Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
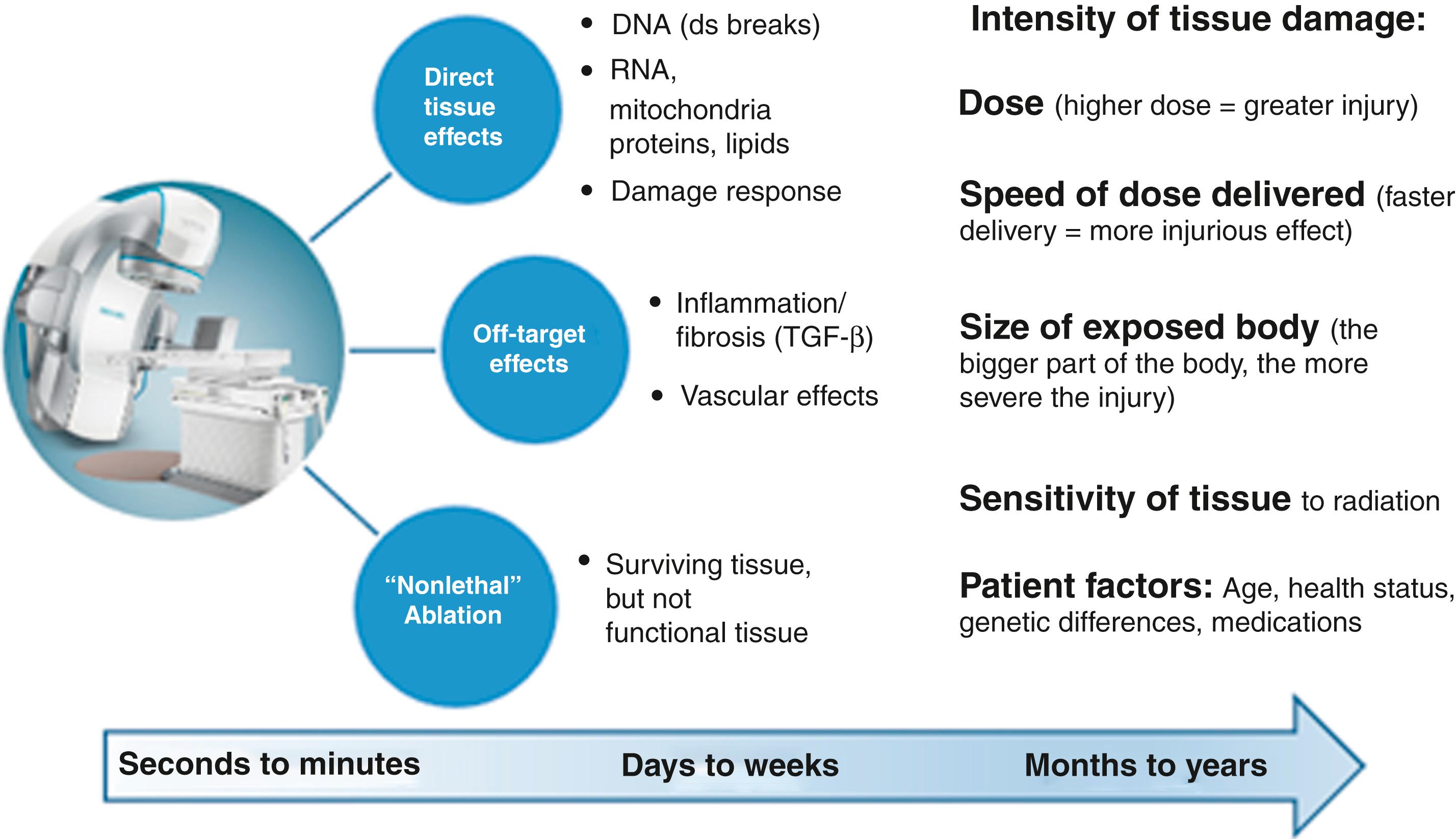
Cardiac ablation using thermal energy is a mainstay of interventional electrophysiology (EP) today. Extremes of heat or cooling can be delivered through the tip of an invasive catheter, causing largely irreversible local tissue destruction. During this process, the thermal energy is first generated at the surface of the heart, either directly with cryoablation or using resistive heating with radiofrequency (RF) ablation. Beyond the first 1 mm of tissue depth, the thermal changes are transferred passively to deeper tissue, using conductive heating, with a goal of achieving tissue ablative effects at depth.
For some tissues, such as accessory pathways and the atrioventricular (AV) node, a shallow ablation is sufficient for clinical success. Other tissues, including most of the left ventricular (LV) myocardium, are considerably thicker than the depth of heat transfer afforded by catheter thermal ablation. Over the past 20 years advances in catheter design, tip size, tip cooling, liquid irrigation salinity, and grounding strategies have allowed incrementally deeper tissue ablation; however, deeper delivery of heat often translates to an increased opportunity for tissue steam pops and risk for severe clinical complications. Nevertheless, even with the most aggressive techniques, the deepest thermal tissue ablation often fails to reach a full-thickness ablative result. Furthermore, most ventricular tachycardias (VTs) caused by structural heart disease occur in areas that contain scar, where the increased fibrillar collagen and adipose tissue and the altered electrical resistivity reduce RF ablation heating. Additionally, both collagen and adipose act as thermal insulators, limiting the thermal conduction to deeper myocardium. As a result, RF catheter ablation in myocardial scar yields much smaller ablation lesions than expected compared with normal myocardial tissue. Inability to achieve deep heating is a common cause of catheter-based VT ablation failure.
To overcome the biophysical limits of heat transfer that plague thermal ablation energies in thicker and scarred heart tissues, lessons can be learned from treating tumors in the body with radiation. External beam therapy, also called external radiation therapy , is a method for delivering a beam or several beams of high-energy x-rays to a patient’s tumor. Beams are generated outside the patient (usually with a linear accelerator) and are targeted at the tumor site.
Stereotactic ablative radiotherapy (SABR) is a type of external beam therapy that uses special equipment to position a patient and precisely deliver radiation to tumors in the body. This type of radiation therapy, also known as stereotactic body radiation therapy (SBRT), delivers thousands of beams of x-ray radiation that enter the body from many angles, allowing for the precise delivery of high doses of radiation to targets in the body with minimal exposure of the normal adjacent tissue ( Fig. 133.1 ). Such treatments have been successfully used to treat malignant and benign tumors at all body sites, most commonly in the brain, lung, liver, and spine. Advances in image guidance and motion management have been central to the success of SABR, particularly for mobile tumors. SABR capabilities are now widespread across academic and nonacademic centers, with a rapid increase in utilization over the past 10 years.
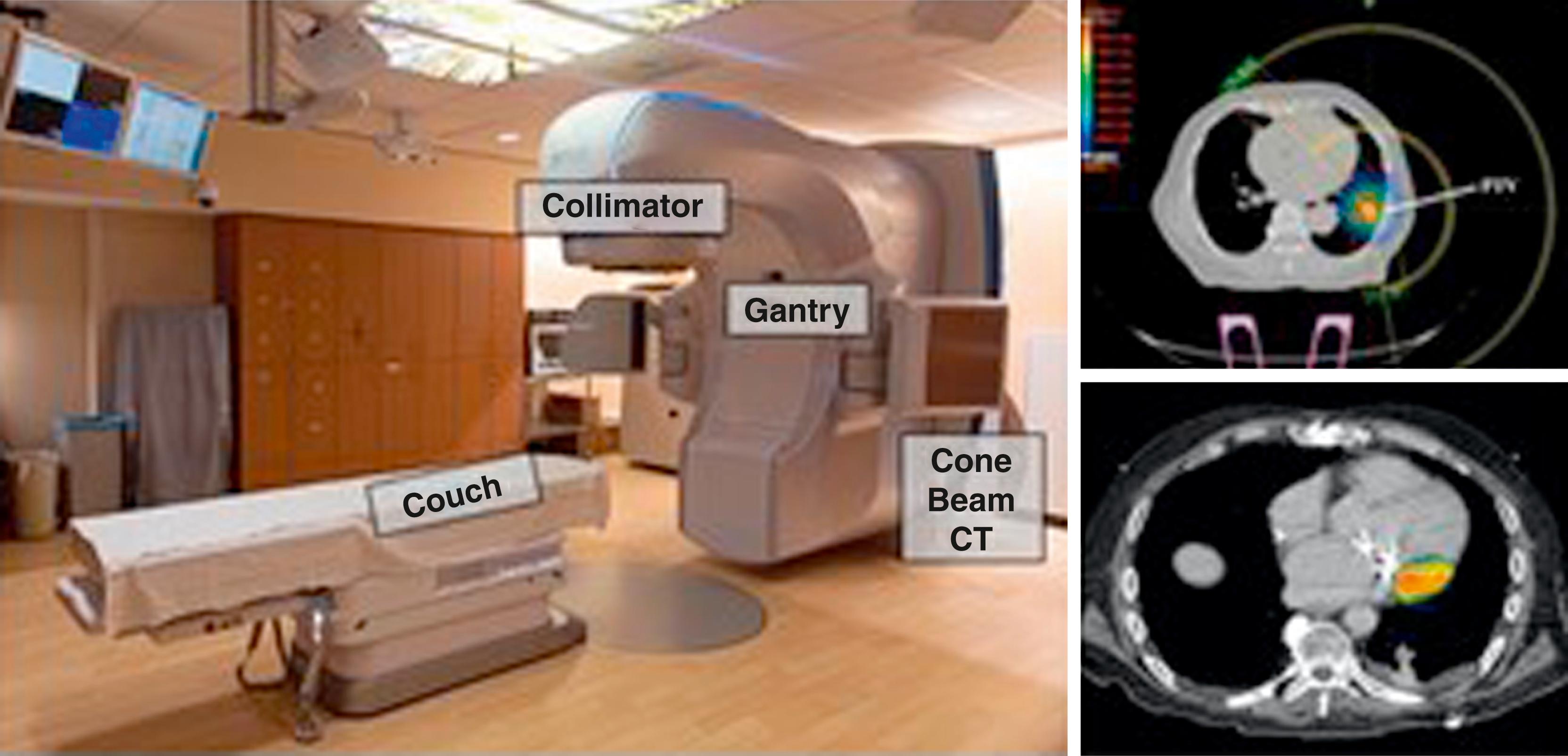
A modern linear accelerator (LINAC) consists of a couch, a gantry, and a collimator. The patient lies on the couch, and the gantry uses high-frequency electromagnetic waves to accelerate charged particles (electrons) to high energies through a linear tube. The x-rays that are produced from the high-energy electrons are filtered and sculpted as they exit the collimator at the head of the gantry.
In short, the SABR process can be divided into (1) proper selection of patients, (2) immobilization, (3) respiratory motion control, (4) computed tomography (CT) simulation, (5) delineation of target volume and organs at risk (OARs), (6) treatment planning, (7) pretreatment verification, and (8) treatment delivery. This process, which is not familiar to most heart rhythm specialists, is outlined in Figs. 133.2 and 133.3 and described in more detail here.
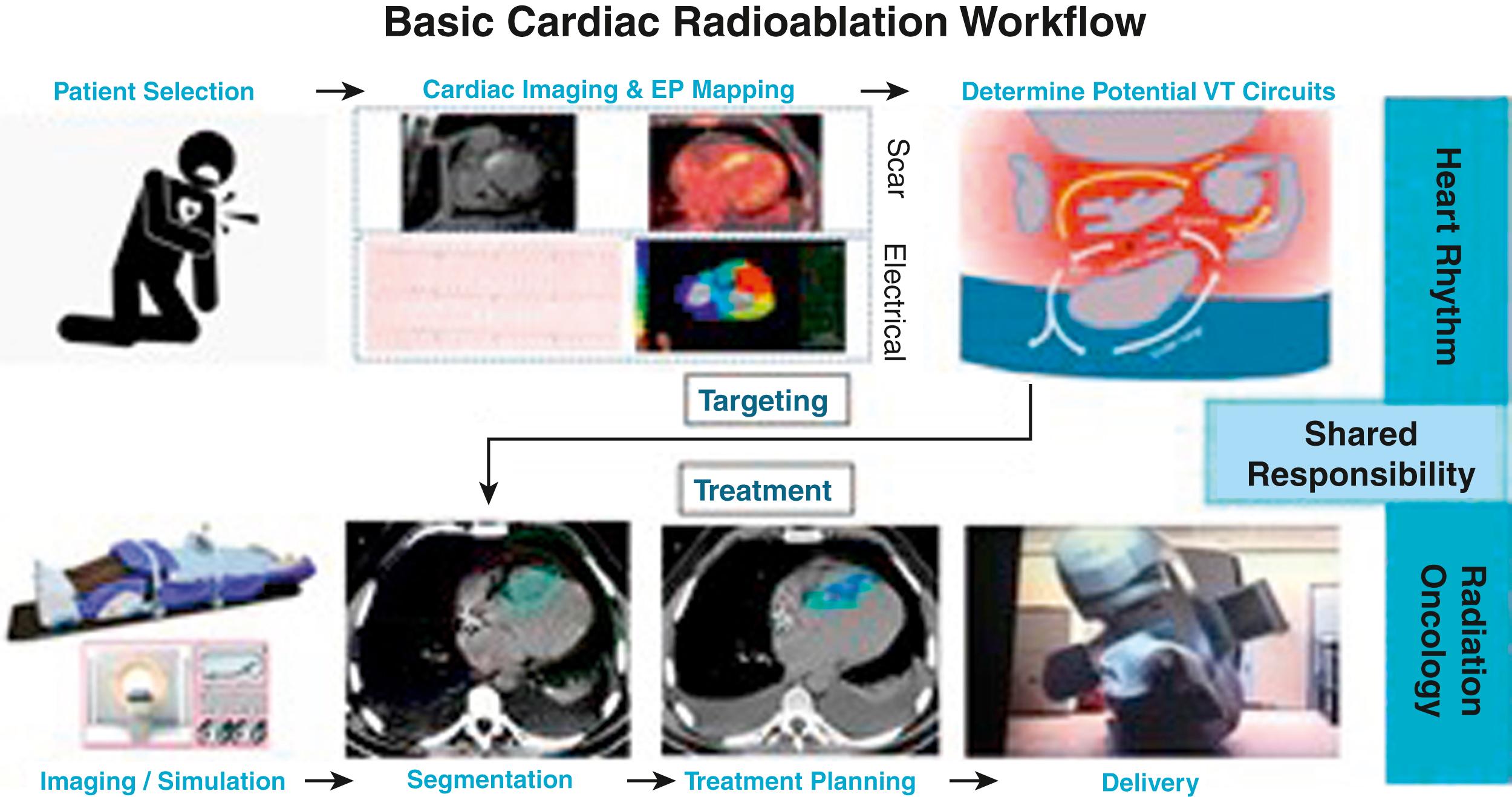
First, patient selection is paramount. Because heart failure is common among VT patients, it is important to ensure the cardiac SABR patient can comfortably lie flat on the couch for the duration of the treatment. Immobilization of the abdomen can be achieved with either rigid external devices, a vacuum cushion, or a body cradle, or by active motion detection and compensation. Respiratory motion control generally occurs with motion dampening and the entire motion envelop of the cardiac target throughout respiratory and cardiac phases is treated (internal target volume [ITV] method). Alternative ways to account for respiratory motion include gating and tracking, but both will result in longer treatment times, which can be difficult for patients with heart failure. A CT simulation scan is performed (free breathing, nongated); contrast injection can greatly facilitate delineation of myocardial contours and vascular OARs. The heart rhythm specialist and radiation oncologist then come together to identify the location on the planning CT scan where the ablative dose of radiation is to be delivered. Once the target is created (cardiac target volume [CTV]), the radiation team can further expand the target to account for respiratory and cardiac motion (ITV) and patient setup uncertainties (planning target volume [PTV]). The treatment is carefully planned, keeping in mind the nearby OARs and dose constraints determined by unique tissue radiosensitivities. When the patient returns on the day of treatment, they are realigned into the exact same spot on the treatment table. The accuracy of this process is confirmed with body surface markers and on-board imaging (cone-beam CT scan). Finally, the prescribed dose is delivered to the prescribed volume as carefully planned.
There are several advantages to using SABR to deliver ablative energy into myocardial scar. First is its well-established record of effectiveness and accuracy. SABR uses image guidance technologies to ablate tumors, and cardiac scar, with millimeter-scale accuracy. As a result, for most cancer indications, local tumor control rates of 90% can be achieved. The ability to both spare healthy tissue and intensify the radiation dose at a myocardial scar is a primary advantage of SABR.
The second advantage is that SABR allows for the flexibility of any three-dimensional treatment volume. VT circuits and myocardial scar exist throughout the full thickness of heart tissue (endocardium, midmyocardium, epicardium), often spanning several centimeters in any dimension. SABR uses a target volume that can be sculpted into any shape or size. It is not delivered as a single-point dose, and it is not restricted by anatomy. As such, the full thickness of the scarred myocardium can be identified as a complex volume and successfully treated in a single session.
Lastly, and most important for patients, unlike catheter-based procedures, cardiac SABR is entirely noninvasive and comfortable for the patient. Treatment is performed while the patient is awake and breathing freely. There is no pain. When the clinical condition allows, the vast majority of cardiac SABR treatments are done as short outpatient visits of 30 minutes to 1 hour, requiring no hospitalization. This process results in an acute procedure that is substantially less risky than invasive catheter ablation procedures. In particular, the importance of the short-term safety is amplified in patients with advanced heart failure, for whom invasive procedures are associated with a 5% risk of death in the first 31 days.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here