion, the dorsal extrinsic ligaments (which attach to the triquetrum) are placed under stress and lead to an avulsion of the dorsal triquetrum. During wrist dorsiflexion and ulnar deviation, it is surmised that the ulnar styloid drives into the triquetrum, leading to a “dorsal chip.” Triquetral body fractures occur with the wrist in extension and ulnar deviation. Body fractures typically follow high-energy mechanisms, and the clinician should suspect related injuries. Perilunate dislocations are present in 12% to 25% of body fractures. Finally, volar cortical injuries are rare and can represent a lunotriquetral ligament avulsion.
Triquetral fractures present with ulnar-sided wrist pain. Associated soft tissue swelling may represent ligamentous injury. Considering the likelihood of concomitant injuries and the close proximity to other ulnar structures such as the triangular fibrocartilage complex, eliciting point tenderness over the triquetrum may be challenging. Direct tenderness and pain with wrist motion are often present with dorsal avulsion injuries.
Radiographs—including PA, lateral, and oblique views—are typically sufficient for the diagnosis of a triquetral fracture. A CT scan can provide additional detail of the fracture pattern. An MRI can be ordered in instances in which there is significant soft tissue swelling or recovery does not progress and a concurrent scapholunate or lunotriquetral tear is suspected.
Fractures of the dorsal cortex and nondisplaced triquetral body fractures are generally considered stable and amenable to nonoperative treatment. Significant soft tissue swelling should raise suspicion for ligamentous injury and requires a longer period of immobilization. Athletes should be counseled regarding fracture fibrous nonunion, a common result with little potential consequence. Surgical treatment is reserved for symptomatic nonunion. Though rare, displaced fractures of the body, as well as body or volar cortical fractures with associated ligamentous injury, are inherently unstable and should be treated surgically.
Triquetral fractures of the dorsal cortex and nondisplaced body fractures can be treated with a brace or a short arm cast for 3 to 6 weeks or longer depending on the condition of the soft tissues ( Fig. 70.2 ). In the event of fibrous nonunion that is symptomatic, surgical excision of the fragment with reattachment of the extrinsic ligaments to the remaining triquetrum may be performed.
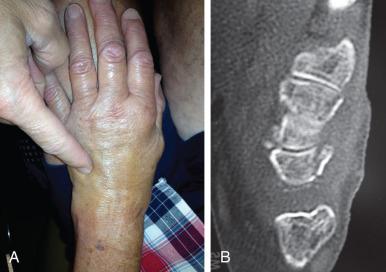
Displaced body fractures may be treated with open reduction and surgical fixation. This can be done using a headless compression screw through a dorsal approach along the extensor carpi ulnaris (ECU) tendon. Treatment of body or volar cortical fractures with associated ligamentous injury focuses on surgical stabilization of the surrounding intercarpal articulations. In cases of symptomatic malunion, nonunion, or pisotriquetral arthritis, excision of the pisiform may provide adequate symptom relief.
For acute fractures of the dorsal cortex with mild to moderate swelling, a cast or splint is applied for 2 to 4 weeks and the athlete is reexamined at weekly intervals until he or she is asymptomatic and ready for unrestricted play. When a triquetral body fracture is associated with a perilunate dislocation, it is our practice to pin the lunotriquetral joint along with the scapholunate joint and midcarpal joints and bypassing fixation of the triquetral fracture.
Postoperatively, triquetral injuries should be immobilized and any Kirschner wires (K-wires) should be left in place for a minimum of 4 weeks.
Most fractures of the triquetral dorsal cortex go on to fibrous nonunion that is asymptomatic. Unstable patterns have more variable outcomes and do best when identified and treated promptly.
The most common complication following fracture of the triquetrum is a delay in diagnosis, which can result in chronic instability due to an untreated associated triangular fibrocartilage complex, perilunate or lunotriquetral ligament injury with associated pain, stiffness, and pisotriquetral arthritis.
Four to 5% of carpal fractures involve the trapezium. Fractures can involve the body or the volar ridge at the attachment of the transverse carpal ligament. Body fractures are most common and are divided into sagittal split, horizontal split, transarticular, dorsoradial tuberosity, and comminuted subtypes. Volar trapezial ridge fractures are subdivided into base (type I) and tip avulsion (type II) fractures.
The mechanism of injury involves an axial load on a dorsiflexed wrist, whereby the thumb metacarpal is driven into the trapezium. Other described mechanisms include a load onto the radially deviated wrist with the thumb abducted and hyperextended, whereby the radial styloid is driven into the trapezium. Most injuries occur as a result of high-energy motor vehicle collisions or falls onto an outstretched hand.
Physical examination reveals point tenderness distal to the scaphoid tubercle at the volar base of the thumb. Pain or weakness can occur with pinch-related activities and may worsen with wrist flexion because of the proximity of the flexor carpi radialis (FCR) tendon to the trapezial ridge ( Fig. 70.3 ). The clinician must evaluate for commonly associated injuries to the distal radius, scaphoid, hamate, and first metacarpal.
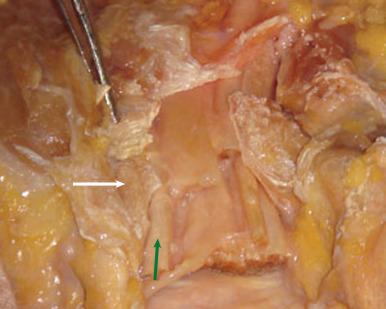
Radiographs—including PA, lateral and oblique views—should be obtained and will typically demonstrate a trapezium fracture when present ( Fig. 70.4 ). Bett's view is an anteroposterior view of the wrist in pronation that allows for better visualization of the trapeziometacarpal articulation. The carpal canal views better depict the volar trapezial ridge. CT is also useful to delineate fracture details and identify concurrent fractures of the neighboring bones.
Nonoperative treatment is reserved for nondisplaced trapezial fractures. Displaced fractures of the volar trapezial ridge are at risk of nonunion due to the pull of the transverse carpal ligament. Trapezial body fractures with displacement greater than 2 mm or carpometacarpal (CMC) subluxation require reduction and surgical fixation.
Nonoperative treatment consists of immobilization in a thumb spica cast for 4 to 6 weeks. Trapezial ridge fractures resulting in symptomatic nonunion can be treated with excision of the fracture fragment ( Fig. 70.5 ). For displaced fractures of the body, open reduction and internal fixation with K-wires or screws may be performed ( Fig. 70.6 ). Comminuted fractures may be amenable to external fixation or oblique dynamic traction splinting for early motion.
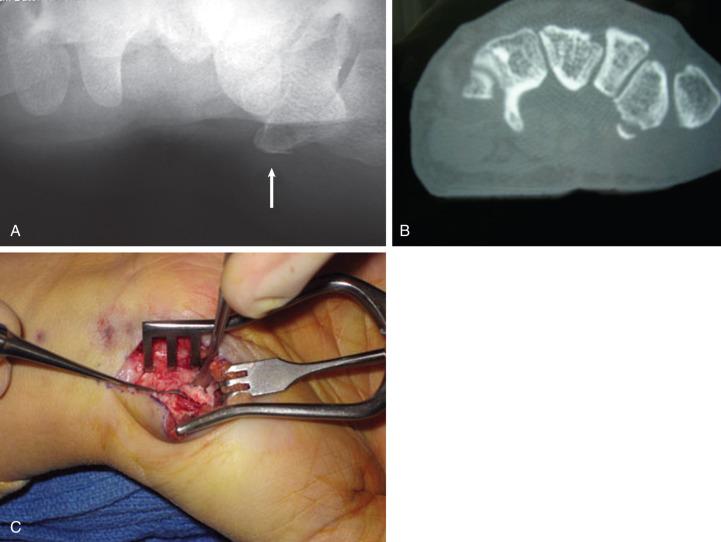
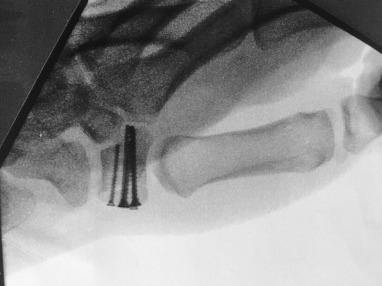
Postoperatively, trapezial fractures should be immobilized and any K-wires should be left in place for a minimum of 4 weeks.
When operative treatment is chosen, we prefer the volar Wagner approach to the trapezium. The skin incision follows the thenar eminence at the junction of the glabrous skin of the palm and the dorsal skin of the wrist and curves in a volar direction. Dissection follows beneath the thenar musculature. Careful attention should be directed to protecting the branches of the radial sensory nerve and the radial artery ( Fig. 70.7 ). Reduction under direct visualization is achieved and fixation established with the use of K-wires or minifragment screws.
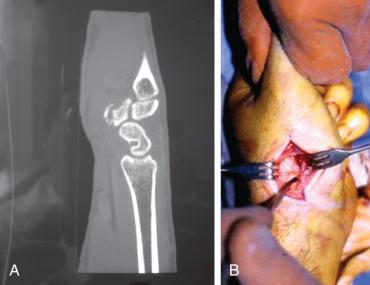
Outcomes studies in the literature are limited to small series of patients. Overall, cast immobilization alone has demonstrated poor outcomes for displaced or comminuted fractures due to the resultant incongruence of the CMC articulation. Surgical treatment with restoration of the articular surface yields good functional outcomes.
Complications of trapezial fractures include nonunion, FCR tendonitis or rupture, carpal tunnel syndrome, stiffness of the CMC joint, posttraumatic arthritis, and loss of pinch strength.
Hamate fractures represent close to 2% of all carpal fractures. The hamate lies in close proximity to surrounding neurovascular structures. It forms the radial border of Guyon canal and serves as an attachment site for the transverse carpal ligament at the ulnar border of the carpal tunnel. As a result, hamate injuries may lead to paresthesias and/or motor dysfunction in the ulnar or median nerve distribution ( Fig. 70.8 ).
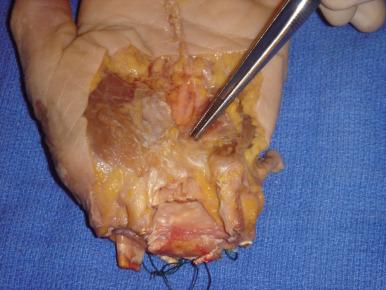
Mechanisms of injury for fractures of the hook of the hamate involve direct compression or shear forces from adjacent tendons during forceful twisting of the wrist. The superficial position of the hook predisposes it to injury from compressive trauma to the palm. The most common activities relating to this injury include baseball and golf, where they affect the nondominant hand, and various racquet-related sports, where the dominant hand is affected. Body fractures of the hamate are rare but may occur as a result of high-energy axial load through the fourth and fifth metacarpals. These fractures are classified as either coronal or transverse. Small finger CMC dislocations may accompany these injuries ( Fig. 70.9 ). Clinical diagnosis can be difficult and these fractures are frequently missed, so a high index of suspicion is needed.
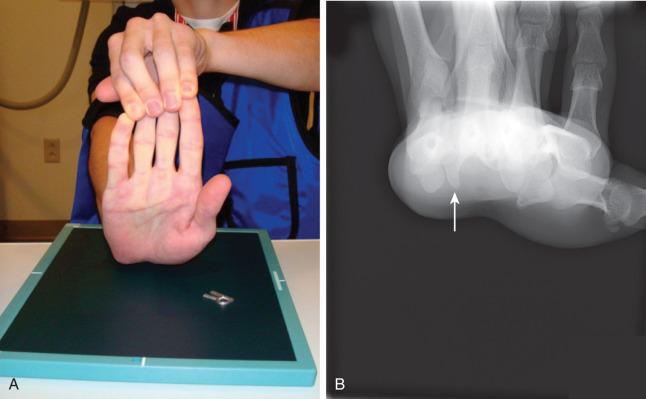
Hamate fractures present as ulnar-sided palm or wrist pain that may be worsened with grasping. Tenderness can often be elicited over the hook. The hook of hamate pull test is performed with resisted flexion of the ring and small fingers and is positive when resultant hamate fracture displacement causes pain. ( Fig. 70.10 ). A thorough motor and sensory examination is important. Irritation of the adjacent ulnar and median nerves may lead to paresthesias. Abrasion of the adjacent flexor tendons against the fractured hook may lead to tendinosis or flexor tendon rupture ( Fig. 70.11 ). Body fractures are often seen with significant swelling and associated CMC joint dislocation.

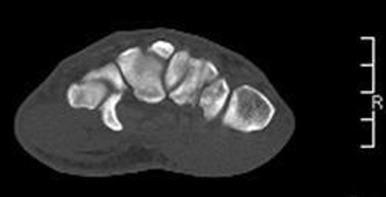
Standard radiographs are often insufficient for detecting hamate fractures. The oblique joint space of the CMC articulation is obliterated in the setting of dislocation. A cortical circular ring, representing the end-on view of the hook of the hamate on PA views, may be missing on injury radiographs. When images demonstrate increased sclerosis around the circular ring, a nondisplaced nonunion should be considered. A CT scan is used to confirm diagnosis, delineate fracture pattern, and distinguish between occult fracture versus an anatomic variant, the os hamuli ( Fig. 70.12 ). Specialized radiographic views can assist with diagnosis and include (1) the carpal tunnel view ( Fig. 70.13 ), (2) the supinated oblique view with the wrist in dorsiflexion, and (3) the lateral view through the first web with the thumb abducted.
Nondisplaced hamate fractures are considered stable, with good healing potential if treated within 1 week of injury. They do, however, require prolonged immobilization (up to 11 weeks). In the athlete, this lengthy period of immobilization and the risk of associated flexor tendon rupture make nonoperative treatment less practical. Furthermore, the tenuous blood supply to the hook of the hamate leads to a high risk of nonunion or avascular necrosis following distal hook fractures. In general, early surgical treatment of all hamate fractures may allow for faster RTP and should be strongly considered.
Nonoperative treatment consists of early cast immobilization with the wrist in slight flexion and the fourth and fifth metacarpophalangeal joints in maximum flexion to minimize shear forces on the hook from the flexor tendons. Thumb immobilization may help to minimize displacing forces from the pull of the transverse carpal ligament. Surgical treatment is most commonly carried out with excision of the fracture fragment via a volar or lateral approach. However, open reduction and internal fixation with either K-wires or screws has also been described.
We prefer hook of hamate excision for both acute fractures and nonunion. When approaching the hook of the hamate, particular attention should be directed to the proximity of the deep motor branch of the ulnar nerve. An S -shaped incision is centered over the hook. We avoid crossing the wrist flexion creases to minimize scar sensitivity and facilitate RTP. The ulnar nerve and artery are dissected proximal to distal toward the hook. The ulnar motor branch, which exits dorsal and ulnar to the ulnar nerve proper, dives deep as it travels in a radial direction toward and around the ulnar border of the hamate hook. Once mobilized and retracted, the hook can be exposed; the periosteum is elevated, and the hook is uncovered ( Fig. 70.14 ). Removal of the entire hook is preferred to avoid irritating the adjacent flexor tendons with residual exposed bone.
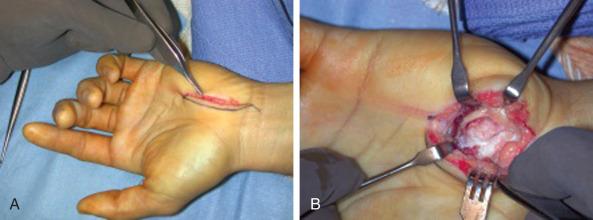
Postoperatively the wrist should be splinted for 3 to 5 days. The wound is checked, and if it has sealed, a soft dressing is applied and the athlete is allowed to perform wrist motion and aerobic workouts. Return to competition is sport-specific. At 2 to 3 weeks, baseball, tennis, and squash players are permitted “dry swings” with a padded glove; golfers are allowed to putt and chip. Contact off a tee in baseball, light volleying in racquet sports, and half swings off a mat in golf may be initiated at 3 weeks, with gradual progression to normal play at 4 to 6 weeks. Athletes can expect scar sensitivity that will improve over time but should be counseled that complete relief may not occur for up to 4 to 6 months after surgery.
Although cadaver studies have demonstrated a loss of grip strength following hamate excision, clinical studies have shown no impact on functional performance in high-level athletes. The literature on outcomes following open reduction with internal fixation is limited to small series and suggests that there may be high rates of nonunion.
Complications of hamate fractures include rupture of either the small or ring finger flexor tendons, which can occur in up to 14% of injuries. Osteonecrosis and nonunion of the hamate body or hook have also been reported. After surgery, the patient should be aware that there is a 3% risk of complication, with nerve injury being the most common untoward event.
Capitate fractures represent 1% to 2% of carpal fractures. They are divided into four types, including transverse pole, transverse body, coronal, and parasagittal. Transverse body fractures are most common.
The capitate can fracture with axial loads directed on the third metacarpal with the wrist in flexion. Other proposed mechanisms include hyperextension with the wrist in ulnar deviation, resulting in dorsal impaction of the distal radius onto the capitate waist. Isolated injuries are rare and more commonly occur in association with greater arc injuries where the force is transmitted through a combination of ligament and bone, including the scaphoid. Wrist hyperextension and radial deviation can lead to transscaphoid, transcapitate, and perilunate fracture dislocations. Scaphocapitate fracture syndrome is a high-energy perilunate injury with dissipation of energy through the scaphoid waist and neck of the capitate, resulting in the 180-degree rotation of the proximal fragment.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here